Abstract
Background
Solena heterophylla Lour. has traditionally been used in the management of diseases pertaining to gastrointestinal, respiratory and vascular system and present study was undertaken to validate its traditional uses.
Methods
The aqueous ethanolic extract of Solena heterophylla Lour (Sh.Cr) was tested in-vitro on isolated rabbit jejunum, tracheal and aorta preparations. The responses of tissues were recorded using isotonic transducers coupled with PowerLab data acquisition system.
Results
The aqueous ethanolic extract of Solena heterophylla Lour (Sh.Cr) (0.03-1.0 mg/ml) on application to spontaneous contractions in isolated rabbit jejunum preparation exerted relaxant effect through decrease in magnitude and frequency of contractions, caused relaxation of K+(80 mM)-induced contractions and shifted the Ca2+ concentration response curves toward right in isolated rabbit jejunum preparations in a manner similar to verapamil (a standard Ca2+ channel blocker), thus confirming its Ca2+ channel blocking activity. The Sh.Cr also caused relaxation of carbachol (1 μM)- and K+(80 mM)-induced contractions in isolated rabbit tracheal preparations in a manner comparable to dicyclomine.
Conclusions
The observed relaxant effect may be outcome of anti-muscarinic and Ca2+ channel blocking activities. The Sh.Cr (0.03-1.0 mg/ml) against phenyephrine (1 μM)- and K+(80 mM)-induced contractions in isolated rabbit aortic preparations exerted a relaxant effect, possibly through Ca2+ channel blocking activity. These findings provide a rationale for the folkloric uses of the plant in the management of ailments pertaining to gastrointestinal, respiratory and vascular system.
Keywords: Solena heterophylla Lour, Spasmolytic activity, Bronchodilator activity, Vasorelaxant activity
Introduction
Solena heterophylla Lour. (Cucurbitaceae) is a climber plant, distributed widely in India, Pakistan, Afghanistan, Indonesia, Malaysia, Myanmar, Nepal, China, Thailand and Vietnam [1-3]. The plant grows in mixed forests, thickets grasslands, roadsides, and mountain slopes at an altitude of 600–2600 m [4]. The roots are fusiform, about 1.5-2 cm in diameter. The stem and branches are glabrous, petiole are slender, 4–10 mm, puberulent at first but subsequently glabrescent. The leaf blade are variable, ovate, oblong, ovate-triangular, or hastate, undivided or 3-5-lobed, leathery; lobes oblong-lanceolate, lanceolate, or triangular, 8–12 × 1–5 cm2, abaxially densely setose or almost glabrous, adaxially densely setose or scabrous, base cordate, margin entire or dentate, apex obtuse or acuminate. Tendrils are slender. The male flowers are umbellate or sub-umbellate; peduncle very short, apically 10-20-flowered; pedicels 2–8 mm; calyx tube 3–5 mm, 3 mm in diameter; segments subulate, 0.2-0.4 mm; corolla yellow or yellow-white; segments triangular, 1–1.5 mm, apex obtuse or acute; filaments filiform, about 3 mm; anther cells curved or conduplicate, puberulent. The female flowers are usually solitary; pedicel 2–10 mm, puberulent; female calyx and corolla are similar to male flowers; ovary ovoid, 2.5-3.5 × 2–3 mm2; stigmas 3. The fruits are red-brown, broadly ovoid, oblong, or sub-globose, 2–6 × 2–5 cm2. The seeds are gray-white or gray-brown, sub-orbicular or obovate, 5–7 × 5–6.5 mm2, smooth or slightly tuberculate. Flowering occurs in May-August and fruiting in June-November [5].
Various parts of this plant possess anti-malarial, anti-diabetic, analgesic, sedative and purgative properties and used to treat toothache, rheumatism and respiratory disorders [6,7]. It is believed to possess invigorating and stimulant properties. The fruits have traditionally been used in the management of common cold, child pneumonia, throat pain and fever; the leaves are applied over inflamed skin, whereas root juice has been used to treat dysuria and spermatorrhoea [4,8]. Phytochemical investigations revealed the presence of behemic acid, columbin and lignoceric acid as plant constituents [4,9]. Scientific investigations on plant extract revealed its hepatoprotective potential, while coumarin and flavonoids isolated from plant were found to inhibit platelet aggregation [10-12]. Moreover, recent study has reported in-vitro and in-vivo antioxidant activity of methanolic extract of Solena whole plant [13] as well as methanolic extract of leaf and stem of S. heterophylla [14]. S. heterophylla has traditionally been used for the management of gastrointestinal, respiratory and cardiovascular ailments [15,16], but no study exists on validation of these activities. As part of series of experiments in our laboratory on validating tagged biological and physiological activities of medicinal plants [17-22], the current study was designed to investigate and validate the therapeutic potential of S. heterophylla in cardiovascular, respiratory and gastrointestinal ailments.
