Abstract
Cardiac mast cells store and release a variety of biologically active mediators, several of which have been implicated in the activation of matrix metalloproteinases in the volume-overloaded heart, while others are involved in the fibrotic process in pressure-overloaded hearts. Increased numbers of mast cells have been reported in explanted human hearts with dilated cardiomyopathy and in animal models of experimentally induced hypertension, myocardial infarction, and chronic cardiac volume overload. Also, there is evolving evidence implicating the cardiac mast cell as having a major role in the adverse remodeling underlying these cardiovascular disorders. Thus, the cardiac mast cell is the focus of this chapter that begins with a historical background, followed by sections on methods for their isolation and characterization, endogenous secretagogues, phenotype, and ability of estrogen to alter their phenotype so as to provide cardioprotection. Finally the role of mast cells in myocardial remodeling secondary to a sustained cardiac volume overload, hypertension, and ischemic injury and future research directions are discussed.
Keywords: Mast cell isolation, Mast cell mediators, Myocardial remodeling, Stem cell factor, Estrogen, Hypertension, Cardiac volume overload, Ischemia–reperfusion, Myocardial infarction, Mast cell secretagogues
1 Introduction
To compensate for a sustained abnormal myocardial stress secondary to injury, disease, or chronic ventricular volume or pressure overload, a progressive, structural remodeling process of the muscular, vascular, and extracellular matrix components of the myocardium is initiated. However, the ability to normalize the elevated stress is limited, and as a result, the ventricle dilates with an inappropriate wall thickness, and eventually the clinical signs and symptoms of heart failure become apparent [1, 2]. Because fibrillar collagen provides a supportive framework which interconnects cardiomyocytes and blood vessels and thereby maintains ventricular size and shape [3, 4], such architectural alterations have to be preceded by a disruption of the collagen network. Co-localized with the interstitial myocardial collagen matrix is a largely latent, matrix metalloproteinase (MMP) system [5], which when activated will cause rapid collagen degradation alterations in the extracellular matrix. Recently cardiac mast cells, which are known to store and release a variety of biologically active mediators including tumor necrosis factor-α (TNF-α) and proteases such as tryptase, chymase, and stromelysin [6–10], have been implicated in the activation of MMPs in the volume-overloaded heart [11]. Mast cells are derived from precursor cells in the bone marrow and locally mature under the influence of the c-Kit ligand, stem cell factor (SCF), with their final phenotype being dependent on the microenvironment in which they reside. Increased numbers of mast cells have been reported in explanted human hearts with dilated cardiomyopathy [12, 13] and in animal models of experimentally induced hypertension [14–16], myocardial infarction [17], and chronic volume overload secondary to aortocaval fistula [11] and mitral regurgitation [18, 19]. Furthermore, there is evolving evidence implicating the cardiac mast cell as having a major role in the adverse remodeling underlying these cardiovascular disorders. Thus, the cardiac mast cell will be the focus of this chapter, which will begin with a historical background, followed by sections on methods for mast cell isolation and characterization, its endogenous secretagogues, its phenotype and the ability of estrogen to alter it, and its role in myocardial remodeling secondary to a sustained cardiac volume overload, hypertension, and ischemic injury.
2 Historical Background
In 1863, Friedrich Daniel von Recklinghausen identified granular cells in the mesentery of the frog [20], which in 1878 Paul Ehrlich named MASTZELLEN or the “well-fed cell” because the cytoplasm of this relatively large cell was stuffed with prominent granules [21]. Surprisingly, articles addressing cardiac mast cells did not appear until 1968. These and several subsequent studies, however, were focused primarily on observations of increased numbers of cardiac mast cells associated with: (1) endomyocardial fibrosis and eosinophilic myocarditis [22, 23], (2) the right ventricle following pulmonary artery banding in rats [16], (3) the subepicardial layer of the infarcted region following experimental myocardial infarction in rats [17], (4) the first week after creation of an infrarenal aortocaval fistula in rats [11], (5) dog hearts 4 months after the onset of experimental mitral regurgitation [18], and (6) explanted hearts from patients with dilated cardiomyopathy [12].
In addition, several articles have been published which addressed the functional role of mast cells in cardiac diseases. In 1986, clear evidence of cardiac mast cell degranulation was correlated with significant interstitial edema in endomyocardial biopsies from two cardiac patients by Ann M. Dvorak [24]. In 1992, Li and his coworkers analyzed serial endomyocardial biopsies from transplanted human hearts and concluded that cardiac mast cells are associated with interstitial and perimyocytic fibrosis [25]. In 1995, Petri T. Kovanen reviewed the accumulating evidence regarding a “cause and effect” role of increased mast cells in atherosclerotic plaque formation and the erosion or rupture of coronary atheromas [26]. In 2002, our laboratory reported a marked, rapid increase in cardiac mast cell density during the first 5 days after creation of an infrarenal aortocaval fistula in rats, which was responsible for MMP activation and subsequent fibrillar collagen degradation [11].
More recently, genetically modified rodent models further demonstrated the adverse functional role of mast cells. For example, in 2002, Hara et al. [27] reported that, in contrast to their wild-type counterpart, heart and lung weights were markedly attenuated, ventricular dilatation was prevented, and fractional shortening was preserved in hypertensive mast cell-deficient mice. Other studies have utilized mast cell-deficient mice to determine the role of mast cells in ischemia–reperfusion injury and myocardial infarction (MI) [28–30]. However, as will be seen below, the data accumulated thus far is somewhat contradictory regarding the role of mast cells in ischemia–reperfusion and MI. In 2007, the mast cell's role in the formation of atherosclerotic plaques was clearly verified using low-density lipoprotein receptor-deficient (Ldlr(−/−)) mast cell-deficient (Kit(W-sh)/(W-sh)) mice [31]. In 2008, we utilized mast cell-deficient rats to demonstrate causality between mast cells and adverse myocardial remodeling. In comparison to the wild-type rat following volume overload, left ventricular dilatation was markedly reduced, MMP-2 activity was not increased, and, thus, collagen degradation was prevented at 5 days and 8 weeks post fistula [32].
From this brief historic overview, it is clear that cardiac mast cell density becomes significantly elevated when subjected to the increased myocardial stress of ischemic injury, cardiomyopathy, and sustained cardiac pressure or volume overload and that an understanding of their role as mediators of ventricular remodeling is beginning to emerge.
3 Cardiac Mast Cell Phenotype, Isolation Techniques, and Endogenous Secretagogues
Two distinct mast cell phenotypes have been identified in the mucosa, skin, and lungs that are classified according to their neutral protease content [8, 33]: the MCT is typically found in mucosal tissue having granules which contain only tryptase, while the MCTC found predominantly in connective tissue contain chymase, cathepsin G, and carboxypeptidase, in addition to tryptase. There are at least three studies that characterize cardiac mast cells as being consistent with the MCTC subtype [34–36]. Mature cardiac mast cells are relatively large and are easily visualized using light microscopy after staining tissue sections with toluidine blue. Cardiac mast cells have also been shown to contain preformed tumor necrosis factor- alpha (TNF-α) [9]. Its role and the roles of other mast cell products including histamine, transforming growth factor-beta (TGF-β), tryptase, and chymase in mast cell-mediated remodeling will be discussed subsequently.
