Summary
Introduction: Fibrin tissue adhesive, which has applications in several areas of medicine, can be prepared by different methods.
Aim: To compare fibrin tissue adhesives prepared by 3 different methods.
Method: In this prospective experimental laboratory study, fibrin tissue adhesives prepared by the use of plasma fibrinogen (group 1), cryoprecipitation (group 2), and precipitation by ammonium sulfate (group 3) were tested on 15 rabbits and 10 fragments of dura mater. The quality of the clots was assessed in terms of the success of the healing process, local toxicity, graft adhesion capacity, and degree of adhesion of 2 fragments of dura mater produced.
Results: All methods produced a clot with high adhesion and no toxicity, but tensile strength testing revealed that the glue produced from the ammonium sulfate-precipitated clot (group 3) was the strongest, requiring 39 g/cm2 to separate the fragments as opposed to 23 g/cm2 for group 2 and 13 g/cm2 for group 1.
Conclusion: All methods produced good results as far as clot formation and non-toxicity, but ammonium sulfate precipitation produced the best tensile strength and was thus the most effective method of preparing fibrin tissue adhesive.
Keywords: Fibrin, Fibrin Tissue Adhesive, Biological Therapy, Fibrinogen, Plasma
Introduction
The search for adjuvant techniques to surgery is incessant, and the evolution of such techniques is no less important than that of the surgical procedure itself. The main goal of current research is the development of materials capable of enhancing the healing process and regulating inflammation.
One of the materials known to perform these functions is fibrin glue. While the use of fibrin glue in surgery is well documented, its availability is limited by the high cost of commercial material1.
In this study, we demonstrate 3 methods of preparing fibrin glue from autologous plasma and compare the clot formation, toxicity, and resistance to traction of the resulting products.
Objective
The preparation of fibrin tissue adhesive in the laboratory, assessment of the properties and characteristics of biological glue prepared by 3 different methods, and comparison of these traits among the methods.
Material
The fibrinogen used to make the adhesive (component I of the glue) was prepared from blood obtained from 2 sources: blood bags from a blood bank (previously citrated to 3.8%) and randomly chosen patients (citrated to 10%). The additional materials used to manufacture component I were commercial sodium citrate (10%), a saturated solution of ammonium sulfate, and calcium chloride (40 mol/l). Component II of the glue, thrombin, was purchased commercially, as were aminocaproic acid and the distilled water used to resuspend the lyophilized powders of the products.
The effects of the products on healing were tested in New Zealand rabbits (3 groups of 5 rabbits; group 1: spin plasma, group 2: cryoprecipitation, and group 3: ammonium sulfate precipitation) and their strength and adhesiveness were investigated using fragments of human dura mater (5 tests with 2 fragments of dura mater.
Methods
Fibrinogen isolation
The glue was prepared by extracting component I (fibrinogen) by 3 different methods. Component II was always the same commercial thrombin diluted in distilled water and mixed with aminocaproic acid (as an antifibrinolytic).
Method 1: Plasma: 36 ml of pre-citrated blood from the blood bank was divided among 4 tubes and centrifuged at 3000 rpm for 3 minutes. The plasma was removed by pipetting and mixed with 1 ml of calcium chloride solution.
Method 2: Plasma Cryoprecipitate: 350 ml of blood in a citrated bag frozen at -18°C was thawed in the refrigerator at 4°C and subjected to cold centrifugation at 5000 rpm for 5 minutes. Calcium chloride solution (1 ml) was added, and the precipitate at the bottom of the bag was used as component I of the glue.
Method 3: Plasma chemoprecipitate: a total of 36 ml of non-citrated blood was collected into 4 tubes containing 1 ml each of sodium citrate solution (10%) and centrifuged at 3000 rpm for 10 minutes. The plasma obtained was mixed with 1.3 ml of a saturated solution of ammonium sulfate in 4 siliconized tubes, which immediately precipitated part of the fibrinogen, and centrifuged at 3000 rpm for 3 minutes. About 1.5 ml of white precipitate was collected by siphoning and mixed with 1 ml of calcium chloride to make component I of the glue.
Tests:
Clot formation: the time to form a highly adhesive clot and the duration of this clot were recorded.
The graft: rabbit skin grafts implanted using the various glues were evaluated for engraftment within 30 days, formation of granulation tissue, dislodgement/displacement of the graft, and scar formation.
Toxicity of the material: local and systemic manifestations of inflammation and/or allergic reaction were assessed.
