Abstract
Objective
Previous studies suggest child abuse and serotonergic polymorphism influence depression susceptibility and anti-depressant efficacy. Polymorphisms of the norepinephrine transporter (NET) may also be involved. Research in the area is possibly clouded by under reporting of abuse in researcher trials.
Methods
Adults (n=51) with major depressive disorder has 8 weeks treatment with escitalopram or venlafaxine. Abuse history was obtained, the ongoing emotional impact of which was measured with the 15-item impact of event scale (IES-15). The 17-item Hamilton Depression Rating Scale (HDRS) was applied serially. Two NET polymorphisms (rs2242446 and rs5569) were assayed, blinded to HDRS ratings and abuse history.
Results
No subjects reporting abuse with high impact in adulthood (IES-15 ≥26, n=12) remitted; whereas 77% reporting low impact (IES-15 <26; n=26) remitted (p<0.001). Subjects reporting high impact abuse (n=12) had a 50-fold (95% confidence interval=4.85–514.6) greater odds of carrying rs2242446-TT genotype, but the small sample size leaves this finding vulnerable to type I error.
Conclusion
The level of persisting impact of child abuse appears relevant to antidepressant efficacy, with susceptibility to such possibly being influence by NET rs2242446 polymorphism. Larger studies may be merited to expand on this pilot level finding given potential for biomarker utility.
Keywords: Abuse, Child, Antidepressants, Norepinephrine transporter, Remission
INTRODUCTION
Prediction of antidepressant response has been an elusive goal.1) Several studies have investigated the relationship between a history of child abuse and differential anti-depressant response in adults suffering from major depression.2–8) Findings have been mixed. In the largest study to date6) 808 patients suffering chronic major depressive disorder (MDD) with clinically significant child abuse (assayed with a trauma questionnaire) had lower response rates to antidepressants. Nemeroff et al.3) studied 681 patients with chronic MDD, with a trauma scale and number of abuse events used to help grade abuse severity. They found that subjects with a history of significant child abuse had preferential response to psychotherapy over the antidepressant nefazodone, with combined treatment not superior to psychotherapy alone. In yet another study, no significant differences in remission rates was identified among 312 adults randomized to interpersonal psychotherapy or selective serotonin reuptake inhibitor, but results were not stratified by abuse severity.7) Finally, among 195 outpatients with MDD a history of child abuse did not help predict response to fluoxetine or nortriptyline.5) A recent meta-analysis9) of the above and other studies concluded that subjects not reporting child abuse had a better response rate to antidepressants (odds ratio=1.43, 95% conference interval [CI] 1.11–1.83). However none of these studies assayed the current emotional impact from prior child abuse. It is possible that the ongoing emotional salience of child abuse in adulthood may be the mediating factor to antidepressant efficacy, in turn possibly mediated by susceptibility polymorphisms.
Emotionally impactful child abuse lacks uniform definition10,11) limiting research in the role of ongoing emotional impacts of abuse into differential antidepressant efficacy and susceptibility genes. Other methodological challenges affect investigating the role of ongoing impacts from child abuse to differential antidepressant efficacy and susceptibility polymorphisms. It is less likely a patient will disclose abuse history to somebody they have not established a trusting alliance with, making the baseline seeking of such personal information prone to false negative reports (Hanson et al.,12) Roesler and Wind13)). This may limit the sensitivity of asking about child abuse as a response predictor from outset of treatment. Additionally, asking about childhood abuse at baseline may act as a psychological stressor for those with such a history, potentially affecting antidepressant response confounding investigation.14–17)
Only a proportion of abused children suffer mental illness in adulthood.18–21) Various protective psychosocial factors have been implicated including personality style, emotional self-regulation, secure attachment relationships, and community supports.20,22,23) Individual features such as personality style and genetic profile may also influence individual resilience or susceptibility to trauma. Investigation of gene environment interactions have demonstrated that childhood trauma is a strong environmental ‘pathogen’ that may be moderated by genetic variation, most notably the serotonin transporter linked promoter region (5HTTLPR) polymorphism.18,24–33) A recent meta-analysis of 52 studies (n=40,749) concluded there is “strong evidence” that the s allele of 5HTTLPR significantly elevated the “depressogenic” effects of childhood maltreatment.34) It is possible a confluence of genetic susceptibilities will moderate the influence of childhood trauma on adult psychopathology beyond the 5HTTLPR polymorphism.35) It is possible that such genetic variability between siblings exposed to similar environmental stressors and resources in part may explain variability in mental health outcomes among them.36) Such vulnerability biomarkers could one day assist targeting resources to children more susceptible to adverse adult outcomes, making this question of potential clinical translational value.
