Abstract
Opioid-induced constipation is a major clinical problem. The effects of morphine, and other narcotics, on the gastrointestinal tract persist over long-term use thus limiting the clinical benefit of these excellent pain relievers. The effects of opioids in the gut, including morphine, are largely mediated by the μ-opioid receptors at the soma and nerve terminals of enteric neurons. Recent studies demonstrate that regional differences exist in both acute and chronic morphine along the gastrointestinal tract. While tolerance develops to the analgesic effects and upper gastrointestinal motility upon repeated morphine administration, tolerance does not develop in the colon with chronic opioids resulting in persistent constipation. Here, we review the mechanisms by which tolerance develops in the small but not the large intestine. The regional differences lie in the signaling and regulation of the μopioid receptor in the various segments of the gastrointestinal tract. The differential role of β-arrestin2 in tolerance development between central and enteric neurons defines the potential for therapeutic approaches in developing ligands with analgesic properties and minimal constipating effects.
Keywords: beta-arrestin, constipation, opioid, tolerance
INTRODUCTION
Opium obtained from the poppy, Papaver somniferum, has been perhaps the most effective medicinal drug available since before the dawn of the twentieth century. Morphine continues to be one of the most frequently prescribed drugs for the treatment of moderate to severe pain with studies indicating an escalating use in recent years.1 However, side-effects associated with its use limit the clinical benefit of this excellent pain reliever in man. Major side-effects of opioids include addiction, tolerance, respiratory depression, and constipation. The mechanisms by which morphine and other opioids affect the gastrointestinal tract have been extensively studied over the last 75 years. However, treatment options for opioid-induced constipation are still limited,2,3 although newer therapeutic approaches including peripheral opioid receptor antagonists and biased ligands (see below) are promising leads.
Localization of the effect of morphine to the neurons within the myenteric plexus was first demonstrated by Paton and Zar.4 Since the early work of Paton,5 the guinea pig longitudinal muscle-myenteric plexus (LMMP) preparation has been the tissue preparation of choice to study the in vitro effects of morphine and related opioids in the gastrointestinal tract. In this preparation, acetylcholine release by electrical field stimulation of the myenteric nerves is depressed by opioids resulting in inhibition of longitudinal muscle contraction. The pharmacological effects on the myenteric neurons of various narcotics correlate with their analgesic potencies, thus making the LMMP an ideal preparation for pharmacological assays. Studies utilizing sharp microelectrodes for intracellular recordings further advanced the cellular basis by which morphine and other opioids affect neurotransmitter release.6 Morphine and other opioids induce membrane hyperpolarization by opioids due to opening of inwardly rectifying potassium channels of enteric and central neurons as the basis for decreased neuronal excitability.7–10 The resulting neuronal hypoexcitability prevents acetylcholine release. More recent studies by patch clamp techniques in isolated mouse enteric neurons have also shown inhibition of sodium channels as a mechanism for decreased neuronal excitability. 11 It should be noted that opioid actions may have distinct functional effects depending on their localization. In the soma, morphine decreases neural excitability, whereas neurotransmitter release is reduced at the terminals. In the myenteric ganglia, presynaptic inhibition results in reduced transmitter release, and decreased excitability when morphine is applied directly to the cell bodies. The clinical effects of morphine are mediated by the seven transmembrane G-protein-coupled receptors. All three opioid receptor types have been demonstrated in the gastrointestinal tract of various species i.e., mu (μ), delta (δ), and kappa (κ) receptors, although overwhelming evidence suggest that the majority of the effects of clinically used opioids, including morphine, are mediated through the μ-opioid receptors. The effects of morphine on GI motility are absent in the μ-opioid receptor knockout mice.12 Each of the receptor subtypes are encoded by separate genes and evidence of alternative splicing resulting in isoforms have also been demonstrated13 that may have therapeutic implications (see below). The coupling of the receptor to the ion channel is through pertussis-toxin sensitive G-proteins.
