Abstract
Neuropathic pain is one of the most difficult consequences of spinal cord injury (SCI). The clinical correlates of the underlying mechanisms responsible for neuropathic pain are not well understood, although methods such as quantitative somatosensory testing (QST) or brain imaging have been used to further a mechanism-based understanding of pain. Our previous SCI study demonstrated a significantly lower glutamate-glutamine/myo-inositol ratio (Glx/Ins) in the anterior cingulate cortex in persons with severe neuropathic pain compared with those with less severe neuropathic pain or pain-free, able-bodied controls, suggesting that a combination of decreased glutamatergic metabolism and glial activation may contribute to the development of severe neuropathic pain after SCI. The present study aimed to determine the relationships between somatosensory function below the level of injury and low thalamic Glx/Ins in persons with intense neuropathic pain after SCI. Participants underwent QST and a 3 Tesla proton magnetic resonance spectroscopy. A cluster analysis including SCI participants resulted in 1 group (n = 19) with significantly (P < 0.001) greater pain intensity (6.43 ± 1.63; high neuropathic pain [HNP], and lower Glx/Ins [1.22 ± 0.16]) and another group (n = 35) with lower pain intensity ratings (1.59 ± 1.52, low neuropathic pain [LNP], and higher Glx/Ins [1.47 ± 0.26]). After correcting for age, QST indicated significantly greater somatosensory function in the HNP group compared with the LNP group. Our results are consistent with research suggesting that damage to, but not abolition of, the spinothalamic tract contributes to development of neuropathic pain after SCI and that secondary inflammatory processes may amplify residual spinothalamic tract signals by facilitation, disinhibition, or sensitization.
Keywords: Magnetic resonance spectroscopy, Spinal cord injury, Neuropathic pain, Somatosensory phenotype, Quantitative sensory testing
1. Introduction
Persistent pain has been reported as one of the most difficult long-term consequences to deal with after a spinal cord injury (SCI).84 Unfortunately, the mechanisms underlying neuropathic pain associated with SCI are multiple and their clinical correlates are not well defined.18,43,88
Development of mechanism-based understanding of pain is dependent on reliable clinical phenotypes and/or surrogate biomarkers of spinal and supraspinal mechanisms. For example, brain-imaging studies in persons with SCI have demonstrated structural and functional abnormalities in brain regions significantly associated with persistent neuropathic pain.30,85,87,89 Noninvasive magnetic resonance spectroscopy (MRS) measures metabolite concentrations in the brain28 that may serve as biomarkers and lead to further understanding of pain mechanisms (for a recent review, see Ref. 9). For example, lower thalamic concentrations of N-acetylaspartate (NAA) have been found in persons with heterogeneous chronic pain,2 heterogeneous neuropathic pain,24 and in neuropathic pain associated with diabetes69 and SCI.57 N-acetylaspartate is primarily located in neurons,68 and a decrease in concentration may indicate loss of neurons or neuronal dysfunction.28 Another metabolite, myo-inositol (Ins), is considered a glial marker with a major role in the volume and osmoregulation of astrocytes,44 and high concentrations may indicate gliosis, glial activation, or increased cell volumes.9 Indeed, high thalamic concentrations of Ins have been associated with neuropathic pain after SCI.57 Because astrocytic and microglial activation can significantly influence transmitter, cytokine, and glutamatergic systems, and has been shown to contribute to the development of neuropathic pain behaviors in animals,35,37,42 decreased Glx (a composite glutamate and glutamine concentration) in combination with Ins may serve as a surrogate marker of these mechanisms. Indeed, a recent study in people with SCI demonstrated that Glx/Ins measured in the anterior cingulate cortex (ACC) was significantly lower in persons with severe neuropathic pain with high psychosocial impact compared to SCI subjects with less severe or no neuropathic pain, and to pain-free, able-bodied (AB) controls.85
Another method, quantitative sensory testing (QST), has been used extensively to facilitate a mechanism-based understanding of pain.3 Results from human QST studies have consistently suggested that injury involving the spinothalamic tract (STT) is related to the presence and/or severity of neuropathic pain after SCI.12,17,19,20,78 Human SCI research also shows that greater residual STT function is significantly related to more intense neuropathic pain.12,17,78 To the best of our knowledge, no previous study has examined to what extent somatosensory function is associated with thalamic Glx/Ins in persons with neuropathic SCI-related pain. Because the thalamus is a brain area critical to the processing, integration, and modulation of sensory nociceptive information,58,91 we hypothesized that lower thalamic Glx/Ins ratios (suggesting decreased glutamatergic metabolism in combination with glial proliferation or activation) and more intense neuropathic pain would be associated with a sensory phenotype with more residual STT function. Thus, the primary purposes of this study were to identify subgroups in persons with SCI based on Glx/Ins and pain intensity and to compare these with respect to somatosensory function.
2. Methods
2.1. Study procedures
All study procedures were approved by the Miami Veterans Affairs Medical Center and the University of Miami, Miller School of Medicine, Institutional Review Boards according to the Declaration of Helsinki. Persons with a complete or incomplete traumatic SCI occurring at least 1 year before study enrollment and pain-free, able-bodied control subjects were recruited through the University of Miami and the Miami Veterans Affairs Medical Center. The first study session consisted of informed consent and screening procedures. Screening was conducted using the Mini-Mental State Examination,23 the Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Non-patient Edition,22 an MRI contraindications checklist, and a brief health questionnaire. Additionally, a neurological examination was performed on subjects with SCI. During the second session, subjects first underwent a questionnaire-based interview regarding demographics, psychosocial factors, and health history, including current use of pain medication and a detailed pain history when appropriate. Subjects with SCI completed additional questions regarding their injury (eg, mode and date of injury). Quantitative sensory testing was also conducted during the second session, followed by an MRS brain scan. A subgroup of SCI subjects completed a third session, approximately 2 to 4 weeks after the second session, which included a repeat of the MRS brain scan. The data used in the present analyses were obtained from a larger set of data obtained from people with SCI with and without neuropathic pain and able-bodied controls. A subset of the larger data set included MRS measurements from the ACC and affective pain measures, the results of which were previously published.85 Our previous publication focused on metabolite concentrations in an area of the brain previously associated with affective factors, the ACC, and the relationship with psychosocial pain impact.
In this study, we analyzed a different set of data points from this sample (eg, thalamic MRS-derived metabolites, comprehensive QST, and pain intensity ratings during MRS) to determine to what extent somatosensory function or sensory pain phenotype differ in individuals with a combination of greater pain ratings and lower thalamic Glx/Ins ratio compared with those with less intense pain and greater thalamic Glx/Ins ratio. We analyzed this relationship using thalamic data because the thalamus is the termination of the spinothalamic tract and is therefore more likely to be directly related to somatosensory function than other brain areas.
2.2. Participants
Subjects were at least 18 years old and able to speak and understand English. Subjects were excluded if they had cognitive impairment (score of <25 on the Mini-Mental State Examination), diagnosis of DSM-IV Axis I disorders, including major depression, current or previous drug or alcohol abuse, and MRI contraindications (eg, metal implants/fragments, pacemaker). Persons with SCI were recruited to include subjects with and without chronic neuropathic pain. Able-bodied (AB) subjects were excluded if they had a chronic pain condition.