Beyond 5HTTLPR genotype, various other genes spanning monoaminergic, inflammatory, and neurotrophic related systems have also been implicated in trauma vulnerability, all be it not yet to a meta-analytic level of evidence.37) Despite the monoamine hypothesis of MDD initially highlighting the role of norepinephrine38) to date little empirical investigation has focussed on the role of norepinephrine transporter (NET) polymorphisms influencing vulnerability to child abuse produce adulthood psychopathology.39) This is surprising given the norepinephrine appears to have a key role in stress responses.40) Studies have demonstrated increased urinary norepinephrine metabolite excretion in both adults and youth who have been exposed to abuse, but findings are inconsistent.41–43)
NET is a sodium dependent transporter re-uptaking nor-epinephrine into the neuron for both cyclic use and termination of the action of norepinephrine at the synapse.44–46) Recently, chronic stress has been shown to up-regulate NET gene expression in rats.47) Human studies have yet to be reported.40) The promoter region of the NET gene is approximately 4.7 kb in length and contains an additional 476 base pair intron containing several potential transcriptional elements.48) The NET182 polymorphism (rs2242446) located in this NET promoter region appears to alter transcription.49) Additionally, a synonymous NET G1287A polymorphism (rs5569) in exon 6 has been linked to alternative mRNA splicing and reduced NET functioning,50) and has also been associated with differential response to noradrenergic antidepressants.51–53) Furthermore, recently the combination of a child abuse history and carrying the TT polymorphism at rs5569 was demonstrated to significantly decreased antidepressant response in a sample of 308 patients,54) and appears commonly polymorphic rather than being a rare variant of limited public health clinical utility.55,56)
The primary a priori study hypothesis was that subjects with persisting high level emotional impact from child abuse exposure (high or low emotional impact assayed with the 15-item impact of event scale [IES-15]) would have reduced antidepressant efficacy. Secondarily–on a post hoc–basis we examine whether two NET polymorphisms (rs2242446 and rs5569) helped predict anti-depressant efficacy stratifying by child abuse history and level of persisting emotional impact. Finally, on a post hoc basis we sought to investigate whether the level of persisting emotional impact from child abuse into adulthood (high or low impact) was associated with NET rs2242446 or rs5569 polymorphisms.
METHODS
Subjects and Ratings
Patients 18 years and over with a principal diagnosis of MDD (Diagnostic and Statistical Manual of Mental Disorders 4th edition [DSM-IV] criteria, semi-structured clinical interview) and baseline HDRS ≥18 were included in the study and studied prospectively for 8 weeks. Treatment refractory cases (≥3 failed medication trails) were excluded as were subjects with co-morbid physical of psychiatric illnesses and those pregnant or breast feeding. Alternative care may have been more appropriate for such patients. There was a five half-life drug washout period for subjects already taking an antidepressant. The study was a limb of a larger study,57,58) with one of the recruitment sites (n=51) obtaining a history of child abuse and rating ongoing emotional impacts with the IES-15. High impact abuse was defined as IES-15 score ≥ 26 following the scale’s validated scoring instructions.59) Clinical Global Impression (CGI) scales for improvement and severity were used to guide clinical dose adjustment.60) All ratings were blinded to genotype, and history of child abuse was assessed at the end of the trial such that HDRS ratings were blind to history of child abuse, and in hopes disclosure rates would be better, and that treatment response would not be confounded by emotional distress reactions from being asked their child abuse history. During the first week all patients received a standard dose of either escitalopram (ESC) 10 mgs or venlafaxine (VEN) 75 mgs. ESC or VEN allocation was on the basis of clinical preference.61) At weeks 1, 4, and 8 of treatment doses were adjusted on a clinical basis, with the dose escalated if there was no improvement on the CGI scale, or the dose reduced if problematic side effects emerged (elevation of the UKU side effects scale with patient intolerance of the reported side effect).62) No other psychotropic medications were given and psychotherapy was not commenced during the study period. The study was approved by an independent research ethics committee (Study 138, The Melbourne Clinic, Richmond, Australia).
Genotyping
DNA was extracted from each sample using QIAamp DNA Mini Kit (QIAGEN Inc., Hilden, Germany) from venous blood or buccal brush samples. Genotypes of candidate (NET rs2242446 and rs5569) and potential confounding (CYP2D6 and CYP2C19 metaboliser status; ABCB1 [P-glycoprotein] rs1045642; and 5HTTLPPR s and l) polymorphisms63) were determined by the polymerase chain reaction followed by single primer extension and analysis on a Sequenom® Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF; Sequenom Inc., San Diego, CA, USA) 384 well genetic analysis system.
Statistical Analysis
Data were analyzed using IBM SPSS Statistics software ver. 19.0 (IBM Co., Armonk, NY, USA). Intention- to-treat analysis was applied. Demographic variables by abuse exposure and impact were examined using Fisher’s exact test and t-tests for categorical and continuous variables respectively. Repeated-measures analysis of variance (ANOVA) was used to determine changes in HDRS scores over the 8-weeks of treatment by abuse exposure and impact as well as NET genotype. Genotype frequencies by abuse exposure and emotional impacts in adulthood were examined using a chi-square analysis with a Bonferroni correction (p=0.025) for the two NET candidate polymorphisms. The CubeX program was applied to detect departures from Hardy Weinberg Equilibrium (HWE) and estimate pairwise linkage disequilibrium (LD) measures r2 and D′.64) Polymorphisms with HWE greater than 0.01 were considered to be in equilibrium. LD was assumed if both NET polymorphisms had r2 and D′ values greater than 0.80. This information would guide the need for haplotype analysis.
RESULTS
No less than 38 (74.5%) of the 51 subjects interviewed for child abuse history at week 8 reported exposure to child abuse (physical, sexual, or emotional). Only 12 (31.6%) of the subjects reporting a history of child abuse had persisting marked emotional impacts (high impact group) from it (IES-15 mean 37.2, standard deviation [SD]=6.8). The other 26 subjects reporting child abuse had much lower scores on the IES-15 (mean=11.2, SD=8.2) suggesting the abuse did not have persisting emotionally impacts into adulthood (low impact group).
Characteristics of subjects by child abuse exposure, level of impact, and NET genotype are displayed in Table 1. Subjects not reporting exposure to child abuse had greater baseline HDRS scores compared to subjects in the high impact exposed group (p=0.043), but there was no significant difference in baseline HDRS between the low and high impact groups. Subjects in the high impact group were less likely to have tertiary level education compared to the low impact (p=0.018) group. No differences were observed by NET genotype stratified by abuse history and impact.
