Abstract
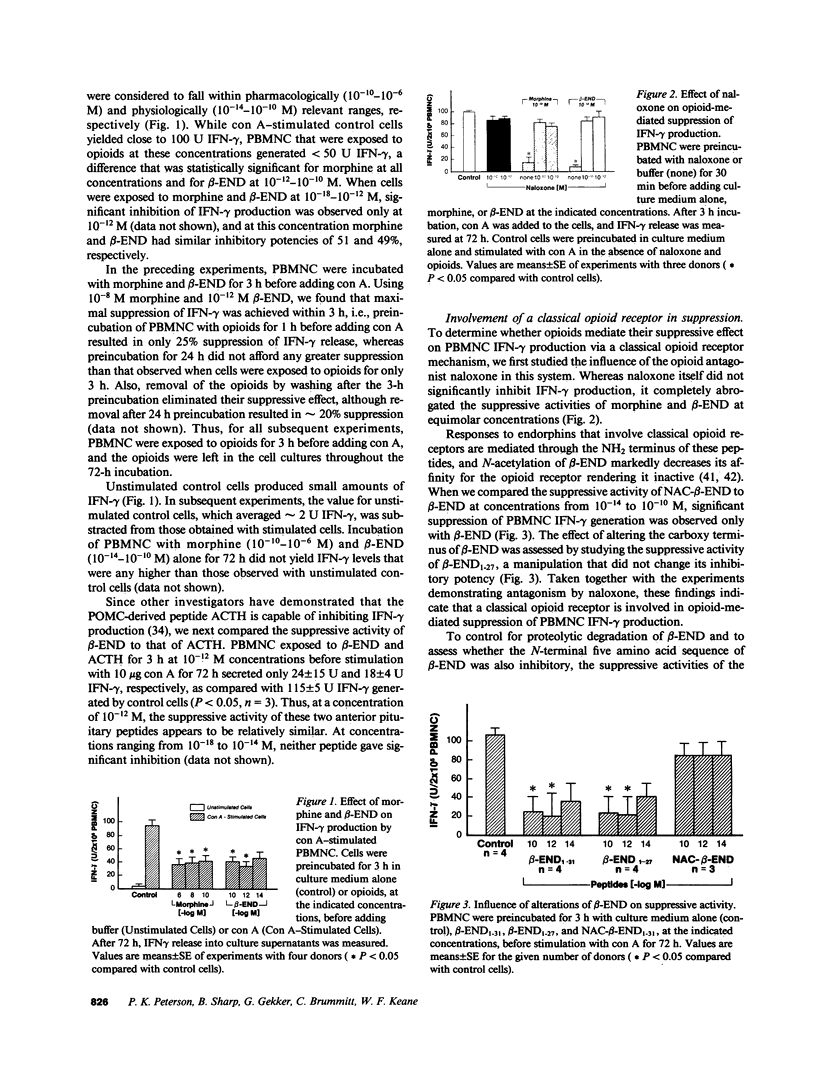
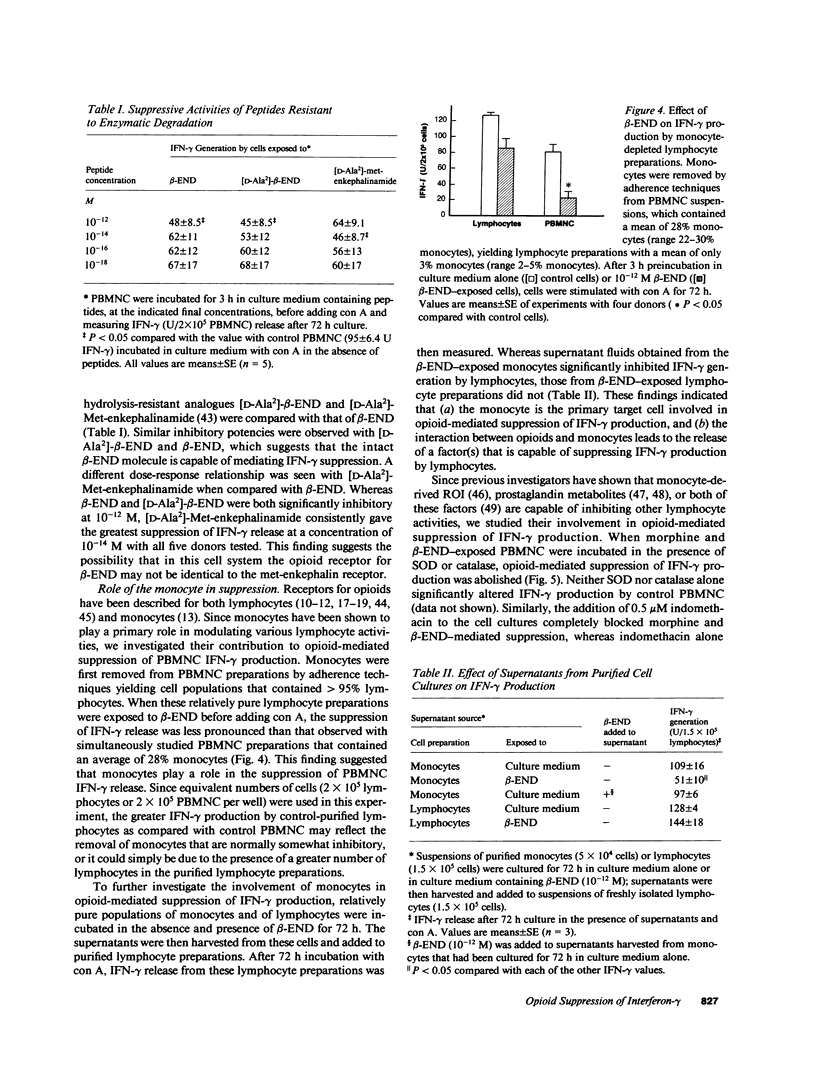
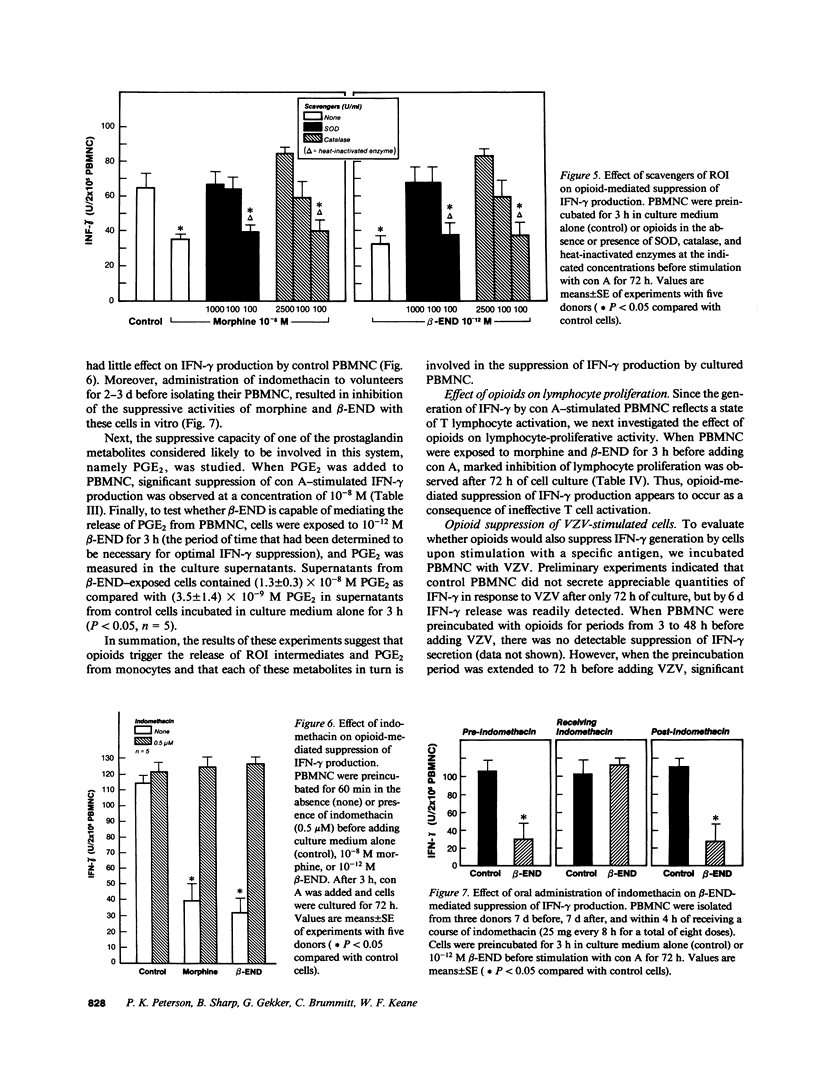
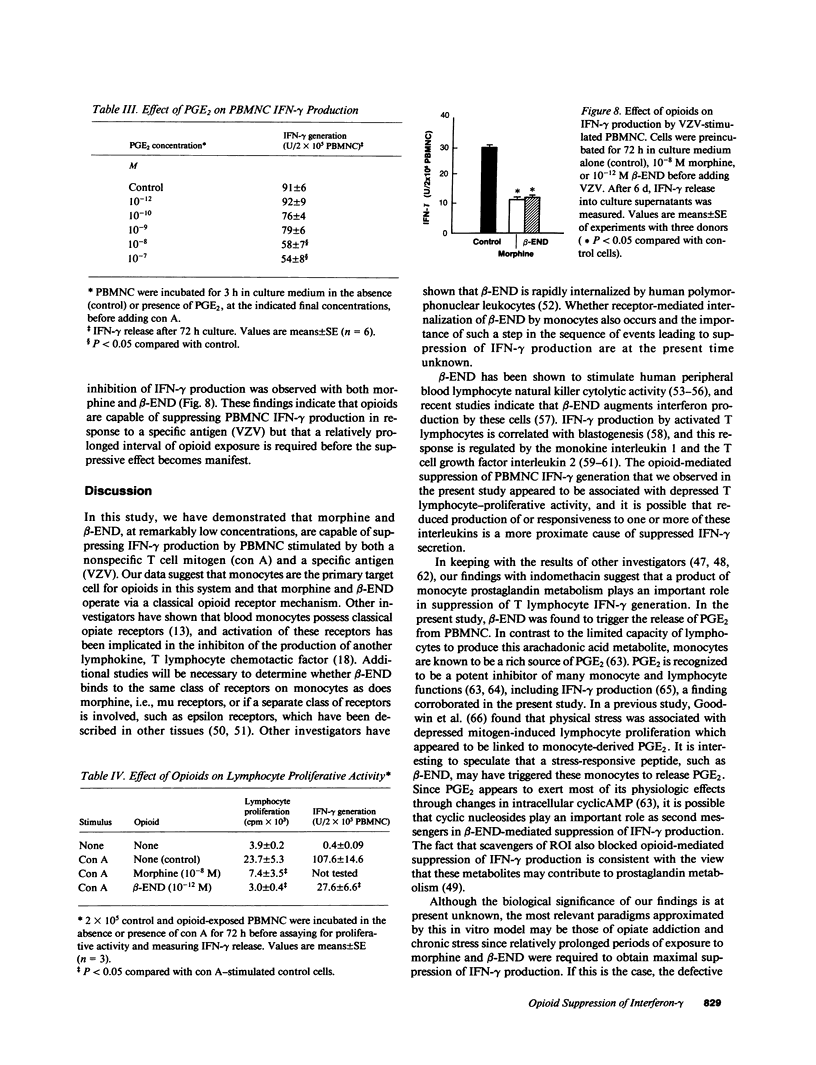
Mounting evidence suggests that opiate addiction and stress are associated with impaired cell-mediated immunity. We tested the hypothesis that morphine and the endogenous opioid beta-endorphin (beta-END), a pituitary peptide released in increased concentrations during stress, can suppress the production of the key macrophage-activating lymphokine interferon-gamma (IFN-gamma) by cultured human peripheral blood mononuclear cells (PBMNC). Using a radioimmunoassay to measure IFN-gamma, we found that exposure of PBMNC to biologically relevant concentrations of both opioids significantly inhibited IFN-gamma generation by cells stimulated with concanavalin A and varicella zoster virus. Studies of the mechanism of suppression revealed (a) a classical opioid receptor is involved (suppression was antagonized by naloxone and was specific for the NH2 terminus of beta-END), (b) monocytes are the primary target cell for opioids (monocyte-depleted lymphocyte preparations showed little suppression), and (c) reactive oxygen intermediates (ROI) and prostaglandin E2 are important mediators (scavengers of ROI and indomethacin eliminated the suppression). Based on these findings we suggest that opioid-triggered release of inhibitory monocyte metabolites may play a role in the immunodeficiency associated with narcotic addiction and stress.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Douglas S. D. Purification of human monocytes on microexudate-coated surfaces. J Immunol. 1978 Apr;120(4):1372–1374. [PubMed] [Google Scholar]
- Akil H., Young E., Watson S. J., Coy D. H. Opiate binding properties of naturally occurring N- and C-terminus modified beta-endorphins. Peptides. 1981 Fall;2(3):289–292. doi: 10.1016/s0196-9781(81)80121-0. [DOI] [PubMed] [Google Scholar]
- Blalock J. E. Proopiomelanocortin-derived peptides in the immune system. Clin Endocrinol (Oxf) 1985 Jun;22(6):823–827. doi: 10.1111/j.1365-2265.1985.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Smith E. M., Meyer W. J., 3rd The pituitary-adrenocortical axis and the immune system. Clin Endocrinol Metab. 1985 Nov;14(4):1021–1038. doi: 10.1016/s0300-595x(85)80087-6. [DOI] [PubMed] [Google Scholar]
