Abstract
The amniote middle ear is a classical example of the evolutionary novelty. Although paleontological evidence supports the view that mammals and diapsids (modern reptiles and birds) independently acquired the middle ear after divergence from their common ancestor, the developmental bases of these transformations remain unknown. Here we show that lower-to-upper jaw transformation induced by inactivation of the Endothelin1-Dlx5/6 cascade involving Goosecoid results in loss of the tympanic membrane in mouse, but causes duplication of the tympanic membrane in chicken. Detailed anatomical analysis indicates that the relative positions of the primary jaw joint and first pharyngeal pouch led to the coupling of tympanic membrane formation with the lower jaw in mammals, but with the upper jaw in diapsids. We propose that differences in connection and release by various pharyngeal skeletal elements resulted in structural diversity, leading to the acquisition of the tympanic membrane in two distinct manners during amniote evolution.
 The evolution of the amniote middle ear remains unclear. Here, the authors show that inactivation of the Edn1-Dlx5/6 cascade during development results in loss of the tympanic membrane in mouse and duplication in chicken, which suggests independent evolution of the tympanic membrane in different amniotes.
The evolution of the amniote middle ear remains unclear. Here, the authors show that inactivation of the Edn1-Dlx5/6 cascade during development results in loss of the tympanic membrane in mouse and duplication in chicken, which suggests independent evolution of the tympanic membrane in different amniotes.
The evolution of an impedance-matching auditory system, in which sound is mechanically transferred to the cochlea via the tympanic membrane (TM) and the middle ear ossicle(s), was a key adaptation to terrestrial life for vertebrates1. Crown synapsids (modern mammals) possess three middle ear ossicles, the malleus, the incus and the stapes. The malleus and incus, which are respectively homologous with the articular and quadrate, form the primary jaw joint (PJJ) between the upper and lower jaws, and are mostly derived from the first pharyngeal arch (PA1), whereas the stapes is derived from the second pharyngeal arch (PA2)2,3,4. In contrast, diapsids (modern reptiles and birds) possess only a single ossicle, the columella auris, which is homologous with parts of the mammalian stapes. Paleontological observation indicates that the hyomandibular of basal amniotes braced between the braincase and the jaw, and did not connect to the TM as seen in crown diapsids5,6; their phylogenetic relationship suggests that mammals, diapsids and probably extinct parareptiles independently acquired the middle ear7,8,9. However, the developmental bases for these different middle ear patterns have remained elusive.
One difficulty in characterizing the distinct middle ears between mammals and diapsids arises from the fact that morphological homology has been established for every skeletal and muscular element2,10,11. Curiously, however, in light of the surrounding soft-tissue anatomy, significant differences have been identified in the relative positions of the TM in these groups2,3. In diapsids the TM attaches to the quadrate, an upper jaw element, while in mammals it spans the tympanic ring, an angular homologue associated with the lower jaw. This raises the possibility that despite the fact that they are derived from histologically comparable elements (endodermal and ectodermal invaginations and intervening tissues), the TMs were independently acquired in synapsids and diapsids, which exhibit distinct developmental topographies2,3. Consistent with this, non-identical positions of the external auditory meati (including the ectodermal component of the TM) have been reported in these lineages12,13. The key to understanding the distinct middle ear patterns may thus lie in the evolutionary relationship between TM-external auditory meatus and skeletal elements, making it crucial to dissect the developmental relations of these components during ontogeny.
In mammals, coupling between the TM and lower jaw components has been demonstrated by Goosecoid (Gsc) loss-of-function experiments14; however, the equivalent experiment has not been performed in chickens. We reasoned that if the evolutionary acquisition of the TM was coupled with patterning of the upper or lower jaw, experimental transformation of the upper/lower jaw should differentially affect the development of TMs between synapsids and diapsids. Endothelin1 (Edn1) signalling regulates Dlx5/6 in the ventral region of the pharyngeal arches to specify the lower jaw and the PJJ by establishing the PA Dlx-code15,16,17. Inactivation of Edn1/Endothelin receptor type-A (Ednra) signalling in the mouse results in loss of lower jaw identity and transformation into a mirror-image duplication of the upper jaw, a phenotype closely resembling that of Dlx5/6-null mice15,18,19,20,21. Conversely, ectopic expression of Edn1, which in turn induces ectopic Dlx5/6 in dorsal PAs, results in duplication of the lower jaw, including the tympanic ring22. Thus, experimental manipulation of Edn1 signalling in PAs may be a suitable approach to the study of TM formation.
Here we show that lower-to-upper jaw transformation induced by inactivation of the Edn1-Dlx5/6 cascade involving Gsc results in loss of the TM in mouse, but causes duplication of the TM in chicken, indicating that the TMs of mammals and diapsids are coupled with the development of the lower and upper jaws, respectively. Furthermore, in contrast to the classical hypothesis that the ventral swelling of the first pharyngeal pouch (PP1) enables acquisition of the lower-jaw-associated TM in mammals23, our comparative anatomical analysis indicates that the dorsal shift of the PJJ towards PP1 leads to differential coupling of the TM to jaw skeletons in avians and mammals. This report endows developmental and molecular evidence to support independent origin of the middle ear in different amniote taxa and the novel insight into the developmental process that led to these distinct middle ear patterns.