Table 1.
Characteristics of sample by exposure to child abuse and its impact in adulthood
| Characteristic | Not exposed (n=13) | Exposed | NET rs2242226 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Low impact (n=26) | High impact (n=12) | Pairwise differences* | CC/TC (n=30) | TT (n=21) | Pairwise differences* | ||
| Baseline HDRS | 25.9±5.7 | 24.0±3.9 | 21.5±3.4 | Low>High | 24.6±4.8 | 22.9±4.0 | – |
| Duration of depression (year) | 6.6±4.5 | 5.2±3.3 | 8.0±5.6 | – | 5.5±3.9 | 7.2±4.7 | – |
| Age (year) | 38.2±11.4 | 43.1±15.6 | 43.8±11.2 | – | 43.9±15.0 | 39.3±11.0 | – |
| Male | 23.1 (3) | 42.3 (11) | 41.7 (5) | – | 46.7 (14) | 23.8 (5) | – |
| Tertiary education | 53.8 (7) | 61.5 (16) | 25.0 (3) | Low>High | 56.7 (17) | 42.9 (9) | – |
| Employed | 100 (13) | 96.2 (25) | 100 (12) | – | 96.7 (29) | 100 (21) | – |
| Medication | |||||||
| Venlafaxine | 46.2 (6) | 42.3 (11) | 58.3 (7) | – | 46.7 (14) | 47.6 (10) | – |
| Escitalopram | 53.8 (7) | 57.7 (15) | 41.7 (5) | – | 53.3 (16) | 52.4 (11) | – |
| Venlafaxine dose at 8 weeks (mg) | 187.5±78.7 | 150.0±82.2 | 203.6±36.6 | – | 160.7±77.0 | 195.0±63.2 | – |
| Escitalopram dose at 8 weeks (mg) | 24.3 (7.7) | 22.7 (7.0) | 22.0 (10.9) | – | 23.1 (7.0) | 22.7 (9.0) | – |
| ABCB1 rs1045642-TT carrier | 30.8 (4) | 34.6 (9) | 25.0 (3) | – | 36.7 (11) | 23.8 (5) | – |
| CYP2C19 extensive metaboliser | 84.6 (11) | 65.4 (17) | 75.0 (9) | – | 70.0 (21) | 76.2 (16) | – |
| CYP2D6 extensive metaboliser | 61.5 (8) | 65.4 (17) | 66.7 (8) | – | 63.3 (19) | 66.7 (14) | – |
| 5HTTLPR l allele carrier | 84.6 (11) | 84.6 (22) | 83.3 (10) | – | 76.6 (23) | 85.7 (18) | – |
Values are presented as mean±standard deviation or percent (number).
Significant at p<0.05.
Effects of Child Abuse Exposure and Persisting Emotional Impacts on Antidepressant Efficacy
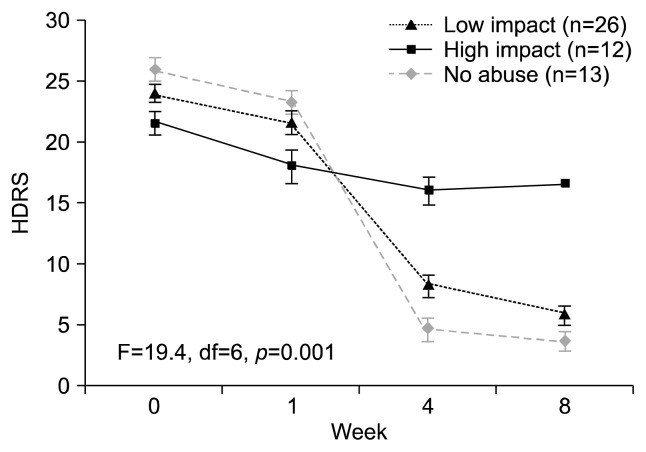
Subjects with no abuse history and low impact abuse had significantly greater HDRS score reduction (p=0.001) compared to subjects with high impact abuse during 8 weeks of antidepressant treatment (Fig. 1). Total of 52.6% of subjects reporting child abuse were in remission (HDRS≥7) from MDD at 8 weeks, whereas 92.3% of subjects without a history of child abuse were in remission from MDD at 8 weeks (p=0.01). Stratification of remission rates by abuse impact revealed subjects with high impact abuse had a zero rate of remission from MDD, while 77% of subjects with low impact abuse remitted (p<0.001). Statistical adjustment for tertiary education did not have an effect on these findings.
Fig. 1.
Seventeen-item Hamilton Depression Rating Scale (HDRS) score change over 8 weeks antidepressant treatment stratified by abuse history and level of persisitng emotional impacts. Subjects with no abuse history and low impact abuse had significantly greater HDRS score reduction (p=0.001) compared to subjects with high impact abuse. df, degree of freedom.
