Abstract
Wing-pattern mimicry in butterflies has provided an important example of adaptation since Charles Darwin and Alfred Russell Wallace proposed evolution by natural selection >150 years ago. The neotropical butterfly genus Heliconius played a central role in the development of mimicry theory and has since been studied extensively in the context of ecology and population biology, behavior, and mimicry genetics. Heliconius species are notable for their diverse color patterns, and previous crossing experiments revealed that much of this variation is controlled by a small number of large-effect, Mendelian switch loci. Recent comparative analyses have shown that the same switch loci control wing-pattern diversity throughout the genus, and a number of these have now been positionally cloned. Using a combination of comparative genetic mapping, association tests, and gene expression analyses, variation in red wing patterning throughout Heliconius has been traced back to the action of the transcription factor optix. Similarly, the signaling ligand WntA has been shown to control variation in melanin patterning across Heliconius and other butterflies. Our understanding of the molecular basis of Heliconius mimicry is now providing important insights into a variety of additional evolutionary phenomena, including the origin of supergenes, the interplay between constraint and evolvability, the genetic basis of convergence, the potential for introgression to facilitate adaptation, the mechanisms of hybrid speciation in animals, and the process of ecological speciation.
Keywords: Heliconius, adaptation, mimicry, speciation
Background
...the study of butterflies—creatures selected as the types of airiness and frivolity—instead of being despised, will someday be valued as one of the most important branches of Biological science” (Bates 1864, p. 413)
Henry Walter Bates discovered mimicry—the evolutionary phenomenon in which natural selection by predators causes unrelated species to appear similar—by collecting and studying butterflies in South America (Bates 1862). It is hard to imagine that anyone in Bates’ time actually “despised” the study of butterflies, and it is clear that this field has not yet risen to the esteemed position that Bates predicted. Yet butterflies, and their mimetic wing patterns in particular, have recently provided a wealth of insight into the genetic basis of adaptation (Joron et al. 2011; Reed et al. 2011; Heliconius Genome Consortium 2012; Martin et al. 2012; Kunte et al. 2014; Timmermans et al. 2014). Animal pigmentation, more generally, has been a staple of research in genetics and evolutionary biology for over a century (Cott 1940; Bennett and Lamoreux 2003; True 2003; Hoekstra 2006; Kronforst et al. 2012). External appearance is so intimately connected to both survival and reproduction that it is frequently the target of intense natural and sexual selection, and the combination of these two evolutionary forces routinely generates great diversity in pigment patterns, both within and between species (True 2003; Caro 2005; Hoekstra 2006; Joron et al. 2006a; Hubbard et al. 2010; Manceau et al. 2010; Kronforst et al. 2012). Perhaps no group of animals is more diverse in terms of pigmentation and patterning than butterflies (Nijhout 1986, 1991; McMillan et al. 2002). Butterflies, including skippers (family Hesperiidae) and butterfly moths (family Hedylidae), are a monophyletic clade within the insect order Lepidoptera (Heikkila et al. 2012). The clade consists of an estimated 18,000 species, and many of these can be identified on the basis of wing pattern alone (Nijhout 1991). Color pattern in butterflies is a morphologically simple phenotype, largely determined by the pigments deposited in tiny scales that line the wing surface like microscopic roofing tiles. In addition, colors like blue, some whites and greens, and features like iridescence are determined by scale structure, which shapes the way light reflects off the wing surface (Ghiradella 1991).
While wing pattern itself is structurally simple, the dizzying array of distinct patterns depicted on these miniature mosaics is exceptionally complex. The reasons for such extreme diversity are manifold because wing pattern plays a role in a variety of biological processes from thermoregulation to predator avoidance and mate attraction (Beldade and Brakefield 2002; McMillan et al. 2002). Evolutionary phenomena that have played particularly important roles in shaping butterfly wing patterns are aposematism and mimicry (Joron and Mallet 1998; Mallet and Joron 1999). Aposematism, or warning coloration, is widespread among butterflies as many species feed on chemically defended host plants and then sequester these compounds themselves to defend against predation. Like other toxic or defended organisms, chemically defended butterflies have subsequently evolved bold warning patterns that likely serve to enhance predator learning and/or allow predators to better distinguish toxic species from co-occurring palatable species. The existence of chemical defense and warning coloration has secondarily generated a permissive environment for the evolution of mimicry. Defensive mimicry is broadly divided into two categories: classic Batesian mimicry, in which an undefended mimic evolves to look like a toxic model (Bates 1862), and Müllerian mimicry, in which mutually defended species converge on a shared warning pattern as a means of enhancing predator learning (Müller 1879).
