Abstract
Case series
Patient: Male, 30 • Female, 44
Final Diagnosis: Post-transplant respiratory infection
Symptoms: Oxidative stress
Medication: —
Clinical Procedure: —
Specialty: Transplantology
Objective:
Challenging differential diagnosis
Background:
Reactive oxygen species function as key metabolites that can impair biological processes. In lung transplantation, severe oxidative stress is expected when ischemia/reperfusion injury, acute allograft rejection, and various infections occur.
Case Report:
Two clinical cases in which serial measurements of the oxidative stress response (levels of diacron-reactive oxygen metabolites) were taken during hospitalization using a Free Radical Elective Evaluator are reported. In the first case, a 30-year-old man underwent right single lung transplantation for juvenile pulmonary emphysema. Immunosuppression was maintained using tacrolimus, mycophenolate mofetil, and steroid. The oxidative stress response fluctuated significantly (p<0.01) during the infections caused by bronchial stenosis compared to the stable condition. No acute rejection was seen during hospitalization. In the second case, a 44-year-old woman underwent right single lung transplantation for lymphangioleiomyomatosis. Immunosuppression was maintained by the same regimen as in case 1. The patient’s postoperative course was uneventful, and there was no allograft rejection or infection. The oxidative stress response remained at the non-stress level.
Conclusions:
The oxidative stress response was measured by the levels of diacron-reactive oxygen metabolites in lung transplantation. High oxidative stress responses were seen during exposure to infections. This might become a noninvasive marker of complications after transplantation.
MeSH Keywords: Immunosuppression, Lung Transplantation, Oxidative Stress
Background
Oxidative stress can be defined as an imbalance between the pro-oxidant and the antioxidant potential of cells, which results either from an overproduction of reactive oxygen species (ROS), insufficient detoxification of ROS by antioxidants, or a combination of both [1]. It is widely known that ROS function as key metabolites that can impair biological processes, resulting in various diseases, including cardiovascular disease, diabetes mellitus, neurological disorders, and cancer [2–4].
In lung transplantation, severe oxidative stress is expected when ischemia/reperfusion injury, acute allograft rejection, and various infections occur, and these are the major obstacles to long-term survival. Currently, blood levels of hydroperoxides, a type of ROS, can be easily and accurately measured in patients as an index of oxidative injury to cellular components. In addition, plasma ferric-reducing ability can be assessed as an index of total antioxidant potential. Both hydroperoxides and ferric-reducing capacity can be measured with a small volume of patients’ blood using a Free Radical Elective Evaluator [4] (FREE® Carpe Diem; Wismerll Co. Ltd., Tokyo, Japan).
However, there have been few clinical reports about the oxidative stress response with lung transplantation. We hypothesized that the measurement of oxidative stress could help anticipate infections or allograft rejections and distinguish them correctly. Two cases of the serial measurement of the oxidative stress response after lung transplantation, which could become a useful tool to identify infection, are reported.
Case Report
Case 1
A 30-year-old Japanese man underwent right single lung transplantation for chronic obstructive pulmonary disease. He had been receiving a standard triple immunosuppressant regimen: tacrolimus (target trough level, 15–20 ng/mL for the first 3 months, followed by 10–15 ng/mL), MMF (1500 mg/day), and prednisolone (0.4 mg/kg/day for the first 6 months, followed by 0.2 mg/kg/day). The prophylactic strategies for fungal and viral infections were oral voriconazole at 400 mg/day, trimethoprim-sulfamethoxazole at 1 g every other day, and valganciclovir at 900 mg/day.
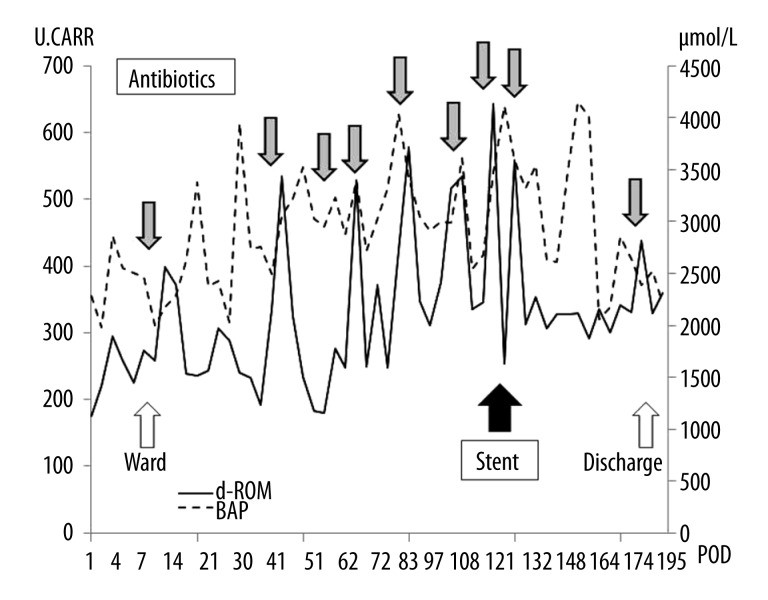
He developed ischemic change of the bronchial anastomosis after transplantation, resulting in bronchial stenosis. This made it difficult for him to expectorate sputum, so frequent bronchoscopic airway cleaning was needed. Infectious episodes were successfully treated by intravenous antibiotics, but the bronchial stenosis worsened gradually (Figure 1). Therefore, a Y-shaped airway silicone stent was inserted 5 months after transplantation. Fortunately, his condition after airway stent insertion improved, and he was discharged without any symptoms after 6 months of hospitalization. No allograft rejection was seen during this period.
