Abstract
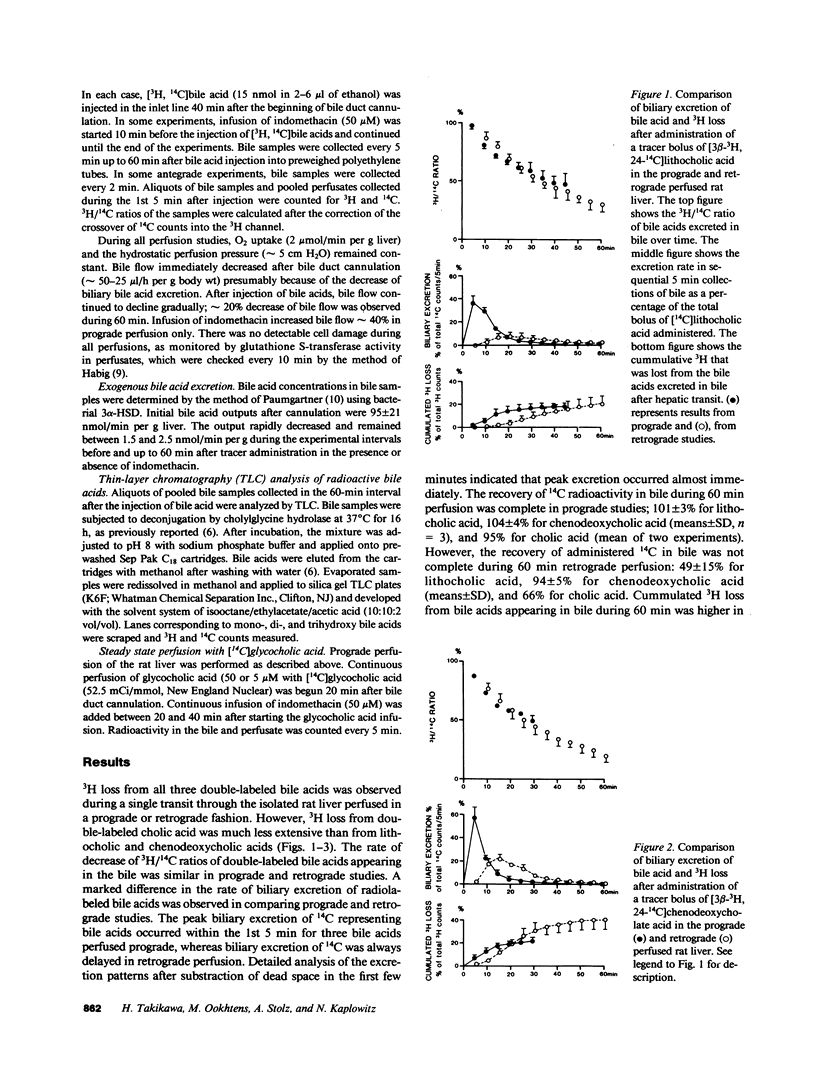
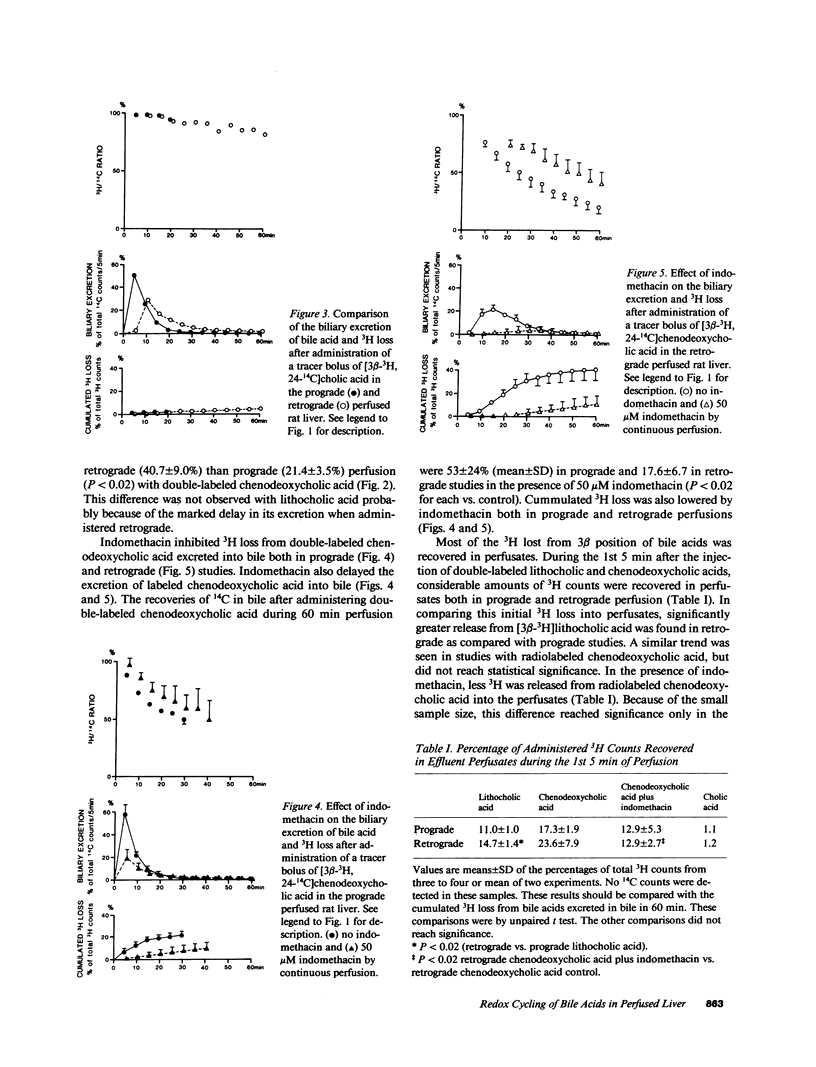
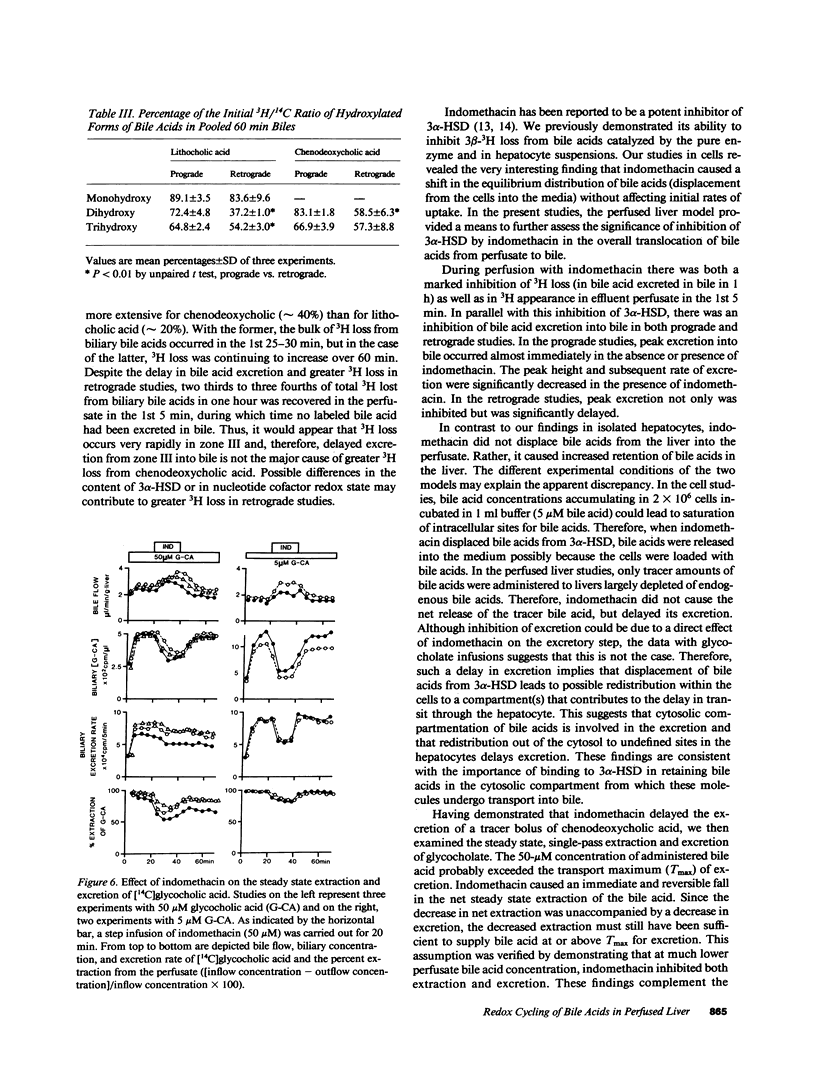
[3 beta-3H, 24-14C]Lithocholic, chenodeoxycholic, and cholic acids were administered in tracer bolus doses either prograde or retrograde in the isolated perfused rat liver. Little 3H loss from cholic acid was observed, whereas with the other bile acids, 20-40% of the administered 3H was lost in a single pass from perfusate to bile. Most of the 3H loss occurred rapidly (5 min) and was recovered as [3H]water in perfusate. Excretion of bile acids was delayed with retrograde administration, and 3H loss was more extensive. In both prograde and retrograde studies, indomethacin markedly inhibited the excretion of the bolus of bile acid into bile. Indomethacin inhibited the extraction of glycocholate (50 microM) during steady state perfusion without affecting transport maximum for excretion. At lower glycocholate concentration (5 microM), indomethacin inhibited both extraction and excretion. A greater effect was seen on excretion in the latter case, which suggests that displacement of bile acid from the cytosolic protein lead to redistribution in the hepatocyte as well as reflux into the sinusoid. These data suggest that binding of bile acids to cytosolic 3 alpha-hydroxysteroid dehydrogenases occurs extensively during hepatic transit and is important in mediating the translocation of bile acids from the sinusoidal to canalicular pole of the cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner U., Miyai K., Hardison W. G. Greater taurodeoxycholate biotransformation during backward perfusion of rat liver. Am J Physiol. 1986 Oct;251(4 Pt 1):G431–G435. doi: 10.1152/ajpgi.1986.251.4.G431. [DOI] [PubMed] [Google Scholar]
