Abstract
Gastric Antral Vascular Ectasia (GAVE) may be an enigmatic source of non-variceal upper GI bleeding associated with various systemic diseases such as connective tissue disorders, liver disease, and chronic renal failure. Successful treatment of GAVE continues to be a challenge and has evolved through the years. Currently, given the rapid response, safety, and efficacy, endoscopic ablative modalities have largely usurped medical treatments as first-line therapy, particularly using argon plasma coagulation. However, other newer ablative modalities such as radiofrequency ablation, cryotherapy, and band ligations are promising. This paper is an overview of GAVE and its various endoscopic and medical therapies.
What is GAVE?
Gastric Antral Vascular Ectasia, or GAVE, can be an obscure cause of upper GI bleeding and was described as early as the 1950 s by Rider et al 1. as ‘fiery red’ hypertrophic antral mucosa with scattered areas of bleeding and blood clots, named veno-capillary ectasia. Later studies detailed the endoscopic appearance of GAVE, coining the term “‘watermelon stomach” to describe the erythematous ectatic vessels in longitudinal stripes along the rugal folds of the antrum 2 or, rarely other parts of the gastric mucosa 3. There are 2 types of GAVE based on distinctive endoscopic appearances. The classic manifestation consists of this “watermelon” appearance of multiple flat, linear, erythematous strips of ectatic vessels radiating from the pylorus to the antrum. The second type is punctate, where the ectasia manifests as diffuse antral angiomas and tends to be more associated with liver cirrhosis 3.
Although GAVE has characteristic endoscopic appearances, it can often be mistaken for other upper GI vascular malformations or sources of GI bleeding. For this reason, diagnostic confirmation is reached through biopsy. Histologically, GAVE appears as dilated, ectatic capillaries in the mucosal and submucosal regions, accompanied by the presence of microthrombi. In addition, there is fibromuscular hyperplasia of the lamina propria in the setting of edema, congestion, and reactive changes of the foveolar epithelium. Generally, very few inflammatory changes are seen. To identify GAVE objectively, a scoring system was devised, which involves the histological factors of ectasia, fibrinolysis, and spindle cell proliferation to differentiate GAVE from portal hypertensive gastropathy with 80 % diagnostic accuracy 4 (Fig. 1).
Fig. 1.
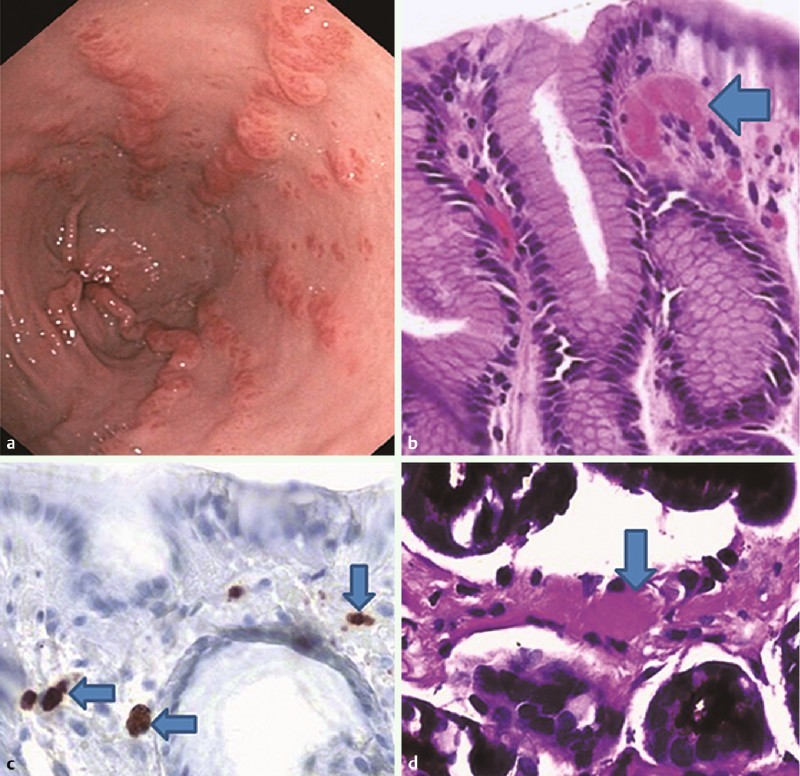
(a) endoscopic image, (b) H&E stained fibrin thrombus, (c) CD61 positive thrombi, (d) PAS positive thrombus. All arrows point to thrombi.
Most cases of GAVE are associated with liver cirrhosis, autoimmune disease, chronic renal failure, heart disease, diabetes, hypothyroidism, and bone marrow transplantation. GAVE accounts for ~ 4 % of non-variceal upper GI bleeding. Seventy-one percent of non-cirrhotic patients with GAVE are women in their early 70 s, and 75 % of cirrhotic patients with GAVE are men in their mid 60 s 2. Given its association with several common comorbidities, GAVE may become increasingly common in the aging population. The actual etiology of GAVE is unknown, however, multiple hypotheses have been proposed by small studies and case reports. Given the diversity of associated conditions, a singular etiology of GAVE is unlikely.
