Abstract
Background
Organ-preserving chemo-radiotherapy (CRT) is the standard of care for non-metastatic anal squamous cell carcinoma (SCC). The optimal dosing schedules are yet to be determined. To improve local control rates, dose escalation has been investigated but found to not increase efficacy at the expense of increased toxicity for an unselected patient population.
Diffusion weighted imaging (DWI) and dynamic contrast enhanced (DCE) Magnetic Resonance Imaging (MRI) performed during CRT have early data suggesting it to be an effective tool in predicting later tumour response for SCC in related body sites.
By performing multi-parametric MRI (mpmMRI) incorporating standard morphological, DWI and DCE sequences, we aim to determine whether the early changes in multi-parametric parameters during CRT can predict for later response in anal SCC. This may create opportunities to investigate treatment adaptation, either intensification or de-escalation, during CRT.
Methods/Design
This protocol describes a prospective non-interventional multi-centre single-arm clinical trial. Twenty eligible patients with histologically confirmed non-metastatic anal SCC will receive standard definitive CRT and undergo multi-parametric MRI’s at the following 4 time points; prior to treatment, during the second and fourth weeks of treatment and 6-8 weeks following treatment.
Complete response will be defined by the absence of tumour persistence or recurrence as determined by clinical examination at 6 months.
Images will be retrospectively analysed to determine the apparent diffusion coefficient and tumour perfusion coefficients (Ktrans and Kep) at each time point. The Mann-Whitney-Wilcoxon Test will be utilised to compare the change in these parameters for responder’s verses non-responders.
Discussion
If validated, mpmMRI, along with other risk factors, can be used to stratify patients and guide radiation dosing in a prospective trial. Informed individualisation of treatment intensity should help us achieve our goals of improved efficacy and reduced toxicity.
Trial registration
Australian New Zealand Clinical Trials Registry (ANZCTR): ACTRN12614001219673 (19/11/2014).
Keywords: Anal neoplasms, Squamous Cell Carcinoma or SCC, Magnetic Resonance Imaging or MRI, Chemoradiotherapy, Diffusion weighted imaging or DWI, Dynamic contrast enhanced or DCE
Background
Anal cancer
Prevalence and risk factors
Anal cancer (AC) is an uncommon malignancy, representing 2.2% of all gastrointestinal cancers. However, the rate of AC is increasing [1]. This is likely due to the rising prevalence of its strongest risk factors – Human Papilloma Virus (HPV) and Human Immunodeficiency Virus (HIV) [2-5]. The majority of anal squamous cell carcinomas (SCCs) are associated with HPV infection and, in particular, the HPV-16 subtype [3,6].
Treatment
Pioneering data from the 1970s found that combined chemo-radiotherapy (CRT) could achieve a complete response in anal cancer [7]. Radical CRT has since become the standard of care for non-metastatic AC [8]. The long-standing combination of radiotherapy, 5-Fluorouracil (5-FU) and Mitomycin-C (MMC) has been validated in a series of large prospective randomised controlled trials [9-13].
Multiparametric MRI
Diffusion Weighted MRI (DW-MRI)
DW-MRI is a functional MRI technique that measures molecular diffusion resulting from normal Brownian motion of water protons within biological tissues [14]. Due to architectural differences, biological tissues are variably restrictive of diffusion. In particular, the densely cellular and disorganised architecture characteristic of cancer results in low molecular diffusion and therefore low signal response. Diffusion is measured quantitatively by the apparent diffusion coefficient (ADC).
Dynamic Contrast Enhanced MRI (dCE-MRI)
DCE-MRI is performed by obtaining sequential MRI images acquired before, during and after intravenous injection of paramagnetic contrast [15]. DCE-MRI measures the rate of contrast movement between the intravascular and extra-cellular extravascular space. This rate reflects tissue microvasculature permeability and perfusion. Cancer, with its abnormal neovasculature, tends to show characteristic changes in the signal intensity compared with normal tissues [16].