Effects of Child Abuse and NET Genotype on Antidepressant Efficacy
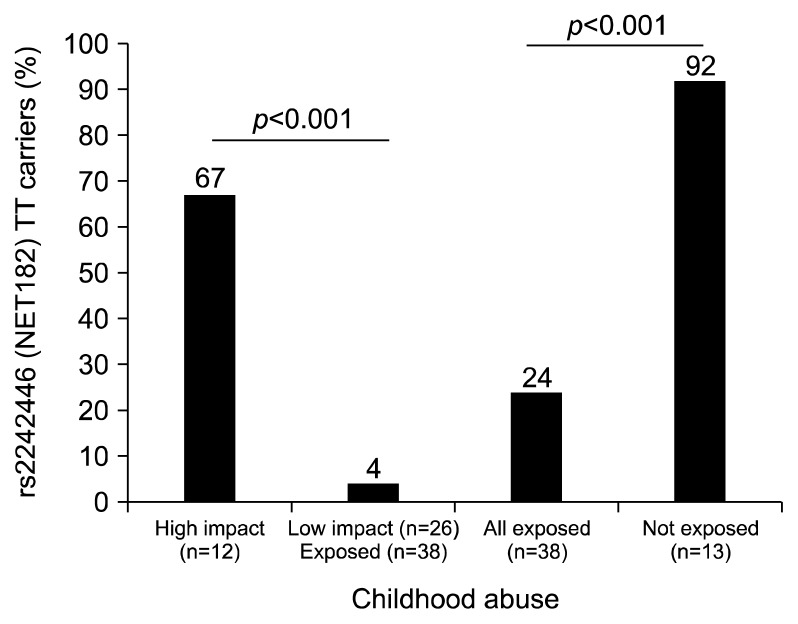
The rs2242226 and rs5569 polymorphisms were not in linkage disequilibrium (D′=0.13, r2=0.02) and neither polymorphism was associated with remission or symptom reduction in the full sample. However, we did observe a non-significant trend for rs2242226 in which C-carriers had greater symptom reduction over 8 weeks antidepressant treatment (F=2.98, degree of freedom [df]=3, p=0.071) but were not more likely to remit (chi-square=1.64, df=1, p=0.162). Subjects reporting a history of child abuse were less likely to have rs2242226 TT genotype compared to subjects reporting no abuse history (24% vs. 92%, p<0.001). Subjects reporting high impact abuse had a 50-fold (95% CI=4.85–514.6) greater odds of being TT genotype at rs2242226 compared to those reporting low impacts abuse (Fig. 2).
Fig. 2.
Proportions of TT carriers at rs2242446 (NET182) stratified by exposure to child abuse and level of persisting emotional impact. Subjects reporting high impact abuse had a 50-fold (95% confidence interval=4.85–514.6) greater odds of being TT genotype at rs2242226 compared to those reporting low impacts abuse.
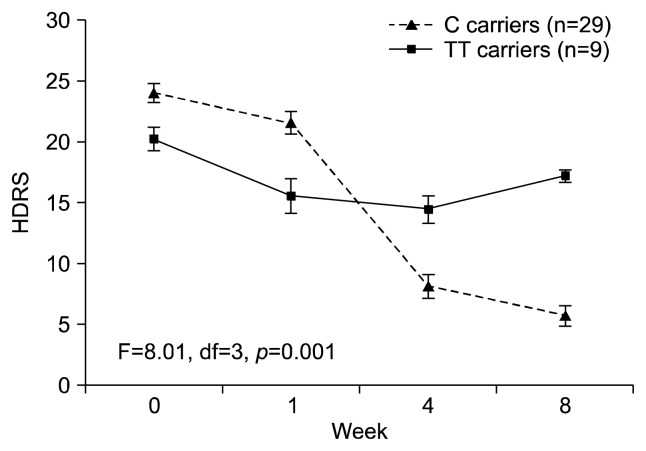
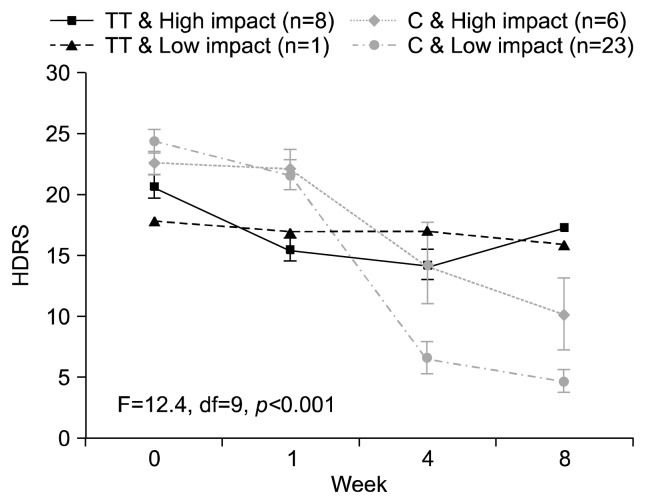
Among subjects reporting exposure to child abuse (n=38), C carriers at rs2242226 had greater symptom reduction over the 8 weeks of treatment compared to TT carriers (p=0.001; Fig. 3). However, symptom reduction for C carriers reporting high impact abuse was attenuated; albeit reductions remained greater than that observed for TT carriers with either high or low impact abuse (p<0.001; Fig. 4). No interaction effects between the other NET polymorphism investigated (rs5569) and child abuse was identified.
Fig. 3.
Seventeen-item Hamilton Depression Rating Scale (HDRS) score over 8 weeks antidepressant treatment stratified by rs2242226 (NET182) genotype among subjects with a history of child abuse. C carriers at rs2242226 had greater symptom reduction over the 8 weeks of treatment compared to TT carriers (p=0.001). df, degree of freedom.
Fig. 4.
Seventeen-item Hamilton Depression Rating Scale (HDRS) score change over 8 weeks antidepressant treatment among subjects reporting a history of child absue stratified by rs2242226 (NET182) genotype and level of persisting emotional impact from child abuse. df, degree of freedom.