GAVE with cirrhosis
Much of the research involving the pathophysiology of GAVE has focused on its relationship with portal hypertension and cirrhosis. Although GAVE is found in many cirrhotic patients, no causal relationship has been established, and portal hypertension was not found to play an etiologic role in GAVE. Nevertheless, GAVE is often confused with portal hypertensive gastropathy (PHG) since both conditions tend to occur in cirrhotic patients. GAVE and PHG have similar endoscopic appearance, but may be distinguished based on location of the vascular ectasia. GAVE appears mostly in the antrum, whereas PHG is mainly manifest in the gastric fundus. Mucosal biopsies may help distinguish GAVE from PHG in cases that are atypical in presentation, such as in diffuse gastric ectasia. PHG histologically presents as mucosal and submucosal vascular dilatation without associated inflammatory changes. Fibrin thrombi, which are typical findings in GAVE, are generally absent in PHG 5. These fibrin thrombi can be highlighted with a simple PAS stain with diastase digestion. Immunohistochemical studies suggest that CD61, a platelet marker, is more readily seen in the fibrin thrombi associated with GAVE. This marker was able to diagnose GAVE more accurately, and was positive in 100 % of patients with a histological diagnosis of GAVE and in 60 % of patients with an endoscopic diagnosis of GAVE. Using CD61, researchers were also able to reclassify incorrectly diagnosed PHG to GAVE and confirm the diagnosis with re-examination of histology. To confirm these findings, researchers used CD31 to determine the mucosal microvessel density, which was found to be significantly higher in cases of GAVE versus PHG (p < 0.01) 6. Despite the similar gross and endoscopic appearance, these two conditions are not the same, and distinction between these two etiologies is important given their differing management strategies. Unlike PHG, GAVE does not respond to a reduction in portal hypertension with the transjugular intrahepatic portosystemic shunt (TIPS) procedure or β blockade 7. A study has also demonstrated the presence of elevated prostaglandin E2 levels, mainly in the corpus and antrum, in cirrhotic patients with GAVE lesions compared to cirrhotic patients without GAVE, as well as healthy controls. Elevated levels of E2 are thought to have vasodilatory effects, in addition to acid inhibitory effects 8.
GAVE without cirrhosis
In non-cirrhotic patients with GAVE, autoimmune disorders and, more specifically, connective tissue diseases are commonly seen. Systemic sclerosis (SSc) is associated with telangiectasias in multiple parts of the GI tract, particularly esophageal (3.9 % of SSc patients) or colonic (5.2 % of SSc patients), but can also present with severe hemorrhage and anemia secondary to GAVE. A recent retrospective multi-center study noted the presence of endoscopic changes consistent with GAVE in 25 % of its 103 subjects with early and severe, diffuse systemic sclerosis 9. The pathophysiology of GAVE in the SSc population is unknown, but there are two leading hypotheses: autoimmune reaction to gastric vessels, or pathophysiological consequence of gastric dysmotility.
In support of an autoimmune reaction to gastric vessels, anti-RNA polymerase III (RNAP III) antibodies, which are highly specific for SSc, have been noted as present in SSc patients with GAVE. A cross-reaction between specific proteins of the vascular tissue of the gastric mucosa and these antibodies has been speculated to result in GAVE. In case reports, RNAP III antibodies have been described in up to 25 % of SSc patients with GAVE 10. While smaller studies showed RNAP III as a positive predictor of GAVE in SSc, a more recent, larger multi-center study showed no association between the two diseases 9.
Further studies at the molecular level have been conducted to understand the pathophysiology of GAVE. Valdez et al. isolated an RNA helicase from an autoimmune antibody of a patient with GAVE. These RNA helicases are part of the DEXD box family, and have been implicated in pre-mRNA splicing, translation, ribosomal processing, cell growth and development 11. The group further identified unique epitopes in RNA helicase II/Gu protein specific to GAVE. The GAVE-specific serum was able to recognize epitopes near the carboxy-terminus of RHII/Gu, whereas antibodies from patients with other connective tissue disease recognized epitopes of the NH2-terminus. Two additional serum samples from patients with GAVE in this study did not recognize the RHII/Gu antigen 12. It is currently unclear whether there is a causal relationship of this unique epitope with the symptoms of GAVE, and further studies are needed to understand the pathophysiological role of such antibodies in the autoimmune subtype of GAVE.
Gastric dysmotility has also been touted as an etiology for GAVE. Prolapse or intussusception of the antral mucosa into the pylorus in a chronic, recurrent fashion can result in trauma, causing fibromuscular hyperplasia and vascular ectasia. The discoordinated gastric antral contraction may cause elongation and dilation of mucosal vessels, resulting in the ectatic vessels of GAVE 13. High levels of gastrin have also been noted in various GAVE cases, which may explain the angiodysplasia. In some studies, GAVE has also been associated with low pepsinogen, and achlorhydria, suggesting a hormonal connection, but conflicting results from other studies have made causal relationships unclear. Although GAVE is also found in many cirrhotic patients, no causal relationship has been established. Vasoactive substances, such as gastrin, 5HT-3, and VIP, are secreted by surrounding neuroendocrine cells and may result in malfunction of precapillary sphincters at high levels 14. This sphincter malfunction can, in turn, result in vasodilatation, ectasia, and a higher propensity for bleeding.