The density of cardiac mast cells in normal hearts is remarkably low across species ranging from 1.4 cells/mm2 in Wistar Kyoto rats [37] to 5.3 cells/mm2 in humans [12]. In chronically stressed or diseased hearts, the cardiac mast cell density has been reported to increase in the range of 1.7- [11] to 6-fold [22]. While cardiac mast cells can be isolated enzymatically, we have shown that enzymatic dispersion methods trigger the spontaneous release of histamine throughout the isolation process without producing harsh perturbations to the plasma membrane sufficient to cause mast cell disruption [36]. As a result, collagenase digestion yields mast cells that are minimally responsive to exogenous secretagogues such as compound 48/80 and calcium ionophore A23187 [38]. This is in contrast to peritoneal or pleural cavity mast cells that remain fully functional when isolated nonenzymatically using injected buffers and mechanical dispersion.
These observations led our group to develop a novel technique for isolating viable epicardial mast cells [39]. In addition to the Morgan et al. article [39], we recently published a detailed video and accompanying text of this procedure [40]. The following is a brief description of this technique using rat hearts. A ventral midline incision is made in the abdomen and extended to the level of the xiphoid cartilage allowing for the dissection of the diaphragm to gain access to the pericardium. The left ventral thoracic wall is then retracted medially to expose the heart still encapsulated by the pericardium. Next, a Teflon® catheter attached to a syringe is inserted into the pericardium at a point in the middle of the sternopericardial ligament (preferably more cranial than caudal). Room temperature Hank's buffer [HBSS composed of (1) Hank's calcium and magnesium free salt solution, (2) HEPES (13 mM), (3) 607 units/ml of deoxyribonuclease, and (4) an antibiotic–anti-mycotic mixture of penicillin G sodium, streptomycin sulfate, and amphotericin B] is then gradually introduced into the pericardial sac, filling it with approximately 3–3.5 ml. At this stage in the isolation, the beating of the heart provides gentle mechanical dispersion. After a short period of time, the HBSS cell solution is gently aspirated from the pericardial sac and stored on ice. This process of buffer injection and aspiration is repeated two to three times. The extracted buffer is then subjected to centrifugation for 10 min at 200 × g (4 °C). After centrifugation the cell pellet is reconstituted in 1 mL of HyClone buffer [HBSS containing magnesium sulfate (1.1 mM), calcium chloride (1.3 mM), and phenol red]. We have characterized the resulting isolate and demonstrated a mixed population of lymphocytes (∼70 %) and macrophages (∼12 %) in addition to mast cells (∼12 %) [41].
By avoiding the enzymatic dispersion of tissue, this technique minimizes spontaneous histamine release attributable to cellular degradation and produces a twofold greater recovery of mast cells from rat hearts (i.e., approximately 110,000 cells) compared to previous observations using enzymatic mast cell isolation [36, 39]. Also, the functional responsiveness of epicardial mast cells is not altered as evidenced by a significant histamine release triggered by concentrations of compound 48/80 as low as 0.3 μg/ml [39]. These findings are in stark contrast to our results obtained using cardiac mast cells isolated by enzymatic methods, in which 10 μg/ ml of compound 48/80 elicited the release of less than 2 % of the histamine from cells [36]. Other studies using enzymatically isolated cardiac mast cells have also reported a negligible response to compound 48/80 (i.e., <2 % histamine release), even after incubating the cells overnight to allow for recovery [35, 38]. Also, in contrast to the existing literature indicating that cardiac mast cells do not respond to substance P [34, 35], a significant release of histamine from these epicardial cells was obtained in response to substance P as well as the calcium ionophore A23187 [39, 42]. Accordingly, the heterogeneity of cardiac mast cells from that of non-cardiac mast cells alluded to in the literature is an artifact of the enzymatic methodology. Nevertheless, the supposition of heterogeneity of connective tissue mast cells is reinforced by our recent study using this new technique which established that cardiac mast cells do not degranulate in response to atrial natriuretic peptide [43] unlike peritoneal mast cells which we and others have shown to be activated by atrial natriuretic peptide [43–45]. While the utility of epicardial mast cell isolates to characterize its response to secretagogues via histamine release is readily apparent, the fact that the extract is a mixed cell population should always be considered when investigating the release of other substances such as TNF-α.
In addition to the neuropeptide, substance P, neurotensin has been shown to be an endogenous cardiac mast cell secretagogue. Neurotensin is found in nerve fibers associated with the coronary vasculature, myocytes, and intracardiac ganglia [46]. While there are only two articles reporting the effect of neurotensin on cardiac mast cells, the findings are rather convincing. One of the studies by Rioux et al. [47] demonstrated that infusion of neurotensin to isolated hearts resulted in a rapid release of histamine, and the other by Pang et al. [48] reported that immobilization stress-induced cardiac mast cell degranulation was prevented by neurotensin receptor blockade.
Recently, endothelin 1 (ET-1) has also been shown to be capable of activating cardiac mast cells. Murray et al. [49] demonstrated that administration of a 20 pg/ml bolus of ET-1 to blood- perfused, isolated rat hearts resulted in cardiac mast cell degranulation, MMP-2 activation, collagen degradation, and moderate ventricular dilatation which was prevented by the mast cell membrane stabilizing compound nedocromil. Nedocromil and cromolyn sodium have been used extensively to study mast cell function. However, they have low oral bioavailability and therefore are typically administered experimentally via an osmotic mini-pump or time release pellets implanted subcutaneously. Another mast cell stabilizing drug that has been used experimentally, ketotifen, can be administered orally.
Evidence is accumulating to indicate that reactive oxygen species can also act as a cardiac mast cell secretagogue. We have found that incubation of isolated rat epicardial mast cells with Na2SO3 induced a concentration-dependent histamine release [50] which could be either prevented or attenuated by the antioxidant compounds ebselen and diphenyleneiodonium, respectively. Further evidence was provided by Masini and colleagues [51]. They reported that the superoxide dismutase mimetic M40403 was able to prevent the occurrence of mast cell degranulation following reperfusion of the ischemic rat heart.
While there are many other known non-cardiac mast cell secretagogues, those discussed above are the few that are known to serve as cardiac mast cell secretagogues. However, this list will undoubtedly expand with future research. For example, IL-33 which is a member of the IL-1 family of cytokines and a functional ligand of the ST2 receptor has been shown to regulate mast cell function in arthritis [52]. Extending this to the heart, we have performed preliminary studies which indicate that IL-33 also activates isolated rat-derived epicardial mast cells (unpublished observations).
4 Source of Cardiac Mast Cells
Mast cells are derived from blood-borne, multipotent hematopoietic progenitor cells that, once located in tissue, differentiate to a final phenotype under the influence of the local microenvironment [53]. As mentioned above, even though the density of cardiac mast cells is normally low, it has been reported to be increased severalfold in the chronically stressed or diseased heart. In the case of chronic volume overload, the increase occurs as early as 12 h after initiating the overload condition [11] and has been shown to be primarily due to a rapid maturation of immature resident mast cells with no evidence of proliferation [54]. Here immature and mature cells were identified according to their granules staining predominantly blue with the alcian blue–safranin reaction or having mostly safranin-positive granules. That is, the alcian blue–safranin reaction distinguishes between weakly sulfated (immature cells) and strongly sulfated mucopolysaccharides (mature cells) by a shift from alcian blue to safranin staining. The stimulus for this maturation process appears to be primarily fibroblast-derived stem cell factor (SCF), which we have recently shown to be transiently increased as early as 6 h after initiating the volume overload condition and to remain elevated by a factor of two through the second day of overload; at day three the level was back to normal. Corresponding to this SCF response was a 58–67 % increase in cardiac mast cell density at days 1 and 3 of volume overload with the peak value occurring on the first day. Furthermore, incubation of left ventricular tissue slices (250 μm thick) with SCF for 16 h resulted in a doubling of mast cell density which occurred concomitantly with a significant decrease in the number of immature mast cells [55]. Thus, the rapid increase in mast cell density in the cardiac volume overload model appears to be related primarily to a SCF-driven maturation of resident immature mast cells as opposed to cell proliferation or migration.
