Abstract
Recent warming significantly advanced leaf onset in the northern hemisphere. This signal cannot be accurately reproduced by current models parameterized by daily mean temperature (Tmean). Here using in situ observations of leaf unfolding dates (LUDs) in Europe and the United States, we show that the interannual anomalies of LUD during 1982–2011 are triggered by daytime (Tmax) more than by nighttime temperature (Tmin). Furthermore, an increase of 1 °C in Tmax would advance LUD by 4.7 days in Europe and 4.3 days in the United States, more than the conventional temperature sensitivity estimated from Tmean. The triggering role of Tmax, rather than the Tmin or Tmean variable, is also supported by analysis of the large-scale patterns of satellite-derived vegetation green-up in spring in the northern hemisphere (>30°N). Our results suggest a new conceptual framework of leaf onset using daytime temperature to improve the performance of phenology modules in current Earth system models.
 Recent warming has significantly advanced leaf onset in the northern hemisphere. Here, the authors show asymmetric effects of daytime and nighttime temperature change on the timing of leaf onset.
Recent warming has significantly advanced leaf onset in the northern hemisphere. Here, the authors show asymmetric effects of daytime and nighttime temperature change on the timing of leaf onset.
Phenology, the timing of periodic events in the life cycle of living organisms, is sensitive to climate1,2,3,4. Phenological changes induced by climate change can alter species interactions5,6 and ecosystem functioning, resulting in changes in the carbon, water and energy balances and, hence, climatic feedbacks7. Data from satellite greenness indices, field observations, and atmospheric CO2 observations all show a trend towards an earlier spring green-up for northern vegetation over recent decades, super-imposed on high interannual variability1,2,8,9. Spring temperature correlates well with this trend and with the interannual variability of spring green-up2,8. Mean temperature is the principal variable used by dynamic global vegetation models (DGVMs) for calculating leaf onset in temperature-limited biomes. These models, however, simulate onset dates that have large systematic errors compared with the in situ and satellite observations3,10, suggesting limitations in their equations describing phenology.
In cold and temperate regions, plants generally require the accumulation of a certain amount of heat to trigger spring leaf onset. Several studies also outline the need for plants to endure cold conditions during their dormancy, which defines chilling requirements11,12. Yet, evidence for a widespread chilling requirement is thin, and statistical models without chilling can predict the leaf onset date. Growing degree days (GDDs), the sum of daily mean temperature (Tmean) above a fixed threshold value, is a common surrogate for the accumulation of heat needed to unfold leaves13. Current phenological models that use daily mean temperature ignore potentially different responses of plants to daytime and nighttime warming (see,for example, refs 14, 15). In other words, if daytime and nighttime temperatures impact distinctly the heat requirement of GDD, statistical and conceptual models of leaf onset must carefully distinguish which temperature should be used. In addition, global warming is increasing nighttime temperatures more than daytime temperatures, which makes the use of mean daily temperature likely impractical for modelling phenology16.
Here, using vegetation green-up date (VGD) diagnosed from satellite observations and in situ observations of leaf unfolding dates (LUDs) in Europe and the United States, we demonstrate that the interannual anomalies of the timing of leaf onset are triggered by daytime more than nighttime temperature across the northern hemisphere.
Results
Evidence from in situ observation
We first compared daytime versus nighttime temperature accumulation for predicting in situ observations of LUD in Europe and the United States over the past 30 years (1982–2011). Twenty-four plant species from 2,400 phenology sites in Europe and lilac (Syringa L.) shrubs from 35 phenology sites in the United States were selected from the European Pan European Phenological Database (hereafter EU) and the USA National Phenology Network (hereafter US) (Supplementary Fig. 1; see Methods), respectively. Temperature data included monthly averaged daily maximum (Tmax) and minimum temperature (Tmin) with a spatial resolution of 0.5° obtained from the Tyndall Centre Climate Research Unit (CRU TS 3.20; see Methods). Both precipitation and cloudiness were included in the partial-correlation analyses, while other variables such as soil moisture and soil temperature were not included, since temperature co-varies with these variables.
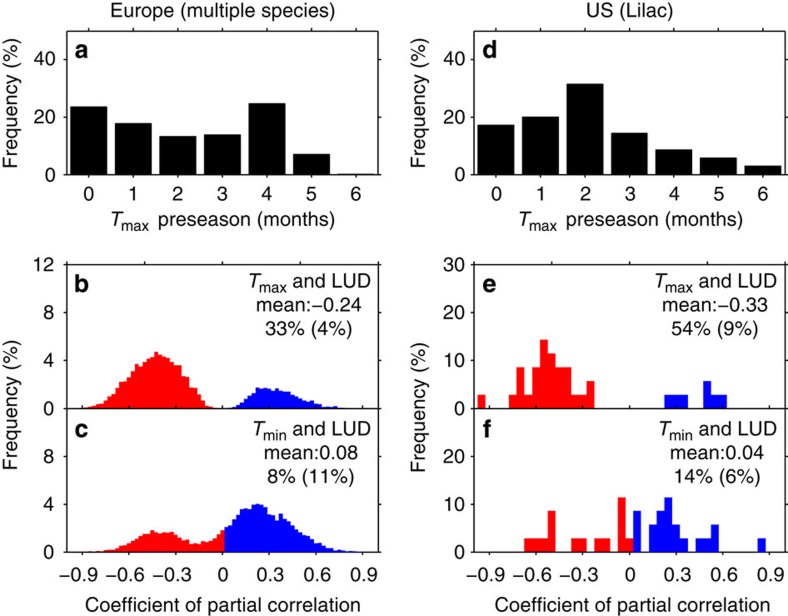
Many lines of evidence show that spring LUD is strongly correlated with temperature of the preceding months (preseason)8. Here we define the length of the preseason for Tmax by Lmax (and by Lmin for Tmin). The value of Lmax is calculated for each site as the period before LUD for which the partial-correlation coefficient between LUD and Tmax is maximized in absolute value (controlling for the effects of Tmin, precipitation and cloudiness; note that the correlation is negative; see Methods) (Supplementary Fig. 2). Lmax ranges from 0 to 3 months across most of the species–site–year combinations for both the phenology data sets (68% for EU and 83% for US; Fig. 1a,d). For the EU network, the partial interannual correlation between LUD and Tmax averaged during Lmax is negative and significant (P<0.05) at 33% of the species–site combinations. By contrast the significantly negative partial interannual correlation between LUD and Lmax-averaged Tmin occurs at <8% of the species–site combinations, as limited as the significantly positive counterpart (Fig. 1b,c). Similarly, the partial interannual correlations between LUD and Lmax-averaged Tmax were found significantly negative at 54% of the US lilac sites (n=35) compared with only 14% if Lmax-averaged Tmin is used (Fig. 1e,f). Similar results were also found with Lmin-averaged variables (Supplementary Fig. 3; see Methods). This observation suggests a predominant role of Tmax rather than Tmin in controlling the interannual variations of LUD in both EU and the US phenological in situ data.