Several treatment modalities have been developed with the main focus on achieving hemostasis, since the underlying pathophysiology of GAVE remains largely unknown. Many patients present with severe gastric bleeding, requiring continuous transfusions. Thus, the first-line therapy is generally endoscopic ablation with medical therapies considered to be adjunctive.
Endoscopic/surgical treatments
APC
The currently embraced endoscopic treatment modality for GAVE is argon plasma coagulation (APC). APC is a thermoablative method, which causes thermocoagulation using a high frequency current that passes through argon gas. Similar to the YAG laser, APC is able to treat large areas of mucosa per treatment session. However, the perforation risk is lower since there is no direct contact with the mucosa and so it presumably avoids deeper mucosal injury 15. Many studies have demonstrated the efficacy of APC, however, most have been single center trials with a low number of subjects 16 17 18 19 20 21. One of the larger trials involving 50 cirrhotic patients with iron deficiency anemia or melena related to GAVE, found an increased mean hemoglobin of 1.35 ± 0.24 g/dl in ~8.5 months of follow-up after the last APC session. Patients were also noted to have undergone a mean of 5.06 ± 1.5 treatment sessions, likely related to the severity of their cirrhotic disease 22. Leclaire et al. corroborated these findings that cirrhotic patients require more sessions of APC to treat GAVE lesions adequately 23. An Arabic study showed similar efficacy in 29 patients with endoscopically proven GAVE, with decreased transfusion requirements and an increase in baseline hemoglobin levels. However, the follow-up time was shorter at 3 months post-APC 24. Most of the early studies on APC showed efficacy in short-term follow-up periods. As a result, additional studies were done to assess long-term efficacy 17 19 25 26 27 28. Nakamura et al. showed that the recurrence-free rate and survival rate after APC declined over time. Cumulative recurrence-free rates were 49.7 % after 1 year, 35.5 % after 2 years, and 35.5 % after 3 years with post-treatment survival rates of 94.4 %, 75.8 %, and 64.9 % at 1, 2, and 3 years, respectively 27. APC has some drawbacks, most notably, sepsis, antral stenosis, and gastric outlet obstruction as other post-procedure complications 29 30. Although deeper mucosal injury is deemed less likely, APC has been shown to result in inflammatory or hyperplastic polyps, which can be additional sources of bleeding 31 32. Financially, APC has a lower initial capital cost, however, the per treatment cost of APC probes is higher given the multiple treatment sessions needed per patient for treatment of GAVE-related hemorrhage 33.
Radiofrequency ablation
Radiofrequency Ablation (RFA) is a thermoablative modality, initially used in the treatment of Barrett esophagus. This technique uses high power energy (11 – 20 J) for short periods of time (less than 1 s) to ablate superficial mucosal lesions and allows for ablation of the muscularis mucosa, resulting in less damage to the submucosal layer 34. The BARRx Halo90 system (Covidien, Sunnyvale, CA) has been used for RFA, and is comprised of an ablative device and energy generator. In addition to its use in esophageal procedures, RFA has also been reported in the treatment of lower GI bleeding secondary to radiation proctitis 35 36. Zhou et al. demonstrated successful use of this modality in patients with lower GI bleeding from ectatic vessels secondary to chronic radiation proctitis, including those patients refractory to other treatment modalities 35. GAVE presents with a similar clinical complication to radiation proctitis in that it results in severe gastric hemorrhage requiring endoscopic ablative treatment. The favorable outcomes and decreased number of complications reported in studies involving the use of RFA in radiation proctitis make RFA an attractive treatment modality for GAVE. In one study, 6 patients with chronic transfusion-dependent bleeding from GAVE, 4 of whom had failed prior treatment with APC, underwent RFA ranging from 1 to 3 ablative treatment sessions. The mean hemoglobin (Hgb) improved from 8.5 to 10.2 and 5 of the 6 subjects were no longer transfusion-dependent. No complications were noted in the study 37. A more recent study on 21 patients with GAVE refractory to APC showed similar efficacy and lack of complications 6 months post-treatment with RFA 38. In addition, a retrospective international study of 8 European centers and 1 U.S. center investigated the use of RFA in patients with GAVE who were mostly refractory to APC (17/18 subjects) and recently presented promising results. Results were significant for treatment of 97 % of lesions without adverse effects, as well as decreased blood transfusion requirements 6 months post-procedure compared with transfusion requirements 6 months prior to RFA 39. It is unclear, however, whether such superficial ablations sufficiently ablate the deeper submucosal vascular network of GAVE, and larger studies with a longer follow-up period are needed. Regardless, the results of these recent studies are promising for the future of RFA in the treatment of GAVE.
Band ligation
Endoscopic Band Ligation (EBL) has been used as standard treatment in other GI vascular disorders, such as esophageal varices, hemorrhoids, and Dieulafoy lesions, and has been shown to be safer than its surgical/thermoablative counterparts. The submucosal obliteration of the vascular network is thought to be safer using EBL than other treatment modalities. Given this background, EBL became a viable option for GAVE as well. A study by Wells et al. demonstrated the efficacy of EBL versus thermoablative modalities, specifically APC. Patients who were treated using EBL showed a higher rate of bleeding cessation (67 % versus 23 %) in fewer treatment sessions (1.9 versus 4.7). In addition, the EBL group needed fewer blood transfusions and demonstrated a higher level of baseline hemoglobin after treatment 40. However, this study reported a higher number of treatment sessions and lower efficacy rate of APC compared with other published studies on APC and may reflect differences in cohort and technique from similar published data. Regardless, many studies and case reports continue to show decreased bleeding recurrence, fewer hospital admissions, and fewer blood transfusions after EBL, as well as better cost-efficacy, making it a more attractive option for health facilities with limited financial resources 41.