In the cardiac volume overload model, there is a definite relation between mast cell activation and an increase in mast cell density which in all likelihood is related to mast cell-derived chymase stimulating the synthesis and release of SCF from fibroblasts in a positive feedback fashion [56, 57]. Accordingly, when activation of mast cells was prevented using either cromolyn [11] or the NK-1 receptor antagonist L732138 [42], the volume overload-induced increase in mast cell density was prevented. Recently, we obtained direct evidence of this relation via the incubation of left ventricular tissue slices with the chemical secretagogue, compound 48/80, for 16 h. As a result of this chemical activation, SCF levels in both the left ventricular slices and media together with mast cell density were increased [55].
As stated earlier, mast cell density is also increased in the hypertensive heart, and, here too, it would appear that SCF is responsible [14]. However, unlike the volume overload condition, the hypertension-related increase does not appear to be the result of mast cell activation. In the spontaneous hypertensive rat (SHR) treated with nedocromil, mast cell density was found to increase [37]. Mast cell density also has been reported to be significantly increased in sympathectomized SHR [41]. Here it is assumed that, as a result of sympathectomy, substance P was depleted thereby preventing mast cell activation. While SCF was not measured in these two studies, the results of Shiota et al. [14] would indicate that it would remain elevated despite the prevention of mast cell degranulation and hence result in an increase in mast cell density.
Mast cell density is also increased in the failing heart regardless of etiology [12–14, 17]. While little is known regarding their source, it seems reasonable to assume that elevated levels of SCF represent the stimulus for both migration into the myocardium and maturation. Shiota's findings of elevated mRNA for soluble SCF and its receptor, c-Kit, as well as a marked increase in mast cell density in elderly SHR who were in congestive heart failure support this assumption [14]. Further support is gleaned from the study of Jahanyar et al. [58]. They found that left ventricular assist device support of patients with congestive heart failure resulted in an increase of SCF and c-Kit gene expression that coincided with a marked increase in the number of mast cells after ventricular unloading.
5 Myocardial Infarction and Ischemia–Reperfusion Injury
The data accumulated thus far in experimental animal studies seem to convincingly suggest an important role for mast cells in ischemia– reperfusion injury. However, due to the conflicting nature of the results between mouse studies, it is far less clear as to the role of mast cells following myocardial infarction without reperfusion. Frangogiannis et al. [59] have shown an increase in mast cell numbers during the healing phase in a canine model of ischemia– reperfusion, with maximum accumulation in areas of collagen deposition. Furthermore, increased numbers of degranulating mast cells were found to co-localize with newly recruited macrophages and neutrophils and were closely associated with vascular structures after 7 days of reperfusion following ischemia in a canine model of MI [60]. Jaggi et al. [61] demonstrated the involvement of mast cells in ischemia–reperfusion injury by subjecting isolated rat hearts treated with ketotifen to 30 min of global ischemia followed by 120 min of reperfusion. They found mast cell degranulation and myocardial injury to be decreased in the treated hearts. In an alternative approach they degranulated mast cells with compound 48/80 effectively removing mast cell mediators from the heart prior to inducing ischemia–reperfusion. This too resulted in attenuation of injury. Recent studies have begun to utilize mast cell (c-Kit)-deficient mice to attempt to determine the role of mast cells in ischemia–reperfusion injury and MI. Using a protocol of 30 min ischemia followed by 6 h of reperfusion in the W/Wv strain of mast cell-deficient mice, Bhattacharya et al. [28] found that the amount of viable myocardium was significantly greater in the reperfused mast cell-deficient mice.
The role of cardiac mast cells is less clear in myocardial infarction without reperfusion. Using female W/Wv mast cell-deficient mice, Cimini et al. [29] reported that these mice have a greater infarct area, ventricular dilatation, and reduced infarct thickness at 14 days post MI. However, they discounted the importance of mast cells to myocardial remodeling due to their small number and concluded that diminished recruitment of myofibroblasts accounted for the impaired healing of the scar. Interestingly, mast cells are known to have a prominent role in regulating myofibroblast function [62]. Ayach et al. [30] examined long-term remodeling and cardiac function in male W/Wv mice as well as W/Wv mice reconstituted with bone marrow cells at 35 days post MI. Their results indicated that W/Wv mice developed larger hearts with more collagen deposition, albeit with an increased stroke volume, even though they had reduced rates of contraction and relaxation. However, there was virtually no difference in survival rate between the wild-type and W/Wv mice 35 days post MI. Improvements were observed in all parameters measured post MI in mast cell-deficient mice reconstituted with bone marrow- derived mast cells. We have also sought to determine the role of mast cells to post-MI myocardial remodeling using W/Wv mice. In contrast to the previously mentioned studies, our preliminary results, conducted at 7 days post MI, indicate that chamber dilatation was significantly greater in the wild-type hearts compared to W/Wv hearts (126 % vs. 73 % increase in end diastolic volume, respectively). Also, the wild-type mice had thinner walls and increased collagen deposition in the viable myocardium. Based on these discrepancies, it would appear that additional research is needed regarding the role of mast cells in myocardial infarction- induced remodeling. Furthermore, given that post-infarction healing is a progressive process which may include mast cell-induced extracellular matrix degradation followed by mast cell-induced fibrosis, temporal studies are warranted. It should be noted that while mast cell-deficient mice are a powerful tool for studying mast cell biology, they are not without confounding variables. The W/Wv and Sl/Sld mice suffer from anemia, sterility, and a lack of hair pigmentation, as well as decreased numbers of bone marrow granulocytes and megakaryocytes. Other problems include the spontaneous development of lymphocytic leukemia, severe ulcerative dermatitis, stomach papillomas, and chronic ulcers of the gastric antrum [63]. Alternatively, the Kit(W-sh/W-sh) mouse is deficient in mast cells, but does not display anemia or sterility [64]. These mice also did not show a high incidence of idiopathic dermatitis, ulcers, or squamous papillomas of the stomach, but do still lack interstitial cells of Cajal in the gut.