Figure 1.
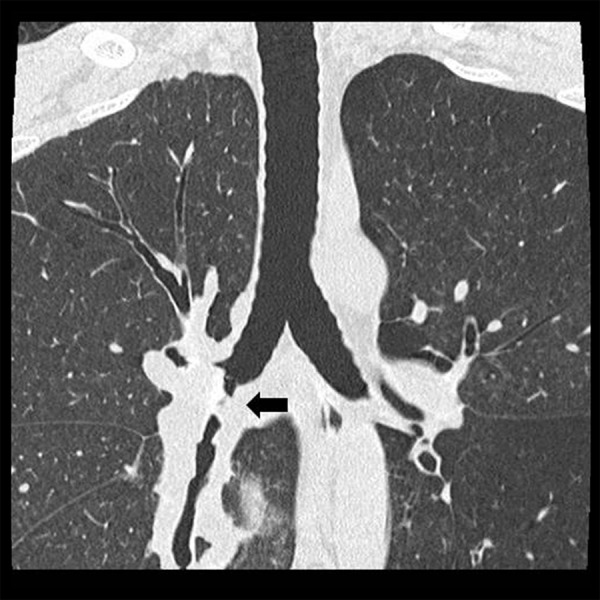
Chest computed tomography in Case 1 shows stenosis of the intermediate bronchus (arrow).
Oxidative stress in this patient was monitored constantly (total 55 times) using a Free Radical Elective Evaluator after transplantation. This study was conducted in accordance with the principles of the Helsinki Declaration and local ethics committee approval for this clinical research was obtained prior to its commencement. The measurement points were determined by the times for routine blood collection in our lung transplant protocol and when clinical symptoms appeared. Briefly, the levels of blood hydroperoxides, called diacron-reactive oxygen metabolites (d-ROMs), a type of ROS, were measured as an index of oxidative injury, and the biological antioxidant activity of plasma (BAP) was also measured. The d-ROMs are expressed in arbitrary units called Carratelli units (U.CARR). The normal values of both d-ROM and BAP were determined as shown in Table 1. The principles and methods of this apparatus have been previously described [4].
Table 1.
Definitions of d-ROM and BAP levels.
| d-ROM (CARR) | Value | BAP (µmol/L) | Value |
|---|---|---|---|
| Normal range | 200–300 | Border-line | 2200–2000 |
| Borderline stress | 301–320 | Slight reduction | 2000–1800 |
| Lows stress | 321–340 | Moderate reduction | 1800–1600 |
| Middle stress | 341–400 | Strong reduction | 1600–1400 |
| High stress | 401–500 | Very strong reduction | >1400 |
| Very high | >501 |
d-ROM – diacron reactive oxygen metabolite; BAP – biological antioxidant potential; CARR – Carretelli units, A single U.CARR corresponds to 0.08 ng/100 mL of H2O2.
The d-ROM levels were significantly (p<0.01) higher during treatment of infection (n=23, 401 U.CARR) than during non-treatment conditions (n=32, 277 U. CARR). On the other hand, the BAP levels were not significantly different (p=0.63, 2960 µmol/L, 2880 µmol/L, respectively). The d-ROM level after airway silicone stent insertion improved to the non-treatment level (Figure 2).
Figure 2.