Tensile strength: tensile test using dura mater fragments: 2 fragments of dura mater were attached with the fibrin glue, and the force required to detach them was measured using the amount of distilled water in the pipette as the weight (Figure 1).
Figure 1.
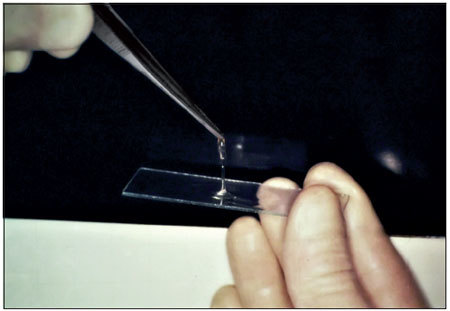
Fibrin glue.
Results
All methods produced a large, adhesive clot that formed within 2 seconds and persisted unchanged for several hours. When the glues were used for skin grafting, group 1 rabbits did not engraft, as the grafts could be moved by gravity alone, while all grafts in groups 2 and 3 were clinically successful after 30 days. The rabbits' sclerae healed well with no evidence of toxicity in all groups. Method 3 produced the best tensile strength results in the dura mater test, requiring 39 g/cm2 to separate the fragments vs. 23 g/cm2 for the product of method 2 and 13 g/cm2 for the product of method 1 (Figures 2 and 3).
Figure 2.
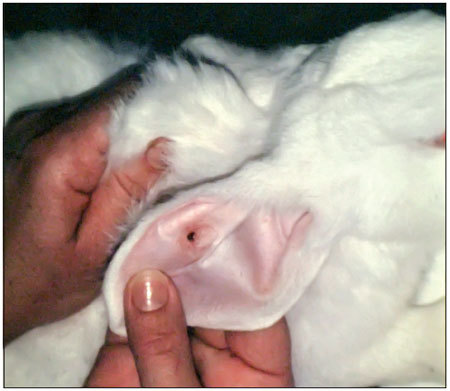
Handle with graft plasma.
Figure 3.
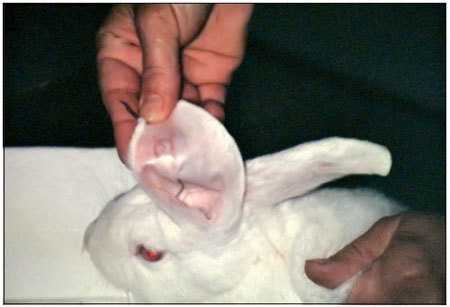
Handle with graft chemoprecipitate.
Discussion
Despite the development of surgical techniques for hemostasis, the search for effective hemostatic agents continues. One such agent is fibrin glue.
Fibrin sealants reproduce the final stages of the enzymatic coagulation cascade, in which fibrinogen is converted to fibrin in the presence of thrombin, factor XIII, fibronectin, and ionic calcium1 2.
Fibrin glue acts by inducing local hemostasis (modulation of the local inflammatory response) and/or by adhering apposed tissues (mechanical support)2.
The use of fibrinogen adhesive remains limited by the high cost.
In this study, we demonstrated 3 methods for obtaining fibrinogen from blood for the production of fibrin glue and compared the products of these methods to determine which was best for surgical use.
Compared with other adhesives, such as cyanoacrylate and gelatin-formaldehyde-resorcinol, fibrin glue has the advantages of not causing tissue rejection reactions or stimulating the growth of fibroblasts; it is also completely biodegradable.
Precipitation of plasma proteins concentrates not only fibrinogen but also other desirable and undesirable components, such as XIII and plasminogen. Plasmin, a derivative of plasminogen, is the major proteolytic enzyme that acts to dissolve the clot. Due to this tendency of plasmin to destroy the fibrin clot, an antifibrinolytic agent such as aminocaproic acid should be added to glue component II in order to retard clot lysis and thus stabilize the tissues to allow scar formation.
Another advantage of fibrin adhesive is its low predisposition to infection. The growth of staphylococci in blood clots is 10 times higher than in fibrin clots and 100 times higher than in fibrin clots containing factor XIII3.
Biological adhesives are used in several surgical procedures: closure of the dura, hemostasis, surgery of the middle and inner ears, septoplasty, facial nerve grafting, cerebrospinal fluid leak sealing CSF leaks, grafting of skin and mucous membranes, fracture stabilization, plastic surgery, and vascular, cardiac, thoracic, urologic, digestive, and dental microsurgery. The scope for its use is virtually unlimited, and the benefits are numerous4 5 6 7 8.