6 Hypertension
A link between increased pressure overload and mast cells in the heart has been established by Olivetti et al. [16] who observed increases in cardiac mast cell density in the right ventricle following pulmonary artery banding in rats. Following that, Panizo and coworkers [15] similarly observed increases in mast cell density in the left ventricle of SHR. This increase in mast cell density strongly correlated with myocardial collagen concentration. In keeping with these findings, Shiota et al. [14] reported cardiac mast cell density to be increased dramatically above control levels at birth and throughout the lifespan of the SHR. Isolated heart studies have also shown that cardiac mast cells can be a significant source of NF-κB and IL-6 expression in the left ventricle of compensated 12-month-old SHR. However, all of these studies while making interesting observations failed to establish a causal relationship between cardiac mast cells and adverse remodeling in the hypertensive heart. To this end, Hara et al. [27] used the aortic banding model of experimentally induced pressure overload in mast cell-deficient mice to focus on the role of mast cells in the progression to heart failure. They found that, in contrast to their wild-type counterpart, heart and lung weights were markedly attenuated, ventricular dilatation was prevented, and fractional shortening was preserved. Alternatively, we have focused our investigation on the role of mast cells in fibrosis in the hypertensive heart. We treated SHR with nedocromil and found that fibrosis was completely prevented [37]. Mast cell stabilization was also able to prevent macrophage recruitment and normalized myocardial tryptase, IL-4, and IFN-γ levels. Interestingly, mast cell stabilization also prevented the decrease in the anti-inflammatory cytokine IL-10 that was observed in untreated SHR.
7 Volume Overload and Heart Failure
Cardiac mast cell density increases in the left ventricle under conditions of volume overload such as that which occurs with mitral regurgitation [19]. Using the aortocaval (AV) fistula model of volume overload, we have been able to demonstrate that mast cell stabilization prevented the increase in myocardial MMP-2 activity and the accompanying reduction in collagen volume fraction that occurs in the first 5 days following induction of volume overload [11, 32]. Treatment of rats with nedocromil for a period of 8 weeks post fistula [65] attenuated left ventricular hypertrophy and pulmonary edema, prevented ventricular dilatation and the increase in compliance, and prevented the decrease in intrinsic contractile function. Most important, there was a significant decrease in mortality. Further studies in mast cell-deficient rats with cardiac volume overload support these findings in that they did not have: (1) elevated MMP activity, (2) collagen degradation, or (3) dilatation of the left ventricle [32]. Further, Chancey et al. [66] administered a bolus of compound 48/80 to normal hearts, using a blood-perfused isolated heart preparation, and found that the subsequent mast cell degranulation produced an increase of 126 % in MMP activity and a nearly 50 % decrease in myocardial collagen volume fraction within 30 min. A tendency for the left ventricle to dilate was also evident despite a significant histamine-induced myocardial edema. The fact that mast cell density is also increased in the left ventricle of dogs with experimentally induced mitral regurgitation [19] demonstrates that mast cell-mediated myocardial remodeling in response to volume overload is not species or model specific.
8 Mast Cell Mediators and Myocardial Remodeling
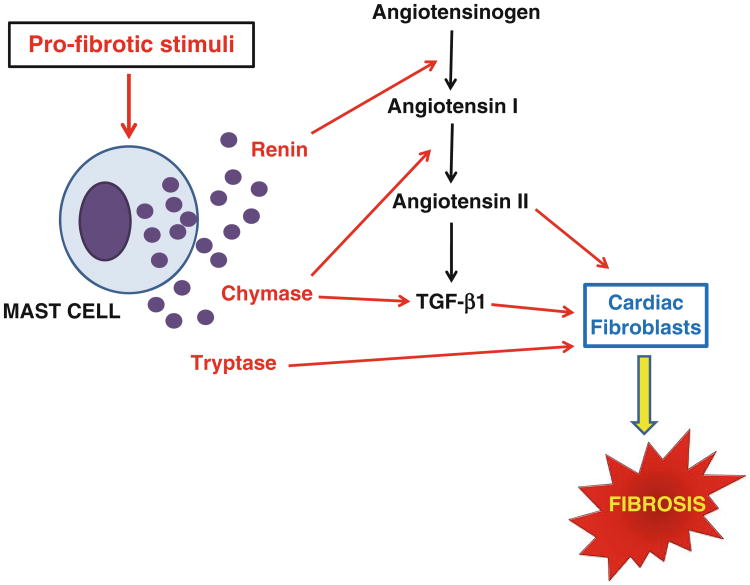
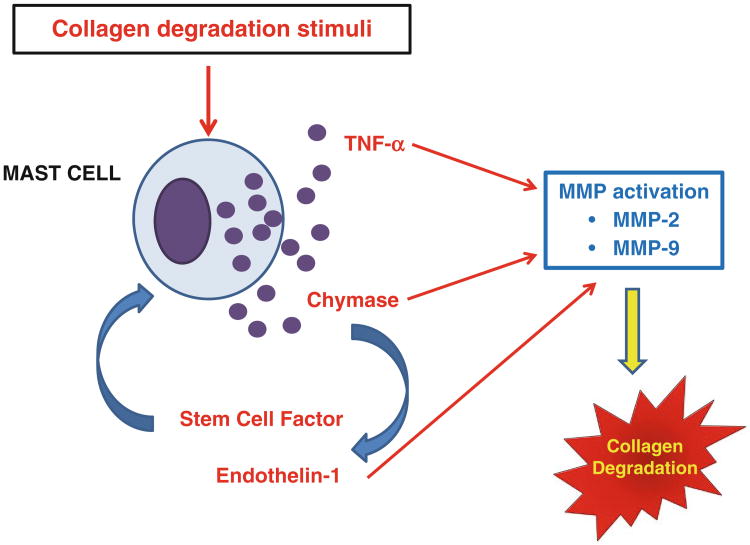
From the previous section of this chapter, it should be clear that mast cells play dual roles to induce remodeling in the heart. They can either stimulate collagen synthesis leading to fibrosis (e.g., in hypertension and ischemia–reperfusion) or induce MMP activation resulting in collagen degradation and ultimately ventricular dilatation. Understanding how mast cells in the heart are regulated to produce these seemingly disparate effects represents the next important phase of cardiac mast cell biology research. The function of mast cells in various cardiac pathologies likely is regulated by signaling pathways emanating from an interaction between myocytes, interstitial cells, and mast cells as well as from a neurohormonal influence which result in the production and release of different mast cell mediators. Below is a brief discussion of several such mast cell mediators which are known to influence remodeling. The role of these mediators in mast cell-induced collagen synthesis and degradation is summarized in Figs. 1 and 2.
Fig. 1.
Depicts the interactions of mast cell products currently thought to be involved in mast cell-induced fibrosis in the heart. The various mediators are discussed in detail in the text
Fig. 2.
Depicts the interactions of mast cell products currently thought to be involved in mast cell-induced degradation of collagen in the heart. The various mediators are discussed in detail in the text
8.1 TNF-α
Several studies have indicated that mast cells are an important source of TNF-α in the heart. Frangogiannis et al. [9] used labeling techniques and found that almost all TNF-α in the canine heart was localized to cardiac mast cells. When assessed again following 1 h of ischemia and 3 h of reperfusion, cardiac mast cells were still the predominant source of TNF-α, and mast cells in the infarct area (but not the remote region) could be seen releasing TNF-α. Gilles et al. [67] also suggested that this was the case, based on the observation that ketotifen and cromolyn sodium prevented the increase in myocardial TNF-α levels following reperfusion. In further agreement with these findings, we found that TNF-α was almost undetectable in the hearts of mast cell-deficient rats following 5 days of volume overload; conversely, wild-type rats had dramatically increased myocardial TNF-α levels in response to cardiac volume overload [32]. TNF-α can activate MMPs [68], and the infusion of TNF-α in rats has been shown to induce collagen degradation and dysfunction in the heart [69]. In cardiac volume overload, inhibition of TNF-α prevented collagen degradation [70]. However, TNF-α may also be pro-fibrotic in hypertension since it can increase angiotensin II-stimulated production of collagen by increasing angiotensin II type 1 receptors [71]. Furthermore, fibrosis and hypertrophy are attenuated in TNF-α knockout mice following transverse aortic constriction [72].