Figure 1. Responses of in situ-observed LUDs to Tmax and Tmin in Europe and the United States during 1982–2011.
The frequency distributions of the length (in months) of Tmax preseason in (a) Europe and (d) the United States are shown. The Tmax preseason is defined as the period with the highest negative partial correlation between LUD and averaged Tmax for the months preceding LUD. Frequency distributions of the highest partial-correlation coefficients between LUDs and preseason Tmax in (b) Europe and (e) the United States after controlling for corresponding Tmin, cloudiness and precipitation. Frequency distributions of partial-correlation coefficients between LUD and Tmin in (c) Europe and (f) the United States during the same preseason as in a after controlling for corresponding Tmax, cloudiness and precipitation. Note that LUDs of multiple species in Europe and only lilacs (Syringa L.) in the United States were analysed. The mean values of partial-correlation coefficients across all phenological stations, the percentages of significantly negative partial correlations and the percentages of significantly positive partial correlations (in parentheses) are provided in b,c,e and f.
To further test the robustness of the results shown in Fig. 1, we performed the same analyses with climate data of weekly and biweekly resolution (see Methods). All analyses produced similar results as shown in Fig. 1 (Supplementary Figs 4–6), confirming the stronger relationship of LUD with daytime temperature rather than with nighttime temperature, which was not affected by the temporal resolution of the climate data sets. Furthermore, we also extended the analyses to include winter temperature as a predictor to account for chilling effects (see Methods). Here winter temperature was defined as the average Tmean during the period from the onset of the preceding dormancy (the time at which daily mean temperature falls below 0 °C, or the default date of 1 November in the year preceding LUD) to the beginning of the Tmax preseason. Including winter temperature did not alter the conclusion that Tmax is a stronger predictor of LUD than Tmin (Supplementary Fig. 7).
Evidence from satellite observation
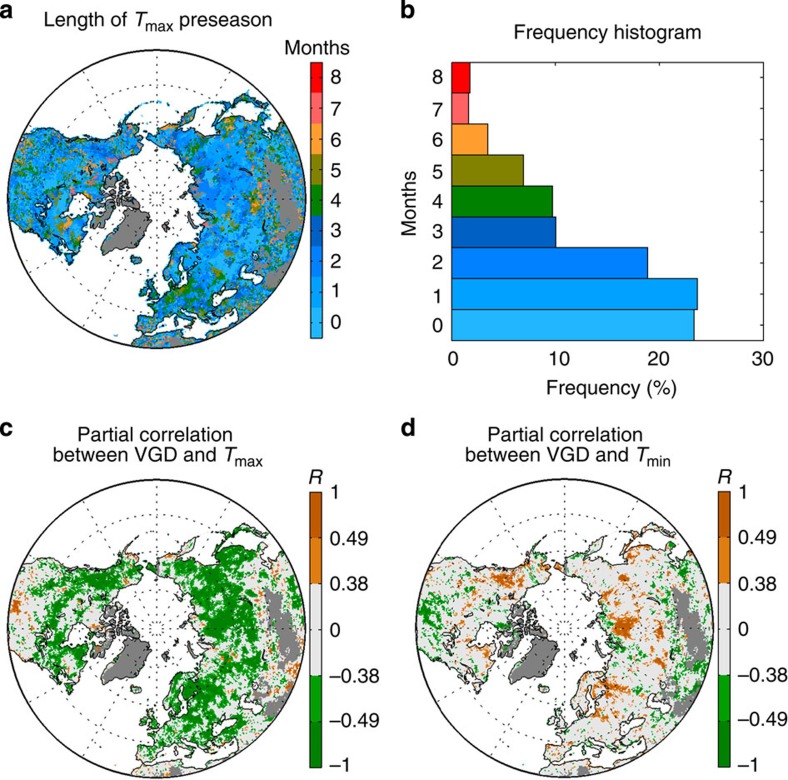
In situ phenology observations cover only a small fraction of world's vegetation types, geographic ranges and climate gradients. To evaluate the generality of the in situ-observed asymmetric temperature effects on leaf onset in Europe and parts of the US, we further analysed the effects of daytime and nighttime temperature changes on satellite-derived VGD in the terrestrial northern hemisphere (> 30°N) over the past 30 years (1982–2011; see Methods). VGD at 0.5 × 0.5° resolution was estimated from time series of the NDVI3g data set (1982–2011) developed by the Global Inventory Modeling and Mapping Studies (GIMMS) group (see Methods). Note the reported VGD here is the average value from four different VGD algorithms: Spline Midpoint, Hants Maximum, Polyfit Maximum and Timesat SG (Savitzky–Golay; see Methods). Similar to the in situ-observation results, satellite-derived Lmax ranged between 0 and 3 months across 76% of the study area (Fig. 2a,b), also in agreement with earlier findings17. Statistically significant (P<0.05) negative partial correlations between VGD and Lmax-averaged Tmax were found in 42% of the study area (Fig. 2c). In contrast, over the same preseason only 11% of the study area showed significantly negative partial correlations between VGD and preseason averaged Tmin, mostly in temperate dry regions (Fig. 2d). Even when using the Tmin preseason, the negative partial correlation between Tmin and VGD remained less prevailing (13% of the study area exhibited significantly negative correlation coefficients, Supplementary Fig. 8b) than that between Tmax and VGD (32% of the study area; Supplementary Fig. 8a).