Materials and methods
Plant material
The aerial parts of Solena heterophylla were collected from hilly areas of Nathia Gali (Abbottabad), Pakistan in August 2011. The plant was authenticated by the expert taxonomist Prof. Dr. Altaf Ahmad Dasti, Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan, Pakistan and a voucher specimen in preserved in the same University. The plant material was rendered free of foreign contamination by manual picking and allowed to be dried in shade. The dried herbal material was grinded into coarse powder by means of a herbal grinder and was subjected to maceration by soaking (1000 g) in aqeous-ethanolic (70%) mixture in amber colour glass container at room temperature for 7 days with occasional shaking. The material was passed through a muslin cloth and fluid obtained was filtered through Whatmann-1 filter paper. The filtrate was evaporated on a rotary evaporator (Büchi R-200 Switzerland) attached with a vacuum pump (Büchi Vac V-500) and re-circulating chiller (B-740) at 37°C under reduced pressure to a thick dark green paste of semi solid consistency, with an approximate yield of 23%. The extract was stored in air tight jar and all the dilutions were made fresh on the day of experiment [23,24].
Chemicals
Acetylcholine chloride, carbachol, potassium chloride, verapamil hydrochloride, phenylephrine, magnesium chloride, ethylene tetra-acetic acid (EDTA) were purchased from Sigma Chemicals Co. (St Louis, MO, USA). Calcium chloride, glucose, magnesium sulphate, potassium dihydrogen phosphate, sodium bicarbonate, sodium dihydrogen phosphate and methanol were obtained from Merck (Darmstadt, Germany). Ammonium hydroxide, sodium chloride, and sodium hydroxide were purchased from BDH Laboratory supplies (Poole, England). The chemicals used in these experiments were of highest purity and reagent analytical grade. Stock solutions and subsequent dilutions were made in distilled water on the day of experiment. The drugs were made soluble in vehicles which were without any effect on tissues in control experiments.
Experimental animals and housing conditions
Animals (♂/♀) used in this study were local strain rabbits (1.0-1.8 kg). These were housed under controlled environmental condition (23–25°C) at the animal house of Faculty of Pharmacy, Bahauddin Zakariya University, Multan. The animals were provided with standard food and tap water ad libitum. The animals were deprived of food 24 hr prior to the experiments but were given free access to water. Rabbits were sacrificed following a blow on back of head to be used for in vitro studies. All the experiments performed complied with the rulings of Institute of Laboratory Animal Resources, Commission on Life Sciences [25]. The experimental protocols regarding current study were submitted to and approved by the ethical committee meeting held on 16-02-2011 via Notification Number EC/04/2011 dated of the Department of Pharmacy, Bahauddin Zakariya University, Multan.
In vitro experiments
The experiments on isolated tissues were performed by procedures previously described [17-21]. Briefly, we used freshly prepared jejunum, tracheal and aortic tissue segments from the rabbit and maintained adequately in the respective buffer solutions. The detailed elaboration of each tissue extraction procedure is described below under the respective heading of tissue of interest.
Isolated rabbit jejunum preparations
The crude ethanolic extract of S. heterophylla (Sh.Cr) was tested for the possible presence of either spasmolytic or spasmogenic activity by using isolated rabbit jejunum preparations. Isolated rabbit jejunum segments of approximately 2 cm in length were suspended in isolated tissue baths containing Tyrode’s solution, at 37°C, aerated with carbogen (95% O2 and 5% CO2). The composition of the Tyrode’s solution (mM) was: KCl (2.68), NaCl (136.9), MgCl2 (1.05), NaHCO3 (11.90), NaH2PO4 (0.42), CaCl2 (1.8) and glucose (5.55). A preload of 1 gm was applied and intestinal responses were recorded through an isotonic transducer by Power Lab Data Acquisition System (AD Instruments, Sydney, Australia) attached to a computer installed with a Lab Chart Software (Version 6). The tissues were allowed to equilibrate for at least 30 min prior to the addition of any drug. Isolated rabbit jejunum preparations exhibit spontaneous rhythmic contractions and allow testing of the antispasmodic (relaxant) effect without application of an agonist [26]. The observed response of the test material was quantified by the application of doses in a cumulative fashion. The relaxant effects on the part of the test substances were taken as the percent change in spontaneous contractions of the preparation recorded immediately before the addition of test substances.
