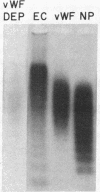
Abstract
The interactions of normal erythrocytes and erythrocytes from patients having hemoglobin S hemoglobinopathies with normal human endothelial cells (EC) were investigated under flow conditions. When EC supernatant, containing 2.8-11.0 U/dl of von Willebrand factor (vWF) antigen and vWF multimeric forms larger than those present in normal plasma, was the red blood cell (RBC)-suspending medium instead of serum-free medium (SFM), the adhesion of sickle RBC, but not normal RBC, to endothelial cells was greatly increased (range of enhancement of sickle RBC adhesion, 2- to 27-fold). Adhesion of sickle RBC to endothelial cells was reduced to near serum-free levels when EC supernatant was immunologically depleted of vWF forms. Sickle RBC suspended in SFM containing 200 U/dl of purified vWF multimers of the type found in normal human plasma or 300 micrograms/ml human fibronectin were only slightly more adhesive to endothelial cells than sickle RBC suspended in SFM alone. These data indicate that unusually large vWF multimers produced by endothelial cells are potent mediators of the adhesion of sickle erythrocytes to endothelial cells. Vaso-occlusive crises in sickle cell anemia may be caused, at least in part, by adhesive interactions between the abnormal surfaces of sickle RBC and the endothelium after the release of unusually large vWF multimeric forms from stimulated or damaged endothelial cells.
Full text
PDF

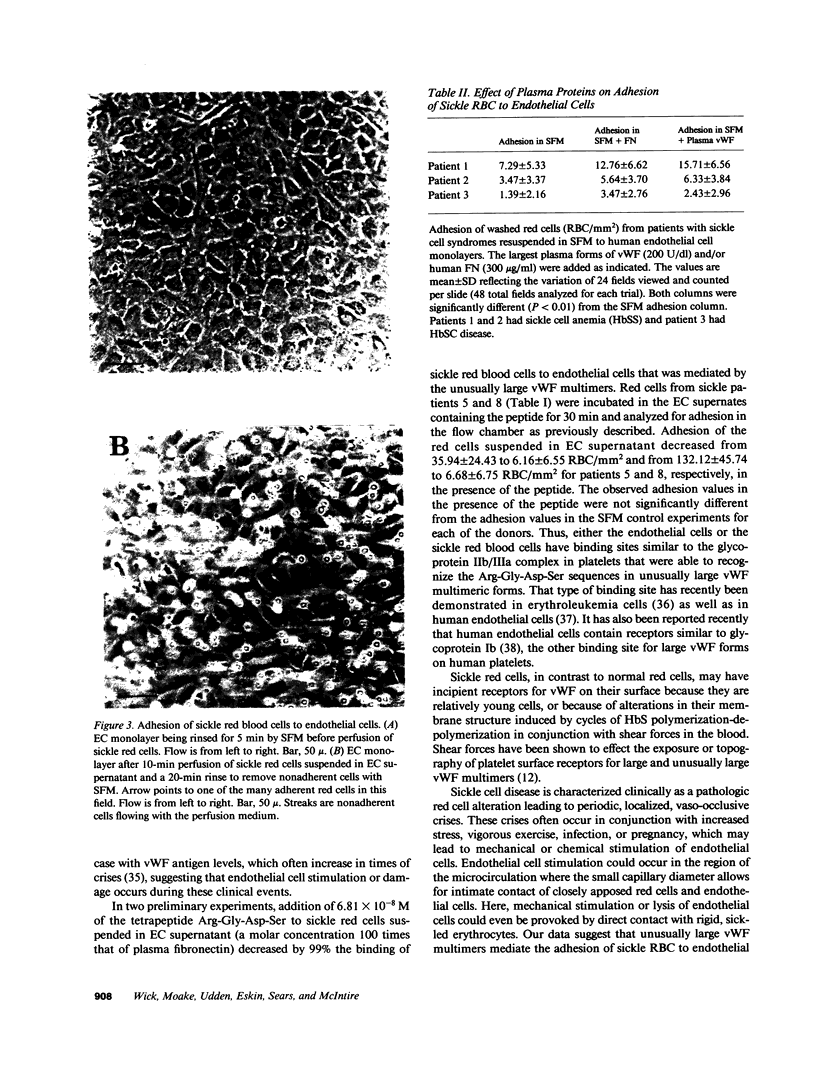


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brittenham G. M., Schechter A. N., Noguchi C. T. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood. 1985 Jan;65(1):183–189. [PubMed] [Google Scholar]
- Charo I. F., Fitzgerald L. A., Steiner B., Rall S. C., Jr, Bekeart L. S., Phillips D. R. Platelet glycoproteins IIb and IIIa: evidence for a family of immunologically and structurally related glycoproteins in mammalian cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8351–8355. doi: 10.1073/pnas.83.21.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983 Jan;61(1):99–110. [PubMed] [Google Scholar]
- Counts R. B. Solid-phase immunoradiometric assay of factor-VIII protein. Br J Haematol. 1975 Dec;31(4):429–436. doi: 10.1111/j.1365-2141.1975.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Handin R. I., Bonthron D. T., Donlon T. A., Bruns G. A., Latt S. A., Orkin S. H. Human von Willebrand factor (vWF): isolation of complementary DNA (cDNA) clones and chromosomal localization. Science. 1985 Jun 21;228(4706):1401–1406. doi: 10.1126/science.3874428. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Boogaerts M. A., Eaton J. W., Steinberg M. H. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980 May 1;302(18):992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Yamada O., Moldow C. F., Jacob H. S., White J. G., Eaton J. W. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980 Jan;65(1):154–160. doi: 10.1172/JCI109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R., Rubin R., Wise G., Warren R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood. 1979 Oct;54(4):872–876. [PubMed] [Google Scholar]