- Groothuis G. M., Hardonk M. J., Keulemans K. P., Nieuwenhuis P., Meijer D. K. Autoradiographic and kinetic demonstration of acinar heterogeneity of taurocholate transport. Am J Physiol. 1982 Dec;243(6):G455–G462. doi: 10.1152/ajpgi.1982.243.6.G455. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Ookhtens M., Hobdy K., Corvasce M. C., Aw T. Y., Kaplowitz N. Sinusoidal efflux of glutathione in the perfused rat liver. Evidence for a carrier-mediated process. J Clin Invest. 1985 Jan;75(1):258–265. doi: 10.1172/JCI111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumgartner G., Horak W., Probst P., Grabner G. Effect of phenobarbital on bile flow and bile salt excretion in the rat. Naunyn Schmiedebergs Arch Pharmakol. 1971;270(1):98–101. doi: 10.1007/BF00997305. [DOI] [PubMed] [Google Scholar]
- Penning T. M., Mukharji I., Barrows S., Talalay P. Purification and properties of a 3 alpha-hydroxysteroid dehydrogenase of rat liver cytosol and its inhibition by anti-inflammatory drugs. Biochem J. 1984 Sep 15;222(3):601–611. doi: 10.1042/bj2220601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning T. M., Talalay P. Inhibition of a major NAD(P)-linked oxidoreductase from rat liver cytosol by steroidal and nonsteroidal anti-inflammatory agents and by prostaglandins. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4504–4508. doi: 10.1073/pnas.80.14.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. The use of perfusion of liver and other organs for the study of microsomal electron-transport and cytochrome P-450 systems. Methods Enzymol. 1978;52:48–59. doi: 10.1016/s0076-6879(78)52005-3. [DOI] [PubMed] [Google Scholar]