Figure 2. The relationship of the satellite-derived onset dates of vegetation green-up with Tmax and Tmin in the northern hemisphere during 1982–2011.
(a) The spatial pattern of the length (in months) of the preseason defined as the period with the highest negative partial correlation between VGD and averaged Tmax for the months preceding VGD. (b) The frequency distribution of the length of the preseason shown in a. (c) Partial-correlation coefficients (R) between preseason Tmax and VGD after controlling for corresponding Tmin, cloudiness and precipitation. (d) Partial-correlation coefficients (R) between Tmin and VGD during the Tmax-derived preseason after controlling for corresponding Tmax, cloudiness and precipitation. The 1% and 5% significance levels of the partial correlations correspond to ±0.49 and ±0.38, respectively.
In addition, we tested the robustness of the satellite-derived results using the four different satellite-derived VGD algorithms instead of the mean VGD from all algorithms (Supplementary Fig. 9), using different climatic data sets that had different time resolutions (Supplementary Figs 10–12) and taking chilling effects into account (Supplementary Fig. 13). All the tests returned similar results. In particular, we analysed data from individual meteorological stations (Supplementary Fig. 14) thereby avoiding any potential bias of spatial extrapolation like in the gridded climatic data sets. Examining the relationship between VGD and weekly, biweekly or monthly Tmax and Tmin at the locations of the 2,510 meteorological stations with >15 years of climatic data available for 1982–2011 (see Methods), we confirmed the statistically significant and negative partial correlations between VGD and Tmax in the preceding 0–3 months at ∼43% of the stations, against only 14–16% of the stations between VGD and Tmin for the same period (Supplementary Fig. 14). In addition, to determine whether the temporal binning of the GIMMS NDVI3g could bias the results, we performed the same analysis using VGD estimated from MODIS NDVI (2000–2010; Supplementary Fig. 15). For comparison, results from GIMMS NDVI3g during the same period (2000–2010) were also presented in Supplementary Fig. 15. The significantly negative correlation between VGD and preseason Tmax was still unambiguously more prevailing (22% of area for GIMMS and 24% for MODIS) than that between VGD and Tmin for the same period (8% of area for GIMMS and 12% for MODIS) although the results were not as apparent as that in Fig. 2 due to the shorter period in the MODIS NDVI time series (Supplementary Fig. 15).
Temperature sensitivity of spring phenology
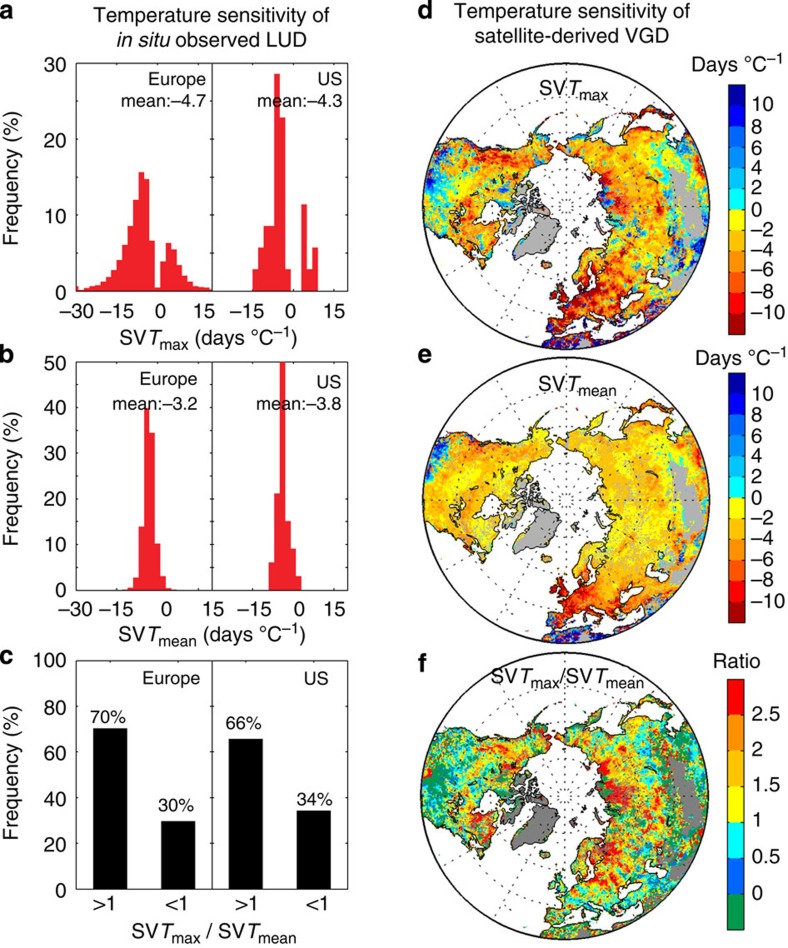
The stronger relationship between LUD (VGD) and Tmax compared with Tmin suggests that Tmax is a better indicator of spring phenology. We therefore calculated the sensitivity (linear regression slope) of both in situ-observed LUD and satellite-derived VGD to preseason Tmax (SVTmax) using multiple linear regressions in which LUD (VGD) is regressed against Tmax, Tmin, precipitation and cloudiness (see Methods). On average, an increase of 1 °C in Tmax would advance LUD by 4.7 days in Europe and 4.3 days in the United States (Fig. 3d,e) during 1982–2011. As for VGD, an increase in Tmax of 1 °C was associated with a 3-day earlier VGD in the northern hemisphere during 1982–2011 (95% confidence interval: −12.0 days °C−1∼9.1days °C−1). The highest Tmax sensitivities of VGD were observed in Europe, northern Siberia and northwestern Canada, where they exceeded −10 days °C−1 in some regions (Fig. 3a). Estimates of the Tmax sensitivity of VGD were robust across the different satellite-derived VGD algorithms used (Supplementary Fig. 16). We note that the satellite-derived Tmax sensitivity is not fully consistent with that derived from in situ observations at the same geographical locations (satellite pixel containing the site) in Europe (−8 ±7 versus −5±9 days °C−1) and in the United States (−3±3 versus −4±6 days °C−1). This small discrepancy between satellite and in situ-observation sensitivities may be due to their different temporal and spatial footprint. Compared with discrete, in situ data, GIMMS NDVI3g satellite observations provide more homogeneous phenological records over 8 by 8 km areas (including different species and sometimes different land cover types)4.