2.3. Magnetic resonance imaging and spectroscopy
All participants were scanned on a 3 Tesla Siemens TIM TRIO MRI System (Erlangen, Germany). An 8-channel, phased-array coil was used to provide optimum signal-to-noise for magnetic resonance imaging and spectroscopy data. Axial, sagittal, and coronal localizer images were acquired using true fast imaging with steady-state precession localizer. T1-weighted sagittal images were acquired using a spin-echo sequence through the midline. The T1-weighted sagittal images were used to align the axial images for the study along the anterior commissure-posterior commissure line. Axial T1-weighted images were acquired using 3-dimensional magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence, which provided isotropic voxel resolution of 1 mm3. The 3-dimensional axial images were used to generate sagittal and coronal images using a multiplanar reconstruction routine provided by the manufacturer. Axial turbo fluid-attenuated inversion recovery (FLAIR) with fat saturation images was acquired. These images used the same slice orientation and offsets as the axial MP-RAGE sequences. Similarly, axial T2-weighted turbo spin-echo images were acquired using a turbo factor of 15.
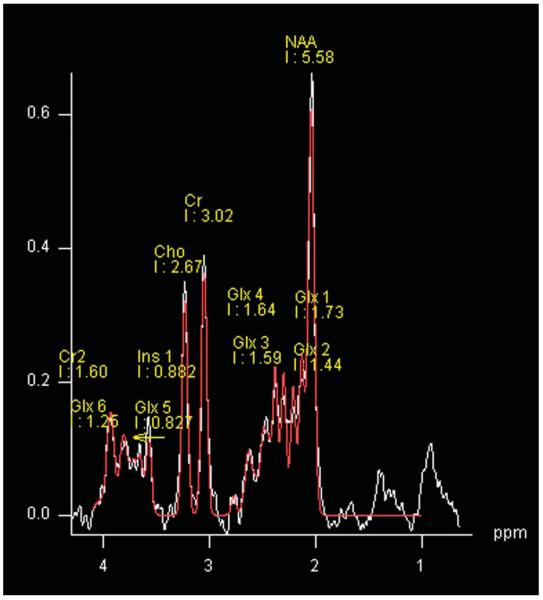
The spectroscopy was performed using a 2-dimensional chemical shift imaging method using a point-resolved spectroscopy sequence (repetition time 5 2000 ms, echo time = 30 ms) with 4 signal averages in the central 8 cm × 8 cm voxel (Fig. 1: within the white outlined square) and 1 signal average in the outer voxels (field of view = 160 mm, slice thickness = 1.5 mm, sampling points = 1024, and bandwidth 5 = kHz). The slice was positioned on the axial image that visualized the thalamus using MP-RAGE images, as well as T2-weighted and FLAIR images (Fig. 1). The offset relative to the center slice that was aligned to the anterior commissure–posterior commissure line was noted for the placement of the chemical shift imaging of the repeat study. A typical spectrum that was obtained with our chemical shift imaging method is shown in Fig. 2, which is from 1 subvoxel. The analysis of the spectral data was performed using the linear combination model (LC Model) software60 from 4.0 ppm to 0.2 ppm. To account for subject-to-subject variability in coil loading, the results were analyzed using ratios of metabolite concentrations relative to creatine (Cr) for: N-acetylaspartate (NAA), choline (Cho), myo-inositol (Ins), and the glutamate–glutamine composite (Glx).
Figure 1.
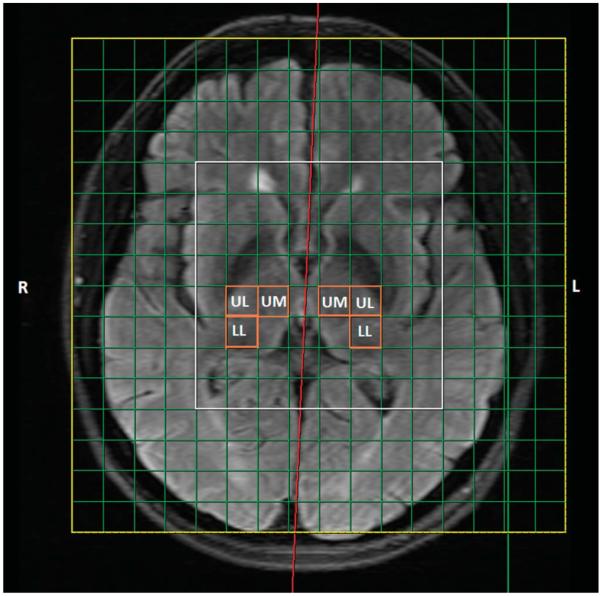
Positioning of the 2-dimensional (chemical shift imaging) slice over the region of left and right thalamus is indicated in the yellow square. The central 8 × 8 voxels highlighted in the white square are processed, and 3 voxels (UL, upper lateral; UM, upper medial; LL, lower lateral) within the region of the right and left thalamus are indicated in the orange squares.
Figure 2.
Quality of the spectrum obtained from 1 subvoxel in the thalamus. Cho, choline; Cr, creatine (Cr 1–2); Glx, glutamate and glutamine (Glx 1–4); Ins, myo-inositol; NAA, N-acetyl-aspartate.
2.4. Neurological examination
For each subject with SCI, a trained examiner conducted a standard neurological examination for SCI and classified their neurological level of injury (LOI) per the guidelines in the reference manual for the International Standards for Neurological Classification of Spinal Cord Injury.1 Per this standard, the LOI is defined as the most caudal segment of the spinal cord with normal sensory and motor function findings on both sides of the body.
2.5. Quantitative sensory testing
Somatosensory function below the level of injury was evaluated with QST. For each subject, a site was selected that was at least 3 dermatomes below the defined LOI. For most subjects, this included a standard site or sites on the medial abdomen (level of the umbilicus), anterior mid-thigh, medial calf, or dorsal foot. To standardize the data, we used data from a database including 50 pain-free, AB subjects (age 34.8 ± 10.9, 37 males [74.0%] and 13 [26.0%] females) to construct normal distributions for each test site and converted threshold values from SCI subjects to z-scores based on these distributions according to the procedures described by Rolke et al.62 Subjects' sensory function was assessed using vibration detection thresholds (VDT), cool detection thresholds (CDT), warm detection thresholds (WDT), cold pain thresholds (CPT), and hot pain thresholds (HPT). Standard instructions were read and a demonstration of procedures was performed for each threshold testing method ahead of actual testing.
The VDT was calculated as the average of 3 trials performed using the ascending method of limits, delivered through the VSA-3000 accessory for the TSA-II (Medoc Ltd, Ramat Yishai, Israel). The VSA-3000 is a hand-held vibratory probe with a 1.22-cm2 tip that can deliver a range of stimulus amplitude from 0 to 130 μm at 100 Hz frequency. The increase in stimulus amplitude was set to 0.5 μm/s using the software accompanying the TSA-II. At least 15 seconds elapsed between each of the 3 trials.
The CDT and WDT were measured using the 1.6- × 1.6-cm square thermal probe (thermode) attached to the TSA-II. Each thermal detection threshold was calculated as the average of 4 trials performed with a stimulus interval of at least 15 seconds. Each trial began with the temperature of the thermode at 32°C (an adaptation temperature approximating skin temperature) and decreased (for CDT) or increased (for WDT) at a rate of 1°C per second. The CPT and HPT were similarly measured using 3 trials, a stimulus interval of at least 30 seconds, and a temperature change of 1.5°C per second. Greater detail regarding all performed QST procedures can be found in the studies of Felix and Widerström-Noga17 and Cruz-Almeida et al.12
2.6. Assessment of pain and associated psychosocial factors
2.6.1. Pain diagnosis
All SCI subjects who reported having any pain condition that was persistent over the past 6 months or more completed a structured pain history interview,79 which included questions regarding the location, onset, duration, and quality of each pain condition the subject reported, as well as factors affecting the severity of pain (eg, movement, specific treatments). Based on these responses and information gathered in the neurological examination,1 SCI subjects were classified as having “neuropathic pain” according to the criteria set forth in Siddall et al.67: (1) pain present for a minimum of 6 months; (2) pain in an area with neurological deficits (ie, below the LOI) described as sharp, shooting, burning, or electric. Subjects who did not report persistent pain or whose pain did not meet the above criteria were classified as having “no neuropathic pain.”