- Stolz A., Takikawa H., Sugiyama Y., Kuhlenkamp J., Kaplowitz N. 3 alpha-hydroxysteroid dehydrogenase activity of the Y' bile acid binders in rat liver cytosol. Identification, kinetics, and physiologic significance. J Clin Invest. 1987 Feb;79(2):427–434. doi: 10.1172/JCI112829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y., Stolz A., Sugimoto M., Kaplowitz N. Evidence for a common high affinity binding site on glutathione S-transferase B for lithocholic acid and bilirubin. J Lipid Res. 1984 Nov;25(11):1177–1183. [PubMed] [Google Scholar]
- Sugiyama Y., Yamada T., Kaplowitz N. Newly identified bile acid binders in rat liver cytosol. Purification and comparison with glutathione S-transferases. J Biol Chem. 1983 Mar 25;258(6):3602–3607. [PubMed] [Google Scholar]
- Takikawa H., Kaplowitz N. Binding of bile acids, oleic acid, and organic anions by rat and human hepatic Z protein. Arch Biochem Biophys. 1986 Nov 15;251(1):385–392. doi: 10.1016/0003-9861(86)90086-x. [DOI] [PubMed] [Google Scholar]
- Takikawa H., Otsuka H., Beppu T., Seyama Y., Yamakawa T. Quantitative determination of bile acid glucuronides in serum by mass fragmentography. J Biochem. 1982 Oct;92(4):985–998. doi: 10.1093/oxfordjournals.jbchem.a134055. [DOI] [PubMed] [Google Scholar]
- Takikawa H., Sugiyama Y., Kaplowitz N. Binding of bile acids by glutathione S-transferases from rat liver. J Lipid Res. 1986 Sep;27(9):955–966. [PubMed] [Google Scholar]