8.2 Histamine
Frangogiannis et al. [9] observed degranulating cardiac mast cells and an ∼twofold increase in histamine levels in cardiac lymph following ischemia–reperfusion in the canine heart. Histamine receptor antagonists in a canine model of ischemia–reperfusion revealed that blockade of the histamine type 2 (H2), but not H1, receptors decreased infarct size regardless of whether the H2 antagonist was administered during ischemia or reperfusion [73]. Interestingly, this did not lead to functional improvements. However, in retrospective and prospective clinical studies, the H2 receptor antagonist famotidine was found to reduce plasma brain natriuretic peptide (BNP) levels (a marker of left ventricular hypertrophy) as well as left ventricular diameter in diastole and systole while improving New York Heart Association (NYHA) functional class [74]. The ultimate effect of histamine on regulation of the extracellular matrix remains unclear. Upregulation of connective tissue growth factor mRNA has been reported in lung fibroblasts [75], while synovial fibroblasts isolated from rheumatoid synovial tissue release MMP-1 and MMP-3 as well as PGE2 in response to histamine [76]. Cardiac fibroblasts release PGE2 as well as the stable metabolite of the cardioprotective PGI2, 6-keto-PGF1α, in response to histamine [77].
8.3 Chymase/Renin/Angiotensin II
Chymase activity has been found to be increased in both the remote and infarcted areas of the myocardium following 1 h of ischemia and 3 h of reperfusion in pigs [78]. Chymase inhibition resulted in a reduction of necrosis in the risk area. The effects of mast cells on the myocardium may ultimately involve the production of angiotensin II. Mast cells contain chymase, which is capable of cleaving inactive angiotensin I to the active angiotensin II [79]. In support of this, an AT1 receptor antagonist had an efficacious effect on mortality post MI in hamsters, while an ACE inhibitor did not [80]. Further, ACE-independent angiotensin II formation was important for the release of norepinephrine from sympathetic nerves following an ischemic event in the human heart [81]. Also significant amounts of angiotensin II were mast cell-derived following ischemia–reperfusion in the guinea pig heart [82] with mast cell-derived angiotensin II being responsible for increased norepinephrine levels and norepinephrine-induced arrhythmias. It is unclear whether chymase can push the balance toward collagen synthesis or collagen degradation in vivo. Neonatal cardiac fibroblasts proliferate and produce collagen in response to chymase by inducing TGF-β production and activation of Smad pathways [83]. More recently, Roberto Levi's group identified cardiac mast cells as a source for renin in the heart [82, 84]. Mast cell production of renin would obviously aid in the production of angiotensin II in concert with chymase to produce a pro-fibrotic outcome. However, chymase can activate MMP-2 and MMP-9 [85], and chymase activity is also elevated in dogs with mitral regurgitation where collagen degradation predominates [19]. In fact, inhibition of chymase in pigs undergoing ischemia–reperfusion resulted in a decrease in MMP-9 activity [78]. Also, chymase is capable of activating SCF [55], converting Big ET-1 to ET-1 [86], and cleaving latent TGF-β to the active form [87, 88].
8.4 Tryptase
Studies linking peritoneal and skin mast cells to tissue remodeling have shown that mast cell tryptase can activate interstitial collagenase (MMP-1) and stromelysin (MMP-3) under in vitro conditions [6, 10]. However, Gruber et al. [89] demonstrated that tryptase was unable to directly activate MMP-1. Instead, tryptase first cleaves proMMP-3, with active MMP-3 then activating MMP-1. In contrast, we have clearly demonstrated that tryptase is pro-fibrotic in the heart. We initially observed that tryptase was increased in the SHR heart [37] and upon further investigation were able to demonstrate that tryptase causes isolated adult rat cardiac fibroblasts to proliferate, convert to a myofibroblast phenotype, and produce collagen [37, 90]. These effects occurred via tryptase activation of protease-activated receptor-2 (PAR-2), which induced ERK1/2 phosphorylation, but not p38 or JNK activation. Cardiac fibroblasts isolated from SHR hearts had this same pattern of selective activation, and blockade of PAR-2 in SHR was able to prevent fibrosis from occurring, suggesting that our in vitro findings were indicative of what was occurring in vivo.
8.5 TGF-β
Mast cell chymase is capable of cleaving latent TGF-β to the active form. The pro-fibrotic effects of TGF-β in the heart have been well documented. As mentioned above, TGF-β and subsequent activation of Smad pathways may mediate the proliferative and collagen-producing effects of chymase on neonatal cardiac fibroblasts [83]. More recently, Zhang et al. [91] co-incubated mast cells with cardiac fibroblasts and found that α-smooth muscle actin expression, proliferation, and collagen messenger RNA expression were all increased in cardiac fibroblasts isolated from mice overexpressing TNF-α when compared to fibroblasts from wild-type controls. However, it is important to consider that this study used MC/9 mast cells, which are derived from murine fetal livers. In view of the fact that the differentiation of mast cells is dependent on their microenvironment, the relevance of MC/9 and other mast cell lines to cardiac mast cells is questionable.
9 Modulation of Cardiac Mast Cell Phenotype by Estrogen
Although there are clear gender differences in the prevalence and severity of cardiovascular disease in humans [92], our understanding of the underlying mechanisms responsible for the lower incidence of cardiac disease in premenopausal females is poor. In this regard, we had made the observation that degranulation of mast cells with compound 48/80 in isolated hearts from ovariectomized female rats caused an increase in MMP-2 activation, which led to collagen degradation and ventricular dilatation when compared to hearts from normal females [93]. Restoration of estrogen to ovariectomized female rats was able to prevent the increase in MMP-2 activation, collagen degradation, and ventricular dilatation. These observations have led us to hypothesize that estrogen may confer cardioprotection in part by modulating cardiac mast cell phenotype. That is, estrogen may downregulate synthesis of or prevent the release of mast cell proteases [94] or other products such as TNF-α [95] as has been shown in non-cardiac mast cells. In support of this, we recently reported that, in contrast to male rat hearts, cardiac mast cell density does not increase in response to volume overload in female rat hearts [96]; as a result, collagen degradation did not occur. However, when female rats were ovariectomized, cardiac mast cell density did increase following 3 days of volume overload leading to collagen degradation at 5 days, which is identical to the response seen in male rats. Further, myocardial TNF-α levels were not increased in intact female rats following volume overload but were increased in ovariectomized female rats following volume overload. The increase in TNF-α in ovariectomized hearts was regulated by mast cells since nedocromil normalized TNF-α values. In keeping with the changes in mast cell density following volume overload, stem cell factor (SCF) increased by almost 50 % in ovariectomized female rats as compared to ∼10 % in female rats that had not been ovariectomized.