DISCUSSION
This study demonstrates an association between child abuse history and poorer remission rates to the anti-depressants ESC and VEN–particularly for subjects for whom child abuse remained highly emotionally impactful in adulthood (IES-15 ≥ 26). The study also suggests a NET functional polymorphism increases the risk of child abuse remaining highly emotionally impactful into adulthood (TT genotype at rs2242226). Other potential gene environment associations have been reported, but none for a polymorphism located in the NET gene.37)
Our finding that individuals reporting high impact abuse had greater odds of being TT carriers at rs2242226 is biologically plausible. The recent finding that chronic stress up-regulates NET expression in animals47) suggests impediments to such up-regulation may prevent normal allostatic adjustment.65) Increased DNA methylation from environmental stress appears to impede gene transcription66–70) and seems to be a mechanism mediating gene environment interactions in depression.71) As the rs2242226 polymorphism is in a promoter region49) it may have an additive effect on reduced NET expression in the setting of stress induced NET hypermethylation, with TT carriers potentially being particularly susceptible to NET under expression when exposed to environmental stress. This may underlie both reduced antidepressant efficacy and an elevated risk of persisting emotional impacts from child abuse among such subjects - possibly akin to the association found for s carriers at the 5HTTLPR.34,72) While our study design could not shed light on whether the rs2242226 TT genotype increased the risk of de novo MDD, it did demonstrate that subjects reporting high impact abuse who also carried the TT genotype had poorest response to antidepressant medication. A converse finding that T carriers at rs2242226 with MDD had better response to the antidepressant milnacipran52) has been reported; however, this study failed to control for child abuse history or impacts possibly confounding results. In fact, in the current study 12 of the 13 subjects reporting no exposure to child abuse carried the TT genotype of which 92% (n=11) remitted. Thus, it seems the detrimental effect of the TT genotype appears to be dependent on child abuse exposure, suggesting a gene-environment interaction. Larger studies will be required to confirm this preliminary gene-environment association between NET rs2242226 and persisting emotional impacts from child abuse into adulthood, and to what extent this gene-environment association predicts antidepressant efficacy.
This study also provides an arguably more refined approach to assaying the clinical relevance of a history of child abuse in clinical care. Subjects with high impact abuse had substantially poorer antidepressant remission, in fact none of these subjects remitted. A strength of the study was that remission was employed as a more robust and clinically relevant measure of antidepressant efficacy given that subjects who respond but do not remit are more likely to relapse, making remission rather than response the goal of treatment and the pathway to recovery from MDD.73)
A key characteristic of this study was that the information about child abuse was collected after the acute treatment phase in hopes to reduce false negative reports of child abuse, and also to prevent an emotional reaction to the asking of abuse history confounding medication efficacy via an adjustment reaction overlay. The reduced false negative report of child abuse may help explain the very high rates of child abuse reported by subjects in this study (74.5% of subjects). Alternatively, this high rate may reflect a concentration of such trauma in complicated MDD cases being managed in a psychiatric clinic form whence subjects were recruited. A limitation of this approach (history of abuse attained after 8 weeks treatment) is that our data cannot shed light on the role of child abuse reported prior to treatment as an antidepressant remission predictive factor. However, it may be the case in clinical practice that rapport is established over several weeks prior to considering an antidepressant trial, thus findings of this study could have some clinical translational utility–subjects with impactful abuse less likely to benefit from an antidepressant making other modes of therapy for MDD potentially more appropriate.
The current study is limited by small sample size and lack of control for various polymorphisms (other than 5HTTLPR genotype) which have been implicated as moderators of persisting emotional impacts from child abuse.35) Having said this, controlling for 5HTTLPR genotype, pharmacokinetic related polymorphisms (P450 and ABCB1), medication dose, clinical features, demographic features, and ongoing impacts of child abuse with the IED-15 were novel strengths of this study.33,63) Finally, variability between protective factors in childhood (e.g., community supports) could have also modified results, but these factors were not controlled for in this study, possibly confounding our findings.
One further limitation that needs to be considered is accuracy of diagnosis. Subjects were assessed in a semi-structured clinical interview by a psychiatrist to determine a principal diagnosis of MDD using DSM-IV criteria. It is possible that some subjects may have had features of conditions such as borderline personality disorder, post traumatic stress disorder, and adjustment disorder with depressed mood which did not meet clinical threshold for diagnosis and may have contributed to differential treatment outcome, potentially confounding our results. Sub-threshold personality disorder for example is likely to modulate treatment response,74) but quantifying such sub-syndromal co-morbidities is difficult.
This is the first study to the authors’ knowledge to suggest that the emotional impacts of child abuse in adulthood may be moderated by a NET polymorphism and those with persisting high emotional impacts from child abuse appear to have a significantly lower odds of remitting to anti-depressant medication. If replicated in larger samples, these findings could help identifying children at higher risk of persisting emotional impacts from childhood abuse, possibly enabling better targeted supports and interventions to children subject to such trauma. These findings–if replicated–may also help guide prescribers throughout the decision making process on whether trialing an antidepressant in individuals with persistently emotionally impactful childhood trauma or other modalities of care are more appropriate.
Acknowledgments
Singh AB: Consultant Psychiatrist, The Geelong Clinic, Geelong, Australia. Senior Clinical Lecturer, School of Medicine, Deakin University, Geelong, Australia. Received a research grant from Pfizer and speaker honoraria from Astra Zeneca Australia, Lilly Australia, Pfizer Australia, Lundbeck Australia, The GP Association of Geelong, and The Mental Health Professionals Network. Served as an advisor to The Australian Federal Government, Mayne Pharma, Pfizer, and Deakin University.