YAG laser coagulation
Earlier endoscopic treatment of GAVE spanning the 1990s – 2000s focused on laser technology, particularly with Nd:YAG lasers which result in thermal destruction of tissue by absorption of laser light. Nd:YAG lasers exhibit deeper mucosal injury to 4 – 6 mm depth compared with other laser modalities, allowing coagulation of superficial and submucosal vessels without direct contact 42. In a 2003 long-term retrospective study, 24 patients with GAVE were treated with Nd:YAG laser over an 18-year period with a median of 2 treatments and follow-up median of 55 months (range 9 – 127) after the last procedure. Twenty patients in this cohort experienced resolution of bleeding, and remained transfusion-free for a median of 16 months 43. Deeper treatment also increases the risk of perforation, which occurred in 1 /24 subjects in this study. Further disadvantages include the higher cost associated with the technology, making other treatment modalities with similar efficacy more attractive.
Cryotherapy
Cryotherapy applies extremely cold temperatures to the area of interest to cause thermal destruction or necrosis of the tissue. Initial studies by Kantsevoy et al. demonstrated success of cryotherapy in patients with refractory GI bleeding secondary to GAVE. Of the 7 patients with GAVE treated, 5 (77 %) had cessation of bleeding with normal mucosal findings at 6 months post-treatment 44. Another study focused on patients with GAVE and iron deficiency anemia, who required a mean number of 4.6 units of blood transfusion 3 months prior to treatment. Of 12 enrolled patients, 6 showed a complete response 4 weeks after completion of 3 treatment sessions as defined by improvement in endoscopic appearance, increase in Hgb level, and no requirement for blood transfusions. Five patients showed a partial response, i. e., incomplete ablation with stable Hgb and a reduced number of transfusions 45. A potential advantage of cryotherapy over APC is the large mucosal areas that can be treated in a 5-min treatment session.
Heater probe/sclerotherapy/mucosal resection
Lesser-studied endoscopic modalities include heater probe, sclerotherapy, and mucosal resection. When using a heater probe, the end of the probe is moved along each area of vascular ectasia with continuous coagulation until the bleeding ceases and mucosa blanches. In the late 1980s, one study showed the efficacy of heater probe therapy with 8/10 transfusion-dependent patients no longer requiring transfusions after treatments 46. A more recent case report also demonstrated, albeit anecdotally, that both heater probe and hot biopsy forceps did not result in the same side effects as APC, namely bleeding, antral scarring, hyperplastic polyps, gastric outlet obstruction, and pneumoperitoneum. The use of hot forceps biopsy was also touted to be more efficient, given its dual ability to biopsy and provide hemostasis when needed during each 20-min session 47. While these studies have provided some encouraging evidence with regard to the endoscopic heater probe, there is insufficient evidence to suggest that this modality has advantages over other endoscopic modalities and requires more study. Similarly, snare coagulation, which involves sweeping a snare over the mucosal surface, has been effective in a case report, but needs further assessment 48. Endoscopic mucosal resection (EMR) has been used mostly for resection of superficial dysplastic lesions of the gastrointestinal tract such as Barrett’s esophagus and adenoma. It has also been used as a novel endoscopic modality for the treatment of GAVE in some case reports, with the thought being that some patients tend to hemorrhage with endoscopic modalities such as sclerotherapy and photocoagulation. This tends to occur more readily when larger vessels are present in the area of treatment 49. While case reports, such as that of Okamoto et al., demonstrate resolution of symptoms after EMR 49, much more investigation is required before it can become a more routine treatment modality.
Surgical antrectomy
Surgical intervention with modalities such as antrectomy have been shown in small studies and case reports to be the definitive treatment for GAVE. Antrectomy is often considered for patients whose disease consists of more extensive vascular malformations, which are refractory to medical or endoscopic therapies 50 51. The morbidity and mortality of the procedure outweigh the benefits, and laparoscopic antrectomy has attempted to decrease the risk involved with a less invasive approach 50. Unfortunately, the morbidity and mortality associated with abdominal surgical procedures are even higher in patients with severe liver disease, such as cirrhosis, owing to increased bleeding risk from abdominal collateral vessels in the setting of severe portal hypertension 51 (Table 1).