Rationale for the proposed study
Multiparametric MRI as a biomarker in anal cancer
Presently, CRT results in local failure rates of 14 to 37%, even in patients with early stage disease [17-19]. Although it is feasible to intensify the radiotherapy dose, this increases toxicity, and a recent randomized trial has shown that this dose escalation strategy is not beneficial for an unselected patient population [10].
Nigro et al. reported local control in 23 of 28 patients who received an intermediate radiation dose of 30 Gy in 15 fractions combined with 1000 mg/m2 of 5-FU delivered on days 1-5 and 29-33, and 15 mg/m2 of Mitomycin-C on day 1 only [20]. More recently, Hu et al. [21] and Hatfield et al. [22] found 30Gy sufficient to treat microscopic and small volume (<2 cm) macroscopic disease.
This suggests there are some anal cancers that require less than the current regimen of approximately 50-55 Gy, and others that require more. Adaptive radiotherapy using a biomarker would allow clinicians to tailor the treatment dose to an individual patient’s tumour response. Such a strategy could see decreased toxicity (perineal skin atrophy and fibrosis, sexual dysfunction, femoral neck fractures and persistent gastrointestinal disturbance such as sphincter dysfunction) and improved local control rates.
Diffusion-weighted MRI (DW-MRI) and dynamic contrast enhanced MRI (dCE-MRI) performed during CRT have early data suggesting it to be an effective tool in predicting later tumour response for both cervical and head & neck cancer [23,24]. As cervical and some head and neck SCCs share a common aetiology with anal SCC via Human Papilloma Virus (HPV), there are grounds to hypothesize that similar tumour response patterns may occur across these various malignancies when treated with organ preserving CRT. Application of this modality to anal cancer may allow adaptation of radiotherapy dosing, both intensification and de-escalation, to compensate for observed tumour biology.
Study hypothesis
DW-MRI and dCE-MRI performed during CRT for AC is predictive of later tumour response and prognostic of outcome.
Methods
Study design
This study is designed to be a single-arm, multicenter, prospective, observational trial to investigate the value of mpmMRI as an imaging biomarker in AC for later response to CRT.
Objectives
Primary study endpoint
Correlation of mpmMRI parameters with tumour response.
Secondary study endpoint
Determine the feasibility of performing mpmMRI during CRT for AC.
Study schematic
All patients will receive standardized CRT and have mpmMRI performed at the four time points of pre-treatment, weeks 2 and 4 of CRT, and then 6-8 weeks following completion of CRT (Figure 1).
Figure 1.
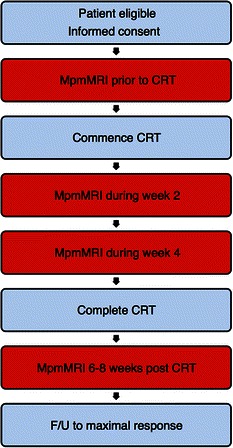
Study Schematic. MpmMRI = Multi-parametric magnetic resonance imaging, CRT = Chemo-radiotherapy, F/U = Follow-up.
Subject selection and withdrawal
Inclusion criteria
Patient capable of providing informed consent
Patient deemed suitable for protocol treatment as assessed by Radiation and Medical Oncologists
Histological diagnosis of invasive primary squamous cell carcinoma of the AC
- TNM Stage: T2-4 N0-3 M0 based on the following diagnostic work-up
- History & physical examination including:
-
i.Digital Rectal Exam (DRE) stating primary size and distance from anal verge
-
ii.Groin examination with documentation of any lymphadenopathy
-
i.