The report by Ono et al.14 illustrates an important aspect regarding the regional differences in the action of morphine in the small and large intestines. The differences in the various segments of the gastrointestinal tract in response to morphine have been subject of much work in recent years. This stems from the clinical findings that patients on long-term morphine develop tolerance to the analgesic effects but not to the constipating effects,15 in as much as many patients refuse opioid treatment for pain relief due to their debilitating effects on the gut. Several clinical surveys strongly suggest that opioid-induced bowel dysfunction compromises the usefulness of opioid analgesics. 16,17 In patients on long-term methadone maintenance, the oral-cecal transit time was significantly lower compared to healthy volunteers and similar to those who received a single dose of morphine leading the authors to conclude that long-term treatment did not result in tolerance to this effect.18 In a prospective study of 214 patients on long-term methadone treatment, 17% of the patients reported a persistent problem with constipation after three or more years of methadone maintenance treatment for narcotic addiction.19 In a recent study, Tuteja et al. 20 noted that constipation was more commonly reported by patients on long-term opiates. Indeed, the study showed that the pervasiveness of constipation increased with duration of treatment and that 79% of patients on long-term opioids (2–10 years) reported constipation. These clinical observations of persistent constipating effects of opioids with chronic use correlate with the findings that tolerance does not develop in the colon.21 In contrast, tolerance occurs in the ileum.22–25 The differences in the development of tolerance between the two segments of the gastrointestinal tract highlight the need to understand the differences in the mechanisms of opioid action in these tissues. Studies of Ono et al.14 provide some insight into this difference. Their studies show that morphine-induced contractions of the circular muscle occur with least strength in the ileum, increasing in force and duration in the proximal—distal colon and maintained tone in the rectum. The contractions in the circular muscle are thought to arise due to a reduction in the inhibitory tone to the muscle as a result of the inhibition of inhibitory transmitter release from the presynaptic terminals21,26,27 and reflect the segmental contractions associated with acute morphine administration. Our studies have shown that chronic morphine administered over 5 days that renders mice tolerant to the antinociceptive effects did not induce tolerance to colonic transit or to circular muscle contractions.21
The first indication of the lack of morphine-induced tolerance toward colonic motility was in fact noted in dogs in 1926.28 In the rat colon, Burks et al.29 demonstrated that continuous infusion of morphine resulted in tolerance in the proximal colon in vivo. In contrast, others have found the lack of tolerance to the anti-transit effects of morphine in mice pretreated twice daily for 10 days30 or over 8 h of continuous infusion.15 The major effects of morphine are enhanced non-propulsive contractions while peristalsis is markedly reduced. Therefore, the ability to maintain responses to morphine in the more distal colon/rectum resulting in non-propulsive contractions account for the hard and dry stools and can lead to fecal impaction.
MECHANISMS FOR MORPHINE TOLERANCE: ROLE OF β-ARRESTIN2
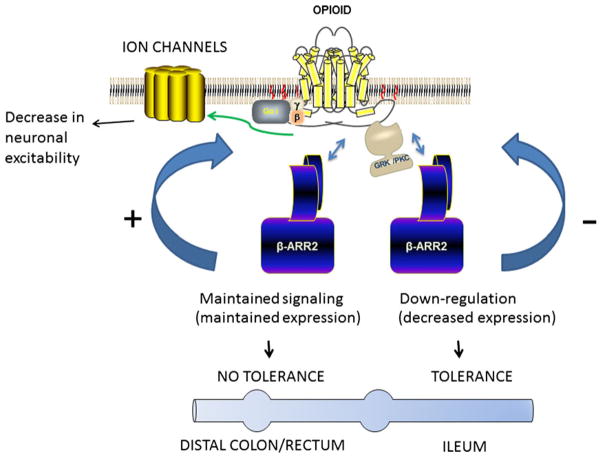
The major drawback to chronic opioid use for pain relief is that increasing the dose to compensate for the loss in efficacy raises the risk of adverse events including overdose, withdrawal and dependence and persistent constipation. The extent of tolerance varies significantly depending on the pharmacological effect e.g., opioid tolerance develops quicker to the analgesic effects, whereas little manifests to constipation. The prolonged activation of opioid receptors results in adaptations that are compensatory in nature and may reflect short-term or long-term tolerance (reviewed by Ref. 31). The acute administration of morphine results in rapid desensitization (seconds to minutes) that may be followed by short-term tolerance (hours). Prolonged opioid use induces long-term tolerance (days to years). The mechanisms underlying morphine tolerance are complex and not fully understood. Studies examining morphine tolerance have focused largely on analgesic tolerance and the neuronal circuitry associated with it in heterologously expressed cell lines. Much less is known with regard to the mechanisms of tolerance development in the enteric neurons of the ileum or the lack of it in colonic neurons. One of the canonical pathways associated with opiate tolerance is the process of desensitization/internalization after receptor phosphorylation by G protein-coupled receptor kinases and the recruitment of β-arrestins. Agonist mediated receptor activation leads to G-protein coupling and inducing receptor conformation that