Bousman CA: Robert Phillip Griffith Research Fellow, The University of Melbourne. Adjunct Research Fellow, Swinburne University of Technology, Centre for Human Psychopharmacology; Research Fellow, Mental Health Research Institute, has received honoraria from Abbott. Ng CH: Served in the Wyeth and Eli Lilly Advisory Boards, received research grant support from Wyeth and Lundbeck and speaker honoraria from Bristol-Myers Squibb, Organon, Eli Lilly, GlaxoSmithKline, Janssen- Cilag, Astra-Zenaca, Wyeth, and Pfizer.
Byron K: Employee of Healthscope Pathology.
Berk M: Professor of Psychiatry, Deakin University, Professorial Research Fellow, Mental Health Research Institute, Orygen Research Centre and the University of Melbourne, consultant to Astra Zeneca, Bristol Meyers Squibb, Eli Lilly, GlaxoSmithKline, Janssen Cilag, Lund-beck, and Servier; is on the speaker’s bureau of Astra Zeneca, Bristol Meyers Squibb, Eli Lilly, GlaxoSmithKline, Janssen Cilag, Lundbeck, Pfizer, Sanofi, Synthlabo, Servier, Solvay, and Wyeth; has received grant/research support from Astra Zeneca, Beyond Blue, Bristol Meyers Squibb, Eli Lilly, GlaxoSmithKline, Janssen Cilag, Lund-beck, Mayne Pharma, MBF Bioscience, National Health and Medical Research Council, Novartis, Organon, Servier, and Stanley Medical Research Foundation; and has received honoraria from Astra Zeneca, Bristol Meyers Squibb, Eli Lilly, GlaxoSmithKline, Janssen Cilag, Lundbeck, Pfizer, Sanofi, Synthlabo, Servier, Solvay, and Wyeth.
REFERENCES
- 1.Dodd S, Berk M. Predictors of antidepressant response: A selective review. Int J Psychiatry Clin Pract. 2004;8:91–100. doi: 10.1080/13651500410005423. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MJ, Klinetob NA. Childhood emotional trauma and chronic posttraumatic stress disorder in adult outpatients with treatment-resistant depression. J Nerv Ment Dis. 2000;188:596–601. doi: 10.1097/00005053-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enns MW, Cox BJ. Psychosocial and clinical predictors of symptom persistence vs remission in major depressive disorder. Can J Psychiatry. 2005;50:769–777. doi: 10.1177/070674370505001206. [DOI] [PubMed] [Google Scholar]
- 5.Johnstone JM, Luty SE, Carter JD, Mulder RT, Frampton CM, Joyce PR. Childhood neglect and abuse as predictors of antidepressant response in adult depression. Depress Anxiety. 2009;26:711–717. doi: 10.1002/da.20590. [DOI] [PubMed] [Google Scholar]
- 6.Klein DN, Arnow BA, Barkin JL, Dowling F, Kocsis JH, Leon AC, et al. Early adversity in chronic depression: clinical correlates and response to pharmacotherapy. Depress Anxiety. 2009;26:701–710. doi: 10.1002/da.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miniati M, Rucci P, Benvenuti A, Frank E, Buttenfield J, Giorgi G, et al. Clinical characteristics and treatment outcome of depression in patients with and without a history of emotional and physical abuse. J Psychiatr Res. 2010;44:302–309. doi: 10.1016/j.jpsychires.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harkness KL, Bagby RM, Kennedy SH. Childhood maltreatment and differential treatment response and recurrence in adult major depressive disorder. J Consult Clin Psychol. 2012;80:342–353. doi: 10.1037/a0027665. [DOI] [PubMed] [Google Scholar]
- 9.Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry. 2012;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 10.Conte JR, Schuerman JR. Factors associated with an increased impact of child sexual abuse. Child Abuse Negl. 1987;11:201–211. doi: 10.1016/0145-2134(87)90059-7. [DOI] [PubMed] [Google Scholar]
- 11.Chaffin M, Shultz SK. Psychometric evaluation of the children’s impact of traumatic events scale-revised. Child Abuse Negl. 2001;25:401–411. doi: 10.1016/S0145-2134(00)00257-X. [DOI] [PubMed] [Google Scholar]
- 12.Hanson RF, Resnick HS, Saunders BE, Kilpatrick DG, Best C. Factors related to the reporting of childhood rape. Child Abuse Negl. 1999;23:559–569. doi: 10.1016/S0145-2134(99)00028-9. [DOI] [PubMed] [Google Scholar]
- 13.Roesler TA, Wind TW. Telling the secret: adult women describe their disclosures of incest. J Interpers Violence. 1994;9:327–338. doi: 10.1177/088626094009003003. [DOI] [Google Scholar]
- 14.Roesler TA. Reactions to disclosure of childhood sexual abuse. The effect on adult symptoms. J Nerv Ment Dis. 1994;182:618–624. doi: 10.1097/00005053-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 15.McNulty C, Wardle J. Adult disclosure of sexual abuse: a primary cause of psychological distress? Child Abuse Negl. 1994;18:549–555. doi: 10.1016/0145-2134(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 16.Everill J, Waller G. Disclosure of sexual abuse and psychological adjustment in female undergraduates. Child Abuse Negl. 1995;19:93–100. doi: 10.1016/0145-2134(94)00102-Z. [DOI] [PubMed] [Google Scholar]
- 17.Ullman SE. Social reactions to child sexual abuse disclosures: a critical review. J Child Sex Abus. 2003;12:89–121. doi: 10.1300/J070v12n01_05. [DOI] [PubMed] [Google Scholar]
- 18.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicchetti D. Resilience under conditions of extreme stress: a multilevel perspective. World Psychiatry. 2010;9:145–154. doi: 10.1002/j.2051-5545.2010.tb00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cicchetti D, Rogosch FA. The role of self-organization in the promotion of resilience in maltreated children. Dev Psychopathol. 1997;9:797–815. doi: 10.1017/S0954579497001442. [DOI] [PubMed] [Google Scholar]