- Anal biopsy
- CT abdomen and pelvis
- Whole Body PET/CT
Age ≥18
Exclusion criteria
ECOG performance status >2
Significant comorbidities that would interfere with the completion of treatment
Renal insufficiency (Creatinine > 150)
Prior radiotherapy to the pelvis
Prior surgery for cancer of the anus that removed all macroscopic cancer
Prior systemic chemotherapy for anal cancer
Evidence of distant metastases (M1) if this precludes radical pelvic treatment
Women who are pregnant or lactating
- Inability to have a MRI due to:
- Implanted magnetic metal e.g. intraocular metal
- Pacemaker/Implantable defibrillator
- Extreme claustrophobia
Radiation therapy
The radiation technique must be one of either:
Intensity Modulated Radiation Therapy (IMRT)
Volumetric Modulated Arc Therapy (VMAT)
Tomotherapy
The treatment plan is at the discretion of the treating Radiation Oncologist and should be determined by analysis of the volumetric dose, the Dose Volume Histograms, planning target volume (PTV) and critical normal structures. An “inverse” planning method will be used with the aim of delivering dose to the PTV while maximally sparing the normal tissues.
Target prescription dose
Dose and fractionation for radical treatment is guided by the Australasian Gastrointestinal Trials Group (AGITG) guidelines [25]. The target prescription dose shall be determined as follows:
For T2N0 disease
The primary tumour PTV will receive 50.4 Gy in 28 fractions at 1.8 Gy per fraction
The uninvolved nodal PTVs will receive 42 Gy in 28 fractions at 1.5 Gy per fraction
For T3-4 N0 disease
The primary tumour PTV will receive 54 Gy in 30 fractions at 1.8 Gy per fraction.
The uninvolved nodal PTVs will receive 45 Gy in 30 fractions at 1.5 Gy per fraction.
For N1-3 disease:
The primary tumour PTV will receive 54 Gy in 30 fractions at 1.8 Gy per fraction.
For involved nodes ≤ 3 cm in maximum dimension, the involved nodal PTV will receive 50.4 Gy in 30 fractions at 1.68 Gy per fraction.
For involved nodes > 3 cm in maximum dimension, the involved nodal PTV will receive 54 Gy in 30 fractions at 1.80 Gy per fraction.
Dose specifications
The following dose specifications are recommended:
98% of the relevant PTV should receive >95% of the prescription dose
No more than 2% of the relevant PTV should receive >107% of the prescription dose
Treatment schedule
Treatment will be delivered once daily on weekdays, 5 days per week except on public holidays. Missed fractions will be made up for at the end of treatment at the discretion of the treating clinician. All PTVs will be treated simultaneously. Treatment breaks will be avoided, if possible, or minimised.
Treatment planning
Target volume definitions are as per ICRU Reports 50, 62 and 83. Treatment planning is as per the Australasian Gastrointestinal Trials Group (AGITG) Contouring Atlas and Planning Guidelines for Intensity-Modulated Radiotherapy in Anal Cancer [25]. This will include elective nodal irradiation of the mesorectum, presacral space, ischiorectal fossa, inguinal, obturator, internal and external iliac lymph nodes,
Gross tumour volumes (GTV)
The gross disease is determined by physical examination, CT, PET and/or MRI.
Clinical target volume (CTV)
The primary CTV must encompass:
GTV
Entire anal canal from the ano-rectal junction to the anal verge
Internal and external anal sphincters
A further 10-20 mm isotropic margin should be added to items (1), (2), and (3) above, to account for microscopic disease, while respecting anatomical boundaries. For the involved nodes or nodal regions, a 10-20 mm margin should be used, respecting anatomical boundaries.
Planning target volume (PTV)
An isotropic 10 mm expansion is recommended on CTVs to generate PTVs. Daily image guidance is recommended, which may allow CTV-PTV margin reduction to 5-7 mm.
Dose constraints
The following normal tissue dose constraints are recommended. Where available, values are taken from the QUANTEC papers. Where not available for that organ, dose constraints are listed as per the RTOG 0529 closed study protocol (Table 1).
Table 1.