2.6.2. Pain intensity
All participants were asked to report their pain intensity just before, at the approximate mid-point, and just after the spectroscopy portion of the study using a 0-to-10 numerical rating scale. The average of these 3 measurements was used in the cluster analysis.
2.6.3. Pain Severity
The Pain Severity subscale of the Multidimensional Pain Inventory-SCI version (MPI-SCI82,83) was used to determine the relationship between Glx/Ins and overall pain severity. This subscale consists of 3 items: (1) level of pain at the present moment, (2) average severity of pain during the past week, and (3) suffering because of pain. The Pain Severity subscore is an average of the 3 item responses.
2.6.4. Spielberger State-Trait Anxiety Inventory
This inventory70 is a valid and reliable56 measure of a person's stable level of anxiety (trait) and their variable, or situation-dependent, anxiety level (state). This measure was used to externally validate the clusters.
2.6.5. Beck Depression Inventory
This amended version4 of the original inventory5 assesses symptoms associated with depression, and has been validated for use in a number of populations, including healthy individuals,49 those with SCI,64 and those with neuropathic pain.36 This measure was used to externally validate the clusters.
2.6.6. Satisfaction with life scale
This measure15 has been used extensively as a self-report measure of overall satisfaction with life. It has been validated in a number of patient groups, including those with SCI.16 This measure was used to externally validate the clusters.
2.7. Statistical analysis
2.7.1. Reliability analysis
The thalamic MRS measurements were obtained in 3 voxels on each side of the thalamus (Fig. 1). We examined the internal consistency among the 6 voxels using Cronbach alpha to determine the appropriateness of averaging them.
For measures to be useful for clinical and research purposes, they should have a certain level of repeatability when they are measured in the same individuals under the same conditions at different time points. Test-retest reliability is a measure of repeatability, and the intraclass correlation coefficient can provide a metric for this purpose, with coefficients ranging from 0.41 to 0.60 considered “fair”, 0.61 to 0.80 “moderate”, and 0.81 to 1.0 “substantial” reliability.66 We calculated 1-way random effect coefficients for each of the thalamic metabolite concentration ratios to determine test–retest reliability between 2 identical study sessions in 30 individuals with SCI and neuropathic pain. To the best of our knowledge, the test–retest reliability of metabolite ratios in the thalamus of persons with SCI has not been previously reported.
2.7.2. Cluster analysis
A 2-step cluster procedure was used to define homogeneous subgroups or “clusters” inherent in the data set based on the thalamic Glx/Ins ratio and average pain intensity. The 2-step clustering procedure has been previously used and described in more detail in our previous work.85 We determined the number of clusters by using the automatic IBM SPSS Statistics 21 for Windows default criterion (Schwarz criterion65). Once the number of clusters was determined, we compared the pain intensity ratings and Glx/Ins ratios to determine the appropriateness of the clusters. After this step, the clusters were statistically compared with regard to other thalamic metabolite concentration ratios of interest, somatosensory function, and demographic and psychosocial factors, using 1-way analysis of variance (ANOVA) with Tukey post hoc adjustments.63 We also performed analysis of covariance (ANCOVA) to compare the clusters on metabolite concentration ratios while controlling for age (with Sidak post hoc adjustments). The nonparametric Kruskal-Wallis and Mann- Whitney tests were used to determine statistically significant differences between the clusters when analyzing dichotomous variables. Independent t test and x2 were used to determine pairwise differences. A probability of less than 0.05 was considered statistically significant.
3. Results
3.1. Reliability analysis of metabolites
3.1.1. Internal consistency
For all individuals, MRS data were acquired from 3 voxels of the left and right thalami. The intervoxel Cronbach alpha coefficients among the 6 voxels for each metabolite concentration ratio were in the moderate range (ie, NAA/Cr = 0.68, Cho/Cr = 0.79, Ins/Cr = 0.65, Glx/Cr = 0.75, Glx/Ins = 0.79, and NAA/Ins = 0.76), with the greatest intervoxel reliability for Glx/Ins. Based on this analysis, the average values for the 6 voxels were calculated for each metabolite concentration ratio and used in the subsequent analyses.
3.1.2. Test–retest reliability
For 30 SCI participants for whom repeat MRS scans were conducted, the intraclass correlation coefficients for the metabolite concentration ratios were in the high-moderate to substantial range (Table 1) indicating adequate test–retest reliability between 2 sessions. The intraclass correlation coefficient for the Glx/Ins ratio was 0.89, indicating excellent test–retest reliability.
Table 1.
Intraclass correlation analyses (1-way random effects) for thalamic metabolite concentration ratios in persons with spinal cord injury (n = 30).
| Metabolite ratio | Scan 1 (mean ± SD) | Scan 2 (mean ± SD) | Intraclass correlation | F, P |
|---|---|---|---|---|
| NAA/Cr | 1.40 ± 0.11 | 1.40 ± 0.10 | 0.78 | 4.63, <0.001 |
| Cho/Cr | 0.27 ± 0.03 | 0.27 ± 0.03 | 0.94 | 17.27, <0.001 |
| Ins/Cr | 0.80 ± 0.09 | 0.78 ± 0.10 | 0.85 | 6.56, <0.001 |
| GIx/Cr | 1.09 ± 0.15 | 1.09 ± 0.19 | 0.74 | 3.79, <0.001 |
| GIx/Ins | 1.40 ± 0.28 | 1.43 ± 0.33 | 0.89 | 8.98, <0.001 |
| NAA/Ins | 1.82 ± 0.27 | 1.85 ± 0.28 | 0.78 | 4.44, <0.001 |
Cho, choline; Cr, creatine; Glx, glutamate and glutamine; Ins, myo-inositol (Ins); NAA, N/-acetylaspartate.
3.2. Cluster analysis based on Glx/Ins and average pain intensity
The cluster analysis included all subjects with SCI and resulted in 2 distinctly different clusters. The pain intensity ratings during the brain scans were used to ensure pain relevant clusters. Another consideration was that these ratings were available for all subjects in the study regardless of pain status. Importantly, the average pain intensity rating during the scan was highly correlated with MPI-SCI Pain Severity subscores in those with neuropathic pain (r = 0.60, P < 0.001).
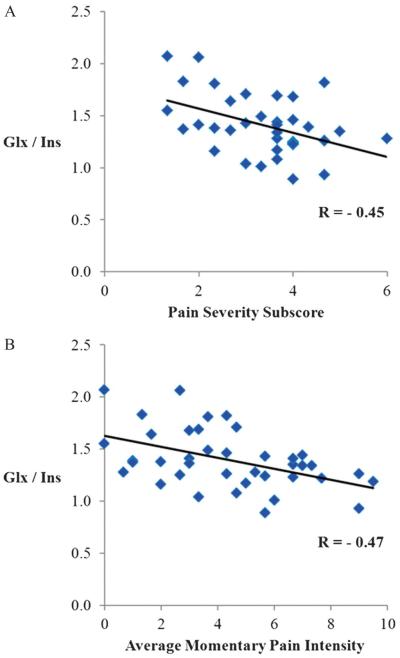
As expected, the groups were significantly different on the clustering variables Glx/Ins and pain intensity ratings. One group (n = 19) was characterized by significantly greater pain intensity (6.43 ± 1.63, ie, high neuropathic pain [HNP], P < 0.001) and lower Glx/Ins ratio (1.22 ± 0.16, P < 0.001) compared with a larger group (n = 35) composed of SCI individuals with lower neuropathic pain intensity ratings or no neuropathic pain (1.59 ± 1.52, ie, low neuropathic pain [LNP]) with a Glx/Ins of 1.47 ± 0.26. Two Pearson correlation analyses also showed pairwise relationships between the Glx/Ins ratio and the Pain Severity subscore of the MPI-SCI (r = −0.45, p = 0.006), and average pain intensity (r = −0.47, p = 0.002) in participants with neuropathic pain (Figs. 3A and 3B, respectively).