Figure 3. The sensitivity of LUD and VGD to Tmax and Tmean during 1982–2011.
The frequency distributions of the temperature sensitivity of LUD to (a) Tmax (SVTmax) and (b) Tmean (SVTmean) and (c) the ratio between SVTmax and SVTmean (SVTmax/SVTmean) in Europe and the United States. In the right panel of figure, the spatial distributions of the sensitivity of VGD are shown (d) Tmax (SVTmax) and (e) Tmean (SVTmean) and (f) the ratio between SVTmax and SVTmean (SVTmax/SVTmean) in the northern hemisphere during 1982–2011.
Discussion
Our results show that both in situ-observed and satellite-derived spring leaf onset is more closely associated with the inter-annual variation of Tmax than with Tmin. Three potential mechanisms may account for these results. First, plants in temperate and boreal regions need a critical level of forcing temperature (for example, GDD) to trigger spring phenology13. Only temperatures above this specific threshold (commonly set at 0 or 5 °C) count in GDD formation11,18. Before the onset of green-up, Tmin is more likely to be below the threshold temperature than Tmax and thus contribute less to fulfil the GDD requirement for green-up. This hypothesis is supported by the multi-year averaged values of Tmax and Tmin during Lmax for VGD (Supplementary Fig. 17a,b). Averaged Tmax was generally >5 °C, while averaged Tmin remained below 0 °C in many areas of the northern hemisphere. Hence, daytime rather than nighttime warming in spring fulfills more efficiently the GDD requirement that triggers leaf onset. Second, photoperiod may also co-regulate spring phenology19. For example, the synchronicity of the daily cycles of light and temperature in spring makes daytime temperature an important determinant of Arabidopsis phenology20. The combined effects of photoperiod and daytime temperature in early spring could, hence, contribute to the stronger relationship with Tmax. Third, since most plant photosynthesis occurs during the daytime but is suspended during the nighttime, daytime temperature rather than nighttime temperature would be more responsible for plant carbon fixation and energy capture and thus produces a stronger effect on the onset of green-up.
In temperate dry regions, by contrast, a weak negative or even a positive interannual correlation between Tmax and VGD is observed, which may be related to spring phenology being delayed by Tmax regulated water stress. It has been suggested that spring phenology of temperate grasslands is co-determined by soil water availability and temperature21. Daytime warming is observed to reduce soil water content by enhancing evaporation14, which may partly or totally offset its advancing effect on VGD. On the other hand, significant negative correlations between Tmin and VGD are observed in temperate dry regions (Fig. 2d), which could be partly attributed to decreased frost risk at higher nighttime temperature. It is also noted that in those areas the preseason average Tmin is at 0 °C or above (Supplementary Fig. 17c) and thus Tmin could contribute to fulfil the heat requirement for spring green-up. In addition, changes in plant community structure and composition in response to rising Tmin22 may also help explain this positive response of satellite-derived VGD to Tmin variations, which need to be further tested.