10 Summary
Mast cells are known to store and release a variety of biologically active mediators which are known to be involved in myocardial remodeling including histamine, cytokines such as TNF-α, proteases such as tryptase and chymase, and growth factors such as TGF-β. Early observations regarding cardiac mast cells were limited to reports of increases in mast cell density in hearts subjected to sustained elevations in myocardial stress or injury; subsequently elevations in stem cell factor and its receptor have been implicated as being the underlying stimuli for this increase via maturation of resident mast cells and possibly enhanced migration of precursor cells. There has been no evidence to indicate the occurrence of mast cell proliferation in the heart. Continuing research has identified the cardiac mast cell as playing a central role in the myocardial remodeling that occurs as a result of pathologic myocardial stress or ischemic damage. Cardiac mast cells cannot be isolated via enzymatic dispersion techniques because spontaneous degranulation has been shown to occur throughout the process. Consequently, the isolated cells are minimally responsive to secretagogues. This problem has been circumvented using a technique whereby epicardial cells are obtained via a pericardial washing. While a mixture of cell types is obtained, the harvested mast cells release significant amounts of histamine in response to activating compounds. However, because of the relatively low density of mast cells in the heart, this technique yields approximately 100,000 mast cells from an adult rat heart, which is usually insufficient for further studies thus requiring the pooling of cells from several hearts or using larger animals. Also, further insight into the functional role of cardiac mast cells can continue to be obtained using mast cell membrane stabilizing drugs and mast cell-deficient rodents. To this end there are numerous questions which remain to be answered. These include: (1) while we know that mast cell secretory products can activate matrix metalloproteinases, it is not known how they mediate pro-fibrotic processes; (2) while there is evidence in other tissue that mast cells interact with other inflammatory cells, it is not known whether such an interaction occurs in the heart; (3) other than ET-1 and substance P, other exogenous secretagogues for cardiac mast cells, if any, remain to be identified; and (4) given that estrogen infers cardioprotection via its effect on mast cells, the influence of male and female hormones on cardiac mast cell phenotype as well as their regulatory pathways need to be investigated in detail.
Acknowledgments
This work was supported in part by grants from NHLBI (to J.S.J.—#-HL-59981, R01-HL-62228, R21-HL-089483 and to S.P.L. R00-HL-093215).
References
- 1.Pfeffer JM, et al. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol. 1991;260:H1406–H1414. doi: 10.1152/ajpheart.1991.260.5.H1406. [DOI] [PubMed] [Google Scholar]
- 2.Grossman W, et al. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borg TK, Caulfield JB. The collagen matrix of the heart. Fed Proc. 1981;40:2037–2041. [PubMed] [Google Scholar]
- 4.Robinson TF, et al. Structure and function of connective tissue in cardiac muscle: collagen types I and III in endomysial struts and pericellular fibers. Scanning Microsc. 1988;2:1005–1015. [PubMed] [Google Scholar]
- 5.Montfort I, Perez-Tamayo R. The distribution of collagenase in normal rat tissues. J Histochem Cytochem. 1975;23:910–920. doi: 10.1177/23.12.172556. [DOI] [PubMed] [Google Scholar]
- 6.Lees M, et al. Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B. Eur J Biochem. 1994;223:171–177. doi: 10.1111/j.1432-1033.1994.tb18980.x. [DOI] [PubMed] [Google Scholar]
- 7.Marone G, et al. Immunological modulation of human cardiac mast cells. Neurochem Res. 1999;24:1195–1202. doi: 10.1023/a:1020776807187. [DOI] [PubMed] [Google Scholar]
- 8.Metcalfe DD. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 9.Frangogiannis NG, et al. Resident cardiac mast cells degranulate and release preformed TNF-α, initiating the cytokine cascade in experimental canine myocardial ischemia/ reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, et al. Activation of precursors for matrix metalloproteinases 1 (interstitial collagenase) and 3 (stromelysin) by rat mast-cell proteinases I and II. Biochem J. 1995;305(Pt 1):301–306. doi: 10.1042/bj3050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brower GL, et al. Cause and effect relationship between myocardial mast cell number and matrix metalloproteinase activity. Am J Physiol. 2002;283:H518–H525. doi: 10.1152/ajpheart.00218.2000. [DOI] [PubMed] [Google Scholar]
- 12.Patella V, et al. Increased cardiac mast cell density and mediator release in patients with dilated cardiomyopathy. Inflamm Res. 1997;46:S31–S32. [PubMed] [Google Scholar]
- 13.Patella V, et al. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97:971–978. doi: 10.1161/01.cir.97.10.971. [DOI] [PubMed] [Google Scholar]
- 14.Shiota N, et al. A role for cardiac mast cells in the pathogenesis of hypertensive heart disease. J Hypertens. 2003;21:1823–1825. doi: 10.1097/00004872-200310000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Panizo A, et al. Are mast cells involved in hypertensive heart disease? J Hypertens. 1995;13:1201–1208. doi: 10.1097/00004872-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Olivetti G, et al. Long-term pressure-induced cardiac hypertrophy: capillary and mast cell proliferation. Am J Physiol Heart Circ Physiol. 1989;257:H1766–H1772. doi: 10.1152/ajpheart.1989.257.6.H1766. [DOI] [PubMed] [Google Scholar]
- 17.Engels W, et al. Transmural changes in mast cell density in rat heart after infarct induction in vivo. J Pathol. 1995;177:423–429. doi: 10.1002/path.1711770414. [DOI] [PubMed] [Google Scholar]
- 18.Dell'Italia LJ, et al. Volume-overload cardiac hypertrophy is unaffected by ACE inhibitor treatment in dogs. Am J Physiol Heart Circ Physiol. 1997;273:H961–H970. doi: 10.1152/ajpheart.1997.273.2.H961. [DOI] [PubMed] [Google Scholar]
- 19.Stewart JA, et al. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol. 2003;35:311–319. doi: 10.1016/s0022-2828(03)00013-0. [DOI] [PubMed] [Google Scholar]
- 20.von Recklinghausen FD. Uber eiterund bindegewebskorperchen. Virchows Arch Pathol Anat Physiol Klin Med. 1863;28:157–197. [Google Scholar]
- 21.Crivellato E, et al. Paul Ehrlich's doctoral thesis: a milestone in the study of mast cells. Br J Haematol. 2003;123:19–21. doi: 10.1046/j.1365-2141.2003.04573.x. [DOI] [PubMed] [Google Scholar]
- 22.Estensen RD. Eosinophilic myocarditis: a role for mast cells? Arch Pathol Lab Med. 1984;108:358–359. [PubMed] [Google Scholar]
- 23.Fernex M. The mast-cell system: its relationship to atherosclerosis, fibrosis and eosinophils. The Williams & Wilkins Company; Baltimore: 1968. pp. 93–95. [Google Scholar]
- 24.Dvorak AM. Mast-cell degranulation in human hearts. N Engl J Med. 1986;315:969–970. doi: 10.1056/nejm198610093151515. [DOI] [PubMed] [Google Scholar]
- 25.Li QY, et al. The relationship of mast cells and their secreted products to the volume of fibrosis in posttransplant hearts. Transplantation. 1992;53:1047–1051. doi: 10.1097/00007890-199205000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Kovanen PT. Role of mast cells in atherosclerosis. Chem Immunol. 1995;62:132–170. [PubMed] [Google Scholar]