allows for receptor phosphorylation. The kinases involved in receptor phosphorylation are ligand dependent with G-receptor kinase—mediated phosphorylation for agonists such as DAMGO, and protein kinase C for e.g., morphine.32 Receptor phosphorylation facilitates recruitment of β-arrestin2. Desensitization may be mediated by the steric hindrance for G protein activation, although β-arrestin2 can also act as a scaffolding protein allowing for receptor function through activation of ERK1/2 MAP-kinase pathway. Internalization of the receptor and recycling can lead to resensitization, while endocytosis and further degradation may lead to down-regulation of functional receptors on the plasma membrane. A major role for β-arrestin2 in the regulation of tolerance was identified in the β-arrestin2 knockout mice. The studies by Bohn et al. demonstrated that antinociceptive tolerance is reduced in β-arrestin2 knockout mice,33 suggesting that maintained levels of β-arrestin2 may be importantly involved in the mechanism of analgesic tolerance. In the gastrointestinal tract, acute morphine did not reduce colonic transit in the β-arrestin2 knockout mice while it reduced upper gastrointestinal transit.34 This has led to the suggestion that morphine signaling requires β-arrestin2 in the colon.35 Kang et al. 36 further investigated whether the difference in morphine tolerance in the ileum and colon was due to differences in the role of β-arrestin2 in these two tissues. Prolonged administration of morphine resulted in the down-regulation of β-arrestin2 in the ileum but not the colon. This is associated with tolerance in the ileum to repeated morphine but not in the colon. In the absence of β-arrestin2 i.e., in the β-arrestin2 knockout mice, tolerance to morphine develops in the colon and the ileum. The central role of β-arrestin2 in the signaling process to tolerance development in the ileum and colon is illustrated in Fig. 1. It is noteworthy that repeated administration of etorphine and fentanyl which are higher efficacy agonists induced tolerance in the colon.37 This may explain the less constipating effects of fentanyl.
Figure 1.
General scheme of the role of β-arrestin2 in the tolerance to morphine in the ileum and colon. Agonist binding to the μ-opioid receptor activates G-protein signaling, and phosphorylation of the receptor by G-protein receptor kinase or protein kinase C dependent on the specific agonist. Phosphorylation leads to recruitment of β-arrestin2. In the ileum, repeated administration of morphine results in down-regulation of β-arrestin2 levels and development of tolerance. In the colon, repeated morphine administration does not affect β-arrestin2 levels and tolerance does not develop. Tolerance in the colon develops in the β-arrestin2 knockout mouse.
Recent studies have demonstrated that β-arrestin2 is localized to choline acetyltransferase and μ-opioid receptor positive neurons in the myenteric plexus.38 These findings suggest that unlike tolerance development in the spinal and supraspinal neurons that mediate antinociception, the regulation of μ-opioid receptors in the enteric neurons are differentially regulated by β-arrestin2. Thus, while β-arrestin2 induces tolerance in the central neurons, it prevents tolerance in the colonic neurons.
SPLICE VARIANTS AS POSSIBLE DIFFERENCES BETWEEN ILEUM AND COLON?
Pharmacological and biochemical studies have supported the concept of multiple variants of the μ-opioid receptor for decades.39–42 Clinical findings of incomplete cross-tolerance among different opioids toward their pain relieving effects coupled with biochemical data demonstrating high and low affinity binding sites led to the designation of μ1 and μ2 receptor sites. Further pharmacological studies utilizing naloxonazine (NLXZ), an irreversible antagonist of the μ opioid receptor, highlighted the differences in the distribution pattern of the two receptor populations. Pretreatment with NLXZ reduced the antinociceptive effects of morphine administered intracereberoventricularly (i.c.v.) but not intrathecally (i.t.), indicating that the antinociceptive effects were mediated via the NLXZ-sensitive μ receptor at the supraspinal level. The existence of multiple type μ receptors was also suggested following studies of centrally mediated effects of morphine on gastrointestinal motility. Studies by Pasternak and colleagues,15,43 and by Heyman et al.44 demonstrated that i.c.v. morphine reduced gastrointestinal transit in a NLXZ insensitive manner, whereas i.t. mediated inhibition of GI transit by morphine was NLXZ sensitive. This is in contrast to the antinociceptive mechanisms raising the possibility that at least two μ opioid receptor types may exist at the spinal and supraspinal levels. It is noteworthy that these early studies of spinal and supraspinal effects of morphine on gastrointestinal function were limited to the small intestine. Recently, Mori et al.45 demonstrated that morphine, oxycodone and fentanyl reduced colonic transit through distinct NLXZ—sensitive and—insensitive receptors at the peripheral and central sites.