Daily mean temperature (Tmean) is currently used as the driver of spring phenology in models. Considering the unequal contribution of Tmax versus Tmin to spring leaf onset, models based on Tmean that includes an ineffective or less effective component of Tmin may give questionable performance in analysing the responses of spring phenology to temperature changes, considering the recent faster warming rate at night than at daytime. For example, in Europe and in the United States, we found that the correlation between LUD and Tmean was weaker than that between LUD and Tmax at >55% of the species–site combinations, although both the correlations were significant for comparable percentages of species–site combinations (Supplementary Fig. 18). Similarly, VGD in northern hemisphere also shows a weaker correlation with Tmean than with Tmax in 65% of the study area, suggesting that Tmax outperforms Tmean as a predictor of spring leaf onset variation. Furthermore, the absolute value of the LUD sensitivity to Tmean, (see,for example, refs 3, 23), was smaller than its sensitivity to Tmax in both Europe (−3.2 versus −4.7 days °C−1) and the United States (−3.8 versus −4.3 days °C−1; Fig. 3d,e). The higher LUD sensitivity to Tmax (SVTmax) than to Tmean (SVTmean) obtained from in situ observations is also corroborated by satellite observations in 60% of the study area, with spatial variation in the magnitude of the positive differences between SVTmax and SVTmean (Fig. 3c). A larger SVTmax than SVTmean is found for the area north of 50°N compared with south of 50°N (Fig. 3c). The largest positive differences between SVTmax and SVTmean were observed in Eastern Europe and north-central Siberia, where SVTmax was one to two times larger than SVTmean. On the other hand, regions where SVTmax is similar or even lower than SVTmean are temperate dry ecosystems, where Tmin rather than Tmax is controlling the interannual variation of VGD as shown in Fig. 2.
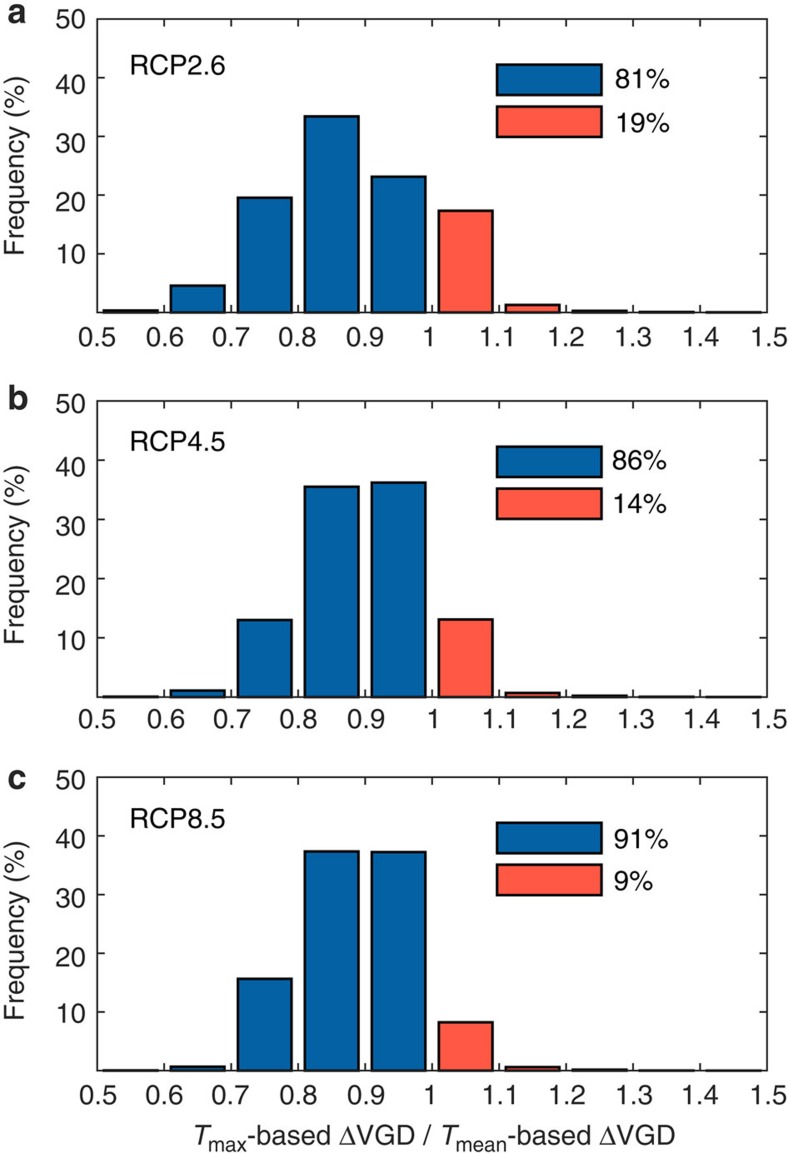
The findings suggest that spring phenology GDD models parameterized by daily mean temperature can be problematic in current vegetation models. To directly translate our findings into spring phenology predictions, we estimated and compared the changes of VGD under future climate and CO2 scenarios using Tmean-based (as used in current vegetation models) and Tmax-based (proposed by this study) GDD models (see Methods). The scenarios include 24 climate models and three radiative forcing trajectories, RCP2.6, RCP4.5 and RCP8.5 (IPCC 2013). We found significant difference between Tmax- and Tmean-based phenology predictions. The advance of VGD caused by warming was larger in the Tmean-based prediction than in the Tmax-based one for 85% of the northern hemisphere across all the climate scenarios (Fig. 4 and Supplementary Fig. 19). This is because in all the climate models analysed, the projected increase of Tmin, and hence of Tmean, is faster than that of Tmax (IPCC 2013). This result suggests that Tmean-based GDD models may overestimate changes in leaf onset, highlighting the need to incorporate the asymmetric phenology effects of daytime and nighttime temperature changes in earth system models.
Figure 4. The ratios of future VGD changes predicted by a Tmax-based GDD concept model to that predicted by a Tmean-based GDD concept model.
Both Tmean-based GDD approaches and Tmax-based GDD models were applied to predict the VGD changes (▵VGD) between 1991–2010 and 2081–2100, using 24 climate models and different climate change scenarios (RCP2.6, RCP4.5 and RCP8.5). For each RCP, the Tmax-based predictions and the Tmean-based predictions were averaged across all models and the distributions of their ratio (Tmax-based predictions/Tmean-based predictions) are shown in (a), (b) and (c). The ratio <1 (blue bar) represents that the future VGD changes predicted by Tmean-based approaches are larger than those predicted by Tmax-based approaches and vice versa (red bar). The percentage of ratios <1 and the percentage of ratios >1 are both provided in a,b and c.