- Bocchini G., Bonanno G., Canevari A. Influence of morphine and naloxone on human peripheral blood T-lymphocytes. Drug Alcohol Depend. 1983 Apr;11(2):233–237. doi: 10.1016/0376-8716(83)90083-2. [DOI] [PubMed] [Google Scholar]
- Brown S. L., Van Epps D. E. Suppression of T lymphocyte chemotactic factor production by the opioid peptides beta-endorphin and met-enkephalin. J Immunol. 1985 May;134(5):3384–3390. [PubMed] [Google Scholar]
- Brown S. M., Stimmel B., Taub R. N., Kochwa S., Rosenfield R. E. Immunologic dysfunction in heroin addicts. Arch Intern Med. 1974 Dec;134(6):1001–1006. [PubMed] [Google Scholar]
- Brummer E., Morrison C. J., Stevens D. A. Recombinant and natural gamma-interferon activation of macrophages in vitro: different dose requirements for induction of killing activity against phagocytizable and nonphagocytizable fungi. Infect Immun. 1985 Sep;49(3):724–730. doi: 10.1128/iai.49.3.724-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D. J., Klimpel G. R. Enhancement of the generation of cytotoxic T cells by endogenous opiates. J Neuroimmunol. 1986 Jul;12(1):75–87. doi: 10.1016/0165-5728(86)90099-8. [DOI] [PubMed] [Google Scholar]
- Celada A., Gray P. W., Rinderknecht E., Schreiber R. D. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984 Jul 1;160(1):55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., McKinney S., Liu V., Kung P. C., Vilcek J., Le J. Use of monoclonal antibodies as sensitive and specific probes for biologically active human gamma-interferon. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5219–5222. doi: 10.1073/pnas.81.16.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J. W., Morgan W. M., Hardy A. M., Jaffe H. W., Darrow W. W., Dowdle W. R. The epidemiology of AIDS: current status and future prospects. Science. 1985 Sep 27;229(4720):1352–1357. doi: 10.1126/science.2994217. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Plotkin S. A., Douglas S. D., Polin R. A. Immune-specific gamma interferon production correlates with lymphocyte blastogenesis. J Clin Microbiol. 