Molecular studies have indeed established alternative splice variants of the μ-opioid receptor gene, Oprm1.46–48 The Oprm1 was initially cloned as MOR-1 containing 4 exons.49 Exons 1, 2, and 3 were suggested to encode for the seven transmembrane segment with exon 4 encoding the intracellular C-terminus. Splice variants have been further identified that differ in the C-terminus due to alternative splicing in the 3′ end, and in the N-terminus due to the utilization of an alternative promoter region in exon 11. At least 17 protein encoding splice variants have been identified, however, all have been cloned from various brain regions. None have been identified in the gut. Given the nature of the difference in opioid tolerance development between the ileum and colon, it is likely that different splice variants exist between the ileum, colon, and central sites. Elucidation and characterization of the splice variants will allow for future therapeutic strategies to target analgesic effects without the debilitating constipating effects of opioids.
PERIPHERAL OPIOID ANTAGONISTS AND BIASED LIGANDS FOR TREATMENT OF OPIOID-INDUCED CONSTIPATION
The conventional approach of treating opioid-induced constipation with laxatives, opioid switching, 5-HT4 agonists, or lubiprostone have had limited satisfaction and therefore newer strategies have been sought. Peripheral μ-opioid receptor antagonists (PAMORA) have recently been developed with some success. Due to their restriction within the periphery, PAMORA prevent or reverse decreased GI transit for patients on opioid therapy for cancer or non-cancer pain. Methyln-altrexone (MNTX) belongs to a new drug class with selective antagonism of peripheral μ-opioid receptors. MNTX is a quaternary derivative of naltrexone, whereby increasing the polarity and reducing lipid solubility prevents the drug from crossing the blood brain barrier. MNTX is administered subcutaneously and indicated in patients with opioid-induced constipation with advanced illness. Abdominal pain and flatulence are major side-effects. Alvimopan has a zwitterionic form with a large molecular weight that limits its gastrointestinal absorption and central nervous system (CNS) penetration. Alvimopan is restricted largely to resolution of postoperative ileus, and is contraindicated in patients who have taken therapeutic doses of opioids for more than 7 days. It is restricted to short-term use. While several clinical trials have demonstrated efficacy for these compounds, particularly in postsurgery setting, it is not known whether they will be effective in patients that require long-term pain relief. Moreover, alvimopan while efficacious for postoperative ileus, may be associated with myocardial infarction and limited in the long-term therapy.50 A pegylated form of naloxone (μ-opioid receptor antagonist), naloxegol has shown significant efficacy in treatment of long-term opioid-induced constipation in a recent phase 3 trial.51,52 Pegylation confers reduced permeability across membranes limiting entry into the CNS. This also increases its bioavailability. Over a 52-week period, naloxegol was well-tolerated, with opioid analgesia being maintained with concomitant opioid therapy. The phase 3 trials indicate that naloxogel has limited adverse effects over the long-term with no indication of CNS penetration. Due to it being a substrate for p-glycoprotein, it is unclear whether drug interaction with inhibitors of p-glycoprotein will induce adverse effects if CNS penetration occurs.
The use of PAMORA requires continued opioid therapy for maintaining analgesia. The concept of biased agonists has evolved from studies, where certain opioid agonists have greater efficacy in G-protein activation than β-arrestin2 recruitment.53 The difference in receptor signaling and regulation may be utilized to define agents that have enhanced analgesic but less constipating effects, thus avoiding the need for combination treatment. TRV130, a compound with strong bias toward G-protein activation and reduced β-arrestin2 recruitment than morphine, induces strong analgesic responses with reduced constipating effects.54 In human trials, TRV130 induced significant greater analgesia than morphine with reduced respiratory drive and nausea,55 although constipation was not examined. Consistent with our findings that down-regulation of β-arrestin2 results in tolerance in the colon, it is tempting to suggest that agents with a bias toward decreased β-arrestin2 recruitment to the receptor, result in tolerance to colonic transit without tolerance development to the analgesic effects.