Wing-pattern mimicry in butterflies, which encompasses hundreds of examples of both Batesian and Müllerian mimicry, has served as an important model of adaptation since the earliest days of modern evolutionary theory. Darwin (1863) himself was amazed by Bates’ discovery of butterfly mimicry, writing: “It is hardly an exaggeration to say, that whilst reading and reflecting on the various facts given in this Memoir, we feel to be as near witnesses, as we can ever hope to be, of the creation of a new species on this earth,” p. 223. More recently, R. A. Fisher (1930) dedicated an entire chapter to mimicry in his classic book, The Genetical Theory of Natural Selection, calling mimicry theory “the greatest post-Darwinian application of natural selection,” p. 146. Since then, butterfly wing patterning, and mimicry in particular, has been explored theoretically and empirically, in the context of Mendelian and population genetics, evolutionary genomics, and evo-devo (Nijhout 1986, 1991; Mallet and Joron 1999; Beldade and Brakefield 2002; McMillan et al. 2002; Papanicolaou et al. 2005; Joron et al. 2006a; Parchem et al. 2007; Beldade et al. 2008; Papa et al. 2008a; Kronforst et al. 2012; Supple et al. 2014).
Butterflies in the genus Heliconius are conspicuous members of neotropical butterfly communities and some of the more influential players in original mimicry theories. From those early days, Heliconius has transformed into something of an emerging model system for the study of ecology, evolution, and behavior, especially in the context of mimicry, leading Turner (1977a) to assert that they were the “best studied terrestrial invertebrates of no economic importance outside the Drosophilidae.” While that is no longer true (e.g., Caenorhabditis elegans), there has been a recent coordinated effort among an international team of researchers to characterize the genes underlying wing-pattern mimicry in Heliconius and then to use this information to address fundamental questions in evolutionary biology. Given the rapid progress that has been made in the past few years, now is an ideal time to review this research program, the history of work that facilitated it, and prospects for new insight in the near future. Here, we briefly introduce the biology of Heliconius and outline historical work on the Mendelian genetics of Heliconius wing patterning that primed it to become the emerging ecological model system that it is today. Then we highlight exciting recent discoveries in which the hunt for wing-patterning genes has come to fruition. Finally, we focus on perhaps the most important issue of all: the critical questions about basic evolutionary processes that can be addressed now that these wing-patterning genes are in hand.
The Biology of Heliconius and Related Genera
Host plants, chemical defense, and pollen feeding
A long history of research focused on Heliconius has revealed a detailed portrait of their fascinating biology (reviewed in Brown 1981). Heliconius, which consists of 43 species, is one of nine genera in the nymphalid tribe Heliconiini (Penz 1999; Beltran et al. 2007), commonly referred to as passion-vine butterflies because of their close affiliation with their Passiflora host plants (Gilbert 1971; Benson et al. 1975; Smiley 1978). Passiflora and Heliconius are cyanogenic, and it is this chemical defense that protects Passiflora from most herbivores and protects Heliconius from predation. Interestingly, a minority of Heliconius species are cyanogenic because they sequester cyanogens (simple monoglycoside cyclopentenyl cyanogens) from their host plants as larvae (Engler et al. 2000). Most Heliconius species actually synthesize aliphatic cyanogenic glycosides de novo from amino acid precursors (Nahrstedt and Davis 1981, 1983), and there is a trade-off in the ability to sequester vs. manufacture cyanogens across the genus (Engler-Chaouat and Gilbert 2007).