Results
Independent developmental origin of the TM in mammals and diapsids
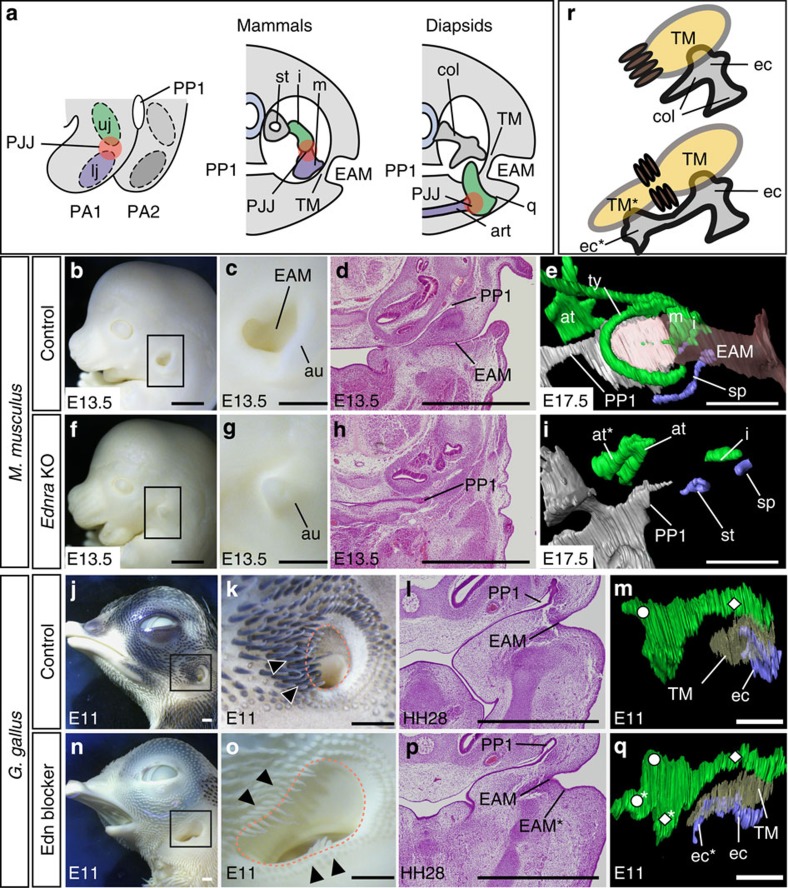
To dissect developmental relationships between TM-external auditory meatus and jaw skeletal elements, we first analysed Ednra-null mice; in these animals, invagination of the external auditory meatus was absent, with loss of the TM (Fig. 1f–i; compare with Fig. 1b–e). The TM of therian mammals consists of two regions, namely the pars tensa and pars flaccida, and some classical studies suggested the homology between the latter and the reptilian TM23. We confirmed, however, the entire TM was lost in the Ednra-null mouse. These observations indicate that formation of the TM in the mouse is coupled with development of the lower jaw.
Figure 1. Comparison of middle ear phenotypes induced by inhibition of the Edn1-Dlx5/6 cascade in mouse and chicken.
(a) Development and morphology of amniote middle ears. Generalized scheme of the first (PA1) and second (PA2) pharyngeal arches (left), transverse section of the middle ear in mammals (middle) and diapsids (right). Three ear ossicles, malleus (m), incus (i) and stapes (st) are present in mammals, while diapsids have only a single ossicle, columella auris (col). The TM comprises the first pharyngeal pouch (PP1) and external auditory meatus (EAM). Left lateral appearance (b,c,f,g), horizontal sections (d,h) and ventrolateral views of the three-dimensional (3D) reconstructed middle ear (e,i) in control (b–e) and Ednra-null (f–i) mice. Higher magnification of boxes in b,f are shown in c,g, respectively. Skeletal components derived from PA1 (green) and PA2 (blue), EAM (pink) and PP1 (grey) are shown in e,i. Left lateral appearance (j,k,n,o), horizontal sections (l,p) and ventrolateral views of the 3D reconstructed middle ear (m,q) in control (j–m) and Edn-blocker-treated (n–q) chickens. (k,o) Higher magnifications of j,n, respectively. Outer edges of EAM is delineated by red dashed lines and ventral auricular feathers are indicated by arrowheads (k,o); they delineate the ventral margin of the EAM in control (k) and the supernumerary EAM was formed ventrally to them (o). Note that pigmentation is also impaired by Edn blockage, which acts on the Ednrb receptor in melanocytes51 (n,o). White circles and lozenges indicate the pterygoid and otic process of the quadrate, respectively, and TM is coloured translucent yellow (m,q). The extracolumella (a lateral part of the columella auris that attaches to the TM in control) is duplicated and attached to the ectopically formed TM in Edn-blocker-treated chicken embryos (m,q,r; also see Supplementary Fig. 1j,n). (r) Summary of the induced middle ear phenotypes in chicken embryos. Dark brown ovals indicate the ventral auricular feathers. art, articular; at, ala temporalis; au, auricle; ec, extracolumella; lj, lower jaw; PJJ, primary jaw joint; q, quadrate; sp, styloid process; ty, tympanic ring; uj, upper jaw; *duplicated elements. Scale bars, 500 μm for c,g, 1 mm for other panels.