Opioid-induced constipation persists in patients on opioid therapy for cancer or non-cancer pain. The lack of tolerance development in the colon21 and the increased acute responses to morphine in the distal portion of the gastrointestinal tract as shown by Ono et al.14 highlight the regional differences in the signaling and regulation of the μ-opioid receptor. Further work on receptor variants in the different segments of the gastrointestinal tract and the associated signaling mechanisms, and development of long-term peripheral opioid antagonists and biased ligands are needed.
Key Messages.
This review describes the importance of β-arrestin2 down-regulation in the development of tolerance in the gastrointestinal tract.
Acknowledgments
The study in the authors laboratories is supported by NIH DA024009, DK046367, and DA036975.
FUNDING
Supported by NIH R01DA024009, R01DK046367, R01DA036975 and P30033934.
Footnotes
DISCLOSURE
No competing interest declared.
References
- 1.Centers for Disease C, Prevention. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–92. [PubMed] [Google Scholar]
- 2.Holzer P. New approaches to the treatment of opioid-induced constipation. Eur Rev Med Pharmacol Sci. 2008;12(Suppl 1):119–27. [PMC free article] [PubMed] [Google Scholar]
- 3.Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol. 2011;106:835–42. doi: 10.1038/ajg.2011.30. quiz 843. [DOI] [PubMed] [Google Scholar]
- 4.Paton WD, Zar MA. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968;194:13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paton WD. The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br J Pharmacol Chemother. 1957;12:119–27. doi: 10.1111/j.1476-5381.1957.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishi S, North RA. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973;231:471–91. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.North RA. Effects of morphine on myenteric plexus neurones. Neuropharmacology. 1976;15:719–21. doi: 10.1016/0028-3908(76)90043-5. [DOI] [PubMed] [Google Scholar]
- 8.North RA. Opiates, opioid peptides and single neurones. Life Sci. 1979;24:1527–45. doi: 10.1016/0024-3205(79)90014-6. [DOI] [PubMed] [Google Scholar]
- 9.North RA, Williams JT. Extracellular recording from the guinea-pig myenteric plexus and the action of morphine. Eur J Pharmacol. 1977;45:23–33. doi: 10.1016/0014-2999(77)90054-1. [DOI] [PubMed] [Google Scholar]
- 10.North RA, Williams JT. On the potassium conductance increased by opioids in rat locus coeruleus neurones. J Physiol. 1985;364:265–80. doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith TH, Grider JR, Dewey WL, Akbarali HI. Morphine decreases enteric neuron excitability via inhibition of sodium channels. PLoS ONE. 2012;7:e45251. doi: 10.1371/journal.pone.0045251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy S, Liu HC, Loh HH. mu-Opioid receptor-knockout mice: the role of mu-opioid receptor in gastrointestinal transit. Brain Res Mol Brain Res. 1998;56:281–3. doi: 10.1016/s0169-328x(98)00051-5. [DOI] [PubMed] [Google Scholar]
- 13.Pasternak GW. Molecular insights into mu opioid pharmacology: from the clinic to the bench. Clin J Pain. 2010;26(Suppl 10):S3–9. doi: 10.1097/AJP.0b013e3181c49d2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono H, Nakamura A, Matsumoto K, Horie S, Sakaguchi G, Kanemasa T. Circular muscle contraction in the mice rectum plays a key role in morphine-induced constipation. Neurogastroenterol Motil. 2014;26:1396–407. doi: 10.1111/nmo.12387. [DOI] [PubMed] [Google Scholar]
- 15.Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45:1627–36. doi: 10.1016/0024-3205(89)90272-5. [DOI] [PubMed] [Google Scholar]
- 16.Grond S, Zech D, Diefenbach C, Bischoff A. Prevalence and pattern of symptoms in patients with cancer pain: a prospective evaluation of 1635 cancer patients referred to a pain clinic. J Pain Symptom Manage. 1994;9:372–82. doi: 10.1016/0885-3924(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 17.Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain. 1995;63:65–76. doi: 10.1016/0304-3959(95)00017-M. [DOI] [PubMed] [Google Scholar]