Recommended organ at risk dose constraints
| ORGAN | CONSTRAINTS: No More than | ||
|---|---|---|---|
| Small Bowel | 195 cc above 45 Gy | 1% of small bowel > 52 Gy | |
| Femoral Head | 50% above 30 Gy | 35% above 40 Gy | 5% above 44 Gy |
| Iliac Crests | 50% above 30 Gy | 35% above 40 Gy | 5% above 50 Gy |
| External Genitalia | 50% above 20 Gy | 35% above 30 Gy | 5% above 40 Gy |
| Bladder | 50% above 55 Gy | ||
| Large Bowel | 50% above 50 Gy | ||
Chemotherapy
Concurrent chemotherapy will begin on the first day of radiotherapy. The second course of chemotherapy will commence on calendar day 29 [4,9].
5-Fluorouracil (5-FU)
5-FU shall be delivered at a dose of 800-1000 mg/m2/day via the IV route for 96 hours continuously starting on day 1 and repeated on day 29. In the instance of an unplanned treatment break, the second cycle of 5-FU shall be delivered on the 29th day of radiotherapy treatment.
Mitomycin-C
Mitomycin-C shall be delivered at a dose of 10 mg/m2 (without exceeding a maximal single dose of 20 mg) via the IV route on day 1 +/- day 29, depending on local practice.
Pathology
All biopsy tissues will be formalin fixed, paraffin embedded and routine H&E stained. Immunohistochemical p16 staining is to be performed for all tumours as recent data suggests that p16 positivity correlates with HPV status and is associated with reduced relapse rates and improved overall survival [26].
Follow-up and surgery
At 6-8 weeks post CRT, the patient will have a mpmMRI performed.
The follow-up schedule is at the discretion of the treating clinician. However, the following suggestions apply:
Progressive disease
Biopsy
○ If negative, reassess in 4 weeks
○ If positive and no evidence of distant disease, consideration of abdominoperineal resection (APR) is recommended
Persistent disease
No biopsy, reassess in 4 weeks
Patients with clinical suspicion of persistent disease at 26 weeks should undergo a biopsy and consideration of APR, if positive.
Complete clinical response
No biopsy
Continue to follow-up at the discretion of treating clinician
Imaging
Imaging schedule
MpmMRI consists of standard morphological MRI, DW-MRI and dCE-MRI. Patients will undergo mpmMRI at the following four time points:
Prior to CRT
During the second week of treatment (fraction days 6-10)
During the fourth week of treatment (fraction days 16-20)
At 6-8 weeks post treatment
Imaging process
MRI’s are performed on a 3 Tesla device. Patients are scanned in the supine position. No rectal coil is used. All patients should have a single IV bolus of Buscopan (20 mg/ml) immediately prior to the first sequence.
Diffusion weighted imaging
- Performed at 4 b-values
- ○ 0, 400, 800 and 1200
Dynamic contrast enhanced imaging
- Contrast injection:
- ○ Magnevist 0.2 ml/kg
- ○ Power Injector (2.5 ml/s)
- ○ 20 ml saline chase at same rate as injection
The eGFR must be checked prior to the MRI to ensure eligibility for full contrast injection. Half doses are not permitted.
Imaging analysis
Standard morphological MRI (SM-MRI)
All images will be assessed independently by two radiologists to determine primary and nodal tumour dimensions. Where there is disagreement, a third will be asked to mediate.
Diffusion weighted MRI (DW-MRI)
A Region Of Interest (ROI) will be placed over primary and involved nodal regions to calculate mean and median primary and nodal ADC values
Dynamic contrast enhanced MRI (dCE-MRI)
A ROI will be placed over the entire primary and involved nodal regions to calculate mean and median primary and nodal Ktrans and Kep values and Relative Signal Intensity (RSI) (Figure 2)
Figure 2.