Figure 3.
Correlations between the Glx/Ins ratio and (A) Pain Severity subscore of the MPI-SCI and (B) Average Momentary Pain Intensity ratings during the brain scan session, in persons with spinal cord injury and neuropathic pain (n = 39). Glx, glutamate and glutamine; Ins, myo-inositol.
3.3. Comparisons between the HNP, LNP, and AB groups
3.3.1. Demographic, injury, and psychosocial factors
There were highly significant differences between the HNP participants and the other 2 groups with respect to some demographic, injury, and psychosocial characteristics (Table 2). After correcting for multiple comparisons, the HNP participants were significantly older than the AB group (P < 0.05), and a greater proportion of the HNP participants had neurologically incomplete cord injuries as compared with the LNP (P < 0.05) group. The HNP group had significantly higher scores on the Spielberger State-Trait Anxiety Inventory (STAI) and lower scores on the Satisfaction With Life Scale (SWLS) than the other 2 groups.
Table 2.
Comparison of demographic, injury, and psychosocial characteristics between the bodied AB, LNP, and HNP groups.
| AB (n = 24) | LNP (n = 35) | HNP (n = 19) | Statistic; P | |
|---|---|---|---|---|
| Age* (mean ± SD) | 34.4 ± 8.6 | 35.7 ± 12.4 | 43.0 ± 12.5 | F = 3.500; 0.035 |
| Time since injury (mean ± SD) | NA | 13.1 ± 9.70 | 12.0 ± 9.66 | F = 0.170; 0.682 |
| Sex (n) | ||||
| Female | 5 | 7 | 3 | χ2 = 0.198; 0.91 |
| Male | 19 | 28 | 16 | |
| Injury severity (n) | ||||
| Incomplete | NA | 11 | 13 | χ2 = 6.825; 0.009 |
| Complete | 24 | 6 | ||
| STAI† (mean ± SD) | 24.5 ± 4.71 | 29.9 ± 8.17 | 36.7 ±11.8 | F = 10.657; 0.001 |
| BDI‡ (mean ± SD) | 1.12 ± 1.36 | 6.80 ± 6.86 | 11.3 ± 9.38 | F = 12.788; 0.001 |
| SWLS§ (mean ± SD) | 28.4 ± 5.35 | 22.1 ± 7.36 | 16.9 ± 8.65 | F = 13.882; 0.001 |
Tukey post hoc correction:
HNP older than AB(P < 0.05).
HNP score significantly higher than AB (P < 0.001) and the LNP (P < 0.001), and LNP score significantly higher than AB (P < 0.05).
HNP score significantly higher than AB (P < 0.001), LNP score significantly higher than AB (P < 0.01).
HNP score significantly lower than AB (P < 0.001) and the LNP (P < 0.05), LNP score significantly lower than AB (P < 0.01).
AB, able-bodied controls; BDI, beck depression inventory; HNP, high neuropathic pain; LNP, low or no neuropathic pain; STAI, State-Trait Anxiety Inventory; SWLS, Satisfaction With Life Scale.
3.3.2. MRS metabolite concentration ratios
The 3 groups were compared on the NAA/Cr, Cho/Cr, Ins/Cr, Glx/Cr, NAA/Ins, and Glx/Ins ratios. The overall ANOVA indicated significant differences among the groups regarding Ins/Cr, Glx/Cr, NAA/Ins, and Glx/Ins. Because age was significantly different among groups and because brain metabolism may be affected by age, we statistically controlled for age (Fig. 4). When correcting for age, the Glx/Cr, NAA/Ins, and Glx/Ins remained significantly different between groups. Post hoc analyses revealed significantly lower NAA/Ins and Glx/Ins ratios in the HNP group compared with the other 2 groups. There were no significant differences in metabolite concentration ratios between the LNP and the AB control groups (P > 0.05).
Figure 4.
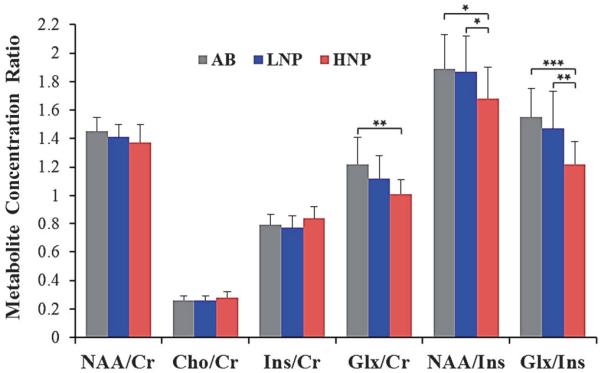
Thalamic metabolite concentrations relative to Cr or Ins (mean and SD) for the AB (n = 24) and the 2 SCI subgroups, LNP (n = 35) and HNP (n = 19) groups. Age-adjusted ANCOVA, F and P values for: NAA/Cr (F = 3.05, P = 0.059); Cho/Cr (F = 2.55, P = 0.152); Ins/Cr (F = 3.48, P = 0.054); Glx/Cra (F = 8.53, P = 0.005); NAA/Insb (F = 5.21, P = 0.008); Glx/Insc (F = 12.4, P = 0.001). Post hoc correction: aHNP significantly lower than AB, (P < 0.01); bHNP significantly lower than AB (P < 0.05) and the LNP, (P < 0.05); cHNP significantly lower than AB (P < 0.001) and LNP (P < 0.01). AB, able-bodied controls; Cho, choline; Cr, creatine; Glx, glutamate and glutamine; HNP, high neuropathic pain; Ins, myo-inositol; LNP, low or no neuropathic pain; NAA, N-acetylaspartate. *P < 0.05, **P < 0.01, ***P < 0.001.
3.3.3. Somatosensory function
Logarithmic transformation was used for VDT, CDT, and WDT data to better approach a normal distribution. Individual threshold values were normalized using data from a sample of 50 AB pain-free controls for each of the somatosensory modalities to create z-scores (see Refs. 12,17,62 for details). These z-scores were obtained from sites below the LOI and were subsequently compared between the HNP and the LNP participants. After correcting for age, we found highly significant differences between the SCI groups, with the HNP group showing greater levels of sensitivity to warm, cool, cold pain, hot pain, and vibratory stimuli compared with the LNP group (Fig. 5). Interestingly, the SDs were much larger for the HNP group compared with the LNP group for the STT-mediated modalities, suggesting sensory subgroups within the HNP group.
Figure 5.
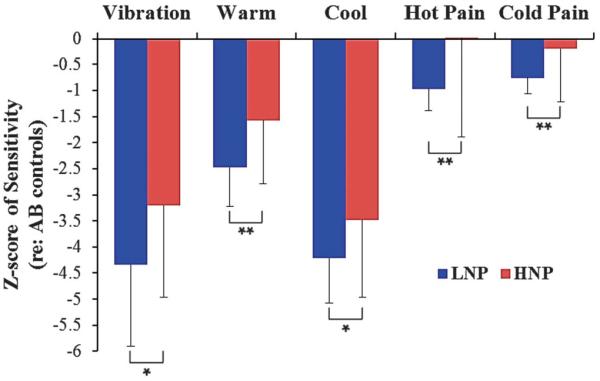
Somatosensory function in the LNP (n = 35) and HNP (n = 19) SCI subgroups. Values are shown as z-scores relative to a normative pain-free sample. Negative values reflect a loss in sensitivity, and positive values reflect a gain in sensitivity, compared with the normative average. Age-adjusted ANCOVA, F, and P values for: vibratory detection (F = 5.06, P = 0.029); warm detection (F = 10.4, P = 0.002); cool detection (F = 5.02, P = 0.029); heat pain detection (F = 8.87, P = 0.004); cold pain detection (F = 7.09, P = 0.010). HNP, high neuropathic pain; LNP, low or no neuropathic pain. *P < 0.05, **P <0.01.