- 18.Yuan CS, Foss JF, O’Connor M, Moss J, Roizen MF. Gut motility and transit changes in patients receiving long-term methadone maintenance. J Clin Pharmacol. 1998;38:931–5. doi: 10.1002/j.1552-4604.1998.tb04389.x. [DOI] [PubMed] [Google Scholar]
- 19.Kreek MJ. Medical safety and side effects of methadone in tolerant individuals. JAMA. 1973;223:665–8. [PubMed] [Google Scholar]
- 20.Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil. 2010;22:424–30. e496. doi: 10.1111/j.1365-2982.2009.01458.x. [DOI] [PubMed] [Google Scholar]
- 21.Ross GR, Gabra BH, Dewey WL, Akbarali HI. Morphine tolerance in the mouse ileum and colon. J Pharmacol Exp Ther. 2008;327:561–72. doi: 10.1124/jpet.108.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rezvani A, Huidobro-Toro JP, Hu J, Way EL. A rapid and simple method for the quantitative determination of tolerance development to opiates in the guinea-pig ileum in vitro. J Pharmacol Exp Ther. 1983;225:251–5. [PubMed] [Google Scholar]
- 23.Leedham JA, Kong JQ, Taylor DA, Johnson SM, Fleming WW. Membrane potential in myenteric neurons associated with tolerance and dependence to morphine. J Pharmacol Exp Ther. 1992;263:15–9. [PubMed] [Google Scholar]
- 24.Collier HO, Cuthbert NJ, Francis DL. Model of opiate dependence in the guinea-pig isolated ileum. Br J Pharmacol. 1981;73:921–32. doi: 10.1111/j.1476-5381.1981.tb08747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein A, Schulz R. Morphine-tolerant longitudinal muscle strip from guinea-pig ileum. Br J Pharmacol. 1973;48:655–66. doi: 10.1111/j.1476-5381.1973.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grider JR, Makhlouf GM. Suppression of inhibitory neural input to colonic circular muscle by opioid peptides. J Pharmacol Exp Ther. 1987;243:205–10. [PubMed] [Google Scholar]
- 27.Iwata H, Tsuchiya S, Nakamura T, Yano S. Morphine leads to contraction of the ileal circular muscle via inhibition of the nitrergic pathway in mice. Eur J Pharmacol. 2007;21(574):66–70. doi: 10.1016/j.ejphar.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Plant OH, Miller GH. Effects of Morphine and some other opium alkaloids on the muscular activity of the alimentary canal 1. Action on the small intestine in unanesthetized dogs and man. J Pharmacol Exp Ther. 1926;27:361–83. [Google Scholar]
- 29.Williams CL, Bihm CC, Rosenfeld GC, Burks TF. Morphine tolerance and dependence in the rat intestine in vivo. J Pharmacol Exp Ther. 1997;280:656–63. [PubMed] [Google Scholar]
- 30.Tan-No K, Niijima F, Nakagawasai O, Sato T, Satoh S, Tadano T. Development of tolerance to the inhibitory effect of loperamide on gastrointestinal transit in mice. Eur J Pharm Sci. 2003;20:357–63. doi: 10.1016/j.ejps.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65:223–54. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance? Trends Pharmacol Sci. 2006;27:558–65. doi: 10.1016/j.tips.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science (New York, NY) 1999;286:2495–8. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 34.Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 35.Raehal KM, Schmid CL, Groer CE, Bohn LM. Functional selectivity at the {micro}-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol Rev. 2011;63:1001–19. doi: 10.1124/pr.111.004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang M, Maguma HT, Smith TH, Ross GR, Dewey WL, Akbarali HI. The role of beta-arrestin2 in the mechanism of morphine tolerance in the mouse and guinea pig gastrointestinal tract. J Pharmacol Exp Ther. 2012;340:567–76. doi: 10.1124/jpet.111.186320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maguma HT, Dewey WL, Akbarali HI. Differences in the characteristics of tolerance to mu-opioid receptor agonists in the colon from wild type and beta-arrestin2 knockout mice. Eur J Pharmacol. 2012;685:133–40. doi: 10.1016/j.ejphar.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maguma HT, De Datta D, Bhave S, Dewey WL, Akbarali HI. Specific localization of beta-arrestin2 in myenteric plexus of mouse gastrointestinal tract. PLoS ONE. 2014;9:e103894. doi: 10.1371/journal.pone.0103894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling GS, Spiegel K, Lockhart SH, Pasternak GW. Separation of opioid analgesia from respiratory depression: evidence for different receptor mechanisms. J Pharmacol Exp Ther. 1985;232:149–55. [PubMed] [Google Scholar]