We next inhibited the Edn1-Dlx5/6 cascade in chicken embryos using bosentan, an Edn receptor antagonist. This resulted in hypoplasia of lower jaw skeletons (Fig. 1j,n and Supplementary Fig. 1g,k), downregulation of Dlx5/6 (Supplementary Fig. 1a,b,d,e) and transformation of the lower jaw morphology, mimicking upper jaw identity (Supplementary Fig. 1g–n) as in the case of the mouse18,19,20. Namely, lower jaw skeletons such as the articular, dentary, angular and the Meckel's cartilage were disturbed, and instead ectopic upper jaw-like skeletons that, because of their relations, correspond to what in the adult we would homologize with the maxilla, jugal, palatine, pterygoid, and otic and pterygoid processes of the quadrate appeared in the lower jaw position in a mirror-image pattern (Supplementary Fig. 1g–n).
In contrast to the mouse, however, a supernumerary external auditory meatus appeared ventral to the original one (Fig. 1j–l,n–p), resulting in duplication of the TM (Fig. 1m,q,r and Supplementary Fig. 1o–r). The supernumerary TM was associated with the duplicated extracolumella (a part of the columella auris attached to the TM) and formed ventral to the typical long feathers that delineate the ventral margin of the control TM (Fig. 1k,m,o,q,r). Thus, although perturbation of the same developmental signalling pathway resulted in similar jaw skeletal patterning phenotypes in mouse and chicken embryos, the resulting TM phenotypes were different.
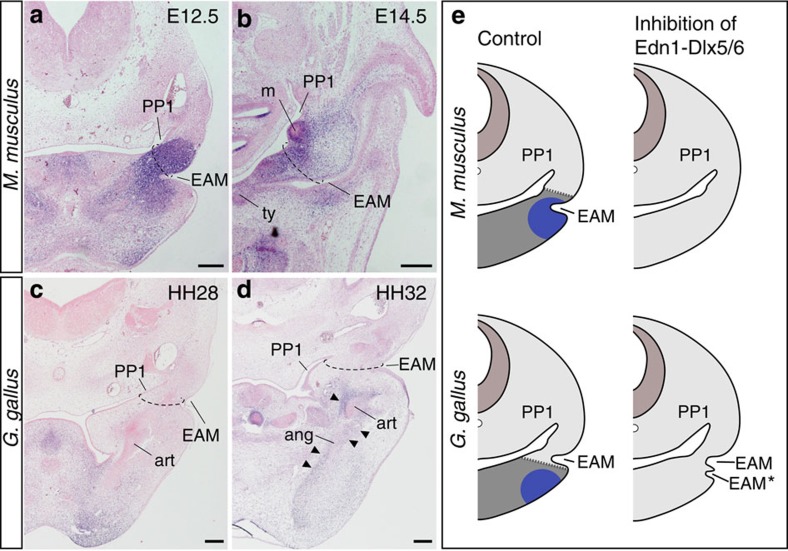
To understand why inhibition of the Edn1-Dlx5/6 cascade induced distinct TM phenotypes, we investigated gene expression patterns involved in jaw and middle ear patterning in both species. Gsc is a downstream target of the Edn1-Dlx5/6 cascade in both mouse24,25 and chicken (Supplementary Fig. 1c,f). In the mouse, Gsc inactivation results in loss of the external auditory meatus and the tympanic ring (homologue of the angular in diapsids)14, suggesting that the external auditory meatus is induced as a lower jaw component. Indeed, in mice Gsc expression was associated with the TM and tympanic ring primordia (Fig. 2a,b). In contrast, chicken Gsc, although also detected in the angular, was not found in the proximity of the forming TM, which is located more dorsally (Fig. 2c,d). The latter expression pattern is also found in gecko (a lepidosaur; see Supplementary Fig. 2), indicating that this expression pattern is conserved in diapsids. These results suggest that although Gsc is expressed in the proximal part of the ventral PA1 in both mammals and diapsids, it plays a role in TM patterning only in mammals. Thus, although the TM of mammals and diapsids are functionally comparable, they are coupled with distinct PA1 components (Fig. 2e).
Figure 2. Expression pattern of Gsc in mouse and chicken.
(a–d) Gsc expression in transverse sections of the mouse embryonic day 12.5 (a) and 14.5 (b), and chicken stage 28 (c) and 32 (d) embryos. The expression signals along the forming articular (art) and angular (ang) in d are indicated by arrowheads. Dashed lines indicate the planes of future TM. (e) The jaw regionalization and the TM formation. The lower jaw region (grey) and expression of Gsc (blue) are shown. EAM, external auditory meatus; m, malleus; PP1, first pharyngeal pouch. Scale bars, 200 μm.