The serial measurement of the oxidative stress response in Case 1 after lung transplantation. The gray bar shows the treatment of infections by intravenous antibiotics. During this period, d-ROM levels are significantly (p<0.01) higher during treatment of infection (n=23, 401) than during stable conditions (n=32, 277), and after airway silicone stent insertion they seem to have improved. d-ROM: diacron-reactive oxygen metabolite, BAP: biological antioxidant activity of plasma, POD: postoperative day, U.CAAR: Carratelli units
Case 2
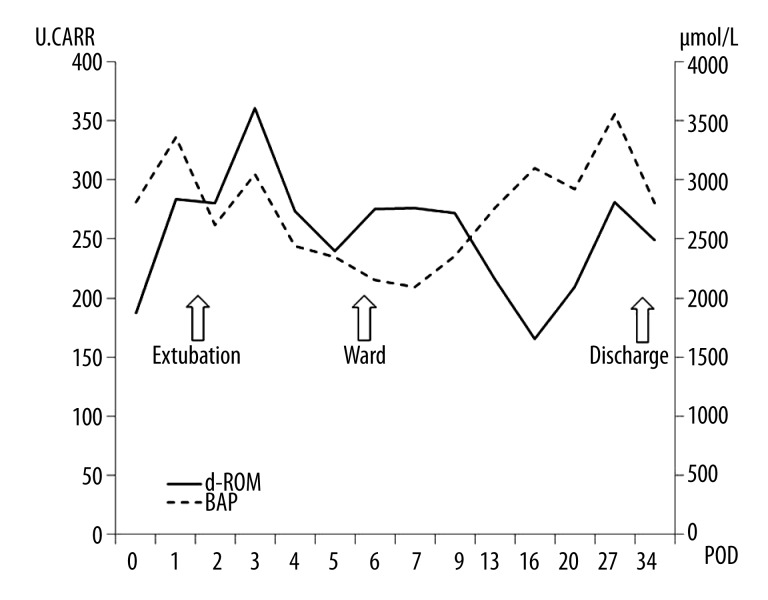
A 44-year-old Japanese woman underwent right single lung transplantation for lymphangioleiomyomatosis. She had been receiving a standard triple immunosuppressant regimen and infectious prophylactic strategies as per our lung transplant protocol described in Case 1. Her postoperative clinical course was uneventful, and no allograft rejection and no infections were seen. The d-ROM and BAP levels were measured as per the protocol for Case 1, and both levels measured during this period (15 times) remained almost within the normal range (Figure 3).
Figure 3.
Both d-ROM and BAP levels measured after transplantation are almost within their normal ranges. No allograft rejection or infections have occurred. d-ROM: diacron-reactive oxygen metabolite, BAP: biological antioxidant activity of plasma, POD: postoperative day, U.CAAR: Carratelli units
Discussion
This report describes 2 clinical cases of the serial measurement of the oxidative stress response after lung transplantation. We found that high oxidative stress occurred during infections, and the serial measurement of oxidative stress could be a useful tool to identify infectious conditions. In lung transplantation, severe oxidative stress is expected when ischemia/reperfusion injury, acute allograft rejection, and various infections occur. These conditions also contribute to the development of bronchiolitis obliterans syndrome (BOS), which prevents long-term survival [5].
There have been several reports [6–10] about oxidative stress in transplant patients by measurements of various kinds of markers, such as lipid peroxidation, oxidized glutathione, and malondialdehyde. Hussein et al. [7] reported that the severity of oxidative stress in pediatric liver transplant patients was correlated with their laboratory data. In addition, malondialdehyde was reported as an early predictive marker of graft dysfunction in kidney transplantation [9]. These reports indicated that the measurement of oxidative stress could enable us to identify the onset and development of many kinds of incidents after transplantation.
Currently, blood levels of hydroperoxides, a type of ROS, can be easily and accurately measured as diacron-reactive oxygen metabolites (d-ROMs). In addition, plasma ferric-reducing ability can be assessed as an index of total antioxidant potential, called the biological antioxidant activity of plasma (BAP). We have previously reported that video-assisted thoracic surgery attenuates perioperative d-ROMs compared to thoracotomy in lung cancer patients [4]. In addition, d-ROM could be a useful index of disease severity, not only for transplant patients, but also for patients with chronic obstructive pulmonary disease [11] and bronchial asthma [12]. However, to date, no clinical evidence has shown the effectiveness of d-ROM and BAP levels in lung transplant patients.
In Case 1, d-ROM levels were significantly (p<0.01) higher during treatment of infection (n=23, 401 CARR) than during non-treatment conditions (n=32, 277 CARR). In contrast, in Case 2, no significant d-ROM level fluctuation was seen under stable conditions. This shows that infectious episodes apparently lead to activation of inflammatory mediators, resulting in increased production of ROS, as Madill reported [5]. Moreover, it is interesting that d-ROM levels decreased to non-treatment levels after infection treatment and after airway silicone stent insertion.
The measurement of d-ROM and BAP is a non-invasive tool; however, the frequency in our study could have been reduced, especially for stable patients.
We cannot reach a firm conclusion about this problem in infection and rejection cases because there are no reports in the literature on the frequency of measurements of d-ROM and BAP.