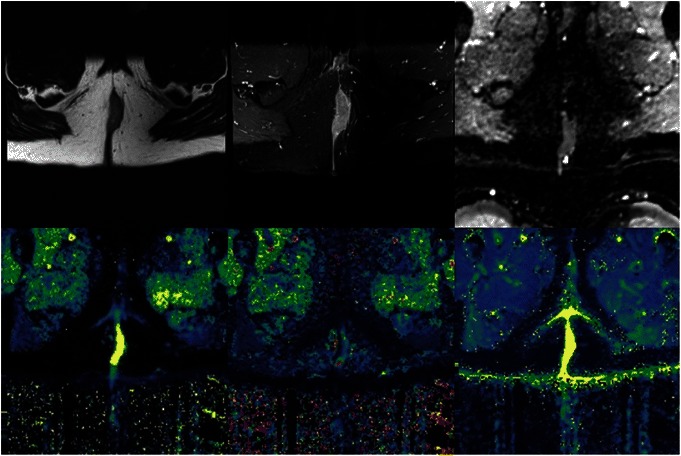
Anal cancer. Morphological MRI (top left and top middle), apparent diffusion coefficient map (top right), dynamic contrast enhanced parametric maps (bottom, from left to right): KTrans, Kep and Ve.
Statistical considerations
Sample size determination
Assuming that 70% of patients are positive responders, then sample sizes of 14 responders and 6 non-responders will achieve between 70% and 80% power to show a difference in mean change (initial to final) in SM-MRI of between 1.2 and 1.4 standard deviations at the 0.05 significance level (alpha) using a two-sided Mann-Whitney-Wilcoxon Test. Previous studies in other body locations have shown a positive result with similar patient numbers. We anticipate recruitment to be achieved within 24 months.
Definition of complete response
No evidence of residual tumour at 26 weeks post CRT
No progression requiring APR prior to 26 weeks
Ethical considerations
This protocol along with the informed consent document and patient information sheet has received ethics approval from the Hunter New England Human Research Ethics Committee (HREC). The protocol also has radiation safety approval.
Study finances
This study has been funded by both the Hunter Translational Cancer Research Unit (HTCRU) and the Royal Australian and New Zealand College of Radiologists (RANZCR), each with a $20,000 competitive research grant (Total = $40,000). Neither the HTCRU nor the RANZCR have been involved in the writing of this protocol or will have any influence on the analysis or publication of the study.
Discussion
There is no consensus on the optimal radiation dose for the treatment of patients with AC. It is very likely that small tumours are often over-treated and large tumours sometimes under-treated. Although TNM staging is highly prognostic for AC, there is still significant heterogeneity in outcomes within a particular stage. Improved prognostication may be achieved with further information such as HPV status and mpmMRI tumour response. If this exploratory phase 2 study finds compelling evidence for an imaging biomarker being independently predictive of later tumour response, a subsequent study would aim to validate this by intensifying radiotherapy dose for tumours with unfavourable biology, and deescalating radiotherapy dose for favorable tumours. If validated, an imaging biomarker for response to CRT would allow clinicians to adapt and personalise treatment, which holds the potential for improved efficacy and reduced toxicity.
Acknowledgements
This study is funded by grants from the Hunter Translational Cancer Research Unit (HTCRU) and Royal Australian and New Zealand College of Radiologists (RANZCR). The granting bodies are not involved in data collection or analysis.
We would also like to thank Dr Mahesh Kumar, Dr Anne Capp, Dr Swetha Sridharan, Dr Mark Lee, Dr Allan Fowler, Prof Steve Ackland, Dr Girish Mallesara, Dr Fiona Day, Dr Peter Lau, Dr Kin Men Leong, Assoc Prof Gary Liney and Dr Christopher Oldmeadow.