Fibrinogen can be precipitated by various methods. Precipitation by the addition of a saturated solution of ammonium sulfate produces greater amounts of fibrinogen in a non-selective manner and brings down some additional proteins that are important in the clotting process9.
In our study, autologous fibrin glues produced by 3 different methods formed clots identically well; however, skin grafting was equally successful when plasma cryoprecipitate or chemoprecipitate was used but unsuccessful when plasma precipitate was used.
The 3 methods were equally satisfactory with respect to local toxicity, as the rabbits' sclerae showed no signs of injury. Laboratory testing of the adhesion of dura mater fragments showed the worst results for glue produced from pure plasma, followed by that made from cryoprecipitate, while the best adhesion was obtained using chemoprecipitated plasma.
Although the adhesion of fibrin glue produced by chemoprecipitation was inferior to that of the commercial glue in the first 10 minutes, it has greater adhesiveness after 30 minutes (Siendentop, 1985) and would therefore be preferable in situations in which the fragments are not subjected to large shifts. Based on our results, fibrinogen prepared by chemoprecipitation from plasma is better for surgical use than that prepared by the other 2 methods.
Some surgeons now utilize the patient's own blood to prepare fibrin glue for the surgical site; this is both less expensive and more convenient for the patient than using commercial glue.
Furthermore, the patient's own blood poses none of the risks for contamination or infection associated with the use of blood from blood banks and also minimizes the risk for allergic reactions.
It is important to remember that the use of any biological or synthetic glue is no substitute for proper surgical technique.
Conclusion
Fibrin glues produced by standard centrifugation, cryoprecipitation, and chemoprecipitation performed equally well at clot formation, and no method produced signs of toxicity in rabbit sclerae. However, the adhesiveness and perioperative graft healing were poor for the glue produced by centrifugation alone. Plasma chemoprecipitation produced the best results for tensile strength testing of adhesion between dural fragments. We therefore conclude that chemoprecipitation is the most effective method for preparing fibrin tissue adhesive.
References
- 1.Dohan S Choukroun J Dohan A Donsimoni J M Gabrieleff D Fioretti F Dohan D, Platelet Rich Fibrin (PRF). Un nouveau biomatériau de cicatrisation. Biotechnologies et fibrine, plaquettes et cytokines, aspects immunitaires, implications thérapeutiques. 1re partie: biotechnologies et fibrine Implantodontie 20041387–97. [Google Scholar]
- 2.Reiss R F Oz M C Autologous Fibrin Glue: Production and Clinical Use Transfusion Medicine Reviews, Vol X, No 2 (April), 1996, 86-92. [DOI] [PubMed] [Google Scholar]
- 3.Almeida C IR, Nina L G, Mello R P. Cola biológica autógena. Rev Bras Otorrinolaringol. 1991;57:115–8. [Google Scholar]
- 4.Bento R F, Miniti. Comparison between fibrin tissue adhesive, epineural suture and natural union in intratemporal facial nerve of cats Acta Oto-Laryngologica 19894651–36. [DOI] [PubMed] [Google Scholar]
- 5.Jessen C, Sharma P. Use of fibrin glue in thoracic surgery. The Annals os Thoracic Surgery. 1985;39(6):521–4. doi: 10.1016/s0003-4975(10)61991-1. [DOI] [PubMed] [Google Scholar]
- 6.Tidrick R T, Seegers W H, Warner E D. Clinical experience with thrombin as na hemostatic agent. Surgery. 1943;14:191–6. [Google Scholar]
- 7.Bento R F, Salomone R, Tsuji R K, Housen M, Neto R B. O Uso de Cola de Fibrina Humana na Anastomose de Lesões Traumáticas Parciais do Nervo Facial. Arq. Int. Otorrinolaringol. 2008;12(2):214–219. [Google Scholar]
- 8.Sterkers O, Becherel P, Sterkers J M. Reparation du nerf facial par colle de fibrine exclusive 56 cas. Annals Otolaryngolo ic Chirurgical Cervicofacial. 1989;106:176–81. [PubMed] [Google Scholar]
- 9.Siedentop K H, Harris D M, Sanchez B. Autologous fibrin tissue. Laryngoscope. 1985;9:1074–76. [PubMed] [Google Scholar]