The possible mechanism of the relaxant activity of the test materials were investigated through the relaxation of the observed sustained spasmodic contractions following the exposure to high concentration of K+(80 mM) [27]. The test materials were applied in a cumulative manner to the sustained contractions to achieve concentration-dependent inhibitory responses [28]. The observed relaxant effect of the test materials on K+ (80 mM)-induced contraction was expressed as percent of the control contractile response.
Calcium channel blocking effect of the test substances were confirmed by the method described by Gilani et al. [26]. The isolated rabbit jejunum preparations were allowed to stabilize in normal Tyrode’s solution, which were subsequently replaced for 30 min with Ca2+-free Tyrode’s solution to which EDTA (0.1 mM) was added in order to remove calcium from the tissues. This bath solution was further replaced with K+-rich and Ca2+-free Tyrode’s solution, having the following composition (mM): KCl (50), NaCl (91.04), MgCl2 (1.05), NaHCO3 (11.90), NaH2PO4 (0.42), glucose (5.55) and EDTA (0.1). Subsequent to an incubation period of 30 min., cumulative Ca2+ concentrations were applied to the tissue bath to obtain control calcium dose–response curves (DRCs). On achievement of the super-imposable control calcium dose–response curves (usually after two cycles), the tissues were then washed and allowed to equilibrated with the plant extract for 1 hr and then the concentration response curves of Ca2+ were recorded and compared to the control curves. The DRCs of Ca2+ were recorded in the presence of different concentrations of the plant extracts in tissue bath.
Isolated rabbit tracheal preparations
The rabbit tracheas were dissected out and kept in Krebs solution with the following composition (mM): NaCl (118.2), NaHCO3 (25.0), CaCl2 (2.5), KCl (4.7), KH2PO4 (1.3), MgSO4 (1.2) and glucose (11.7). The trachea was cleaned free from the surrounding fatty tissues and rings of 2–3 mm width containing 2–3 cartilages were prepared. Each ring was opened by a longitudinal incision on the ventral side opposite to the smooth muscles layer to form a strip with smooth muscles layer in middle and cartilages on both sides. These tracheal preparations were mounted in 20 ml organ bath containing Krebs solution being maintained at 37°C and aerated with carbogen. A preload tension of 1 g was applied and tissue preparations were allowed to be equilibrated for 1 hour prior to any challenge by the drug. Tissue preparations were stabilized by repeated applications of carbachol (1 μM) until constant responses were recorded. The carbachol (1 μM)- and high K+(80 mM)-induced sustained contractions were subsequently used for testing of different doses of the test material in a cumulative fashions. The isometric responses were recorded through a Power Lab Data Acquisition System (AD Instruments, Sydney, Australia) attached to a computer installed with a Lab Chart Software (Version 6). The standard drug with Ca2+ channel blocking effect (verapamil) was tested on high K+(80 mM)- and carbachol- induced spastic contractions in order to confirm the possible mechanism of action.
Isolated rabbit aorta preparation
The effect of Sh.Cr on systemic vascular resistance was assessed on isolated rabbit aorta preparations. Rabbits of either sex were sacrificed by a blow on the back of head and descending thoracic aorta was dissected out and kept in the normal Krebs solution having composition as above described. It was then cut vertically in 2–3 mm width segments. Each isolated tissue segment was then hung in a tissue organ bath (Radnoti) containing Kreb’s solution aerated with carbogen (95% oxygen and 5% carbon dioxide) at 37°C. A pre-load of 2 g was applied to each preparation and allowed to equilibrate for a period of 1 hr. After equilibration, tissue was stabilized by repeated exposure to K+ (80 mM) or phenylephrine (1 μM) depending upon the protocol of the experiment. The vasorelaxant/vasoconstrictive effects of the test substances were studied by addition in tissue organ baths containing pre-stabilized tissue in a cumulative manner. Changes in isometric tension of aortic rings were obtained via fa orce-displacement transducer (Model FORT100, WPI, USA) coupled to a Power Lab data acquisition system (AD Instruments, Sydney, Australia) and a computer running Lab Chart software (version 6).
Statistical analysis
Data are expressed as mean ± S.E.M. (n = 5 of individual experiments) and median effective concentration (EC50) are given with 95% confidence intervals (CI) and the logarithmic dose response curves of different treatments were then plotted using the Computer software “Graphpad Prism” (Graph Pad Software, San Diego, CA, USA).