The relative positions of the PJJ and PP1 led to the distinct TM formation
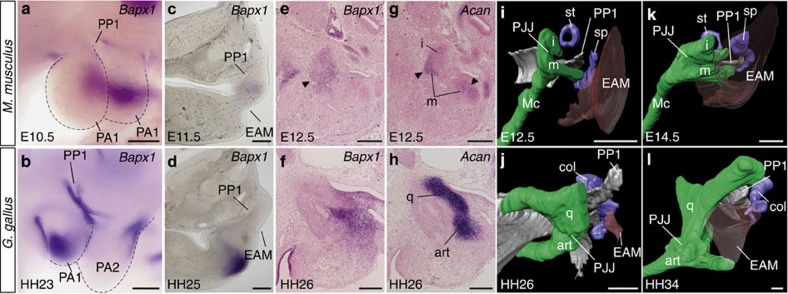
The different roles of Gsc in mouse and chicken embryos appear to reflect differences in the relative topographical relationships between the TM (reflecting the position of PP1) and the PJJ in these species. In embryos of both species, PP1 forms in the same relative position between PA1 and PA2 (Supplementary Fig. 3). We examined expression of Bapx1, a marker gene for PJJ mesenchyme26,27, and found that in the mouse embryo it was expressed in proximity of the PP1 more dorsally than in the chicken embryo (Fig. 3a–d). This difference became more pronounced during development, resulting in the formation of the procartilaginous PJJ adjacent to PP1 in mouse and ventral to PP1 in chicken (Fig. 3e–j); the external auditory meatus invaginates ventral to the PJJ in mouse and dorsal to the PJJ in chicken (Fig. 3i–l). Notably, dorsoventral positional relationships between the external auditory meatus and PP1 did not show significant differences; the external auditory meati invaginate slightly ventral to PP1 in both species (Fig. 3i–l). Thus, the relative positions of skeletal and TM components differ in mammals and diapsids.
Figure 3. Comparison of development of the TM and PJJ in mouse and chicken.
(a–d) Bapx1 expression in pharyngeal arches of the embryonic day 10.5 mouse (a), stage 23 chicken (b) in lateral view and transverse sections of the embryonic day 11.5 mouse (c) and stage 25 chicken (d). Bapx1 and Aggrecan (Acan) expression in sagittal serial sections of embryonic day 12.5 mouse (e,g) and stage 26 chicken (f,h). The expression signals in the incus (i) and malleus (m) are indicated by arrowheads (e,g). (i–l) Three-dimensional reconstruction of forming the pharyngeal skeleton and TM of embryonic day 12.5 (i) and 14.5 (k) mouse, and stage 26 (j) and 34 (l) chicken (coloured same as in Fig. 1). art, articular; col, columella auris; EAM, external auditory meatus; Mc, Meckel's cartilage; PA1–2, pharyngeal arches 1–2; PJJ, primary jaw joint; PP1, first pharyngeal pouch; q, quadrate; sp, styloid process; st, stapes. Scale bars, 200 μm.
To explain the inconsistent positioning of TMs in mammals and diapsids, classical hypotheses assumed either a ventral shift of the ancestral TM28,29 or a de novo acquisition of the TM, associated with a hypothetical ventral swelling of the middle ear cavity in mammals23. The latter hypothesis is inconsistent with the mammal-specific distribution of neural crest cell-derived epithelium in the middle ear cavity30. We showed that both TMs differentiate from PP1, an embryonic anlage developmentally equivalent in mouse and chicken, and that there is no secondary ventral swelling of PP1 in the mouse (Fig. 3i,k). Rather, PJJ primordia develop in different positions relatively to PP1 in avian and mammalian embryos (Fig. 3). The most plausible explanation would simply be that a topographical frameshift between PP1 and the PJJ developmentally leads to different TMs and middle ears in mammals and diapsids.
Discussion
Morphological evolution of the mammalian middle ear has long remained a conundrum2,3,4. To provide a developmental basis for the recent paleontological hypothesis that mammals and diapsids independently acquired the middle ear5,6,7,8, we analysed the dorsoventral patterning of TMs through a developmental biological approach in mouse and chicken embryos. Given that inactivation of the Edn1-Dlx5/6 cascade resulted in comparable skeletal phenotypes18,19,20 (Supplementary Fig. 1), and Bapx1 and Gsc are expressed in a homologous set of skeletal elements in these animals (Figs 2 and 3), it appears that the gene regulatory networks of PAs are highly conserved, to assure the morphological homologies of PA skeletal elements in amniotes17. However, the TMs of mammals and diapsids are topologically distinct, although both are generated through interactions among PP1, the external auditory meatus and the surrounding mesenchyme31, and early PP1 is homologous in these animals (Fig. 3 and Supplementary Fig. 3). These findings collectively indicate that the difference in developmental mechanisms for these TMs should not be regarded as caused by a simple developmental system drift32,33, but rather reflects unshared evolutionary events underlying their acquisition. We thus propose that the developmental programmes for skeletal specification and TM formation are decoupled primarily, and that TM formation was secondarily coupled with lower and upper PA1 components in mammals and diapsids, respectively.