1986 May;23(5):911–915. doi: 10.1128/jcm.23.5.911-915.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin J. F., Doströvsky J. O., Smyth D. G. Influence of N-terminal acetylation and C-terminal proteolysis on the analgesic activity of beta-endorphin. Biochem J. 1980 Sep 1;189(3):501–506. doi: 10.1042/bj1890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoe R. M., Madden J. J., Hollingsworth F., Shafer D., Falek A. Morphine depression of T cell E-rosetting: definition of the process. Fed Proc. 1985 Jan;44(1 Pt 1):95–99. [PubMed] [Google Scholar]
- Faith R. E., Plotnikoff N. P., Murgo A. J. Effects of opiates and neuropeptides on immune functions. NIDA Res Monogr. 1984;54:300–311. [PubMed] [Google Scholar]
- Falke N. E., Fischer E. G. Opiate receptor mediated internalization of 125I-beta-endorphin in human polymorphonuclear leucocytes. Cell Biol Int Rep. 1986 Jun;10(6):429–435. doi: 10.1016/0309-1651(86)90038-x. [DOI] [PubMed] [Google Scholar]
- Fischer E. G., Falke N. E. Beta-endorphin modulates immune functions. A review. Psychother Psychosom. 1984;42(1-4):195–204. doi: 10.1159/000287845. [DOI] [PubMed] [Google Scholar]
- Froelich C. J., Bankhurst A. D. The effect of beta-endorphin on natural cytotoxicity and antibody dependent cellular cytotoxicity. Life Sci. 1984 Jul 16;35(3):261–265. doi: 10.1016/0024-3205(84)90109-7. [DOI] [PubMed] [Google Scholar]
- Gemsa D., Leser H. G., Deimann W., Resch K. Suppression of T lymphocyte proliferation during lymphoma growth in mice: role of PGE2-producing suppressor macrophages. Immunobiology. 1982 Apr;161(3-4):385–391. doi: 10.1016/s0171-2985(82)80096-x. [DOI] [PubMed] [Google Scholar]
- Gilman S. C., Schwartz J. M., Milner R. J., Bloom F. E., Feldman J. D. beta-Endorphin enhances lymphocyte proliferative responses. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4226–4230. doi: 10.1073/pnas.79.13.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Bromberg S., Staszak C., Kaszubowski P. A., Messner R. P., Neal J. F. Effect of physical stress on sensitivity of lymphocytes to inhibition by prostaglandin E2. J Immunol. 1981 Aug;127(2):518–522. [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Grabstein K., Dower S., Gillis S., Urdal D., Larsen A. Expression of interleukin 2, interferon-gamma, and the IL 2 receptor by human peripheral blood lymphocytes. J Immunol. 1986 Jun 15;136(12):4503–4508. [PubMed] [Google Scholar]