- 27.Hara M, et al. Evidence for a role of mast cells in the evolution to congestive heart failure. J Exp Med. 2002;195:375–381. doi: 10.1084/jem.20002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharya K, et al. Mast cell deficient W/Wv mice have lower serum IL-6 and less cardiac tissue necrosis than their normal littermates following myocardial ischemia- reperfusion. Int J Immunopathol Pharmacol. 2007;20:69–74. doi: 10.1177/039463200702000108. [DOI] [PubMed] [Google Scholar]
- 29.Cimini M, et al. c-Kit dysfunction impairs myocardial healing after infarction. Circulation. 2007;116:I–77. doi: 10.1161/CIRCULATIONAHA.107.708107. [DOI] [PubMed] [Google Scholar]
- 30.Ayach BB, et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:2304–2309. doi: 10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J, et al. Mast cells promote atherosclerosis by releasing proinflammatory cyto-kines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 32.Levick SP, et al. Protection from adverse myocardial remodeling secondary to chronic volume overload in mast cell deficient rats. J Mol Cell Cardiol. 2008;45:56–61. doi: 10.1016/j.yjmcc.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galli SJ. The Paul Kallos memorial lecture. The mast cell: a versatile effector cell for a challenging world. Int Arch Allergy Immunol. 1997;113:14–22. doi: 10.1159/000237497. [DOI] [PubMed] [Google Scholar]
- 34.Patella V, et al. Human heart mast cells: a definitive case of mast cell heterogeneity. Int Arch Allergy Immunol. 1995;106:386–393. doi: 10.1159/000236871. [DOI] [PubMed] [Google Scholar]
- 35.Patella V, et al. Human heart mast cells. Isolation, purification, ultrastructure, and immunologic characterization. J Immunol. 1995;154:2855–2865. [PubMed] [Google Scholar]
- 36.Forman MF, et al. Spontaneous histamine secretion during isolation of rat cardiac mast cells. Inflamm Res. 2004;53:453–457. doi: 10.1007/s00011-004-1275-z. [DOI] [PubMed] [Google Scholar]
- 37.Levick SP, et al. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension. 2009;53:1041–1047. doi: 10.1161/HYPERTENSIONAHA.108.123158. [DOI] [PubMed] [Google Scholar]
- 38.Ali H, Pearce FL. Isolation and properties of cardiac and other mast cells from the rat and guinea-pig. Agents Actions. 1985;16:138–140. doi: 10.1007/BF01983121. [DOI] [PubMed] [Google Scholar]
- 39.Morgan LG, et al. A novel technique for isolating functional mast cells from the heart. Inflamm Res. 2008;57:1–6. doi: 10.1007/s00011-007-7059-5. [DOI] [PubMed] [Google Scholar]
- 40.McLarty JL, et al. Isolation of functional cardiac immune cells. J Vis Exp. 2011;58 doi: 10.3791/3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levick SP, et al. Sympathetic nervous system modulation of inflammation and remodeling in the hypertensive heart. Hypertension. 2010;55:270–276. doi: 10.1161/HYPERTENSIONAHA.109.142042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melendez GC, et al. Substance P induces adverse myocardial remodeling via a mechanism involving cardiac mast cells. Cardiovasc Res. 2011;92:420–429. doi: 10.1093/cvr/cvr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray DB, et al. Response of cardiac mast cells to atrial natriuretic peptide. Am J Physiol Heart Circ Physiol. 2007;293:H1216–H1222. doi: 10.1152/ajpheart.01388.2006. [DOI] [PubMed] [Google Scholar]
- 44.Opgenorth TJ, et al. Atrial peptides induce mast cell histamine release. Peptides. 1990;11:1003–1007. doi: 10.1016/0196-9781(90)90024-y. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida H, et al. Histamine release induced by human natriuretic peptide from rat peritoneal mast cells. Regul Pept. 1996;61:45–49. doi: 10.1016/0167-0115(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 46.Reinecke M, et al. Localization of neurotensin immunoreactive nerve fibers in the guinea-pig heart: evidence derived by immunohistochemistry, radioimmunoassay and chromatography. Neuroscience. 1982;7:1785–1795. doi: 10.1016/0306-4522(82)90036-7. [DOI] [PubMed] [Google Scholar]
- 47.Rioux F, et al. Characterization of the histamine releasing effect of neurotensin in the rat heart. Peptides. 1985;6:121–125. doi: 10.1016/0196-9781(85)90087-7. [DOI] [PubMed] [Google Scholar]
- 48.Pang X, et al. A neurotensin receptor antagonist inhibits acute immobilization stress- induced cardiac mast cell degranulation, a corticotropin- releasing hormone-dependent process. J Pharmacol Exp Ther. 1998;287:307–314. [PubMed] [Google Scholar]
- 49.Murray DB, et al. Effects of nonselective endothelin-1 receptor antagonism on cardiac mast cell-mediated ventricular remodeling in rats. Am J Physiol Heart Circ Physiol. 2008;294:H1251–H1257. doi: 10.1152/ajpheart.00622.2007. [DOI] [PubMed] [Google Scholar]
- 50.Melendez GC, et al. Oxidative stress mediated cardiac mast cell degranulation. Toxicol Environ Chem. 2010;92:1293–1301. [Google Scholar]
- 51.Masini E, et al. Protective effects of M40403, a selective superoxide dismutase mimetic, in myocardial ischaemia and reperfusion injury in vivo. Br J Pharmacol. 2002;136:905–917. doi: 10.1038/sj.bjp.0704774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu D, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crivellato E, et al. The history of the controversial relationship between mast cells and basophils. Immunol Lett. 2011;141(1):10–17. doi: 10.1016/j.imlet.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Forman MF, et al. Rat cardiac mast cell maturation and differentiation following acute ventricular volume overload. Inflamm Res. 2006;55:408–415. doi: 10.1007/s00011-006-6016-z. [DOI] [PubMed] [Google Scholar]
- 55.Li J, et al. Stem cell factor is responsible for the rapid response in mature mast cell density in the acutely stressed heart. J Mol Cell Cardiol. 2012;53:469–474. doi: 10.1016/j.yjmcc.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomimori Y, et al. Mast cell chymase regulates dermal mast cell number in mice. Biochem Biophys Res Commun. 2002;290:1478–1482. doi: 10.1006/bbrc.2002.6365. [DOI] [PubMed] [Google Scholar]
- 57.Longley BJ, et al. Chymase cleavage of stem cell factor yields a bioactive, soluble product. Proc Natl Acad Sci U S A. 1997;94:9017–9021. doi: 10.1073/pnas.94.17.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jahanyar J, et al. Increased expression of stem cell factor and its receptor after left ventricular assist device support: a potential novel target for therapeutic interventions in heart failure. J Heart Lung Transplant. 2008;27:701–709. doi: 10.1016/j.healun.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 59.Frangogiannis NG, et al. Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation. 1998;98:687–698. doi: 10.1161/01.cir.98.7.687. [DOI] [PubMed] [Google Scholar]
- 60.Somasundaram P, et al. Mast cell tryptase may modulate endothelial cell phenotype in healing myocardial infarcts. J Pathol. 2005;205:102–111. doi: 10.1002/path.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaggi AS, et al. Cardioprotective effects of mast cell modulators in ischemia-reperfusion- induced injury in rats. Methods Find Exp Clin Pharmacol. 2007;29:593–600. doi: 10.1358/mf.2007.29.9.1161005. [DOI] [PubMed] [Google Scholar]
- 62.Gailit J, et al. The differentiation and function of myofibroblasts is regulated by mast cell mediators. J Invest Dermatol. 2001;117:1113–1119. doi: 10.1046/j.1523-1747.2001.15211.x. [DOI] [PubMed] [Google Scholar]