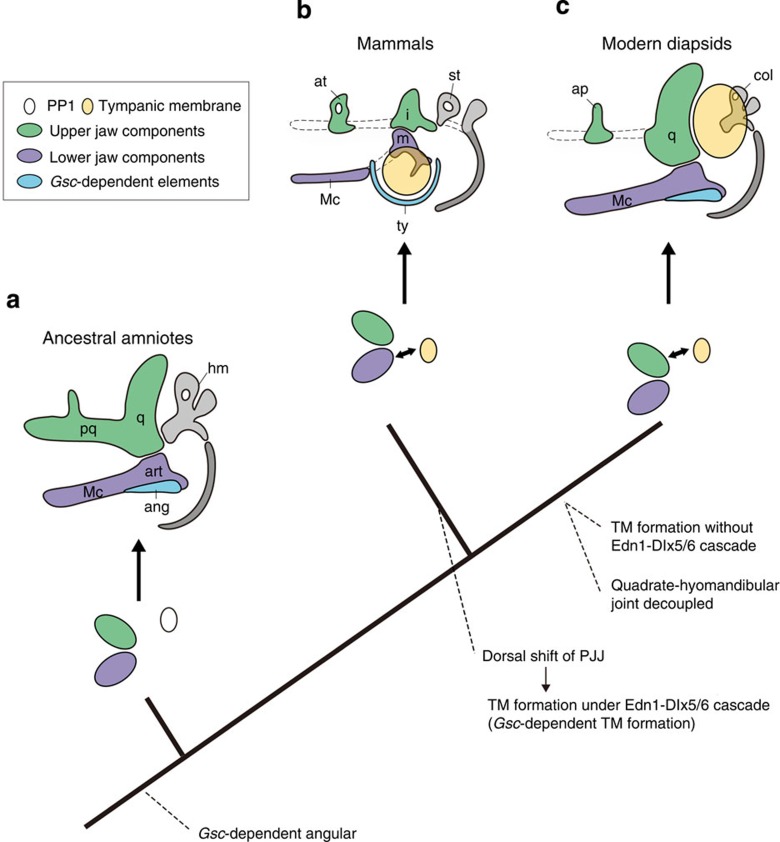
The above noted developmental mechanism of the middle ear has a shallower history than the split of the mammalian and diapsid lineages, which occurred 50 million years after vertebrate terrestrialization34,35. Developmental analyses in outgroups of amniotes (axolotl and shark; Supplementary Fig. 4) and the similarity of adult morphology to basal amniotes5,36,37,38 suggest that the positional interrelationship between PP1 and PJJ in crown diapsids represents the plesiomorphic state for amniotes (Fig. 4a). Stem synapsids and stem diapsids exhibited jaw suspension using the hyomandibular articulated with the quadrate, which later became relaxed due to the rearrangement of bony connections in the skull of the diapsid lineage, and ultimately loss of the quadrate-hyomandibular articulation39. In the synapsid lineage, however, the original articulation among the hyomandibular, quadrate and articular was retained, disabling the possession of hyomandibular-associated TM7. The dorsal positional shift of the PJJ in embryonic development would have permitted the formation of the unique lower jaw-associated TM in mammalian ancestors. Thus, differences in the connection and release among these skeletal elements may have served as a key innovation, marking this watershed in the subsequent evolution of two different origins of middle ears and TMs during amniote evolution as a typical case of convergence (Fig. 4b,c). It is fascinating to see how such functionally and histologically similar structures can arise during evolution, based not only on shifted sets of embryonic anlagen, but on non-equivalent developmental programmes as well.
Figure 4. Hypothesized scenario of the TM and middle ear evolution in amniotes.
(a) In ancestral amniotes, the position of PJJ is thought to have been distant from PP1. The TM had not yet been obtained. (b) In mammalian ancestors, the PJJ shifted dorsally to the proximity of PP1, leading to the coupling of the TM and lower jaw specifications to form the Edn1-Dlx5/6-dependent TM in the lower jaw domain. This TM spans the angular, dependent on Gsc expression. (c) In modern diapsids, the position of the PJJ retains the ancestral state. The hyomandibular has been decoupled from the quadrate and established a connection with the Edn1-Dlx5/6-independent TM in the upper jaw domain. ang, angular; art, articular; ap, ascending process; at, ala temporalis; col, columella auris; hm, hyomandibular; i, incus; m, malleus; Mc, Meckel's cartilage; PP1, first pharyngeal pouch; pq, palatoquadrate; q, quadrate; st, stapes; ty, tympanic ring.
Methods
Sample collection
Mouse Mus musculus embryos were collected and staged according to Theiler40, considering the noon of the day on which vaginal plugs were detected after timed mating as E0.5, from 8- to 40-week-old females. Ednra-null (EdnraGFP) locus was generated by insertion of an EGFP cassette into the Ednra locus, using Cre recombinase-mediated cassette exchange system on Ednraneo/+ embryonic stem cells, which contain exchangeable floxed site in the Ednra locus41. Mice were maintained on a mixed C57BL/6J × ICR background and housed in an environmentally controlled room at 23±2 °C, with a relative humidity of 50%–60% and under a 12-h light:12-h dark cycle. Fertilized eggs of chicken Gallus gallus were incubated in a humidified atmosphere at 37 °C –38 °C and staged according to Hamburger and Hamilton42. Embryos of axolotl Ambystoma mexicanum, catshark Scyliorhinus torazame and gecko Paroedura picta (formerly P. pictus) were collected and staged according to previous studies43,44,45. All animals were analysed without distinction of sex. All animal experiments were performed in accordance with the institutional guidelines of the Animal Care and Use Committees of the University of Tokyo, RIKEN CDB and the Iwate Medical University.