According to the International Heart and Lung Transplantation Registry [13], within the first year post-transplantation, infections contribute to early mortality. Thus, it is desirable for clinicians to correctly and promptly distinguish and diagnose infections from allograft rejection and treat them. Given the present results, serial measurement of d-ROMs could be an effective tool to identify and monitor the effect of treatment for infections, similar to the ImmuKnow assay reported previously [14].
Several limitations must be considered when interpreting the present findings: only 2 cases were reported, and follow-up was limited to the hospitalization period. Therefore, further accumulation of clinical cases and follow-up is needed to determine the effectiveness of measurement oxidative stress in lung transplant patients.
Conclusions
We reported 2 clinical cases of the serial measurement of the oxidative stress response after lung transplantation, which might become a non-invasive marker of complications after transplantation.
Footnotes
Disclosures and freedom of investigation
The authors have no personal conflicts of interest to declare, and no outside support was received for this research.
References:
- 1.Salzman R, Pacal L, Kankova K, et al. High perioperative level of oxidative stress as a prognostic tool for identifying patients with a high risk of recurrence of head and neck squamous cell carcinoma. Int J Clin Oncol. 2010;15:565–70. doi: 10.1007/s10147-010-0108-z. [DOI] [PubMed] [Google Scholar]
- 2.Kanaoka Y, Inagaki E, Hamanaka S, et al. Analysis of reactive oxygen metabolites (ROMs) after cardiovascular surgery as a marker of oxidative stress. Acta Med Okayama. 2010;64:323–30. doi: 10.18926/AMO/40508. [DOI] [PubMed] [Google Scholar]
- 3.Tsukioka T, Nishiyama N, Iwata T, et al. Preoperative serum oxidative stress marker as a strong indicator of nodal involvement in clinical stage I lung adenocarcinoma. Int J Clin Oncol. 2012;17(3):250–55. doi: 10.1007/s10147-011-0283-6. [DOI] [PubMed] [Google Scholar]
- 4.Miyazaki T, Takagi K, Mine M, et al. Video-assisted thoracic surgery attenuates perioperative oxidative stress response in lung cancer patients: a preliminary study. Acta Med Nagasaki. 2014;59:19–25. [Google Scholar]
- 5.Madill J, Aghdassi E, Arendt B, et al. Lung transplantation: does oxidative stress contribute to the development of bronchiolitis obliterans syndrome? Transplant Rev. 2009;23:103–10. doi: 10.1016/j.trre.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Madill J, Aghdassi E, Arendt BM, et al. Oxidative stress and nutrition intake in lung patients with bronchiolitis obliterans syndrome. Transplant Proc. 2009;41:3838–44. doi: 10.1016/j.transproceed.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Hussein MH, Hashimoto T, Daoud GA, et al. Oxidative stress after living related liver transplantation subsides with time in pediatric patients. Pediatr Surg Int. 2011;27:17–22. doi: 10.1007/s00383-010-2721-3. [DOI] [PubMed] [Google Scholar]
- 8.Minz M, Heer M, Arora S, et al. Oxidative status in stable renal transplantation. Transplant Proc. 2006;38:2020–21. doi: 10.1016/j.transproceed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca I, Reguengo H, Almeida M, et al. Oxidative stress in kidney transplantation: malondialdehyde is an early predictive marker of graft dysfunction. Transplantation. 2014;97:1058–65. doi: 10.1097/01.TP.0000438626.91095.50. [DOI] [PubMed] [Google Scholar]
- 10.Kamijyo Y, Wang L, Matsumoto A, et al. Long-term improvement of oxidative stress via kidney transplantation ameliorates serum sulfatide levels. Clin Exp Nephrol. 2012;16:959–67. doi: 10.1007/s10157-012-0634-2. [DOI] [PubMed] [Google Scholar]
- 11.Markoulis N, Gourgoulianis KI, Moulas A, et al. Reactive oxygen metabolites as an index of chronic obstructive pulmonary disease severity. Panminerva Med. 2006;48:209–13. [PubMed] [Google Scholar]
- 12.Suzuki S, Matsukura S, Takeuchi H, et al. Increase in reactive oxygen metabolite in acute exacerbations of asthma. Int Arch Allegy Immunol. 2008;146(Suppl.):67–72. doi: 10.1159/000126064. [DOI] [PubMed] [Google Scholar]
- 13.Yusen RD, Christie JD, Edwards LB, et al. The registry of the International Society for Heart and Lung transplantation: thirtieth adult lung and heart-lung transplant report-2013; focus theme: age. J Heart Lung Transplant. 2013;32:965–78. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki T, Tagawa T, Yamasaki N, et al. Two case reports of successful withdrawal of mycofenolate mofetil after living donor lobar lung transplantation. Transplant Proc. 2013;45:356–59. doi: 10.1016/j.transproceed.2012.09.112. [DOI] [PubMed] [Google Scholar]