3.4. Relationship between average thermal pain sensitivity and Glx/Ins
Z-scores for pain threshold measures of hot and cold stimuli were highly intercorrelated (r = 0.72, P < 0.001). Therefore, we averaged these 2 measurements of thermal pain sensitivity. The average thermal pain sensitivity was then correlated with the Glx/Ins ratio in people with SCI and neuropathic pain to determine the inter-relationship between these 2 factors. The Pearson correlation coefficient was −0.35, p = 0.031, which indicated a significant relationship between a low Glx/Ins ratio and a greater sensitivity to thermal pain stimuli among persons with SCI and neuropathic pain.
3.5. Current use of pain medication
Although most SCI participants did not currently use pain medication, a greater number of individuals in the HNP group reported using pain medication compared with the LNP group. The current use of opioids was 7/19 (36.8%) in the HNP group and 5/35 (14.3%) in the LNP group; antidepressants was 6/19 (31.6%) in the HNP group and 3/35 (8.6%) in the LNP group; and anticonvulsants was 5/19 (26.3%) in the HNP group and 4/35 (11.4%) in the LNP group.
Because pain medication use was more prevalent in the HNP group, we performed a hierarchical linear regression analysis in 54 individuals with SCI with the purpose of controlling for the potential confounding effect of medication on the relationship between Glx/Ins and PS. The regression analysis with PS as the dependent variable and independent variables were entered in 2 steps—Step 1: current use of opioids, antidepressants, or anticonvulsants; and step 2: thalamic Glx/Ins. The final model including all variables was statistically significant (F = 4.540, P = 0.003). The only variable significantly contributing to the final model was Glx/Ins (t = −2.020, P = 0.049), whereas the other variables did not significantly contribute (opioids [t = 1.189, p = 0.240], antidepressants [t = 1.673, p = 0.101], and anticonvulsants [t = 1.193, p = 0.239]). Thus, we do not expect the medication use to have significantly confounded our results.
4. Discussion
Chronic pain after SCI is a serious problem that is often associated with depression, catastrophizing, and affective distress,40,45,54,81 significant psychosocial impact,46,61,72 and reduced quality of life.52,74,86 Unfortunately, many pharmacological and non-pharmacological treatments are tried with only limited success.7,11,55,77,80 To improve the management of these refractory pain types, a better understanding of the mechanisms responsible for the development and maintenance of neuropathic pain is needed.
This study investigated the combination of 2 approaches that have been used in other studies to elucidate pain mechanisms in various chronic pain populations, ie, brain imaging and QST. The brain area of focus in this study, the thalamus, is a critical area associated with neuropathic pain,26 processing, and modulation of nociceptive information and is the termination for the spinothalamic tract. The thalamus has a significant role in pain modulation, and therefore thalamic hypoactivity and loss of inhibition,31 possibly due to sensory deafferentation, may be a factor important for the development and maintenance of neuropathic pain.25
The intraclass correlation coefficient of thalamic Glx/Ins was excellent, suggesting that this metabolite ratio may be useful to monitor metabolic thalamic changes in longitudinal studies and in clinical trials involving persons with SCI and neuropathic pain. The comparison among the HNP, LNP, and AB groups indicated that the HNP group had significantly lower Glx/Ins and NAA/Ins ratios compared with the LNP and AB groups. The Glx/Ins ratio was significantly and inversely associated with both momentary pain intensity ratings during the scan and average pain severity. These results support the idea that this metabolite ratio is primarily related to neuropathic pain and not to SCI itself. Our results are consistent with a recent study of individuals with SCI and neuropathic pain31 suggesting that neuropathic pain after SCI (and not injury per se) was associated with both altered thalamic structure and function. In contrast to the study by Gustin et al.,31 we did not find thalamic NAA/Cr by itself to be significantly different among the groups. However, the low NAA/Ins ratio in the HNP group indicated that decline or dysfunction of thalamic neurons in combination with glial proliferation or activation is an underlying mechanism of severe neuropathic pain after SCI. Consistent with these observations, Pattany et al.57 found reduced NAA and NAA/Ins in the thalamus in those with neuropathic pain and SCI compared with those with SCI and no neuropathic pain. Similarly, neuronal dysfunction including a decrease in the gray matter has been observed in the thalamus and cortex of patients with chronic pain.24,29
Of great importance was the finding that the HNP group had a sensory phenotype significantly different from the LNP group, with more residual function of both the spinothalamic tract and dorsal column medial lemniscal pathway below the level of injury compared with the LNP group. Although both groups had greater loss of sensory function compared with the normative sample, the loss of sensory function was significantly larger in the LNP group. Interestingly, the large SDs in the HNP group suggest that sensory subgroups with gain of function exist within the HNP group. This is consistent with the findings of a recent study by Finnerup et al.,21 where sensory hypersensitivity to cold stimuli measured 1 month after SCI predicted the development of below-level neuropathic pain. Our results also concur with previous studies where severity of neuropathic pain symptoms in persons with SCI was associated with greater sensitivity to thermal nonpainful and painful stimuli below the level of injury.12,17
It is clear that damage to the STT by itself is not a significant predictor of neuropathic pain in persons with SCI13,19,20; however, residual STT function after SCI injury may be a factor contributing to severe neuropathic pain.90 For example, Wasner et al.78 showed that only persons with SCI and neuropathic pain (and not those with SCI without neuropathic pain) had residual STT transmission and Hari et al.38 found that enhanced spontaneous recovery of STT function was associated with the development of neuropathic pain. Thus, our results are consistent with previous research suggesting that damage to, but not abolition of, the STT is one of the factors contributing to the development of central neuropathic pain.78
The lower thalamic Glx/Ins ratio observed in those with more intense neuropathic pain suggests that lower glutamatergic metabolism, glial proliferation, glial hypertrophy, or activation may be factors significantly contributing to intense neuropathic pain after SCI. Although it has been well established that glutamate is the major excitatory neurotransmitter of sensory and cortical inputs to the thalamus, glial modulation of glutamatergic neurotransmission is also important.71 As discussed in a recent review39 of the glutamate—glutamine (GABA) cycle, the glia produces glutamine from glutamate, which transfers to neurons that release it as either transmitter glutamate (excitatory) or GABA (inhibitory). Decreased function of the glutamate—glutamine (GABA) cycle in the spinal cord after SCI is one of the hypothesized mechanisms leading to loss of inhibitory neurons and neuronal hyperexcitability.32
Glial activation and central sensitization involving intracellular signaling processes in the injured spinal cord have been strongly implicated as mechanisms responsible for neuronal hyperexcit-ability and neuropathic pain.10,32,37,93 Although less evidence is available for thalamic glial involvement in neuropathic pain in animals14,73,92 and in humans after SCI,57 it is likely that thalamic glial activation contributes to neuronal hyperexcitability in residual STT neurons and to neuropathic pain by similar mechanisms as those within the injured spinal cord. Wang and Thompson76 showed in a rodent model of central pain that lesioning of the STT results in hyperexcitability of thalamic neurons. These authors hypothesized that the thalamic hyperexcitability was caused by loss of normal STT inputs due to the SCI. Similarly, Lenz et al.48 observed that partial loss of thalamic input by deafferented STT neurons led to significant thalamic reorganization in humans. Consistent with these findings, animal SCI models of neuropathic pain demonstrate changes in supraspinal processing of somato-sensory information including changes in the functional properties of thalamic neurons.27,41,53,92 Because of the strong implications that neuroinflammatory mechanisms involving glia and/or astrocytes significantly contribute to the development and maintenance of neuropathic pain after SCI, there has been a growing interest in the development of treatments that target these mechanisms. For example, research in SCI animal models demonstrate that reducing glial activation after SCI also decreases hyperexcitability of dorsal horn neurons induced by either nonnoxious or noxious stimuli.32,33,34 Multiple approaches designed to reduce the neuroinflammation have been explored in SCI animal models (for a recent comprehensive review, see Ref. 75). One intervention that has shown to reduce pain-related behaviors by targeting neuroinflammation is the administration of IL-10 either by systemic injection59 or through viral vectors.47 However, subsequent clinical trials including IL-10 have not been successful because of lack of effectiveness and impaired normal immune function.6 Other approaches that have shown benefit in animal models include minocycline, COX inhibitors, and approaches suppressing TNF-α and proinflammatory interleukins including IL-6.75 In addition, non-pharmacological interventions may reduce pain behaviors and glial activation. For example, a recent study in a mouse neuropathic pain model showed that early administration of transcutaneous electrical nerve stimulation (within a day of injury) reduced hyperalgesia and inhibited glial activation.51
A potential limitation of this study includes averaging the 6 thalamic metabolite ratios from 3 different regions of both right and left thalami. However, this decision was based both on power considerations and on the fact that Cronbach alpha values suggested high intercorrelations between the different thalamus voxels. Although it is well known that different areas of the thalamus represent different functions, we did not evaluate this because of the reasons mentioned above.