Molecular cloning
Total RNA was extracted from a whole embryo of P. picta, using TRIZOL reagent (Life Technologies). Degenerate reverse transcriptase–PCR and rapid amplification of complementary DNA ends PCR were performed to amplify fragments of Gsc using the GeneRacer Kit (Life Technologies). These fragments were sequenced using an ABI 3130XL automated sequencer (Applied Biosystems). A putative amino acid sequence of the clone was aligned with Gsc family genes in representative species. The molecular phylogenetic tree was inferred using the neighbour-joining method46.
Pharmacological inactivation of Edn signalling in ovo
The method has been described previously47,48. Bosentan (Actelion, Ltd) was suspended in the corn oil (Sigma) in 37 °C water bath for 30 min at the 5 mg ml−1. A small window was opened in the shell of 48-h-incubated eggs and 30 μl bosentan-diluted oil drop was introduced onto the shell membrane, while only oil was given to the control group. The shell opening was sealed with tape and eggs were incubated again until dissection.
Skeletal staining
Skeletal staining of embryos with alizarin red/alcian blue staining was performed according to previously described protocols49, with slight modifications. Samples were fixed in 95% ethanol for 1 week, placed in acetone for 2 days and incubated with 0.015% alcian blue 8GS, 0.005% alizarin red S and 5% acetic acid in 70% ethanol for 3 days. After washing in distilled water, the samples were cleared in 1% KOH for several days and in 1% KOH glycerol series until the surrounding tissues turned transparent. The specimens were stored in glycerol.
In situ hybridization
Digoxigenin (DIG)-labelled riboprobes for mouse Bapx1, Pax1, Aggrecan, chicken Dlx5, Dlx6, Bapx1, Gsc, shark Bapx1 and gecko Gsc were generated by DIG RNA labelling kit (Roche) based on the nucleotide sequences NM_007524, NM_008780, L07049, NM_204159, AY_640308, NM_204137, NM_205331, AB293587 and AB985798 deposited in GenBank, respectively. The nucleotide sequence of mouse Gsc, chicken Aggrecan, and quail Pax1 were gifts from Gen Yamada (Kumamoto University), Atsushi Kuroiwa (Nagoya University) and Hirohiko Aoyama (Hiroshima University), respectively. Embryos were fixed with Serra's fixative or 4% paraformaldehyde, dehydrated, embedded in paraffin and sliced into 6–10 μm sections. In situ hybridization for the sections were performed by a Ventana Discovery XT system, using a BlueMap NBT/BCIP substrate kit and a Red counterstain II (Roche-Ventana Medical Systems). Whole-mount in situ hybridization was performed as previously described50, with slight modifications. Embryos were fixed with 4% paraformaldehyde, dehydrated and treated with hydrogen peroxide solution in methanol (1:5) overnight. The specimens were rehydrated in PBT (PBS containing 0.1% Tween 20), digested with 20 μg ml−1 proteinase K (Roche) and refixed with 4% PFA/PBT containing 0.2% glutaraldehyde. The samples were incubated in hybridization buffer with 0.1–1 μg ml−1 DIG-labelled riboprobe for 24 h at 65 °C. After hybridization, the specimens were incubated with 50 μg ml−1 RNaseA for 30 min at room temperature, washed with 2 × SSC and 0.2 × SSC containing 0.3% CHAPS at 65 °C. The embryos were blocked with 1.5% blocking reagent (Roche) and incubated with alkaline phosphatase-conjugated anti-DIG Fab fragments (diluted 1:4,000; Roche) at 4 °C overnight. The specimens were washed 12 times with Maleic acid buffer (0.15 M Maleic acid, 0.15 M NaCl, 0.1% Tween 20) at room temperature. Alkaline phosphatase activity was detected with NBT/BCIP (Roche). Following in situ hybridization, the whole-mount samples hybridized with Bapx1 riboprobe were embedded in gelatin and sections (120 μm) were generated with a vibrating microtome.
Histological analysis and 3D reconstruction
Each paraffin-embedded section (6–12 μm) was stained by haematoxylin and eosin. For the stage 34 chicken and embryonic day 14.5 mouse embryos, histological sections were stained with 0.1% alcian blue after haematoxylin and eosin staining. Procartilaginous development in histological sections of the stage 26 chicken and embryonic day 12.5 mouse were detected by in situ hybridization with Aggrecan riboprobe. For three-dimensional reconstruction, digital images of the stained sections were loaded into Amira (Visage Imaging, Inc.) with a voxel size appropriate to section thickness.
Author contributions
T.K., M.T., S.K. and H. Kur. designed the study. T.K., H. Kum., K.M., Y.K. and S.M.-T. performed experiments for bosentan-treated chicken embryos. T.K., N.N., H. Kum. and G.L. collected data from Ednra-null mouse embryos. M.T., T. Hirai., J.H. and S.K. collected data from wild-type mouse and chicken embryos. T. Hirasawa., T. Hirai. and M.T. performed the gecko analysis. N.A. and M.T. collected data, using shark embryos. M.T. obtained data from axolotl embryos. T.K., M.T., T. Hirasawa, G.L., H. Kur. and S.K. wrote the manuscript. All of the authors discussed the results and commented on the manuscript.
Additional information
Accession codes: The Gecko Gsc sequences generated in this study have been deposited in DNA Data Bank Japan (DDBJ) under accession code AB985798.
How to cite this article: Kitazawa, T. et al. Developmental genetic bases behind the independent origin of the tympanic membrane in mammals and diapsids. Nat. Commun. 6:6853 doi: 10.1038/ncomms7853 (2015).