- Hoyer L. W., Shainoff J. R. Factor VIII-related protein circulates in normal human plasma as high molecular weight multimers. Blood. 1980 Jun;55(6):1056–1059. [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Mosher D. F. Synthesis of fibronectin by cultured human endothelial cells. J Exp Med. 1978 Jun 1;147(6):1779–1791. doi: 10.1084/jem.147.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul D. K., Fabry M. E., Nagel R. L. Vaso-occlusion by sickle cells: evidence for selective trapping of dense red cells. Blood. 1986 Nov;68(5):1162–1166. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Loscalzo J., Inbal A., Handin R. I. von Willebrand protein facilitates platelet incorporation in polymerizing fibrin. J Clin Invest. 1986 Oct;78(4):1112–1119. doi: 10.1172/JCI112668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie I., Bull H., Brozović M. Altered Factor VIII complexes in sickle cell disease. Br J Haematol. 1980 Nov;46(3):499–502. doi: 10.1111/j.1365-2141.1980.tb06000.x. [DOI] [PubMed] [Google Scholar]
- Moake J. L., Byrnes J. J., Troll J. H., Rudy C. K., Weinstein M. J., Colannino N. M., Hong S. L. Abnormal VIII: von Willebrand factor patterns in the plasma of patients with the hemolytic-uremic syndrome. Blood. 1984 Sep;64(3):592–598. [PubMed] [Google Scholar]
- Moake J. L., Turner N. A., Stathopoulos N. A., Nolasco L. H., Hellums J. D. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest. 1986 Dec;78(6):1456–1461. doi: 10.1172/JCI112736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Evans E. Sickle erythrocyte adherence to vascular endothelium. Morphologic correlates and the requirement for divalent cations and collagen-binding plasma proteins. J Clin Invest. 1985 Oct;76(4):1605–1612. doi: 10.1172/JCI112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman R., Levine R., Jaffe E. A. Synthesis of factor VIII antigen by cultured guinea pig megakaryocytes. J Clin Invest. 1977 Oct;60(4):914–921. doi: 10.1172/JCI108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. I., Gralnick H. R. Identification of platelet glycoprotein IIb/IIIa as the major binding site for released platelet-von Willebrand factor. Blood. 1986 Sep;68(3):732–736. [PubMed] [Google Scholar]
- Patel V. P., Ciechanover A., Platt O., Lodish H. F. Mammalian reticulocytes lose adhesion to fibronectin during maturation to erythrocytes. Proc Natl Acad Sci U S A. 1985 Jan;82(2):440–444. doi: 10.1073/pnas.82.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. P., Lodish H. F. The fibronectin receptor on mammalian erythroid precursor cells: characterization and developmental regulation. J Cell Biol. 1986 Feb;102(2):449–456. doi: 10.1083/jcb.102.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., McEver R. P., Coller B. S., Woods V. L., Jr, Marguerie G. A., Ginsberg M. H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985 Sep;66(3):724–727. [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Mannucci P. M., Lombardi R., Federici A. B., Zimmerman T. S. Multimeric composition of factor VIII/von Willebrand factor following administration of DDAVP: implications for pathophysiology and therapy of von Willebrand's disease subtypes. Blood. 1982 Jun;59(6):1272–1278. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Smith B. D., La Celle P. L. Erythrocyte-endothelial cell adherence in sickle cell disorders. Blood. 1986 Nov;68(5):1050–1054. [PubMed] [Google Scholar]
- Sporn L. A., Marder V. J., Wagner D. D. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986 Jul 18;46(2):185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- Thorell L., Blombäck B. Purification of the factor VIII complex. Thromb Res. 1984 Aug 15;35(4):431–450. doi: 10.1016/0049-3848(84)90235-4. [DOI] [PubMed] [Google Scholar]
- Turitto V. T. Blood viscosity, mass transport, and thrombogenesis. Prog Hemost Thromb. 1982;6:139–177. [PubMed] [Google Scholar]
- Turitto V. T., Weiss H. J., Zimmerman T. S., Sussman I. I. Factor VIII/von Willebrand factor in subendothelium mediates platelet adhesion. Blood. 1985 Apr;65(4):823–831. [PubMed] [Google Scholar]