Results
Effect of Sh.Cr on isolated rabbit jejunum preparations
The aqueous ethanolic extract of Solena heterophylla Sh.Cr when applied to spontaneous contractions in isolated rabbit jejunum preparations, exerted a relaxant effect in tissue bath concentration-dependent manner, in concentration range of 0.03-1.0 mg/ml, with an EC50 value of 0.07002 mg/ml (95% CI: 0.08482-0.1644 mg/ml, n = 5) (Figures 1 and 2). The application of Sh.Cr to K+ (25 mM)-induced spastic contractions in isolated rabbit jejunum preparations, resulted in minor relaxant response with an EC50 value of 0.3226 mg/ml (95% CI: 0.01782-0.3679 mg/ml, n = 5) (Figure 2a and c), whereas the application to K+ (80 mM)-induced spastic contractions caused a complete relaxation, with an EC50 value of 0.06824 mg/ml (95% CI: 0.1459-0.2781, n = 5) (Figure 2b and c). Similarly, verapamil also relaxed the spontaneous and K+ (80 mM)-induced contractions in isolated rabbit jejunum preparations, with respective EC50 values of 0.795 μM (95% C.I: 0.588- 1.105 μM; n = 5) and 0.4511 μM (95% C.I: 0.2944-0.6787 μM; n = 5) (Figure 2d). Moreover, the pretreatment of isolated rabbit jejunum preparations with Sh.Cr (0.1-1.0 mg/ml) caused a rightward shift of Ca2+ concentration response curves in a manner similar to that produced by verapamil (Figure 3).
Figure 1.
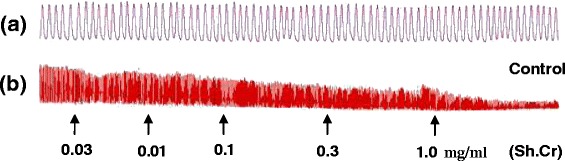
Tracings showing (a) the spontaneous contraction of isolated rabbit jejunum and (b) the spasmolytic effect of the crude ethanol extract of Solena heterophylla (Sh.Cr). Plant extract was added in cumulative manner and values listed were the final tissue bath concentrations, (n = 5).
Figure 2.
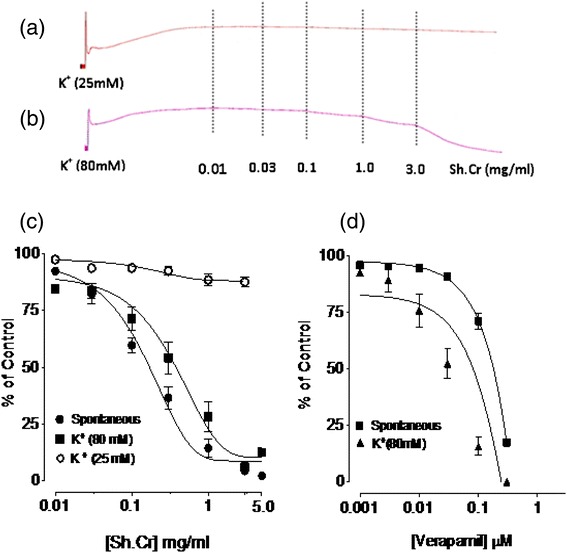
Concentration dependent effect of (a,b,c) the ethanol extract of Solena heterophylla (Sh.Cr) and (d) verapamil on spontaneous, low K+ (25 mM) and high K + (80 mM)-induced contractions in isolated rabbit jejunum preparations. Values are the mean ± SEM, n = 5.
Figure 3.
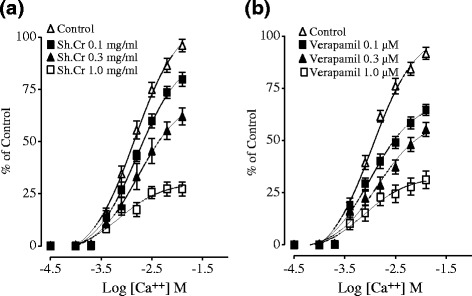
Effect of (a) the ethanol extract of Solena heterophylla Lour (Sh.Cr) and (b) verapamil on concentration response curves of Ca2+ in isolated rabbit jejunum preparations. Values are the mean ± SEM, n = 5.
Effect of Sh.Cr on isolated rabbit tracheal preparations
The application of Sh.Cr on isolated rabbit tracheal preparations did not produce any response (not shown), however, it exerted a relaxant effect on the carbachol (CCh; 1 μM) and K+ (80 mM) induced contractions (Figure 4a,b and c) with respective EC50 values of 0.06550 mg/ml (95% CI: 0.09267- 0.1721 mg/ml, n = 5) and 0.06926 mg/ml (95% CI: 0.1422-0.2761 mg/ml, n = 5). The comparison of the above mentioned values shows that EC50 of Sh.Cr for CCh-induced contractions is numerically minor if compared to EC50 value of Sh.Cr for K+ (80 mM)-induced contractions in isolated rabbit tracheal preparations. For this reason, it is possible that some components of Sh.Cr exerted their relaxant effect through the blockade of muscarinic receptors, whereas remaining components may contribute to the relaxant effect through th eblockade of Ca2+ channels in a manner comparable to dicyclomine, which caused relaxation of CCh and K+ (80 mM) –induced contractions with EC50 values of 0.08764 μM (95% CI: 0.05573-0.1317; n = 5) and 0.08846 μM (95% CI: 0.04268-0.09845; n = 5), respectively (Figure 4).