- Hasler F., Bluestein H. G., Zvaifler N. J., Epstein L. B. Analysis of the defects responsible for the impaired regulation of EBV-induced B cell proliferation by rheumatoid arthritis lymphocytes. II. Role of monocytes and the increased sensitivity of rheumatoid arthritis lymphocytes to prostaglandin E. J Immunol. 1983 Aug;131(2):768–772. [PubMed] [Google Scholar]
- Hazum E., Chang K. J., Cuatrecasas P. Specific nonopiate receptors for beta-endorphin. Science. 1979 Sep 7;205(4410):1033–1035. doi: 10.1126/science.224457. [DOI] [PubMed] [Google Scholar]
- Hirsch R. L., Panitch H. S., Johnson K. P. Lymphocytes from multiple sclerosis patients produce elevated levels of gamma interferon in vitro. J Clin Immunol. 1985 Nov;5(6):386–389. doi: 10.1007/BF00915335. [DOI] [PubMed] [Google Scholar]
- Ho W. K., Leung A. The effect of morphine addiction on concanavalin A-mediated blastogenesis. Pharmacol Res Commun. 1979 May;11(5):413–419. doi: 10.1016/s0031-6989(79)80005-3. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Schmeling D., Peterson P. K. Phagocytosis, bacterial killing, and metabolism by purified human lung phagocytes. J Infect Dis. 1981 Jul;144(1):61–71. doi: 10.1093/infdis/144.1.61. [DOI] [PubMed] [Google Scholar]
- Höllt V. Opioid peptide processing and receptor selectivity. Annu Rev Pharmacol Toxicol. 1986;26:59–77. doi: 10.1146/annurev.pa.26.040186.000423. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Torres B. A., Smith E. M., Dion L. D., Blalock J. E. Regulation of lymphokine (gamma-interferon) production by corticotropin. J Immunol. 1984 Jan;132(1):246–250. [PubMed] [Google Scholar]
- Kay N., Allen J., Morley J. E. Endorphins stimulate normal human peripheral blood lymphocyte natural killer activity. Life Sci. 1984 Jul 2;35(1):53–59. doi: 10.1016/0024-3205(84)90151-6. [DOI] [PubMed] [Google Scholar]