Heliconius also interact intimately with other plants, specifically those in the cucurbit genera Gurania and Psiguira, which provide a specialized resource to adult Heliconius in the form of pollen (Gilbert 1975). Heliconius are unique among butterflies in that they feed on pollen as adults, in addition to nectar (Gilbert 1972; Boggs et al. 1981). While Heliconius collect pollen from many flowers (Estrada and Jiggins 2002), they show a specialized, coevolved relationship with Gurania and Psiguira, plants that provide substantial pollen rewards to Heliconius and appear to be largely pollinated by Heliconius (Gilbert 1975). The nutritional benefit provided by pollen feeding has significant impacts on many aspects of Heliconius biology, such as cyanogenesis, reproduction, and longevity (Dunlap-Pianka et al. 1977; Brown et al. 1991). Other notable aspects of Heliconius biology include home-range behavior (Mallet 1986 a,b; Gilbert 1991), male production of specialized “anti-aphrodisiac” compounds (Gilbert 1976; Schulz et al. 2007, 2008), the unique pupal-mating behavior of some Heliconius species (Deinert et al. 1994; Estrada and Gilbert 2010; Estrada et al. 2010), and their pronounced visual acuity (Zaccardi et al. 2006), which is in part a product of a Heliconius-specific UV opsin duplication (Briscoe et al. 2010; Yuan et al. 2010).
Müllerian mimicry
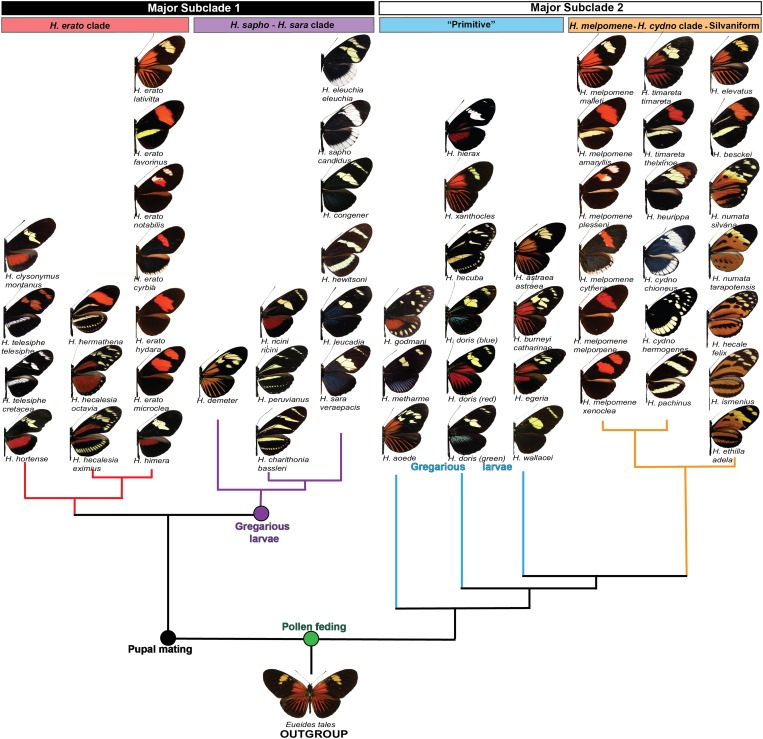
Wing-pattern mimicry is one of the most-studied aspects of Heliconius biology (Brown 1981; Mallet and Joron 1999; Joron et al. 2006a; Papa et al. 2008a; Kronforst et al. 2012). Many examples of mimicry in Heliconius involve convergence between species within the genus, and frequently between pairs of species. These co-mimetic pairs generally consist of distantly related species within the genus (Eltringham 1916; Turner 1976), with one member coming from each of the two major Heliconius subclades (Figure 1). For example, species and subspecies in the H. cydno group generally mimic species from the H. sara/H. sapho clade, while H. melpomene always mimics H. erato. There are exceptions to these patterns, such as species in the silvaniform clade, which are members of larger mimicry rings (groups of co-mimetic taxa) that include other Heliconius and more distantly related taxa (Brown and Benson 1974). In addition, mimicry in certain regions of the Amazon basin stands out as an extreme example of convergence, where Heliconius species from across the phylogeny, as well as a variety of other butterflies and even day-flying moths, have converged on the same red rayed wing pattern (Mallet 1999).
Figure 1.
Phenotypic diversity across Heliconius butterflies. Phylogenetic relationships among all major Heliconius clades are shown (Kozak et al. 2015). Names and color patterns of representative species and subspecies are depicted, together with behavioral traits that characterize phylogenetic nodes across the genus.