Abbreviations
- 5-FU
5-fluorouracil
- AC
Anal cancer
- ADC
Apparent diffusion coefficient
- AGITG
Australasian gastro-intestinal trials group
- APR
Abdomino-pelvic resection
- CRT
Chemo-radiotherapy
- CT
Computed tomography
- CTV
Clinical target volume
- DCE
Dynamic contrast enhanced
- DRE
Digital Rectal examination
- DWI
Diffusion weighted imaging
- ECOG
Eastern cooperative oncology group performance status
- eGFR
Estimated glomerular filtration rate
- GTV
Gross tumour volume
- HIV
Human influenza virus
- HPV
Human papilloma virus
- HREC
Human resource ethics committee
- HTCRU
Hunter translational cancer research unit
- IMRT
Intensity modulated radiotherapy
- MMC
Mitomycin-C
- mpmMRI
Multi-parametric MRI
- MRI
Magnetic resonance imaging
- NS
Normal saline
- PET
Positron emission tomography
- PICC
Peripherally inserted central catheter
- PTV
Planning target volume
- QUANTEC
Quantitative analyses of normal tissue effects in the clinic
- RANZCR
Royal Australian and New Zealand college of radiologists
- ROI
Region of interest
- RSI
Relative signal intensity
- RTOG
Radiation therapy oncology group
- SCC
Squamous cell carcinoma
- SIA
Subject identification number
- SM
Standard morphological
- VMAT
Volumetric modulated arc therapy
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MJ wrote the study protocol. JM conceived of the study, and participated in its design and coordination and helped to draft the manuscript. PS and JA designed the imaging protocol. SG is the principle trial coordinator. GH is the principle investigator at the Chris O’Brien Lifehouse. KW is the principle investigator at the Liverpool Hospital. All authors participated in the design of the study. All authors read and approved the final manuscript.
Contributor Information
Michael Jones, Email: mpjones85@gmail.com.
George Hruby, Email: George.Hruby@lh.org.au.
Peter Stanwell, Email: peter.stanwell@newcastle.edu.au.
Sarah Gallagher, Email: Sarah.Gallagher@calvarymater.org.au.
Karen Wong, Email: Karen.Wong@sswahs.nsw.gov.au.
Jameen Arm, Email: jameen.arm@hnehealth.nsw.gov.au.
Jarad Martin, Email: jarad.martin@calvarymater.org.au.
References
- 1.Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer. 2004;101:281–8. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 2.Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–80. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 3.Frisch M, Glimelius B, Van den Brule AJ, Wohlfahrt J, Meijer CJ, Walboomers JM, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med. 1997;337:1350–8. doi: 10.1056/NEJM199711063371904. [DOI] [PubMed] [Google Scholar]
- 4.Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med. 2000;342:792–800. doi: 10.1056/NEJM200003163421107. [DOI] [PubMed] [Google Scholar]
- 5.Uronis HE, Bendell JC. Anal cancer: an overview. Oncologist. 2007;12:524–34. doi: 10.1634/theoncologist.12-5-524. [DOI] [PubMed] [Google Scholar]
- 6.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124:2375–83. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 7.Nigro ND, Vaitkevicius VK, Considine B. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum. 1974;17:354–6. doi: 10.1007/BF02586980. [DOI] [PubMed] [Google Scholar]
- 8.Glynne-Jones R, Northover JMA, Cervantes A, on behalf of the ESMO Guidelines Working Group Anal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v87–92. doi: 10.1093/annonc/mdq171. [DOI] [PubMed] [Google Scholar]
- 9.UKCCCR Anal Cancer Trial Working Party Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–54. doi: 10.1016/S0140-6736(96)03409-5. [DOI] [PubMed] [Google Scholar]
- 10.Peiffert D, Tournier-Rangeard L, Gerard JP, Lemanski C, Francois E, Giovannini M, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol. 2012;30:1941–8. doi: 10.1200/JCO.2011.35.4837. [DOI] [PubMed] [Google Scholar]