In summary, our results provide information that can be used to improve the performance of current phenological module in DGVMs. The statistical analyses presented in this study, however, require more information for the accumulation of triggering energy and acclimation mechanisms. In this study, most of the preseason nighttime temperature does not contribute to the accumulation of a critical heat amount required for triggering spring phenology in the mid and high latitudes of the northern hemisphere. The heat requirement for spring green-up calculated based on daily mean temperature becomes problematic when one wants to predict future phenology changes based on past heat requirement, given the asymmetric warming rates between daytime and nighttime. While here we used maximum daytime and minimum nighttime temperature, which are not the same as the average daytime and nighttime temperature, our work suggests that temperature accumulation for spring green-up calculated at finer temporal resolutions, such as hourly or every 3 h, may be more appropriate. In addition, the impact of temperature on spring phenology has been found to be non-linear24, which further adds to the difficulty in using a statistical relationship established between current temperature and phenology to predict phenology under future climate scenarios. The non-linear impacts have been noticed and incorporated in early model development, such as the Spring Indices phenological models25. Finally, it should be noted that while daily weather can be a very random event, some synoptic-scale unusually warm daily events may also be critical in determining the timing of spring phenology25,26. The underlying mechanism through daytime and nighttime temperature affects spring phenology remains poorly understood in temperate and boreal ecosystems. Well-designed manipulation experiments therefore are needed to improve our understanding of the interaction between spring leaf unfolding phenology and daytime temperature, and ultimately result in more accurate simulations of spring phenology and better understanding of global carbon balance and ecosystem feedbacks to the ongoing climate change.
Methods
In situ-observation data set
We used in situ observations of LUD from two independent phenology data sets. One is the Pan European Phenological Database (PEP725; http://www.pep725.eu/ ), which is an open-access database with long-term plant phenological observations from 19,608 sites and 78 species across 25 European countries. This data set has been widely used for studying the relationships between spring phenology and climatic changes, especially global warming8,27. To exclude potential biases caused by outliers and inadequate degrees of freedom, we removed species–site compositions with the dates of LUD later than June (180 DOY: day of the year) and focused on the sites with >15 years records over the period 1982–2011. In total, 2,400 phenological sites and 24 plant species from PEP725 were used in this study. We also used in situ phenology observations from the USA National Phenology Network (USA–NPN; https://www.usanpn.org/results/data)28, and only shrubs of the Lilac genus had sufficient station records for LUD. Similarly, after excluding the data with the dates of LUD later than June (180 DOY) or with >15 years of records for 1982–2011, we analysed Lilac LUD data from 35 phenological sites in the United States. The distribution of selected phenological stations is shown in Supplementary Fig. S1. It should be noted that the first leafing date from USA–NPN was regarded as an equivalent of LUD here since USA–NPN does not include the exact phenological event of LUD as those defined in PEP725.
Satellite-derived date of onset of green-up
The temporal cycle of NDVI is an indicator of the seasonal growth of vegetation and can be used for investigating vegetation phenology over large regions1,29,30. The GIMMS NDVI3g data set (1982–2011) with a spatial resolution of 1/12° and a 15-day interval has been used to monitor the phenological cycle of ecosystems2,30. Areas with sparse vegetation, that is, multi-year NDVIs <0.1, were excluded from the analyses. Using NDVI3g data sets, we applied four methods (Spline Midpoint, HANTS maximum, Polyfit maximum and Timesat SG) to estimate the VGD. Detailed information about the four VGD-deriving algorithms and the uncertainty in VGD estimation from the 15-day interval NDVI data set have been documented by refs 15, 30. Follow the previous study15, we applied a Bayesian constraint in each method to rule out the influence of snow cover and limit the VGD within the thermal growing season (5day average temperature >0 °C). The average VGDs of the four algorithms were used in this study (Supplementary Fig. 20), unless otherwise noted.
Climatic data
Monthly data for Tmax, Tmin, Tmean, precipitation and cloudiness were obtained from CRU TS 3.20 and are available for a regular 0.5° latitude/longitude grid for 1982–2011 (ref. 31). Due to the lack of solar-radiation data in the CRU data set during the study period, we used cloudiness data. To independently validate the results based on the CRU data set, we also used 0.5 × 0.5° latitude/longitude gridded 3-h climatic data applying WATCH Forcing Data Methodology to ERA-Interim data (WFDEI, 1982–2011)32, a 3-h global meteorological forcing data set (1982–2008)33 and the station-level global-surface summary of day product (GSOD) by the National Climatic Data Center. Daily weather can be a very random event, and the satellite data are of biweekly resolution, so the climatic data from WFDEI, Sheffield and GSOD were then rescaled to weekly, biweekly and monthly resolutions. For the GSOD data, we only considered the 2,510 stations with >15 years of available data for 1982–2011 and with NDVIs larger than 0.1 for the 0.5° latitude/longitude grids containing the stations. The data for short-wave radiation was obtained from WFDEI.
Analyses
We used partial-correlation analyses to explore the effects of Tmax and Tmin on observed LUDs. With this approach, we could exclude the confounding effects of other climatic variables (precipitation and solar radiation) and of covariate effects between Tmax and Tmin (ref. 14). Temperature during the preseason dormancy period is arguably the most dominant factor for spring phenology12, and current phenology models in most DGVMs are solely based on temperature. It is therefore the aim of this study to identify the most appropriate temperature variables for use in phenology models. However, other environmental drivers in addition to temperature, such as precipitation, can also be involved in controlling the complex vegetation seasonality. Hence, in exploring the temperature effect on spring phenology, we have excluded the confounding effects of precipitation and cloudiness (radiation) in the partial-correlation analyses; while other variables such as soil moisture and soil temperature were not explicitly excluded, since temperature also indirectly influence them.