Supplementary Material
Supplementary Figures 1-4 and Supplementary References
Acknowledgments
We express our gratitude to M. Depew, E. Allin and J. Meng for their helpful discussion. We are also grateful to A. Kuroiwa, H. Aoyama and G. Yamada for giving us materials for in situ hybridization, H. Nagashima, F. Sugahara, F. Nagao, Y. Fujisawa and S. Kushiyama for technical assistance, and R. Ladher and D. Sipp for critical reading of this manuscript. We also thank the Animal Resource Unit of, RIKEN CDB, for the gift of gecko embryos, and Katsumi Shirato for his help in catching adult sharks. Our gratitude is extended to Actelion Pharmaceuticals Ltd for the bosentan usage. T.K. is a Research Fellow of the JSPS (PD). This work was supported in part by Global COE Program (Integrative Life Science based on the Study of Biosignaling Mechanisms), KAKENHI and Platform for Dynamic Approaches to Living System from the MEXT, Japan; grants-in-aid for scientific research from the Ministry of Health, Labour and Welfare, Japan; CREST from JST, Japan.
References
- Hidebrand M. & Goslow G. Analysis of Vertebrate Structure Wiley (2001). [Google Scholar]
- Gaupp E. Die Reichertsche theorie (Hammer,. Ambos-, und Kieferfrage). Arch. Anat. Physiol. Suppl. 1, 416 (1912). [Google Scholar]
- Takechi M. & Kuratani S. History of studies on mammalian middle ear evolution: a comparative morphological and developmental biology perspective. J. Exp. Zool. B Mol. Dev. Evol. 314, 417–433 (2010). [DOI] [PubMed] [Google Scholar]
- Anthwal N., Joshi L. & Tucker A. S. Evolution of the mammalian middle ear and jaw: adaptations and novel structures. J. Anat. 222, 147–160 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer A. S. & Price L. I. Review of the Pelycosauria. Geol. Soc. Am. Spec. Pap. 28, 1–538 (1940). [Google Scholar]
- Gardner N. M., Holliday C. M. & O'Keefe F. R. The braincase of Youngina capensis (Reptilia, Diapsida): new insights from high-resolution CT scanning of the holotype. Palaeont. Elect. 13, 19A (2010). [Google Scholar]
- Lombard R. E. & Bolt J. R. Evolution of the tetrapod ear: an analysis and reinterpretation. Biol. J. Linn. Soc. 11, 19–76 (1979). [Google Scholar]
- Laurin M. The importance of global parsimony and historical bias in understanding tetrapod evolution. Part I. Systematics, middle ear evolution and jaw suspension. Ann. Sci. Nat. Zool. 19, 1–42 (1998). [Google Scholar]
- Tsuji L. A., Muller J. & Reisz R. R. Anatomy of Emeroleter levis and the phylogeny of the nycteroleter parareptiles. J. Vert. Paleontol. 32, 45–67 (2012). [Google Scholar]
- Allin E. F. Evolution of the mammalian middle ear. J. Morphol. 147, 403–437 (1975). [DOI] [PubMed] [Google Scholar]
- Barghusen H. R. in: The Ecology and Biology of Mammal-like Reptiles eds Hotton N. III, MacLean P. D., Roth J. J., Roth E. C. 253–262Smithsonian Institution Press (1986). [Google Scholar]
- Minoux M. et al. Mouse Hoxa2 mutations provide a model for microtia and auricle duplication. Development 140, 4386–4397 (2013). [DOI] [PubMed] [Google Scholar]
- Mall F. P. Entwickelung der Branchialbogen und -Spalten des Hühnchens. Arch. f. Anat. u. Physiol. 1–34 (1887). [Google Scholar]
- Yamada G. et al. Targeted mutation of the murine goosecoid gene results in craniofacial defects and neonatal death. Development 121, 2917–2922 (1995). [DOI] [PubMed] [Google Scholar]
- Depew M. J., Lufkin T. & Rubenstein J. L. Specification of jaw subdivisions by Dlx genes. Science 298, 381–385 (2002). [DOI] [PubMed] [Google Scholar]
- Minoux M. & Rijli F. M. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 137, 2605–2621 (2010). [DOI] [PubMed] [Google Scholar]
- Medeiros D. M. & Crump J. G. New perspectives on pharyngeal dorsoventral patterning in development and evolution of the vertebrate jaw. Dev. Biol. 371, 121–135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y. et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature 368, 703–710 (1994). [DOI] [PubMed] [Google Scholar]
- Ozeki H., Kurihara Y., Tonami K., Watatani S. & Kurihara H. Endothelin-1 regulates the dorsoventral branchial arch patterning in mice. Mech. Dev. 121, 387–395 (2004). [DOI] [PubMed] [Google Scholar]
- Ruest L. B., Xiang X., Lim K. C., Levi G. & Clouthier D. E. Endothelin-A receptor-dependent and -independent signaling pathways in establishing mandibular identity. Development 131, 4413–4423 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverdam A. et al. Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past? Genesis 34, 221–227 (2002). [DOI] [PubMed] [Google Scholar]
- Sato T. et al. An endothelin-1 switch specifies maxillomandibular identity. Proc. Natl Acad. Sci. USA 105, 18806–18811 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westoll T. S. The hyomandibular of Eusthenopteron and the tetrapod middle ear. Proc. R. Soc. B 131, 393–414 (1943). [Google Scholar]