- 21.Cicchetti D, Toth SL. A developmental psychopathology perspective on child abuse and neglect. J Am Acad Child Adolesc Psychiatry. 1995;34:541–565. doi: 10.1097/00004583-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Beeghly M, Cicchetti D. Child maltreatment, attachment, and the self system: Emergence of an internal state lexicon in toddlers at high social risk. Dev Psychopathol. 1994;6:5–30. doi: 10.1017/S095457940000585X. [DOI] [Google Scholar]
- 23.Haskett ME, Nears K, Ward CS, McPherson AV. Diversity in adjustment of maltreated children: factors associated with resilient functioning. Clin Psychol Rev. 2006;26:796–812. doi: 10.1016/j.cpr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds. Dev Psychopathol. 2007;19:1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- 27.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, et al. Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychol Med. 2009;39:1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- 29.Aslund C, Leppert J, Comasco E, Nordquist N, Oreland L, Nilsson KW. Impact of the interaction between the 5HTTLPR polymorphism and maltreatment on adolescent depression. A population-based study. Behav Genet. 2009;39:524–531. doi: 10.1007/s10519-009-9285-9. [DOI] [PubMed] [Google Scholar]
- 30.Benjet C, Thompson RJ, Gotlib IH. 5-HTTLPR moderates the effect of relational peer victimization on depressive symptoms in adolescent girls. J Child Psychol Psychiatry. 2010;51:173–179. doi: 10.1111/j.1469-7610.2009.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumsta R, Stevens S, Brookes K, Schlotz W, Castle J, Beckett C, et al. 5HTT genotype moderates the influence of early institutional deprivation on emotional problems in adolescence: evidence from the English and Romanian Adoptee (ERA) study. J Child Psychol Psychiatry. 2010;51:755–762. doi: 10.1111/j.1469-7610.2010.02249.x. [DOI] [PubMed] [Google Scholar]
- 32.Sugden K, Arseneault L, Harrington H, Moffitt TE, Williams B, Caspi A. Serotonin transporter gene moderates the development of emotional problems among children following bullying victimization. J Am Acad Child Adolesc Psychiatry. 2010;49:830–840. doi: 10.1016/j.jaac.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 36.De Bellis MD, Zisk A. The biological effects of childhood trauma. Child Adolesc Psychiatr Clin N Am. 2014;23:185–222. vii. doi: 10.1016/j.chc.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uher R. Genes, environment, and individual differences in responding to treatment for depression. Harv Rev Psychiatry. 2011;19:109–124. doi: 10.3109/10673229.2011.586551. [DOI] [PubMed] [Google Scholar]
- 38.Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61( Suppl 6):4–6. [PubMed] [Google Scholar]
- 39.Strawn JR, Geracioti TD., Jr Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25:260–271. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- 40.Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, Bradley-Davino B. PTSD and gene variants: new pathways and new thinking. Neuropharmacology. 2012;62:628–637. doi: 10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Bellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, et al. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. J Clin Endocrinol Metab. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- 42.Girdler SS, Sherwood A, Hinderliter AL, Leserman J, Costello NL, Straneva PA, et al. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom Med. 2003;65:849–856. doi: 10.1097/01.PSY.0000088593.38201.CD. [DOI] [PubMed] [Google Scholar]
- 43.Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosom Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Uhl GR, Johnson PS. Neurotransmitter transporters: three important gene families for neuronal function. J Exp Biol. 1994;196:229–236. doi: 10.1242/jeb.196.1.229. [DOI] [PubMed] [Google Scholar]
- 45.Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 46.Béïque JC, Lavoie N, de Montigny C, Debonnel G. Affinities of venlafaxine and various reuptake inhibitors for the serotonin and norepinephrine transporters. Eur J Pharmacol. 1998;349:129–132. doi: 10.1016/S0014-2999(98)00241-6. [DOI] [PubMed] [Google Scholar]
- 47.Chen P, Fan Y, Li Y, Sun Z, Bissette G, Zhu MY. Chronic social defeat up-regulates expression of norepinephrine transporter in rat brains. Neurochem Int. 2012;60:9–20. doi: 10.1016/j.neuint.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim CH, Kim HS, Cubells JF, Kim KS. A previously undescribed intron and extensive 5′ upstream sequence, but not Phox2a-mediated transactivation, are necessary for high level cell type-specific expression of the human norepinephrine transporter gene. J Biol Chem. 1999;274:6507–6518. doi: 10.1074/jbc.274.10.6507. [DOI] [PubMed] [Google Scholar]
- 49.Zill P, Engel R, Baghai TC, Juckel G, Frodl T, Müller-Siecheneder F, et al. Identification of a naturally occurring polymorphism in the promoter region of the norepinephrine transporter and analysis in major depression. Neuropsychopharmacology. 2002;26:489–493. doi: 10.1016/S0893-133X(01)00386-4. [DOI] [PubMed] [Google Scholar]