- 63.Galli SJ, Kitamura Y. Genetically mast-cell- deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol. 1987;127:191–198. [PMC free article] [PubMed] [Google Scholar]
- 64.Grimbaldeston MA, et al. Mast cell-deficient W-sash c-kit Mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brower GL, Janicki JS. Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J Card Fail. 2005;11:548–556. doi: 10.1016/j.cardfail.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Chancey AL, et al. Cardiac mast cell-mediated activation of gelatinase and alteration of ventricular diastolic function. Am J Physiol. 2002;282:H2152–H2158. doi: 10.1152/ajpheart.00777.2001. [DOI] [PubMed] [Google Scholar]
- 67.Gilles S, et al. Release of TNF-α during myocardial reperfusion depends on oxidative stress and is prevented by mast cell stabilizers. Cardiovasc Res. 2003;60:608–616. doi: 10.1016/j.cardiores.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 68.Seguin CA, et al. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine. 2008;33:356–365. doi: 10.1097/BRS.0b013e3181642a5e. [DOI] [PubMed] [Google Scholar]
- 69.Bozkurt B, et al. Pathophysiologically relevant concentrations of tumor necrosis factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–1391. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 70.Jobe LJ, et al. TNF-α inhibition attenuates adverse myocardial remodeling in a rat model of volume overload. Am J Physiol Heart Circ Physiol. 2009;297:H1462–H1468. doi: 10.1152/ajpheart.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gurantz D, et al. IL-1β and TNF-α upregulate angiotensin II type 1 (AT1) receptors on cardiac fibroblasts and are associated with increased AT1 density in the post-MI heart. J Mol Cell Cardiol. 2005;38:505–515. doi: 10.1016/j.yjmcc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 72.Sun M, et al. Tumor necrosis factor-α mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation. 2007;115:1398–1407. doi: 10.1161/CIRCULATIONAHA.106.643585. [DOI] [PubMed] [Google Scholar]
- 73.Asanuma H, et al. Blockade of histamine H2 receptors protects the heart against ischemia and reperfusion injury in dogs. J Mol Cell Cardiol. 2006;40:666–674. doi: 10.1016/j.yjmcc.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 74.Kim J, et al. Impact of blockade of histamine H2 receptors on chronic heart failure revealed by retrospective and prospective randomized studies. J Am Coll Cardiol. 2006;48:1378–1384. doi: 10.1016/j.jacc.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 75.Kunzmann S, et al. Connective tissue growth factor expression is regulated by histamine in lung fibroblasts: potential role of histamine in airway remodeling. J Allergy Clin Immunol. 2007;119:1398–1407. doi: 10.1016/j.jaci.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 76.Tetlow LC, Woolley DE. Effect of histamine on the production of matrix metalloproteinases- 1, -3, -8 and -13, and TNFalpha and PGE(2) by human articular chondrocytes and synovial fibroblasts in vitro: a comparative study. Virchows Arch. 2004;445:485–490. doi: 10.1007/s00428-004-1109-y. [DOI] [PubMed] [Google Scholar]
- 77.Linssen MC, et al. Production of arachidonic acid metabolites in adult rat cardiac myocytes, endothelial cells, and fibroblast-like cells. Am J Physiol Heart Circ Physiol. 1993;264:H973–H982. doi: 10.1152/ajpheart.1993.264.3.H973. [DOI] [PubMed] [Google Scholar]
- 78.Oyamada S, et al. Chymase inhibition reduces infarction and matrix metalloproteinase- 9 activation and attenuates inflammation and fibrosis after acute myocardial ischemia/ reperfusion. J Pharmacol Exp Ther. 2011;339:143–151. doi: 10.1124/jpet.111.179697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caughey GH, et al. Angiotensin II generation by mast cell α- and β-chymases. Biochim Biophys Acta. 2000;1480:245–257. doi: 10.1016/s0167-4838(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 80.Jin D, et al. Possible roles of cardiac chymase after myocardial infarction in hamster hearts. Jpn J Pharmacol. 2001;86:203–214. doi: 10.1254/jjp.86.203. [DOI] [PubMed] [Google Scholar]
- 81.Maruyama R, et al. Angiotensin-converting enzyme-independent angiotensin formation in a human model of myocardial ischemia: modulation of norepinephrine release by angiotensin type 1 and angiotensin type 2 receptors. J Pharmacol Exp Ther. 2000;294:248–254. [PubMed] [Google Scholar]
- 82.Mackins CJ, et al. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest. 2006;116:1063–1070. doi: 10.1172/JCI25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao XY, et al. Chymase induces profibrotic response via transforming growth factor- beta 1/Smad activation in rat cardiac fibroblasts. Mol Cell Biochem. 2008;310:159–166. doi: 10.1007/s11010-007-9676-2. [DOI] [PubMed] [Google Scholar]
- 84.Silver RB, et al. Mast cells: a unique source of renin. Proc Natl Acad Sci U S A. 2004;101:13607–13612. doi: 10.1073/pnas.0403208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tchougounova E, et al. A key role for mast cell chymase in the activation of pro- matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- 86.Wypij DM, et al. Role of mast cell chymase in the extracellular processing of big-endothelin- 1 to endothelin-1 in the perfused rat lung. Biochem Pharmacol. 1992;43:845–853. doi: 10.1016/0006-2952(92)90252-e. [DOI] [PubMed] [Google Scholar]
- 87.Taipale J, et al. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995;270:4689–4696. doi: 10.1074/jbc.270.9.4689. [DOI] [PubMed] [Google Scholar]
- 88.Lindstedt KA, et al. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J. 2001;15:1377–1388. doi: 10.1096/fj.00-0273com. [DOI] [PubMed] [Google Scholar]
- 89.Gruber BL, et al. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989;84:1657–1662. doi: 10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McLarty JL, et al. Tryptase/protease-activated receptor 2 interactions induce selective mitogen-activated protein kinase signaling and collagen synthesis by cardiac fibroblasts. Hypertension. 2011;58:264–270. doi: 10.1161/HYPERTENSIONAHA.111.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang W, et al. The development of myocardial fibrosis in transgenic mice with targeted overexpression of tumor necrosis factor requires mast cell-fibroblast interactions. Circulation. 2011;124:2106–2116. doi: 10.1161/CIRCULATIONAHA.111.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hayward CS, et al. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000;46:28–49. doi: 10.1016/s0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 93.Chancey AL, et al. Modulation of cardiac mast cell-mediated extracellular matrix degradation by estrogen. Am J Physiol Heart Circ Physiol. 2005;289:H316–H321. doi: 10.1152/ajpheart.00765.2004. [DOI] [PubMed] [Google Scholar]
- 94.Harnish DC, et al. Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G118–G125. doi: 10.1152/ajpgi.00024.2003. [DOI] [PubMed] [Google Scholar]
- 95.Kim MS, et al. Estrogen regulates cytokine release in human mast cells. Immunopharmacol Immunotoxicol. 2001;23:495–504. doi: 10.1081/iph-100108596. [DOI] [PubMed] [Google Scholar]
- 96.Lu H, et al. Prevention of adverse cardiac remodeling to volume overload in female rats is the result of an estrogen-altered mast cell phenotype. Am J Physiol Heart Circ Physiol. 2011;302:H811–H817. doi: 10.1152/ajpheart.00980.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]