- Layon J., Idris A., Warzynski M., Sherer R., Brauner D., Patch O., McCulley D., Orris P. Altered T-lymphocyte subsets in hospitalized intravenous drug abusers. Arch Intern Med. 1984 Jul;144(7):1376–1380. [PubMed] [Google Scholar]
- Le J., Lin J. X., Henriksen-DeStefano D., Vilcek J. Bacterial lipopolysaccharide-induced interferon-gamma production: roles of interleukin 1 and interleukin 2. J Immunol. 1986 Jun 15;136(12):4525–4530. [PubMed] [Google Scholar]
- Lewis J. W., Shavit Y., Terman G. W., Gale R. P., Liebeskind J. C. Stress and morphine affect survival of rats challenged with a mammary ascites tumor (MAT 13762B). Nat Immun Cell Growth Regul. 1983;3(1):43–50. [PubMed] [Google Scholar]
- Lolait S. J., Clements J. A., Markwick A. J., Cheng C., McNally M., Smith A. I., Funder J. W. Pro-opiomelanocortin messenger ribonucleic acid and posttranslational processing of beta endorphin in spleen macrophages. J Clin Invest. 1986 Jun;77(6):1776–1779. doi: 10.1172/JCI112501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolait S. J., Lim A. T., Toh B. H., Funder J. W. Immunoreactive beta-endorphin in a subpopulation of mouse spleen macrophages. J Clin Invest. 1984 Jan;73(1):277–280. doi: 10.1172/JCI111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopker A., Abood L. G., Hoss W., Lionetti F. J. Stereoselective muscarinic acetylcholine and opiate receptiors in human phagocytic leukocytes. Biochem Pharmacol. 1980 May 15;29(10):1361–1365. doi: 10.1016/0006-2952(80)90431-1. [DOI] [PubMed] [Google Scholar]
- Mandler R. N., Biddison W. E., Mandler R., Serrate S. A. beta-Endorphin augments the cytolytic activity and interferon production of natural killer cells. J Immunol. 1986 Feb 1;136(3):934–939. [PubMed] [Google Scholar]
- Mannering G. J., Deloria L. B. The pharmacology and toxicology of the interferons: an overview. Annu Rev Pharmacol Toxicol. 1986;26:455–515. doi: 10.1146/annurev.pa.26.040186.002323. [DOI] [PubMed] [Google Scholar]
- Mathews P. M., Froelich C. J., Sibbitt W. L., Jr, Bankhurst A. D. Enhancement of natural cytotoxicity by beta-endorphin. J Immunol. 1983 Apr;130(4):1658–1662. [PubMed] [Google Scholar]