- 50.Sogawa C, Mitsuhata C, Kumagai-Morioka K, Sogawa N, Ohyama K, Morita K, et al. Expression and function of variants of human catecholamine transporters lacking the fifth transmembrane region encoded by exon 6. PLoS One. 2010;5:e11945. doi: 10.1371/journal.pone.0011945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higuchi H, Yoshida K, Takahashi H, Naito S, Kamata M, Ito K, et al. Milnacipran plasma levels and antidepressant response in Japanese major depressive patients. Hum Psychopharmacol. 2003;18:255–259. doi: 10.1002/hup.484. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida K, Takahashi H, Higuchi H, Kamata M, Ito K, Sato K, et al. Prediction of antidepressant response to milnacipran by norepinephrine transporter gene polymorphisms. Am J Psychiatry. 2004;161:1575–1580. doi: 10.1176/appi.ajp.161.9.1575. [DOI] [PubMed] [Google Scholar]
- 53.Kim H, Lim SW, Kim S, Kim JW, Chang YH, Carroll BJ, et al. Monoamine transporter gene polymorphisms and anti-depressant response in koreans with late-life depression. JAMA. 2006;296:1609–1618. doi: 10.1001/jama.296.13.1609. [DOI] [PubMed] [Google Scholar]
- 54.Dong C, Wong ML, Licinio J. Sequence variations of ABCB1, SLC6A2, SLC6A3, SLC6A4, CREB1, CRHR1 and NTRK2: association with major depression and antidepressant response in Mexican-Americans. Mol Psychiatry. 2009;14:1105–1118. doi: 10.1038/mp.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belfer I, Phillips G, Taubman J, Hipp H, Lipsky RH, Enoch MA, et al. Haplotype architecture of the norepinephrine transporter gene SLC6A2 in four populations. J Hum Genet. 2004;49:232–245. doi: 10.1007/s10038-004-0140-9. [DOI] [PubMed] [Google Scholar]
- 56.Stöber G, Nöthen MM, Pörzgen P, Brüss M, Bönisch H, Knapp M, et al. Systematic search for variation in the human norepinephrine transporter gene: identification of five naturally occurring missense mutations and study of association with major psychiatric disorders. Am J Med Genet. 1996;67:523–532. doi: 10.1002/(SICI)1096-8628(19961122)67:6<523::AID-AJMG3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 57.Singh AB, Bousman CA, Ng CH, Byron K, Berk M. ABCB1 polymorphism predicts escitalopram dose needed for remission in major depression. Transl Psychiatry. 2012;2:e198. doi: 10.1038/tp.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh AB, Bousman CA, Ng CH, Byron K, Berk M. Psychomotor depressive symptoms may differentially respond to venlafaxine. Int Clin Psychopharmacol. 2013;28:121–126. doi: 10.1097/YIC.0b013e32835f1b9f. [DOI] [PubMed] [Google Scholar]
- 59.Sundin EC, Horowitz MJ. Impact of Event Scale: psychometric properties. Br J Psychiatry. 2002;180:205–209. doi: 10.1192/bjp.180.3.205. [DOI] [PubMed] [Google Scholar]
- 60.Berk M, Ng F, Dodd S, Callaly T, Campbell S, Bernardo M, et al. The validity of the CGI severity and improvement scales as measures of clinical effectiveness suitable for routine clinical use. J Eval Clin Pract. 2008;14:979–983. doi: 10.1111/j.1365-2753.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- 61.Fries JF, Krishnan E. Equipoise, design bias, and randomized controlled trials: the elusive ethics of new drug development. Arthritis Res Ther. 2004;6:R250–R255. doi: 10.1186/ar1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 63.Porcelli S, Drago A, Fabbri C, Gibiino S, Calati R, Serretti A. Pharmacogenetics of antidepressant response. J Psychiatry Neurosci. 2011;36:87–113. doi: 10.1503/jpn.100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaunt TR, Rodríguez S, Day IN. Cubic exact solutions for the estimation of pairwise haplotype frequencies: implications for linkage disequilibrium analyses and a web tool ‘CubeX’. BMC Bioinformatics. 2007;8:428. doi: 10.1186/1471-2105-8-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, et al. Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Dev Psychopathol. 2011;23:1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at SLC6A4 is linked to family history of child abuse: an examination of the Iowa Adoptee sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:710–713. doi: 10.1002/ajmg.b.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- 68.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 69.McGowan PO, Sasaki A, Huang TC, Unterberger A, Suderman M, Ernst C, et al. Promoter-wide hypermethylation of the ribosomal RNA gene promoter in the suicide brain. PLoS One. 2008;3:e2085. doi: 10.1371/journal.pone.0002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schroeder M, Hillemacher T, Bleich S, Frieling H. The epigenetic code in depression: implications for treatment. Clin Pharmacol Ther. 2012;91:310–314. doi: 10.1038/clpt.2011.282. [DOI] [PubMed] [Google Scholar]
- 72.Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol. 2012;22:239–258. doi: 10.1016/j.euroneuro.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. J Clin Psychiatry. 1999;60( Suppl 22):7–11. [PubMed] [Google Scholar]
- 74.Zimmerman M, Chelminski I, Young D, Dalrymple K, Martinez J. Does the presence of one feature of borderline personality disorder have clinical significance? Implications for dimensional ratings of personality disorders. J Clin Psychiatry. 2012;73:8–12. doi: 10.4088/JCP.10m06784. [DOI] [PubMed] [Google Scholar]