In conclusion, our results suggest that intense neuropathic pain in persons with SCI is associated with thalamic neuronal dysfunction and glial activation and greater residual sparing of STT-mediated function compared with those with less intense neuropathic pain. Indeed, evidence in animals and humans suggests that thalamic dysfunction will significantly impact neurotransmission at several levels of the neural axis including somatosensory function.8,50
Acknowledgements
The authors acknowledge Drs Alberto Martinez-Arizala, Diana Cardenas, and Qing He for conducting neurological examinations, and Dr. Salome Perez for performing clinical screening for mental disorders. Mr James Adcock, Ms Letitia Fisher, and Ms Maydelis Escalona for conducting pain and sensory assessments and for data processing.
Conflict of interest statement This work was funded by the US Department of Veterans Affairs Rehabilitation Research and Development Service (Merit Review 5023R), Craig Neilsen Foundation #164678 (normative data), and The Miami Project to Cure Paralysis. The authors have no financial or other relationships that inappropriately influenced this work. The sponsors had no involvement in the collection, analysis, and interpretation of data, nor in the writing or the decision to submit this article for publication.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].American Spinal Injury Association . Reference manual of the International Standards for Neurological Classification of Spinal Cord Injury. American Spinal Injury Association; Chicago, IL: 2003. [Google Scholar]
- [2].Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- [3].Baron R, Förster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol. 2012;11:999–1005. doi: 10.1016/S1474-4422(12)70189-8. [DOI] [PubMed] [Google Scholar]
- [4].Beck AT, Steer RA. Manual for the Beck Depression Inventory. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- [5].Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- [6].Bijjiga E, Martino AT. Interleukin 10 (IL-10) regulatory cytokine and its clinical consequences. J Clin Cell Immunol. 2013;S1:007:1–7. [Google Scholar]
- [7].Cardenas DD, Jensen MP. Treatments for chronic pain in persons with spinal cord injury: A survey study. J Spinal Cord Med. 2006;29:109–17. doi: 10.1080/10790268.2006.11753864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Casey KL, Morrow TJ, Lorenz J, Minoshima S. Temporal and spatial dynamics of human forebrain activity during heat pain: analysis by positron emission tomography. J Neurophysiol. 2001;85:951–59. doi: 10.1152/jn.2001.85.2.951. [DOI] [PubMed] [Google Scholar]
- [9].Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol. 2013;8:576–93. doi: 10.1007/s11481-013-9460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp Neurol. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- [11].Cruz-Almeida Y, Martinez-Arizala A, Widerström-Noga EG. Chronicity of pain associated with spinal cord injury: A longitudinal analysis. J Rehabil Res Dev. 2005;42:585–94. doi: 10.1682/jrrd.2005.02.0045. [DOI] [PubMed] [Google Scholar]
- [12].Cruz-Almeida Y, Felix ER, Martinez-Arizala A, Widerström-Noga EG. Decreased spinothalamic and dorsal column medial lemniscus-mediated function is associated with neuropathic pain after spinal cord injury. J Neurotrauma. 2012;29:2706–15. doi: 10.1089/neu.2012.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Defrin R, Ohry A, Blumen N, Urca G. Characterization of chronic pain and somatosensory function in spinal cord injury subjects. PAIN. 2001;89:253–63. doi: 10.1016/s0304-3959(00)00369-9. [DOI] [PubMed] [Google Scholar]
- [14].Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol. 2008;212:337–47. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction with life scale. J Pers Assess. 1985;49:71–5. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- [16].Dijkers MP. Correlates of life satisfaction among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:867–76. doi: 10.1016/s0003-9993(99)90076-x. [DOI] [PubMed] [Google Scholar]
- [17].Felix ER, Widerström-Noga EG. Reliability and validity of quantitative sensory testing in persons with spinal cord injury and neuropathic pain. J Rehabil Res Dev. 2009;46:69–83. [PubMed] [Google Scholar]
- [18].Finnerup NB, Baastrup C. Spinal cord injury pain: mechanisms and management. Curr Pain Headache Rep. 2012;16:207–16. doi: 10.1007/s11916-012-0259-x. [DOI] [PubMed] [Google Scholar]
- [19].Finnerup NB, Johannesen IL, Fuglsang-Frederiksen A, Bach FW, Jensen TS. Sensory function in spinal cord injury patients with and without central pain. Brain. 2003;126:57–70. doi: 10.1093/brain/awg007. [DOI] [PubMed] [Google Scholar]
- [20].Finnerup NB, Sorensen L, Biering-Sorensen F, Johannesen IL, Jensen TS. Segmental hypersensitivity and spinothalamic function in spinal cord injury pain. Exp Neurol. 2007;207:139–49. doi: 10.1016/j.expneurol.2007.06.001. [DOI] [PubMed] [Google Scholar]
- [21].Finnerup NB, Norrbrink C, Trok K, Piehl F, Johannesen IL, SØrensen JC, Jensen TS, Werhagen L. Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J Pain. 2014;15:40–8. doi: 10.1016/j.jpain.2013.09.008. [DOI] [PubMed] [Google Scholar]
- [22].First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- [23].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [24].Fukui S, Matsuno M, Inubushi T, Nosaka S. N-Acetylaspartate concentrations in the thalami of neuropathic pain patients and healthy comparison subjects measured with (1)H-MRS. Magn Reson Imaging. 2006;24:75–9. doi: 10.1016/j.mri.2005.10.021. [DOI] [PubMed] [Google Scholar]
- [25].Fukumoto M, Ushida T, Zinchuk VS, Yamamoto H, Yoshida S. Contralateral thalamic perfusion in patients with reflex sympathetic dystrophy syndrome. Lancet. 1999;354:1790–1. doi: 10.1016/S0140-6736(99)03746-0. [DOI] [PubMed] [Google Scholar]
- [26].Garcia-Larrea L, Peyron R. Pain matrices and neuropathic pain matrices: a review. PAIN. 2013;154(suppl l):S29–43. doi: 10.1016/j.pain.2013.09.001. [DOI] [PubMed] [Google Scholar]
- [27].Gerke MB, Duggan AW, Xu L, Siddall PJ. Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience. 2003;117:715–22. doi: 10.1016/s0306-4522(02)00961-2. [DOI] [PubMed] [Google Scholar]
- [28].Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–53. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- [29].Grachev ID, Fredrickson BE, Apkarian AV. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. PAIN. 2000;89:7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- [30].Gustin SM, Wrigley PJ, Siddall PJ, Henderson LA. Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb Cortex. 2010;20:1409–19. doi: 10.1093/cercor/bhp205. [DOI] [PubMed] [Google Scholar]