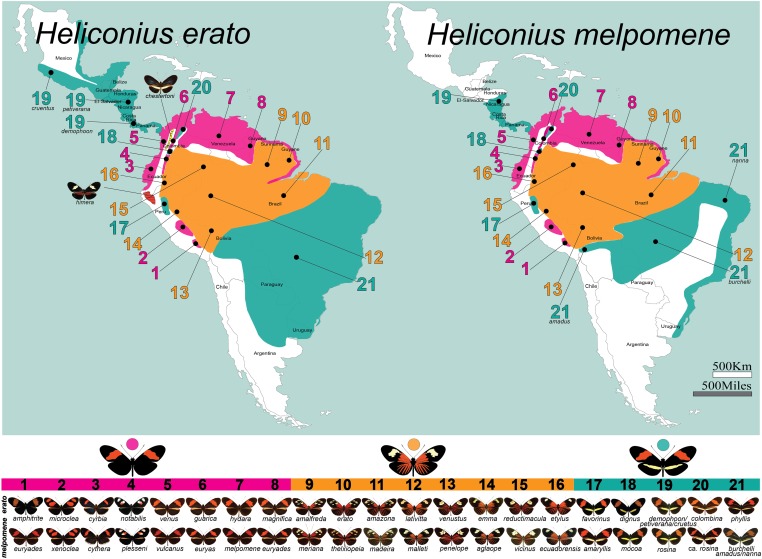
The dynamics of Heliconius mimicry appear to play out at a fine spatial scale, yielding both striking convergence among species as well as incredible diversity within species. Perhaps the most extreme example of this is the case of H. melpomene and H. erato (Brown et al. 1974; Sheppard et al. 1985; Brower 1994, 1996b; Turner and Mallet 1996; Flanagan et al. 2004; Quek et al. 2010; Hines et al. 2011; Nadeau et al. 2014). These two species are distributed throughout much of South and Central America, and they mimic one another wherever they co-occur. Like many other examples of pairwise mimicry in Heliconius, one of these species comes from each of the two major within-Heliconius subclades. Strikingly, the shared wing patterns of H. melpomene and H. erato switch geographically, in tandem, producing a patchwork of mimicry phenotypes across their range (Figure 2). This has resulted in >20 named wing-pattern races in each species, but there are common themes among phenotypes that cluster them into three general categories: red-banded, rayed, and “postman.” The evolutionary history of this shared diversity between H. melpomene and H. erato remains a bit of a mystery. Historically, Sheppard, Turner, Brown, and others believed that this geographical patchwork emerged from a strict co-evolutionary process whereby H. melpomene and H. erato radiated in parallel, over time and space, and that this was driven by populations of each species becoming isolated in Pleistocene forest refugia (Brown et al. 1974; Brown 1981; Sheppard et al. 1985; Brower 1996b; Turner and Mallet 1996). However, recent phylogeographic work suggests that this may not be the case. For example, the radiation of H. erato seems to predate that of H. melpomene by almost 1 million years (3.1 vs. 2.1 MYA) (Quek et al. 2010; Hill et al. 2013; but see Cuthill and Charleston 2012). This time discrepancy may be reflective of a larger trend as divergence events throughout the clade that includes H. erato, H. sara, and H. sapho appear to be older than those in the clade that includes H. melpomene, H. cydno, and the silvaniforms (Kozak et al. 2015). The emerging picture is that H. erato likely radiated first, and it was this established diversity that served as a template for a subsequent H. melpomene radiation. If so, the ultimate source of H. erato diversification remains an open question although there is speculation that genetic drift may have played a role (Turner and Mallet 1996; Mallet 2010).
Figure 2.
Geographic distributions of the H. erato and H. melpomene convergent radiations. Approximate distributions of the three major phenotypes and locations of many described subspecies, or “races,” of the Müllerian co-mimics H. erato and H. melpomene. Matching wing-color-pattern variation and subspecies names are shown below the maps. Two incipient species that are part of the H. erato radiation, H. himera and H. erato chestertoni, are also presented.