- 40.Pasternak GW, Childers SR, Snyder SH. Opiate analgesia: evidence for mediation by a subpopulation of opiate receptors. Science. 1980;208:514–6. doi: 10.1126/science.6245448. [DOI] [PubMed] [Google Scholar]
- 41.Pasternak GW, Snyder SH. Identification of novel high affinity opiate receptor binding in rat brain. Nature. 1975;253:563–5. doi: 10.1038/253563a0. [DOI] [PubMed] [Google Scholar]
- 42.Wolozin BL, Pasternak GW. Classification of multiple morphine and enkephalin binding sites in the central nervous system. Proc Natl Acad Sci U S A. 1981;78:6181–5. doi: 10.1073/pnas.78.10.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poonyachoti S, Portoghese PS, Brown DR. Characterization of opioid receptors modulating neurogenic contractions of circular muscle from porcine ileum and evidence that delta- and kappa-opioid receptors are coexpressed in myenteric neurons. J Pharmacol Exp Ther. 2001;297:69–77. [PubMed] [Google Scholar]
- 44.Heyman JS, Williams CL, Burks TF, Mosberg HI, Porreca F. Dissociation of opioid antinociception and central gastrointestinal propulsion in the mouse: studies with naloxonazine. J Pharmacol Exp Ther. 1988;245:238–43. [PubMed] [Google Scholar]
- 45.Mori T, Shibasaki Y, Matsumoto K, Shibasaki M, Hasegawa M, Wang E, Masukawa D, Yoshizawa K, et al. Mechanisms that underlie mu-opioid receptor agonist-induced constipation: differential involvement of mu-opioid receptor sites and responsible regions. J Pharmacol Exp Ther. 2013;347:91–9. doi: 10.1124/jpet.113.204313. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Xu M, Rossi GC, Pasternak GW, Pan YX. Identification and characterization of seven new exon 11-associated splice variants of the rat mu opioid receptor gene, OPRM1. Mol Pain. 2011;7:9. doi: 10.1186/1744-8069-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan L, Xu J, Yu R, Xu MM, Pan YX, Pasternak GW. Identification and characterization of six new alternatively spliced variants of the human mu opioid receptor gene, Oprm. Neuroscience. 2005;133:209–20. doi: 10.1016/j.neuroscience.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 48.Oldfield S, Braksator E, Rodriguez-Martin I, Bailey CP, Donaldson LF, Henderson G, Kelly E. C-terminal splice variants of the mu-opioid receptor: existence, distribution and functional characteristics. J Neurochem. 2008;104:937–45. doi: 10.1111/j.1471-4159.2007.05057.x. [DOI] [PubMed] [Google Scholar]
- 49.Thompson RC, Mansour A, Akil H, Watson SJ. Cloning and pharmacological characterization of a rat mu opioid receptor. Neuron. 1993;11:903–13. doi: 10.1016/0896-6273(93)90120-g. [DOI] [PubMed] [Google Scholar]
- 50.Bream-Rouwenhorst HR, Cantrell MA. Alvimopan for postoperative ileus. Am J Health Syst Pharm. 2009;66:1267–77. doi: 10.2146/ajhp080445. [DOI] [PubMed] [Google Scholar]
- 51.Webster L, Chey WD, Tack J, Lappalainen J, Diva U, Sostek M. Randomised clinical trial: the long-term safety and tolerability of naloxegol in patients with pain and opioid-induced constipation. Aliment Pharmacol Ther. 2014;40:771–9. doi: 10.1111/apt.12899. [DOI] [PubMed] [Google Scholar]
- 52.Chey WD, Webster L, Sostek M, Lappalainen J, Barker PN, Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387–96. doi: 10.1056/NEJMoa1310246. [DOI] [PubMed] [Google Scholar]
- 53.Raehal KM, Bohn LM. beta-arrestins: regulatory role and therapeutic potential in opioid and cannabinoid receptor-mediated analgesia. Handb Exp Pharmacol. 2014;219:427–43. doi: 10.1007/978-3-642-41199-1_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344:708–17. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 55.Soergel DG, Subach RA, Sadler B, Connell J, Marion AS, Cowan CL, Violin JD, Lark MW. First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 2014;54:351–7. doi: 10.1002/jcph.207. [DOI] [PubMed] [Google Scholar]