- Clouthier D. E. et al. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 125, 813–824 (1998). [DOI] [PubMed] [Google Scholar]
- Depew M. J. et al. Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 126, 3831–3846 (1999). [DOI] [PubMed] [Google Scholar]
- Tucker A. S., Watson R. P., Lettice L. A., Yamada G. & Hill R. E. Bapx1 regulates patterning in the middle ear: altered regulatory role in the transition from the proximal jaw during vertebrate evolution. Development 131, 1235–1245 (2004). [DOI] [PubMed] [Google Scholar]
- Miller C. T., Yelon D., Stainier D. Y. & Kimmel C. B. Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development 130, 1353–1365 (2003). [DOI] [PubMed] [Google Scholar]
- Goodrich E. S. The chorda tympani and middle ear in reptiles, birds, and mammals. Quart. J. Microsc. Sci. 61, 137–160 (1914). [Google Scholar]
- Goodrich E. S. Studies on the Structure and Development of Vertebrates McMillan (1930). [Google Scholar]
- Thompson H. & Tucker A. S. Dual origin of the epithelium of the mammalian middle ear. Science 339, 1453–1456 (2013). [DOI] [PubMed] [Google Scholar]
- Mallo M. Formation of the middle ear: recent progress on the developmental and molecular mechanisms. Dev. Biol. 231, 410–419 (2001). [DOI] [PubMed] [Google Scholar]
- True J. R. & Haag E. S. Developmental system drift and flexibility in evolutionary trajectories. Evol. Dev. 3, 109–119 (2001). [DOI] [PubMed] [Google Scholar]
- Roth V. L. In: Ontogeny and Systematics ed. Humphries C. J. 1–26Columbia University Press (1988). [Google Scholar]
- Benton M. J. & Donoghue P. C. Paleontological evidence to date the tree of life. Mol. Biol. Evol. 24, 26–53 (2007). [DOI] [PubMed] [Google Scholar]
- Daeschler E. B., Shubin N. H. & Jenkins F. A. Jr A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Nature 440, 757–763 (2006). [DOI] [PubMed] [Google Scholar]
- Sushkin P. P. On the modifications of the mandibular and hyoid arches and their relations to the brain-case in the early Tetrapoda. Palaont. Z 8, 263–321 (1927). [Google Scholar]
- Carroll R. L. A middle pennsylvanian captorhinomorph and the interrelationships of primitive reptiles. J. Paleontol. 43, 151–170 (1969). [Google Scholar]
- Reisz R. R. In: Major Transitions in Vertebrate Evolution eds Anderson J. S., Sues H. -D. 228–252Indiana University Press (2007). [Google Scholar]
- Clack J. A. Patterns and processes in the early evolution of the tetrapod ear. J. Neurobiol. 53, 251–264 (2002). [DOI] [PubMed] [Google Scholar]
- Theiler K. The House Mouse. Atlas of Embryonic Development Springer-Verlag (1989). [Google Scholar]
- Asai R. et al. Endothelin receptor type A expression defines a distinct cardiac subdomain within the heart field and is later implicated in chamber myocardium formation. Development 137, 3823–3833 (2010). [DOI] [PubMed] [Google Scholar]
- Hamburger V. & Hamilton H. L. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 (1951). [PubMed] [Google Scholar]
- Bordzilovskaya N. P., Dettlaff T. A., Duhon S. T. & Malacinski G. M. In: Developmental Biology of the Axolotl eds Armstrong J. B., Malacinski G. M. 201–219Oxford University Press (1989). [Google Scholar]
- Adachi N. & Kuratani S. Development of head and trunk mesoderm in the dogfish, Scyliorhinus torazame: I. Embryology and morphology of the head cavities and related structures. Evol. Dev. 14, 234–256 (2012). [DOI] [PubMed] [Google Scholar]
- Noro M., Uejima A., Abe G., Manabe M. & Tamura K. Normal developmental stages of the Madagascar ground gecko Paroedura pictus with special reference to limb morphogenesis. Dev. Dyn. 238, 100–109 (2009). [DOI] [PubMed] [Google Scholar]
- Saitou N. & Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987). [DOI] [PubMed] [Google Scholar]
- Arima Y. et al. Preotic neural crest cells contribute to coronary artery smooth muscle involving endothelin signalling. Nat. Commun. 3, 1267 (2012). [DOI] [PubMed] [Google Scholar]
- Kempf H., Linares C., Corvol P. & Gasc J. M. Pharmacological inactivation of the endothelin type A receptor in the early chick embryo: a model of mispatterning of the branchial arch derivatives. Development 125, 4931–4941 (1998). [DOI] [PubMed] [Google Scholar]
- McLeod M. J. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22, 299–301 (1980). [DOI] [PubMed] [Google Scholar]
- Murakami Y. et al. Identification and expression of the lamprey Pax6 gene: evolutionary origin of the segmented brain of vertebrates. Development 128, 3521–3531 (2001). [DOI] [PubMed] [Google Scholar]
- Hosoda K. et al. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell 79, 1267–1276 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-4 and Supplementary References