Figure 4.
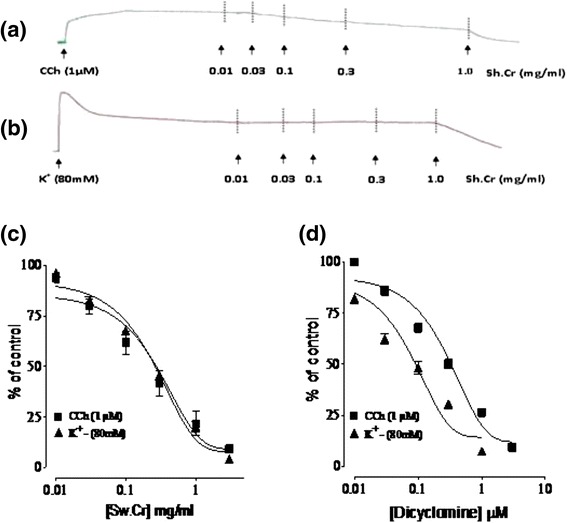
Concentration dependent inhibitory effect of (a, b and c) the ethanol extract of Solena heterophylla (Sh.Cr) and (d) dicyclomine on carbachol (1 μM)- and high K+ (80 mM)- induced contractions in isolated rabbit tracheal preparations. Values are the mean ± SEM, n = 5.
Effect of Sh.Cr on isolated rabbit aorta preparations
Sh.Cr did not exert any effect on isolated rabbit aorta preparation in isolated tissue bath concentration range of 0.01-5 mg/ml (Figure 5a). However, it relaxed phenylephrine (1 μM) and K+ (80 mM)-induced contractions with respective EC50 values of 0.05571 mg/ml (95% CI: 0.04482-0.07642 mg/ml, n = 5) and 0.04955 mg/ml (95% CI: 0.09620-0.1537, n = 5) (Figure 5b, c and d). As the EC50 value of Sh.Cr for phenylephrine-induced contractions was found to be high in comparison with the EC50 value of Sh.Cr for K+(80 mM)-induced contractions, it is possible that the relaxant effect of Sh.Cr on phenylephrine- and K+(80 mM)-induced contractions may be mediated through the blockade of Ca2+ channels in a manner similar to verapamil, exerting ra elaxant effect on phenyleprine- and K+(80 mM)-induced contractions with respective EC50 values of of 0.87 μM (95% CI:0.037-5.62; n = 5) and 0.47 μM (95% CI: 0.033-2.11; n = 5) (Figure 5e).
Figure 5.
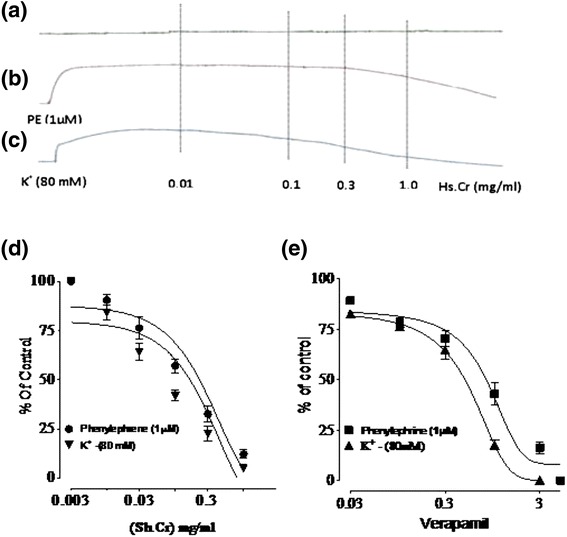
Effect of Sh.Cr on isolated rabbit aorta preparations. (a) Unstimulated aortic tissue as control. Concentration dependant inhibitory effect of (b,c and d) ethanol extract of Solena heterophylla (Sh.Cr) and (e) verapamil on phenylephrine (1 μM)- and high K + (80 mM)- induced contractions in isolated rabbit aorta preparations. Values are the mean ± SEM, n = 5.