Spring phenological changes are highly associated with the temperatures in the preceding months8. To determine the length of the preseason whose average Tmax had the largest influence on LUD, we calculated the partial-correlation coefficients between LUD and mean Tmax during the 0, 1, 2, 3 … k months preceding LUD, controlling for corresponding average Tmin, accumulated precipitation and cloudiness (all variables non-detrended). The maximum k corresponded to the length of the period from the month of mean LUD (1982–2011) to the onset of preceding dormancy, defined as the month when the multi-year averaged mean temperature dropped to 0 °C, or November as a default value. The preceding months with the highest absolute partial-correlation coefficients were then considered as the Tmax-derived ‘preseason', in which Tmax had the largest influence on the timing of green-up. Similarly, by replacing Tmax with Tmin, we also obtained the Tmin-derived preseason.
To assess the robustness of our results, we also used grided climatic data sets at different temporal resolutions instead of the CRU monthly climatic data sets. In addition, to determine if winter chilling affected the responses of LUD to Tmax and Tmin, we performed the same partial-correlation analysis with Tmax, Tmin, precipitation, cloudiness and winter temperature as independent variables. Winter temperature was defined as the average Tmean during the period from the onset of the preceding dormancy (the time at which daily mean temperature falls below 0 °C, or the default date of 1 November in the year preceding LUD) to the beginning of the Tmax preseason.
Our results indicated that the interannual variation in LUD was more strongly associated with changes in Tmax than that in Tmin, so we then only estimated the sensitivity of LUD to Tmax based on multiple linear regressions with LUD as the dependent variable and Tmax, Tmin, precipitation and cloudiness as independent variables (all variables non-detrended). We used the monthly averaged values of each independent variable during the Tmax-derived preseason. We estimated the sensitivity of LUD to Tmean based on the same multiple linear regressions but replacing Tmax and Tmin with Tmean. Accordingly, the monthly averaged values of each independent variable during the Tmean-derived preseason were used in this analysis.
The same partial-correlation and sensitivity analyses were applied to satellite-derived observations, with preseason defined separately. To spatially match satellite data (1/12° spatial resolution) with climatic data (0.5° spatial resolution), we used averaged VGDs within each grid of the climatic data set. Besides the same robustness tests as those in the species–site level analysis (see above), we also performed additional robustness tests by using VGDs derived from individual algorithms instead of the multi-method averaged VGD, using station-level climate data set at different time resolution instead of CRU monthly climatic data sets, as well as using VGDs derived from MODIS NDVI instead of AVHRR NDVI estimated VGDs. The partial-correlation coefficients and temperature sensitivities derived from satellite and in situ observations were further compared with all the pixels covered by both the data sources.
Future prospects
To directly translate our findings into spring phenology predictions, we performed phenology prediction tests with Tmean (used in current DGVMs) and Tmax (proposed by this study) approaches, respectively. First, we calculated the mean GDD requirement for each pixel over the period of 1991–2010 using both daily Tmax and Tmean from WFDEI climate data sets (Supplementary Fig. 21). The GDD requirement here is defined as an integration of temperature above 0 °C from 1 January to the satellite-derived VGD of each year. Second, we applied these two mean GDD values (GDDTmax, GDDTmean) separately as the threshold to predict the VGD of each year over two periods, that is, 1991–2010 and 2081–2100, using 24 climate models and three climate scenarios (RCP2.6, RCP4.5 and RCP8.5). For each climate model and RCP scenario, the difference between the mean VGD of the two periods (mean_VGD2081–2100 minus mean_VGD1991–2010) was then calculated for both Tmax- and Tmean-based GDD models. Finally, under each RCP scenario, the mean values of those differences across all climate models were calculated for each vegetated pixel. For comparison, we calculated the ratio of VGD changes predicted by GDDTmax to that predicted by GDDTmean. The spatial distribution of the ratios is shown in Supplementary Fig. 19.
Author contributions
S. Piao designed research; J.T. and Q.L. performed analysis; S. Piao, J.T. and A.C. wrote the draft; and all the authors contributed to the interpretation of the results and the writing of the paper. S. Piao and J.T contributed equally to this work.
Additional information
How to cite this article: Piao, S. et al. Leaf onset in the northern hemisphere triggered by daytime temperature. Nat. Commun. 6:6911 doi: 10.1038/ncomms7911 (2015).
Supplementary Material
Supplementary Figures 1-21
Acknowledgments
This study was supported by a Strategic Priority Research Program (B) of the Chinese Academy of Sciences (grant no.XDB03030404), the National Basic Research Program of China (grant no. 2013CB956303), the National Natural Science Foundation of China (41125004) and the 111 Project (B14001). P.C., I.J. and J.P. were supported by the European Research Council Synergy grant ERC-2013-SyG-610028 IMBALANCE-P. S.V. is a postdoctoral research associate of the Fund for Scientific Research-Flanders.