Table 1. Endoscopic treatments.
| References | Treatment modality | n | Power settings | Mean # sessions | Response rate | Mean Hgb increase | Follow-up duration (months) | Complications |
| Mathou et al. 43 | Nd:YAG laser | 24 | 20 – 30 W | Median 2 | 20 /24 (83 %) | N/A | Range 9 – 127 | Gastric perforation – 1; Pyloric stenosis – 2 |
| Petrini and Johnston 46 | Heater probe | 12 | 4 | 10 /12 (83 %) | 20 | N/A | ||
| Komiyama et al. 47 | Heater probe | 1 | 80 W | 2 | 1 /1 (case report) | N/A | None | |
| Sebastian et al. 21 | Argon plasma coagulation | 12 | 1.5 L/min, 40 W | Median 2 (1 – 5) | 12 /12 showed improvement; 2 recurrences at 4 and 9 months | + 4.07 | Range 6 – 30 | None |
| Naga et al. 24 | Argon plasma coagulation | 29 | 2.8 – 4 L/min, 60 – 80 W | (1 – 3) | 22 /25 CR | + 2.7 | 3 | N/A |
| Roman et al. 29 | Argon plasma coagulation | 21 | 0.8 L/min; 50 – 80 W | 2.81 (1 – 5) | 6 /21; 2 recurrences, 11 unrelated deaths, 2 lost to follow-up. | + 2.23 | Mean 14.9 (1 – 60.6) | Hematemesis – 2; septicemia – 1 |
| Fuccio et al. 31 | Argon plasma coagulation | 20 | Median 3 (1 – 10) | 14 /20 CR; 6 recurrences | Mean 28 | Hyperplastic polyps – 3 | ||
| Wells et al. 40 | Endoscopic band ligation | 9 | N/A | 1.9 | 5 /9 (56 %) | + 2.8 | 10.1 months | Post-procedure nausea/vomiting – 1 |
| Sato et al. 41 | Endoscopic band ligation | 12 | N/A | 3 (range 2 – 4) | 11 /12 (91.7 %) | 14.6 months | None | |
| Gross et al. 37 | Radiofrequency ablation | 6 | N/A | 1.7 | 5 /6 (83 %) | 1.6 | 2 months | None |
| McGorisk et al. 38 | Radiofrequency ablation | 21 | N/A | 4 | 18 /21 (86 %) | 2.4 | 6 months | None |
Medical therapies
Many medical therapies have been proposed over the years as a non-invasive alternative for the treatment of GAVE-related hemorrhage. Therapies, such as cyclophosphamide, estrogen, progesterone, corticosteroids, tranexamic acid, octreotide, cyproheptadine, and thalidomide, have shown positive results in case reports and small clinical trials, but have not shown sufficient efficacy to function as alternatives to endoscopic modalities. In addition, certain agents, such as estrogen/progesterone, corticosteroids, tranexamic acid, and cyproheptadine may result in unnecessary side effects. As a result, these medical therapies are still considered experimental and are generally not used as standards of care for GAVE-induced hemorrhage 52 (Table 2).
Table 2. Medical therapy.
| Reference | Type of therapy | n | Response rate | Duration of follow-up, months | Complications |
| Shulz et al., 2009 53 | IV Cyclophosphamide | 3 | 3/3 (100 %) CR | 8 – 36 | N/A |
| Soykan et al., 2003 54 | Cyproheptadine | 1 | 1/1 (100 %) | 8 | Delirium in elderly |
| Ge et al., 2011 55 | Thalidomide | 78 (3 GAVE patients) | 20/28 (71.4 %); GAVE patients were not distinguished | 8 – 52 | Leukopenia – 1, somnolence – 1, peripheral edema – 4, bitter taste – 2, thrombopenia – 1, bradycardia – 1, headache – 1, tremor – 1, rash – 1, tinnitus – 1, blurred vision – 1, herpes zoster – 1, pruritis – 1 |
| Nardone et al., 2001 56 | Octreotide | 17; 3 GAVE patients | 1/3 (33 %) CR; 2/3 (66 %) PR | 36 – 48 | None |
| Barbara et al., 1998 57 | Octreotide | 1 | 0/1 (0 %) | 24 | Continuous melena |
| Calam et al., 1980 58 | Prednisolone | 1 | 1/1 (100 %) | 6 | None |
| Jabbari et al. 2 | Prednisolone | 1 | 1/1 (100 %); PR | N/A | |
| Tran et al., 1999 59 | Estrogen/progesterone | 6 | 1/6 (16.7 %) no response | 3 – 12 | Gynecomastia – 2, metrorrhagia – 1 |
| Moss et al., 1992 60 | Estrogen/progesterone | 1 | 1/1 (100 %) | 12 | Cyclical uterine bleeding |
Conclusion
GAVE is uncommon, but encountered by most endoscopists as a cause of severe upper gastrointestinal bleeding. There is no consensus for the optimal therapeutic approach. Data reviewed here favor the use of endoscopic ablation over medical treatments, given their more rapid effect and reported success. However, larger and controlled trials are lacking comparing endoscopic to medical therapies alone or to addition of medical therapies as adjunct. Moreover, there is no consensus with regard to the optimal endoscopic modality for the treatment of GAVE, largely because of the very small number of head-to-head comparative studies among these modalities to date. While APC is generally thought to be the gold standard, based on the relatively large amount of data available, new treatment modalities, such as RFA, also show promising results because of the broader treatment field and more controlled depth of ablation. The use of RFA may be beneficial for more diffuse cases of GAVE, similar to experiences in the treatment of chronic radiation proctocolitis. RFA may be more suited for ablation given its broader area of treatment coverage and more controlled uniform superficial ablation, which may conceivably avoid ulcerations and strictures. However, the concern remains whether such superficial treatments are adequate in impacting on the deeper submucosal vascular network of GAVE, as suggested for band ligations. Clearly, larger controlled trials are needed to assess and compare these treatment modalities, with longer follow-up periods to evaluate durability and safety.