Discussion
Cardiovascular, respiratory and gastrointestinal problems are some of most common ailments that people face globally in both developing and developed countries. Botanical therapies are still considered as safe by indigenous communities to treat such ailments in developing countries. The current study is aimed to rationalize the folk use of a medicinal plant to cure these ailments. The spasmolytic properties of Sh.Cr were evaluated by its application to spontaneous contractions of isolated rabbit jejunum preparation because these spontaneous rhythmic contractions are suitable for direct testing of relaxant activity without using any agonist [21]. The Sh.Cr exhibited relaxant activity on rhythmic contractions in isolated rabbit jejunum preparations, thus demonstrating its antispasmodic potential.
The contractile activities in smooth muscle preparations are function of increase/decrease in free Ca2+ concentration within cytoplasm [29] and the cellular free Ca2+ concentration is increased by influx on either via voltage dependent Ca2+ channels (VDCs) or release of Ca2+ from sarcoplasmic stores [30]. Morevoer, the spontaneous rhythmic contractions in isolated rabbit jejunum preparations are outcome of periodic depolarization subsequent to repolarization, permitting a rapid influx of Ca2+ through VDCs at the peak of depolarization [31]. The observed spasmolytic effect of Sh.Cr is likely to be attributed to a decrease in cytoplasmic Ca2+ due to the blockade of VDCs or opening of K+ channels. The sustained contractions in isolated rabbit jejunum preparations subsequent to K+(25 mM) exposure were not relaxed on treatment with Sh.Cr, indicating that the spasmolytic activity of Sh.Cr was independent of K+-channels. However, K+(80 mM)-induced contractions in isolated rabbit jejunum were found to be relaxed following the treatment with Sh.Cr, indicating that the spasmolytic effect may due to the blockade of rapid influx of extracellular Ca2+ through opened VDCs [24,32]. These findings agree with previous studies on medicinal plants [17,33]. These considerations were further confirmed as the previous treatment of isolated rabbit jejunum preparation with Sh.Cr caused decrease in contractile response to Ca2+ and rightward shifting of the concentration response curves for Ca2+ in a manner similar to verapamil, a standard Ca2+ channel blocker [34]. The Ca2+ channel blockers are an established class of therapeutic agents and are known to be effective in hyperactive diseases of the gut [35].
The Sh.Cr caused relaxation of carbachol (1 μM)- and K+(80 mM)-induced contractions in isolated rabbit tracheal preparations in a manner comparable to dicyclomine and is mediated possibly through antagonism of muscarinic receptors as well as the blockade of Ca2+ channels. The anti-muscarinic agents as well as Ca2+ channel blockers are useful bronchodilator in conditions of increased sensitivity of the airway [30,31,36,37]. This study provided a scientific basis to validate the traditional uses of the plant in the management of respiratory disorders including asthma, cough, and bronchitis.
The Sh.Cr exerted relaxant effect on phenylephrine (1 μM)- and K+(80 mM)-induced contractions in isolated rabbit aorta preparations, but phenylephrine-induced contractions were relaxed at increased tissue bath concentrations, indicating that the observed relaxant effect may possibly be mediated through the blockade of voltage dependent Ca2+ channel [32,38]. The relaxant effect on isolated rabbit aorta preparations may provide a scientific basis to validate the use of Solena heterophylla in the management of hypertension.
Conclusions
The observed relaxant effect may be outcome of anti-muscarinic and Ca2+ channel blocking activities. The Solena heterophylla exerted a relaxant effect against phenyephrine (1 μM)- and K+(80 mM)-induced contractions in isolated rabbit aortic preparations, possibly through Ca2+ channel blocking activity. These findings provide a rationale for the folkloric uses of the plant in the management of ailments pertaining to gastrointestinal, respiratory and vascular system. However, more detailed studies are required to establish the safety, efficacy and toxicity of this plant and to isolate the bioactive constituents.
Acknowledgements
We are thankful to laboratory staff of Faculty of Pharmacy, Bahauddin Zakariya University Multan, Pakistan for their help in smooth running of this experiment.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KHJ, TA, FS, and II designed and carried out the experimental work. MZUH, CJ and MM analyzed the statistical data and interpretation of results. VDF drafted and critically evaluated the manuscript. All authors read and approved the final manuscript.
Contributor Information
Khalid Hussain Janbaz, Email: khjanbaz@hotmail.com.
Tashfeen Akhtar, Email: pharmacistgenius@yahoo.com.
Fatima Saqib, Email: fatima2saqib@yahoo.com.
Imran Imran, Email: imran.ch@bzu.edu.pk.
Muhammad Zia-Ul-Haq, Email: ahirzia@gmail.com.