- McCain H. W., Lamster I. B., Bozzone J. M., Grbic J. T. Beta-endorphin modulates human immune activity via non-opiate receptor mechanisms. Life Sci. 1982 Oct 11;31(15):1619–1624. doi: 10.1016/0024-3205(82)90054-6. [DOI] [PubMed] [Google Scholar]
- McDonough R. J., Madden J. J., Falek A., Shafer D. A., Pline M., Gordon D., Bokos P., Kuehnle J. C., Mendelson J. Alteration of T and null lymphocyte frequencies in the peripheral blood of human opiate addicts: in vivo evidence for opiate receptor sites on T lymphocytes. J Immunol. 1980 Dec;125(6):2539–2543. [PubMed] [Google Scholar]
- Mehrishi J. N., Mills I. H. Opiate receptors on lymphocytes and platelets in man. Clin Immunol Immunopathol. 1983 May;27(2):240–249. doi: 10.1016/0090-1229(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Macrophage-mediated suppression. I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980 Feb;124(2):983–988. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. W. Leukotrienes and prostaglandins in the immune system. Adv Prostaglandin Thromboxane Leukot Res. 1986;16:113–134. [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Biagiotti R., Almerigogna F., Mingari C., Maggi E., Liang C. M., Moretta L. B cell growth factor activity of interferon-gamma. Recombinant human interferon-gamma promotes proliferation of anti-mu-activated human B lymphocytes. J Immunol. 1986 May 15;136(10):3513–3516. [PubMed] [Google Scholar]
- Schindler B. A. Stress, affective disorders, and immune function. Med Clin North Am. 1985 May;69(3):585–597. doi: 10.1016/s0025-7125(16)31034-3. [DOI] [PubMed] [Google Scholar]
- Sharp B. M., Keane W. F., Suh H. J., Gekker G., Tsukayama D., Peterson P. K. Opioid peptides rapidly stimulate superoxide production by human polymorphonuclear leukocytes and macrophages. Endocrinology. 1985 Aug;117(2):793–795. doi: 10.1210/endo-117-2-793. [DOI] [PubMed] [Google Scholar]
- Shavit Y., Lewis J. W., Terman G. W., Gale R. P., Liebeskind J. C. Opioid peptides mediate the suppressive effect of stress on natural killer cell cytotoxicity. Science. 1984 Jan 13;223(4632):188–190. doi: 10.1126/science.6691146. [DOI] [PubMed] [Google Scholar]