Footnotes
Competing interests: None
References
- 1.Rider J A, Klotz A P, Kirsner J B. Gastritis with veno-capillary ectasia as a source of massive gastric hemorrhage. Gastroenterology. 1953;24:118–123. [PubMed] [Google Scholar]
- 2.Jabbari M, Cherry R, Lough J O. et al. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology. 1984;87:1165–1170. [PubMed] [Google Scholar]
- 3.Stotzer P O, Willén R, Kilander A F. Watermelon stomach: not only an antral disease. Gastrointest Endosc. 2002;55:897–900. doi: 10.1067/mge.2002.124558. [DOI] [PubMed] [Google Scholar]
- 4.Gilliam J H III, Geisinger K R, Wu W C. et al. Endoscopic biopsy is diagnostic in gastric antral vascular ectasia. The “watermelon stomach”. Dig Dis Sci. 1989;34:885–888. doi: 10.1007/BF01540274. [DOI] [PubMed] [Google Scholar]
- 5.Payen J L, Calès P, Voigt J J. et al. Severe portal hypertensive gastropathy and antral vascular ectasia are distinct entities in patients with cirrhosis. Gastroenterology. 1995;108:138–144. doi: 10.1016/0016-5085(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 6.Westerhoff M, Tretiakova M, Hovan L. et al. CD61, CD31, and CD34 improve diagnostic accuracy in gastric antral vascular ectasia and portal hypertensive gastropathy: An immunohistochemical and digital morphometric study. Am J Surg Pathol. 2010;34:494–501. doi: 10.1097/PAS.0b013e3181d38f0a. [DOI] [PubMed] [Google Scholar]
- 7.Burak K W, Lee S S, Beck P L. Portal hypertensive gastropathy and gastric antral vascular ectasia (GAVE) syndrome. Gut. 2001;49:866–872. doi: 10.1136/gut.49.6.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saperas E, Perez Ayuso R M, Poca E. et al. Increased gastric PGE2 biosynthesis in cirrhotic patients with gastric vascular ectasia. Am J Gastroenterol. 1990;85:138–144. [PubMed] [Google Scholar]
- 9.Hung E W, Mayes M D, Sharif R. et al. Gastric antral vascular ectasia and its clinical correlates in patients with early diffuse systemic sclerosis in the SCOT trial. J Rheumatol. 2013;40:455–460. doi: 10.3899/jrheum.121087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceribelli A, Cavazzana I, Airò P. et al. Anti-RNA polymerase III antibodies as a risk marker for early gastric antral vascular ectasia (GAVE) in systemic sclerosis. J Rheumatol. 2010;37:1544. doi: 10.3899/jrheum.100124. [DOI] [PubMed] [Google Scholar]
- 11.Valdez B C, Henning D, Busch R K. et al. A nucleolar RNA helicase recognized by autoimmune antibodies from a patient with watermelon stomach disease. Nucleic Acids Res. 1996;24:1220–1224. doi: 10.1093/nar/24.7.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia M C, Zhou J, Henning D. et al. Unique epitopes in RNA helicase II/Gu protein recognized by serum from a watermelon stomach patient. Mol Immunol. 2000;37:351–359. doi: 10.1016/s0161-5890(00)00062-6. [DOI] [PubMed] [Google Scholar]
- 13.Sallam H, McNearney T A, Chen J D. Systematic review: pathophysiology and management of gastrointestinal dysmotility in systemic sclerosis (scleroderma) Aliment Pharmacol Ther. 2006;23:691–712. doi: 10.1111/j.1365-2036.2006.02804.x. [DOI] [PubMed] [Google Scholar]
- 14.Lowes J R, Rode J. Neuroendocrine cell proliferations in gastric antral vascular ectasia. Gastroenterology. 1989;97:207–212. doi: 10.1016/0016-5085(89)91437-6. [DOI] [PubMed] [Google Scholar]
- 15.Ripoll C, Garcia-Tsao G. The management of portal hypertensive gastropathy and gastric antral vascular ectasia. Dig Liver Dis. 2011;43:345–351. doi: 10.1016/j.dld.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Yew B S, Ng K Y, Ang D S. et al. Gastric antral vascular ectasia successfully controlled by argon plasma coagulation. Ann Acad Med Singapore. 2007;36:702–703. [PubMed] [Google Scholar]
- 17.Yusoff I, Brennan F, Ormonde D. et al. Argon plasma coagulation for treatment of watermelon stomach. Endoscopy. 2002;34:407–410. doi: 10.1055/s-2002-25287. [DOI] [PubMed] [Google Scholar]
- 18.Herrera S, Bordas J M, Llach J. et al. The beneficial effects of argon plasma coagulation in the management of different types of gastric vascular ectasia lesions in patients admitted for GI hemorrhage. Gastrointest Endosc. 2008;68:440–446. doi: 10.1016/j.gie.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Kwan V, Bourke M J, Williams S J. et al. Argon plasma coagulation in the management of symptomatic gastrointestinal vascular lesions: experience in 100 consecutive patients with long-term follow-up. Am J Gastroenterol. 2006;101:58–63. doi: 10.1111/j.1572-0241.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 20.Chiu Y C, Lu L S, Wu K L. et al. Comparison of argon plasma coagulation in management of upper gastrointestinal angiodysplasia and gastric antral vascular ectasia hemorrhage. BMC Gastroenterol. 2012;12:67. doi: 10.1186/1471-230X-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastian S, McLoughlin R, Qasim A. et al. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia (watermelon stomach): long-term results. Dig Liver Dis. 2004;36:212–217. doi: 10.1016/j.dld.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Bhatti M A, Khan A A, Alam A. et al. Efficacy of argon plasma coagulation in gastric vascular ectasia in patients with liver cirrhosis. J Coll Physicians Surg Pak. 2009;19:219–222. [PubMed] [Google Scholar]