- [31].Gustin SM, Wrigley PJ, Youssef AM, McIndoe L, Wilcox SL, Rae CD, Edden RA, Siddall PJ, Henderson LA. Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. PAIN. 2014;155:1027–36. doi: 10.1016/j.pain.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gwak YS, Crown ED, Unabia GC, Hulsebosch CE. Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. PAIN. 2008;138:410–22. doi: 10.1016/j.pain.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience. 2009;161:895–903. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gwak YS, Unabia GC, Hulsebosch CE. Activation of p-38alpha MAPK contributes to neuronal hyperexcitability in caudal regions remote from spinal cord injury. Exp Neurol. 2009;220:154–61. doi: 10.1016/j.expneurol.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gwak YS, Kang J, Unabia GC, Hulsebosch CE. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol. 2012;234:362–72. doi: 10.1016/j.expneurol.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA, Iannetti GD, Jensen TS, Kauppila T, Nurmikko TJ, Rice AS, Rowbotham M, Serra J, Sommer C, Smith BH, Treede RD. NeuPSIG guidelines on neuropathic pain assessment. PAIN. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- [37].Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–17. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hari AR, Wydenkeller S, Dokladal P, Halder P. Enhanced recovery of human spinothalamic function is associated with central neuropathic pain after SCI. Exp Neurol. 2009;216:428–30. doi: 10.1016/j.expneurol.2008.12.018. [DOI] [PubMed] [Google Scholar]
- [39].Hertz L. The Glutamate-Glutamine (GABA) Cycle: importance of late postnatal development and potential reciprocal interactions between biosynthesis and degradation. Front Endocrinol (Lausanne) 2013;4:59. doi: 10.3389/fendo.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hoffman JM, Bombardier CH, Graves DE, Kalpakjian CZ, Krause JS. A longitudinal study of depression from 1 to 5 years after spinal cord injury. Arch Phys Med Rehabil. 2011;92:411–18. doi: 10.1016/j.apmr.2010.10.036. [DOI] [PubMed] [Google Scholar]
- [41].Hubscher CH, Johnson RD. Chronic spinal cord injury induced changes in the responses of thalamic neurons. Exp Neurol. 2006;197:177–88. doi: 10.1016/j.expneurol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- [42].Hulsebosch CE. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp Neurol. 2008;214:6–9. doi: 10.1016/j.expneurol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–13. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Isaacks RE, Bender AS, Kim CY, Norenberg MD. Effect of osmolality and myo-inositol deprivation on the transport properties of myo-inositol in primary astrocyte cultures. Neurochem Res. 1997;22:1461–9. doi: 10.1023/a:1021950311308. [DOI] [PubMed] [Google Scholar]
- [45].Jensen MP, Kuehn CM, Amtmann D, Cardenas DD. Symptom burden in persons with spinal cord injury. Arch Phys Med Rehabil. 2007;88:638–45. doi: 10.1016/j.apmr.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kennedy P, Lude P, Taylor N. Quality of life, social participation, appraisals and coping post spinal cord injury: a review of four community samples. Spinal Cord. 2006;44:95–105. doi: 10.1038/sj.sc.3101787. [DOI] [PubMed] [Google Scholar]
- [47].Lau D, Harte SE, Morrow TJ, Wang S, Mata M, Fink DJ. Herpes simplex virus vector-mediated expression of interleukin-10 reduces below-level central neuropathic pain after spinal cord injury. Neurorehabil Neural Repair. 2012;26:889–97. doi: 10.1177/1545968312445637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lenz FA, Kwan HC, Martin R, Tasker R, Richardson RT, Dostrovsky JO. Characteristics of somatotopic organization and spontaneous neuronal activity in the region of the thalamic principal sensory nucleus in patients with spinal cord transection. J Neurophysiol. 1994;72:1570–87. doi: 10.1152/jn.1994.72.4.1570. [DOI] [PubMed] [Google Scholar]
- [49].Lightfoot SL, Oliver JM. The Beck Inventory: psychometric properties in university students. J Pers Assess. 1985;49:434–6. doi: 10.1207/s15327752jpa4904_12. [DOI] [PubMed] [Google Scholar]
- [50].Lorenz J, Casey KL. Imaging of acute versus pathological pain in humans. Eur J Pain. 2005;9:163–5. doi: 10.1016/j.ejpain.2004.07.009. [DOI] [PubMed] [Google Scholar]
- [51].Matsuo H, Uchida K, Nakajima H, Guerrero AR, Watanabe S, Takeura N, Sugita D, Shimada S, Nakatsuka T, Baba H. Early transcutaneous electrical nerve stimulation reduces hyperalgesia and decreases activation of spinal glial cells in mice with neuropathic pain. PAIN. 2014;155:1888–901. doi: 10.1016/j.pain.2014.06.022. [DOI] [PubMed] [Google Scholar]
- [52].Middleton J, Tran Y, Craig A. Relationship between quality of life and self-efficacy in persons with spinal cord injuries. Arch Phys Med Rehabil. 2007;88:1643–8. doi: 10.1016/j.apmr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- [53].Morrow TJ, Paulson PE, Brewer KL, Yezierski RP, Casey KL. Chronic, selective forebrain responses to excitotoxic dorsal horn injury. Exp Neurol. 2000;161:220–6. doi: 10.1006/exnr.1999.7246. [DOI] [PubMed] [Google Scholar]
- [54].Nicholson Perry K, Nicholas MK, Middleton J. Spinal cord injury-related pain in rehabilitation: a cross-sectional study of relationships with cognitions, mood and physical function. Eur J Pain. 2009;13:511–7. doi: 10.1016/j.ejpain.2008.06.003. [DOI] [PubMed] [Google Scholar]
- [55].Norrbrink Budh C, Kowalski J, Lundeberg T. A comprehensive pain management programme comprising educational, cognitive and behavioural interventions for neuropathic pain following spinal cord injury. J Rehabil Med. 2006;38:172–80. doi: 10.1080/16501970500476258. [DOI] [PubMed] [Google Scholar]
- [56].Oei TP, Evans L, Crook GM. Utility and validity of the STAI with anxiety disorder patients. Br J Clin Psychol. 1990;29:429–32. doi: 10.1111/j.2044-8260.1990.tb00906.x. [DOI] [PubMed] [Google Scholar]
- [57].Pattany PM, Yezierski RP, Widerström-Noga EG, Bowen BC, Martinez-Arizala A, Garcia BR, Quencer RM. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. 2002;23:901–5. [PMC free article] [PubMed] [Google Scholar]
- [58].Paulson PE, Morrow TJ, Casey KL. Bilateral behavioral and regional cerebral blood flow changes during painful peripheral mononeuropathy in the rat. PAIN. 2000;84:233–45. doi: 10.1016/s0304-3959(99)00216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Plunkett JA, Yu CG, Easton JM, Bethea JR, Yezierski RP. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol. 2001;168:144–54. doi: 10.1006/exnr.2000.7604. [DOI] [PubMed] [Google Scholar]
- [60].Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- [61].Richards JS, Meredith RL, Nepomuceno C, Fine PR, Bennett G. Psychosocial aspects of chronic pain in spinal cord injury. PAIN. 1980;8:355–66. doi: 10.1016/0304-3959(80)90079-2. [DOI] [PubMed] [Google Scholar]