- Simon E. J. Recent studies on opioid receptors: heterogeneity and purification. Ann N Y Acad Sci. 1986;463:31–45. doi: 10.1111/j.1749-6632.1986.tb21501.x. [DOI] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecoma E. S., Huey L. Y. Psychic distress and the immune response. Life Sci. 1985 May 13;36(19):1799–1812. doi: 10.1016/0024-3205(85)90152-3. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Yip Y. K., Vilcek J. Interferon-gamma enhances expression of cellular receptors for tumor necrosis factor. J Immunol. 1986 Apr 1;136(7):2441–2444. [PubMed] [Google Scholar]
- Tubaro E., Borelli G., Croce C., Cavallo G., Santiangeli C. Effect of morphine on resistance to infection. J Infect Dis. 1983 Oct;148(4):656–666. doi: 10.1093/infdis/148.4.656. [DOI] [PubMed] [Google Scholar]
- Wakasugi N., Virelizier J. L., Arenzana-Seisdedos F., Rothhut B., Huerta J. M., Russo-Marie F., Fiers W. Defective IFN-gamma production in the human neonate. II. Role of increased sensitivity to the suppressive effects of prostaglandin E. J Immunol. 1985 Jan;134(1):172–176. [PubMed] [Google Scholar]
- Walker C., Kristensen F., Bettens F., deWeck A. L. Lymphokine regulation of activated (G1) lymphocytes. I. Prostaglandin E2-induced inhibition of interleukin 2 production. J Immunol. 1983 Apr;130(4):1770–1773. [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Westly H. J., Kleiss A. J., Kelley K. W., Wong P. K., Yuen P. H. Newcastle disease virus-infected splenocytes express the proopiomelanocortin gene. J Exp Med. 1986 Jun 1;163(6):1589–1594. doi: 10.1084/jem.163.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Appelboom T., Famaey J. P., Govaerts A. Suggestive evidence for receptors for morphine and methionine-enkephalin on normal human blood T lymphocytes. J Immunol. 1979 Sep;123(3):1068–1070. [PubMed] [Google Scholar]
- Wybran J. Enkephalins and endorphins as modifiers of the immune system: present and future. Fed Proc. 1985 Jan;44(1 Pt 1):92–94. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Zoschke D. C., Messner R. P. Suppression of human lymphocyte mitogenesis mediated by phagocyte-released reactive oxygen species: comparative activities in normals and in chronic granulomatous disease. Clin Immunol Immunopathol. 1984 Jul;32(1):29–40. doi: 10.1016/0090-1229(84)90040-0. [DOI] [PubMed] [Google Scholar]