- 23.Lecleire S, Ben-Soussan E, Antonietti M. et al. Bleeding gastric vascular ectasia treated by argon plasma coagulation: a comparison between patients with and without cirrhosis. Gastrointest Endosc. 2008;67:219–225. doi: 10.1016/j.gie.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Naga M, Esmat S, Naguib M. et al. Long-term effect of argon plasma coagulation (APC) in the treatment of gastric antral vascular ectasia (GAVE) Arab J Gastroenterol. 2011;12:40–43. doi: 10.1016/j.ajg.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Probst A, Scheubel R, Wienbeck M. Treatment of watermelon stomach (GAVE syndrome) by means of endoscopic argon plasma coagulation (APC): long-term outcome. Z Gastroenterol. 2001;39:447–452. doi: 10.1055/s-2001-15722. [DOI] [PubMed] [Google Scholar]
- 26.Chaves D M, Sakai P, Oliveira C V. et al. Watermelon stomach: clinical aspects and treatment with argon plasma coagulation. Arq Gastroenterol. 2006;43:191–195. doi: 10.1590/s0004-28032006000300007. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura S, Mitsunaga A, Konishi H. et al. Long-term follow up of gastric antral vascular ectasia treated by argon plasma coagulation. Dig Endosc. 2006;18:128–133. [Google Scholar]
- 28.Shibukawa G, Irisawa A, Sakamoto N. et al. Gastric antral vascular ectasia (GAVE) associated with systemic sclerosis: relapse after endoscopic treatment by argon plasma coagulation. Intern Med. 2007;46:279–283. doi: 10.2169/internalmedicine.46.6203. [DOI] [PubMed] [Google Scholar]
- 29.Roman S, Saurin J C, Dumortier J. et al. Tolerance and efficacy of argon plasma coagulation for controlling bleeding in patients with typical and atypical manifestations of watermelon stomach. Endoscopy. 2003;35:1024–1028. doi: 10.1055/s-2003-44594. [DOI] [PubMed] [Google Scholar]
- 30.Farooq F T, Wong R C, Yang P. et al. Gastric outlet obstruction as a complication of argon plasma coagulation for watermelon stomach. Gastrointest Endosc. 2007;65:1090–1092. doi: 10.1016/j.gie.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Fuccio L, Zagari R M, Serrani M. et al. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia-related bleeding in patients with liver cirrhosis. Digestion. 2009;79:143–150. doi: 10.1159/000210087. [DOI] [PubMed] [Google Scholar]
- 32.Baudet J S, Salata H, Soler M. et al. Hyperplastic gastric polyps after argon plasma coagulation treatment of gastric antral vascular ectasia (GAVE) Endoscopy. 2007;39:E320. doi: 10.1055/s-2007-966802. [DOI] [PubMed] [Google Scholar]
- 33.Rosenfeld G, Enns R. Argon photocoagulation in the treatment of gastric antral vascular ectasia and radiation proctitis. Can J Gastroenterol. 2009;23:801–804. doi: 10.1155/2009/374138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muguruma N, Okamoto K, Kimura T. et al. Endoscopic ablation therapy for gastrointestinal superficial neoplasia. Dig Endosc. 2012;24:139–149. doi: 10.1111/j.1443-1661.2011.01227.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhou C, Adler D C, Becker L. et al. Effective treatment of chronic radiation proctitis using radiofrequency ablation. Therap Adv Gastroenterol. 2009;2:149–156. doi: 10.1177/1756283X08103341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rustagi T, Mashimo H. Endoscopic management of chronic radiation proctitis. World J Gastroenterol. 2011;17:4554–4562. doi: 10.3748/wjg.v17.i41.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross S A, Al-Haddad M, Gill K R. et al. Endoscopic mucosal ablation for the treatment of gastric antral vascular ectasia with the HALO90 system: a pilot study. Gastrointest Endosc. 2008;67:324–327. doi: 10.1016/j.gie.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 38.McGorisk T, Krishnan K, Keefer L. et al. Radiofrequency ablation for refractory gastric antral vascular ectasia (with video) Gastrointest Endosc. 2013;78:584–588. doi: 10.1016/j.gie.2013.04.173. [DOI] [PubMed] [Google Scholar]
- 39.Dray X, Repici A, Gonzalez P. et al. 1040 Radiofrequency Ablation Treatment of Gastric Antral Vascular Ectasia: Results From an International Collaborative Study. Gastrointest Endosc. 2013;77:AB180. [Google Scholar]