References
- Schwartz M. D., Ahas R. & Aasa A. Onset of spring starting earlier across the Northern Hemisphere. Glob. Change Biol. 12, 343–351 (2006). [Google Scholar]
- Barichivich J. et al. Large-scale variations in the vegetation growing season and annual cycle of atmospheric CO2 at high northern latitudes from 1950 to 2011. Glob. Change Biol. 19, 3167–3183 (2013). [DOI] [PubMed] [Google Scholar]
- Richardson A. D. et al. Terrestrial biosphere models need better representation of vegetation phenology: results from the North American Carbon Program Site Synthesis. Glob. Change Biol. 18, 566–584 (2012). [Google Scholar]
- Cleland E. E. et al. Shifting plant phenology in response to global change. Trends Ecol. Evol. 22, 357–365 (2007). [DOI] [PubMed] [Google Scholar]
- CaraDonna P. J., Iler A. M. & Inouye D. W. Shifts in flowering phenology reshape a subalpine plant community. Proc. Natl Acad. Sci. USA 111, 4916–4921 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylianakis J. M., Didham R. K., Bascompte J. & Wardle D. A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363 (2008). [DOI] [PubMed] [Google Scholar]
- Richardson A. D. et al. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meterol. 169, 156–173 (2013). [Google Scholar]
- Menzel A. et al. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 12, 1969–1976 (2006). [Google Scholar]
- Keeling R. F., Piper S. C. & Heimann M. Global and hemispheric CO2 sinks deduced from changes in atmospheric O2 concentration. Nature 381, 218–221 (1996). [Google Scholar]
- Kucharik C. J. et al. A multiyear evaluation of a Dynamic Global Vegetation Model at three AmeriFlux forest sites: vegetation structure, phenology, soil temperature, and CO2 and H2O vapor exchange. Ecol. Modell. 196, 1–31 (2006). [Google Scholar]
- Harrington C. A., Gould P. J. & St. Clair J. B. Modeling the effects of winter environment on dormancy release of Douglas-fir. For. Ecol. Manage. 259, 798–808 (2010). [Google Scholar]
- Hänninen H. & Kramer K. A framework for modelling the annual cycle of trees in boreal and temperate regions. Silva Fenn. 41, 167–205 (2007). [Google Scholar]
- Chuine I. A unified model for budburst of trees. J. Theor. Biol. 207, 337–347 (2000). [DOI] [PubMed] [Google Scholar]
- Peng S. S. et al. Asymmetric effects of daytime and night-time warming on Northern Hemisphere vegetation. Nature 501, 88–92 (2013). [DOI] [PubMed] [Google Scholar]
- Fu Y. H. et al. Unexpected role of winter precipitation in determining heat requirement for spring vegetation green-up at northern middle and high latitudes. Glob. Change Biol. 20, 3743–3755 (2014). [DOI] [PubMed] [Google Scholar]
- IPCC. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge Univ. Press (2013). [Google Scholar]
- Jeong S. J., Ho C. H., Gimi H. J. & Brown M. E. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Glob. Change Biol. 17, 2385–2399 (2011). [Google Scholar]
- Hänninen H. Modelling bud dormancy release in trees from cool and temperate regions. Acta For. Fenn 213, 1–47 (1990). [Google Scholar]
- Körner C. & Basler D. Phenology under global warming. Science 327, 1461–1462 (2010). [DOI] [PubMed] [Google Scholar]
- Chew Y. H. et al. An augmented Arabidopsis phenology model reveals seasonal temperature control of flowering time. N. Phytol. 194, 654–665 (2012). [DOI] [PubMed] [Google Scholar]
- Yu F., Price K. P., Ellis J. & Shi P. Response of seasonal vegetation development to climatic variations in eastern central Asia. Remote Sens. Environ. 87, 42–54 (2003). [Google Scholar]
- Alward R. D., Detling J. K. & Milchunas D. G. Grassland vegetation changes and nocturnal global warming. Science 283, 229–231 (1999). [DOI] [PubMed] [Google Scholar]
- Wolkovic E. M. et al. Warming experiments underpredict plant phenological responses to climate change. Nature 485, 494–497 (2012). [DOI] [PubMed] [Google Scholar]
- Pope K. S. et al. Detecting nonlinear response of spring phenology to climate change by Bayesian analysis. Glob. Change Biol. 19, 1518–1525 (2013). [DOI] [PubMed] [Google Scholar]
- Schwartz M. D., Ahas R. & Aasa A. Onset of spring starting earlier across the northern hemisphere. Glob. Change Biol. 12, 343–351 (2006). [Google Scholar]
- Schwartz M. D. & Marotz G. A. Synoptic events and spring phenology. Phys. Geogr. 9, 151–161 (1988). [Google Scholar]
- Cook B. I. et al. Sensitivity of spring phenology to warming across temporal and spatial climate gradients in two independent databases. Ecosystems 15, 1283–1294 (2012). [Google Scholar]
- Schwartz M. D., Betancourt J. L. & Weltzin J. F. From Caprio's lilacs to the USA National Phenology Network. Front. Ecol. Environ. 10, 324–327 (2012). [Google Scholar]
- Piao S. L. et al. Evidence for a weaking relationship between interannual temperature variability and northern vegetataion activity. Nat. Commun. 5, 5018 (2014). [DOI] [PubMed] [Google Scholar]
- White M. A. et al. Intercomparison, interpretation, and assessment of spring phenology in North America estimated from remote sensing for 1982-2006. Glob. Change Biol. 15, 2335–2359 (2009). [Google Scholar]
- Mitchell T. D. & Hulme M. New, climate data for political areas. Area 34, 109–112 (2002). [Google Scholar]
- Weedon G. P. et al. The WFDEI meteorological forcing dataset: WATCH forcing data methodology applied to ERA-Interim reanalysis data. Water Resour. Res. 50, 7505–7514 (2014). [Google Scholar]
- Sheffield J. G. & Wood E. F. Development of a 50-yr high-resolution global dataset of meteorological forcings for land surface modeling. J. Climate 19, 3088–3111 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-21