Chaweeewan Jansakul, Email: chaweewan.j@psu.ac.th.
Vincenzo De Feo, Email: defeo@unisa.it.
Marius Moga, Email: moga.og@gmail.com.
References
- 1.Chakravarty HL. Cucurbitaceae. In: Jain SK, editor. Fascicles of Flora of India. Calcutta, India: Botanical Survey of India; 1982. [Google Scholar]
- 2.Lu A, Huang L, Chen SK, Jeffrey C. Cucurbitaceae. In: Wu ZY, Raven PH, Hong DY, editors. Flora of China. St. Louis, USA: Missouri Botanical Garden Press; 2011. [Google Scholar]
- 3.Renner SS, Pandey AK. The Cucurbitaceae of India: Accepted names, synonyms, geographic distribution, and information on images and DNA sequences. Phyto Keys. 2013;20:53–118. doi: 10.3897/phytokeys.20.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khare CP. Indian medicinal plants: an illustrated dictionary. Berlin, Germany: Springer-Verlag; 2007. p. 729. [Google Scholar]
- 5.Ambasta SP, Ramchandran K, Kashyapa K, Chand R. The useful plants of India. New Delhi, India: Council of Science and Industrial Research; 1992. [Google Scholar]
- 6.Bhattarai NK. Traditional phytotherapy among the Sherpas of Helambu, central Nepal. J Ethnopharmacol. 1989;27:45–54. doi: 10.1016/0378-8741(89)90076-7. [DOI] [PubMed] [Google Scholar]
- 7.Bhattarai NK. Folk medicinal use of plants for respiratory complaints in central Nepal. Fitoterapia. 1993;64:163–70. [Google Scholar]
- 8.Rajbhandari KR. Ethnobotany of Nepal. Kathmandu, Nepal: Ethnobotanical Society of Nepal; 2001. p. 189. [Google Scholar]
- 9.Rastogi RP, Mehrotra BN. A compendium of Indian medicinal plants. New Delhi, India: CDRI-Lucknow and Publications and Information Directorate; 1993. [Google Scholar]
- 10.Iman RA, Priya BL, Chithra R, Shalini K, Sharon V, Chamundeeswari D, et al. In- vitro antiplatelet activity-guided fractionation of aerial parts of Melothria maderaspatana. Indian J Pharm Sci. 2006;68:668–70. doi: 10.4103/0250-474X.29646. [DOI] [Google Scholar]
- 11.Kunwar RM, Nepal BK, Kshhetri HB, Rai SK, Bussmann RW. Ethnomedicine in Himalaya: a case study from Dolpa, Humla, Jumla and Mustang districts of Nepal. J Ethnobiol Ethnomed. 2006;2:27–33. doi: 10.1186/1746-4269-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunwar RM, Shrestha KP, Bussmann RW. Traditional herbal medicine in Far-west Nepal: a pharmacological appraisal. J Ethnobiol Ethnomed. 2010;6:35–53. doi: 10.1186/1746-4269-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkateshwarlu E, Raghuram RA, Goverdhan P, Swapna RK, Jayapal RG. In Vitro and In Vivo antioxidant activity of methanolic extract of Solena amplexicaulis (Whole Plant) Int J Pharm Bio Sci. 2011;1:522–33. [Google Scholar]
- 14.Karthila K, Paulsamy S, Jamuna S. Evaluation of in vitro antioxidant potential of methanolic leaf and stem extracts of Solena amplexicaulis (Lam.) Ghandi. J Chem Pharm Res. 2012;4:3254–8. [Google Scholar]
- 15.Pant S, Samant SS. Ethanobotanical observations in the mornaula reserve forest of Kumoun, West Himalaya India. Ethnobot Leafl. 2010;14:193–217. [Google Scholar]
- 16.Anonymous . The Wealth of India. New Delhi: Publications and Information Directorate, CSIR; India; 1962. p. 335. [Google Scholar]
- 17.Imran I, Hussain L, Zia-Ul-Haq M, Janbaz KH, Gilani AH, De Feo V. Gastrointestial and respiratory activities of Acacia leucophloea. J Ethnopharmacol. 2011;138:676–82. doi: 10.1016/j.jep.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhary MA, Imran I, Bashir S, Mehmood MH, Rehman NU, Gilani AH. Evaluation of gut modulatory and bronchodilator activities of Amaranthus spinosus Linn. BMC Complement Altern Med. 2012;12:166–73. doi: 10.1186/1472-6882-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janbaz KH, Haider S, Imran I, Zia-Ul-Haq M, De Martino L, De Feo V. Pharmacological evaluation of Prosopis cineraria (L.) Druce in gastrointestinal, respiratory, and vascular disorders. Evid Based Complement Altern Med. 2012;2012:1–8. doi: 10.1155/2012/735653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janbaz KH, Nisa M, Saqib F, Imran I, Zia-Ul-Haq M, De Feo V. Bronchodilator, vasodilator and spasmolytic activities of methanolic extract of Myrtus communis L. J Physiol Pharmacol. 2013;64:479–84. [PubMed] [Google Scholar]