- 40.Wells C D, Harrison M E, Gurudu S R. et al. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc. 2008;68:231–236. doi: 10.1016/j.gie.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Sato T, Yamazaki K, Akaike J. Endoscopic band ligation versus argon plasma coagulation for gastric antral vascular ectasia associated with liver diseases. Dig Endosc. 2012;24:237–242. doi: 10.1111/j.1443-1661.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez S A, Douglas G. American Society for Gastrointestinal Endoscopy Technology Committee . Mucosal ablation devices. Gastrointest Endosc. 2008;68:1031–1042. doi: 10.1016/j.gie.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Mathou N G, Lovat L B, Thorpe S M. et al. Nd:YAG laser induces long-term remission in transfusion-dependent patients with watermelon stomach. Lasers Med Sci. 2004;18:213–218. doi: 10.1007/s10103-003-0284-4. [DOI] [PubMed] [Google Scholar]
- 44.Kantsevoy S V, Cruz-Correa M R, Vaughn C A. et al. Endoscopic cryotherapy for the treatment of bleeding mucosal vascular lesions of the GI tract: a pilot study. Gastrointest Endosc. 2003;57:403–406. doi: 10.1067/mge.2003.115. [DOI] [PubMed] [Google Scholar]
- 45.Cho S, Zanati S, Yong E. et al. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest Endosc. 2008;68:895–902. doi: 10.1016/j.gie.2008.03.1109. [DOI] [PubMed] [Google Scholar]
- 46.Petrini J L Jr, Johnston J H. Heat probe treatment for antral vascular ectasia. Gastrointest Endosc. 1989;35:324–328. doi: 10.1016/s0016-5107(89)72802-9. [DOI] [PubMed] [Google Scholar]
- 47.Komiyama M, Fu K, Morimoto T. et al. A novel endoscopic ablation of gastric antral vascular ectasia. World J Gastrointest Endosc. 2010;2:298–300. doi: 10.4253/wjge.v2.i8.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chong V H. Snare coagulation for gastric antral vascular ectasia ablation. Gastrointest Endosc. 2009;69:1195. doi: 10.1016/j.gie.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 49.Okamoto T, Okayama Y, Hirai M. et al. Gastric vascular ectasia treated by endoscopic mucosal resection. Dig Endosc. 2002;14:9–11. [Google Scholar]
- 50.Sherman V, Klassen D R, Feldman L S. et al. Laparoscopic antrectomy: a novel approach to treating watermelon stomach. J Am Coll Surg. 2003;197:864–867. doi: 10.1016/S1072-7515(03)00600-8. [DOI] [PubMed] [Google Scholar]
- 51.Spahr L, Villeneuve J P, Dufresne M P. et al. Gastric antral vascular ectasia in cirrhotic patients: absence of relation with portal hypertension. Gut. 1999;44:739–742. doi: 10.1136/gut.44.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuccio L, Mussetto A, Laterza L. et al. Diagnosis and management of gastric antral vascular ectasia. World J Gastrointest Endosc. 2013;51:6–13. doi: 10.4253/wjge.v5.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schulz S W, O’Brien M, Maqsood M. et al. Improvement of severe systemic sclerosis-associated gastric antral vascular ectasia following immunosuppressive treatment with intravenous cyclophosphamide. J Rheumatol. 2009;36:1653–1656. doi: 10.3899/jrheum.081247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soykan I, Toruner M, Idilman R. et al. Reversal of iron deficiency anemia in a patient with gastric antral vascular ectasia treated with cyproheptadine. J Clin Gastroenterol. 2003;36:183–184. doi: 10.1097/00004836-200302000-00021. [DOI] [PubMed] [Google Scholar]
- 55.Ge Z Z Chen H M Gao Y J et al. Efficacy of thalidomide for refractory gastrointestinal bleeding from vascular malformation Gastroenterology 20111411629–1637.e1–e4 [DOI] [PubMed] [Google Scholar]
- 56.Nardone G, Rocco A, Balzano T. et al. The efficacy of octreotide therapy in chronic bleeding due to vascular abnormalities of the gastrointestinal tract. Aliment Pharmacol Ther. 1999;13:1429–1436. doi: 10.1046/j.1365-2036.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- 58.Barbara G, De Giorgio R, Salvioli B. et al. Unsuccessful octreotide treatment of the watermelon stomach. J Clin Gastroenterol. 1998;26:345–346. doi: 10.1097/00004836-199806000-00029. [DOI] [PubMed] [Google Scholar]
- 57.Calam J, Walker R J. Antral vascular lesion, achlorhydria, and chronic gastrointestinal blood loss: response to steroids. Dig Dis Sci. 1980;25:236–239. doi: 10.1007/BF01308145. [DOI] [PubMed] [Google Scholar]
- 59.Tran A, Villeneuve J P, Bilodeau M. et al. Treatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with estrogen-progesterone in cirrhotic patients: an open pilot study. Am J Gastroenterol. 1999;94:2909–2911. doi: 10.1111/j.1572-0241.1999.01436.x. [DOI] [PubMed] [Google Scholar]
- 60.Moss S F, Ghosh P, Thomas D M. et al. Gastric antral vascular ectasia: maintenance treatment with oestrogen-progesterone. Gut. 1992;33:715–717. doi: 10.1136/gut.33.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]