- [62].Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [63].Rosner B. Fundamentals of biostatistics. 3rd Edition PWS-Kent; Boston, Massachusetts, USA: 1990. [Google Scholar]
- [64].Sakakibara BM, Miller WC, Orenczuk SG, Wolfe DL, SCIRE Research Team A systematic review of depression and anxiety measures used with individuals with spinal cord injury. Spinal Cord. 2009;47:841–51. doi: 10.1038/sc.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Schwarz GE. Estimating the dimension of a model. Ann Stat. 1978;6:461–4. [Google Scholar]
- [66].Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- [67].Siddall PJ, Taylor DA, Cousins MJ. Classification of pain following spinal cord injury. Spinal Cord. 1997;35:69–75. doi: 10.1038/sj.sc.3100365. [DOI] [PubMed] [Google Scholar]
- [68].Simmons ML, Frondoza CG, Coyle JT. Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience. 1991;45:37–45. doi: 10.1016/0306-4522(91)90101-s. [DOI] [PubMed] [Google Scholar]
- [69].Sorensen L, Siddall PJ, Trenell MI, Yue DK. Differences in metabolites in pain-processing brain regions in patients with diabetes and painful neuropathy. Diabetes Care. 2008;31:980–1. doi: 10.2337/dc07-2088. [DOI] [PubMed] [Google Scholar]
- [70].Spielberger CD, Gorsuch RL, Lushene RE. The State-Trait Anxiety Inventory (Test Manual) Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- [71].Struzyńska L, Sulkowski G. Relationships between glutamine, glutamate, and GABA in nerve endings under Pb-toxicity conditions. J Inorg Biochem. 2004;98:951–8. doi: 10.1016/j.jinorgbio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- [72].Summers JD, Rapoff MA, Varghese G, Porter K, Palmer RE. Psychosocial factors in chronic spinal cord injury pain. PAIN. 1991;47:183–9. doi: 10.1016/0304-3959(91)90203-A. [DOI] [PubMed] [Google Scholar]
- [73].Toth CC, Jedrzejewski NM, Ellis CL, Frey WH., 2nd Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. Mol Pain. 2010;6:16. doi: 10.1186/1744-8069-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vassend O, Quale AJ, Roise O, Schanke AK. Predicting the long-term impact of acquired severe injuries on functional health status: the role of optimism, emotional distress and pain. Spinal Cord. 2011;49:1193–7. doi: 10.1038/sc.2011.70. [DOI] [PubMed] [Google Scholar]
- [75].Walters ET. Neuroinflammatory contributions to pain after SCI: roles for central glial mechanisms and nociceptor-mediated host defense. Exp Neurol. 2014;258:48–61. doi: 10.1016/j.expneurol.2014.02.001. [DOI] [PubMed] [Google Scholar]
- [76].Wang G, Thompson SM. Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions. J Neurosci. 2008;28:11959–69. doi: 10.1523/JNEUROSCI.3296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Warms CA, Turner JA, Marshall HM, Cardenas DD. Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin J Pain. 2002;18:154–63. doi: 10.1097/00002508-200205000-00004. [DOI] [PubMed] [Google Scholar]
- [78].Wasner G, Lee BB, Engel S, McLachlan E. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain. 2008;131:2387–400. doi: 10.1093/brain/awn169. [DOI] [PubMed] [Google Scholar]
- [79].Widerström-Noga EG. Evaluation of clinical characteristics of pain and psychosocial factors after spinal cord injury. In: Yezierski RP, Burchiel KJ, editors. Spinal Cord Injury Pain: Assessment, Mechanisms, Management Vol 23, Progress in Pain Research and Management. IASP Press; Seattle WA: 2002. pp. 53–82. [Google Scholar]
- [80].Widerström-Noga EG, Turk DC. Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: influence of pain and psychosocial characteristics. Spinal Cord. 2003;41:600–9. doi: 10.1038/sj.sc.3101511. [DOI] [PubMed] [Google Scholar]
- [81].Widerström-Noga EG, Cruz-Almeida Y, Felix ER, Adcock JP. Relationship between pain characteristics and pain adaptation type in persons with SCI. J Rehabil Res Dev. 2009;46:43–56. [PubMed] [Google Scholar]
- [82].Widerström-Noga EG, Cruz-Almeida Y, Martinez-Arizala A, Turk DC. Internal consistency, stability, and validity of the spinal cord injury version of the multidimensional pain inventory. Arch Phys Med Rehabil. 2006;87:516–23. doi: 10.1016/j.apmr.2005.12.036. [DOI] [PubMed] [Google Scholar]
- [83].Widerström-Noga EG, Duncan R, Felipe-Cuervo E, Turk DC. Assessment of the impact of pain and impairments associated with spinal cord injuries. Arch Phys Med Rehabil. 2002;83:395–404. doi: 10.1053/apmr.2002.28028. [DOI] [PubMed] [Google Scholar]
- [84].Widerström-Noga EG, Felipe-Cuervo E, Broton JG, Duncan RC, Yezierski RP. Perceived difficulty in dealing with consequences of spinal cord injury. Arch Phys Med Rehabil. 1999;80:580–6. doi: 10.1016/s0003-9993(99)90203-4. [DOI] [PubMed] [Google Scholar]
- [85].Widerström-Noga E, Pattany P, Cruz-Almeida Y, Felix ER, Perez S, Cardenas DD, Martinez-Arizala A. Metabolite concentrations in the anterior cingulate cortex predict high neuropathic pain impact after spinal cord injury. PAIN. 2013;154:204–12. doi: 10.1016/j.pain.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wollaars MM, Post MW, van Asbeck FW, Brand N. Spinal cord injury pain: the influence of psychologic factors and impact on quality of life. Clin J Pain. 2007;23:383–91. doi: 10.1097/AJP.0b013e31804463e5. [DOI] [PubMed] [Google Scholar]
- [87].Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. PAIN. 2009;141:52–9. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- [88].Yezierski RP. Spinal cord injury pain: spinal and supraspinal mechanisms. J Rehabil Res Dev. 2009;46:95–107. [PubMed] [Google Scholar]
- [89].Yoon EJ, Kim YK, Shin HI, Lee Y, Kim SE. Cortical and white matter alterations in patients with neuropathic pain after spinal cord injury. Brain Res. 2013;1540:64–73. doi: 10.1016/j.brainres.2013.10.007. [DOI] [PubMed] [Google Scholar]
- [90].Zeilig G, Enosh S, Rubin-Asher D, Lehr B, Defrin R. The nature and course of sensory changes following spinal cord injury: predictive properties and implications on the mechanism of central pain. Brain. 2012;135:418–30. doi: 10.1093/brain/awr270. [DOI] [PubMed] [Google Scholar]
- [91].Zhang YQ, Tang JS, Yuan B, Jia H. Inhibitory effects of electrical stimulation of thalamic nucleus submedius area on the rat tail flick reflex. Brain Res. 1995;696:205–12. doi: 10.1016/0006-8993(95)00856-l. [DOI] [PubMed] [Google Scholar]
- [92].Zhao P, Waxman SG, Hains BC. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine-cysteine chemokine ligand 21. J Neurosci. 2007;27:8893–902. doi: 10.1523/JNEUROSCI.2209-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. PAIN. 2005;114:149–59. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]