- 21.Janbaz KH, Arif J, Saqib F, Imran I, Ashraf M, Zia-Ul-Haq M, et al. In-vitro and in-vivo validation of ethnopharmacological uses of methanol extract of Isodon rugosus Wall. ex Benth (Lamiaceae) BMC Complement Altern Med. 2014;14:71–82. doi: 10.1186/1472-6882-14-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zia-Ul-Haq M, Ahmad S, Bukhari SA, Amarowicz R, Ercisli S, Jaafar HZE. Compositional studies and biological activities of some mash bean (Vigna mungo (L.) Hepper) cultivars commonly consumed in Pakistan. Bio Res. 2014;47:23. doi: 10.1186/0717-6287-47-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zia-Ul-Haq M, Cavar S, Qayum M, Khan I, Ahmad S. Compositional studies and antioxidant potential of Acacia leucophloea Roxb. Acta Bot Croat. 2013;72:27–31. [Google Scholar]
- 24.Zia-Ul-Haq M, Ahmad S, Qayum M, Ercişli S. Compositional studies and antioxidant potential of Albizia lebbeck (L.) Benth. Turk J Bio. 2013;37:25–32. [Google Scholar]
- 25.National Research Council . Guide for the Care and Use of Laboratory Animals. 8. Washington, DC, USA: National Academy Press; 2011. [Google Scholar]
- 26.Gilani AH, Shah AJ, Ghayur MN, Majeed K. Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci. 2005;76:3089–105. doi: 10.1016/j.lfs.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Farre AJ, Colombo M, Fort M, Gutierrez B. Differential effects of various Ca2+ antagonists. Gen Pharmacol. 1991;22:177–81. doi: 10.1016/0306-3623(91)90331-Y. [DOI] [PubMed] [Google Scholar]
- 28.VanRosum JM. Cumulative dose–response curves. II. Technique for the making of dose response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Therap. 1963;143:299–330. [PubMed] [Google Scholar]
- 29.Karaki H, Weiss B. Mini-review: calcium release in smooth muscle. Life Sci. 1983;42:111–22. doi: 10.1016/0024-3205(88)90674-1. [DOI] [PubMed] [Google Scholar]
- 30.Godfraind T, Miller R, Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986;38:321–416. [PubMed] [Google Scholar]
- 31.Brading AF. How do drugs initiate contraction in smooth muscles? Trend Pharm Sci. 1981;2:261–5. doi: 10.1016/0165-6147(81)90334-5. [DOI] [Google Scholar]
- 32.Bolton TB. Mechanism of action of transmitters and other substances on smooth muscles. Physiol Rev. 1979;59:606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- 33.Gilani AH, Ghayur MN, Khalid A, Choudhary MI. Presence of antispasmodic, antidiarrheal, antisecretory, calcium antagonist and acetylcholinesterase inhibitory steroidal alkaloids in Sarcoccas aligna. Planta Med. 2005;71:1–6. doi: 10.1055/s-2005-837777. [DOI] [PubMed] [Google Scholar]
- 34.Fleckenstein A. Specific Pharmacology of Ca++ in myocardium, cardiac pacemakers and vascular smooth muscles. Rev Pharm Toxic. 1977;17:149–66. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- 35.Brunton LL. Agents effecting gastrointestinal water flux and motility, emesis and antiemesis, bile acids and pancreatic enzymes. In: Gooodman LS, Limbird LE, Milinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman’s: The Pharmacological Basis of Therapeutics. 9. New York, USA: McGraw-Hill; 1996. [Google Scholar]
- 36.Gross NJ, Skorodin MS. Anticholinergic, antimuscarinic bronchodilators. Am Rev Respir Dis. 1984;129:856–70. doi: 10.1164/arrd.1984.129.5.856. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed T. Calcium antagonists: potential for asthma therapy. Choices Resp Manag. 1992;22:41–3. [Google Scholar]
- 38.Graham RM, Perez DM, Hwa J, Piascik MI. α1-Adrenergic receptor subtypes molecular structure, function, and signalling. Circulation Res. 1996;78:737–49. doi: 10.1161/01.RES.78.5.737. [DOI] [PubMed] [Google Scholar]