- 11.Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, III, Thomas CR, Jr, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal. JAMA. 2008;299:1914–21. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 12.Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–39. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 13.Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–9. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 14.Koh D, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. Am J Roentgenol. 2007;188:1622–35. doi: 10.2214/AJR.06.1403. [DOI] [PubMed] [Google Scholar]
- 15.Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293–8. doi: 10.1200/JCO.2006.06.8080. [DOI] [PubMed] [Google Scholar]
- 16.Zahra MA, Hollingsworth KG, Sala E, Lomas DJ, Tan LT. Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncol. 2007;8:63–74. doi: 10.1016/S1470-2045(06)71012-9. [DOI] [PubMed] [Google Scholar]
- 17.Martenson JA, Lipsitz SR, Lefkopoulou M, Engstrom PF, Dayal YY, Cobau CD, et al. Results of combined modality therapy for patients with anal cancer (E7283): an Eastern Cooperative Oncology Group study. Cancer. 1995;76:1731–6. doi: 10.1002/1097-0142(19951115)76:10<1731::AID-CNCR2820761009>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Peiffert D, Seitz JF, Rougier P, Francois E, Cvitkovic F, Mirabel X, et al. Preliminary results of a phase II study of high-dose radiation therapy and neoadjuvant plus concomitant 5-fluorouracil with CDDP chemotherapy for patients with anal canal cancer: a French cooperative study. Ann Oncol. 1997;8:575–81. doi: 10.1023/A:1008295119573. [DOI] [PubMed] [Google Scholar]
- 19.Gerard JP, Ayzac L, Hun D, Romestaing P, Coquard R, Ardiet JM, et al. Treatment of anal canal carcinoma with high dose radiation therapy and concomitant fluorouracil-cisplatinum. Long-term results in 95 patients. Radiother Oncol. 1998;46:249–56. doi: 10.1016/S0167-8140(97)00192-8. [DOI] [PubMed] [Google Scholar]
- 20.Nigro ND, Seydel HG, Considine B, Vaitkevicius VK, Leichman L, Kinzie JJ. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer. 1983;51:1826–9. doi: 10.1002/1097-0142(19830515)51:10<1826::AID-CNCR2820511012>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Hu K, Minsky BD, Cohen AM, Kelsen DP, Guillem JG, Paty PP, et al. 30 Gy may be an adequate dose in patients with anal cancer treated with excisional biopsy followed by combined-modality therapy. J Surg Oncol. 1999;70:71–7. doi: 10.1002/(SICI)1096-9098(199902)70:2<71::AID-JSO2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 22.Hatfield P, Cooper R, Sebag-Montefiore D. Involved-field, low-dose chemoradiotherapy for early-stage anal carcinoma. Int J Radiat Oncol Biol Phys. 2008;70:419–24. doi: 10.1016/j.ijrobp.2007.06.072. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Loevner L, Quon H, Sherman E, Weinstein G, Kilger A, et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to Chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15:986–94. doi: 10.1158/1078-0432.CCR-08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somoye G, Harry V, Semple S, Plataniotis G, Scott N, Gilbert FJ, et al. Early diffusion weighted magnetic resonance imaging can predict survival in women with locally advanced cancer of the cervix treated with combined chemo-radiation. Eur Radiol. 2012;22:2319–27. doi: 10.1007/s00330-012-2496-0. [DOI] [PubMed] [Google Scholar]
- 25.Ng M, Leong T, Chander S, Chu J, Kneebone A, Carroll S, et al. Australasian Gastrointestinal Trials Group (AGITG) contouring atlas and planning guidelines for intensity-modulated radiotherapy in anal cancer. Int J Radiat Oncol Biol Phys. 2012;83:1455–62. doi: 10.1016/j.ijrobp.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert DC, Williams A, Allan K, Stokoe J, Jackson T, Linsdall S, et al. p16INK4A, p53, EGFR expression and KRAS mutation status in squamous cell cancers of the anus: correlation with outcomes following chemo-radiotherapy. Radiother Oncol. 2013;109:146–51. doi: 10.1016/j.radonc.2013.08.002. [DOI] [PubMed] [Google Scholar]