Measuring selection on wing patterns
Butterfly mimicry is an appealing evolutionary study system because, unlike many natural systems, we have a good understanding of the targets of selection (wing-pattern traits that match mimicry models) as well as the agents of selection (predators). Furthermore, for Heliconius specifically, there are abundant and varied empirical data documenting natural selection on wing pattern as well as quantitative estimates of the strength of selection. An important early experiment revealed the strength of purifying selection on Heliconius wing patterns. Benson (1972) altered the wing pattern of H. erato in Costa Rica by obscuring the red forewing band on a minority of individuals in the population. Subsequent recapture results revealed that the altered phenotypes disappeared from the population rapidly, and those that were re-caught, showed a higher frequency of bird beak marks on their wings. Mallet and Barton (1989) followed this with a reciprocal transplant experiment, moving H. erato butterflies across a racial hybrid zone. Subsequent recapture revealed rapid disappearance of the foreign morph, combined with elevated instances of bird attack, yielding a selection coefficient estimate of 0.51 overall, or s ∼ 0.17 per mimicry locus. In this same hybrid zone, and the coincident hybrid zone between matching races of H. melpomene, Mallet et al. (1990) measured cline widths and linkage disequilibrium among mimicry loci to estimate selection on wing pattern, resulting in estimates of s ∼ 0.23 per mimicry locus for H. erato and s ∼ 0.25 per mimicry locus for H. melpomene. These are large selection coefficients, as predicted by the very narrow hybrid zones (∼10 km).
While effectively documenting selection on wing patterns, this work focused on purifying selection within species rather than the convergence between species predicted by mimicry theory. Kapan (2001) filled this gap by experimenting with a Heliconius mimicry system in western Ecuador that contained two divergent and monomorphic species, white-winged H. sapho and yellow-winged H. eleuchia, and a third polymorphic species, H. cydno alithea. Heliconius cydno alithea exists as two morphs, a white form that mimics H. sapho and a yellow form that mimics H. eleuchia. Kapan (2001) moved white and yellow H. cydno alithea morphs among sites in western Ecuador, where the abundances of H. sapho and H. eleuchia varied. Consistent with previous work, H. cydno individuals that differed from the locally abundant model disappeared from the population faster than those that matched the model. Furthermore, Kapan (2001) showed that increasing the density of experimentally released individuals reduced the effect, presumably as a result of predator learning. These results provided the first field test of Müllerian mimicry theory, showing natural selection for convergence between different toxic species.
Mimicry and speciation
The evolution of mimicry in Heliconius has a further, second-order effect on biodiversity because divergent natural selection for mimicry appears to play an important role in the early stages of speciation (Jiggins 2008). Given the strong selection on wing patterns documented in previous experiments, what happens when a subpopulation of one species leaps the adaptive valley to join a different mimicry ring? The prediction is that divergence in color pattern should generate reproductive isolation because recombinant wing patterns are nonmimetic and thus likely to be sampled by predators. This is a form of hybrid incompatibility, but in contrast to intrinsic incompatibilities such as hybrid sterility or inviability, this incompatibility is extrinsic because it is generated by the fit (or lack thereof) between a hybrid and the environment. Recently, Merrill et al. (2012) tested this hypothesis using two different approaches—behavioral assays of field-caught birds when presented with live butterflies and estimates of natural predation using clay butterfly models—and found that predators do indeed attack hybrids more often than the parental types. Other work has also shown that the parental species are not attracted to hybrids as potential mates (Naisbit et al. 2001). The result is that divergent natural selection to match different mimicry rings immediately generates, as a by-product, pronounced extrinsic, postygotic isolation as well as disruptive sexual selection.
Mimicry appears to interact with mate preference in an even more fundamental way, which further contributes to reproductive isolation. Multiple studies have shown that Heliconius species and subspecies generally mate assortatively, with wing pattern serving as a critical cue in mate selection (McMillan et al. 1997; Jiggins et al. 2001, 2004; Naisbit et al. 2001; Kronforst et al. 2006b, 2007; Mavarez et al. 2006; Chamberlain et al. 2009; Melo et al. 2009; Merrill et al. 2011a,b, 2014; Finkbeiner et al. 2014). For example, H. cydno and H. melpomene rarely hybridize in nature, despite being broadly sympatric and partially interfertile (Naisbit et al. 2002), and that appears to be due, in part, to a strong preference for conspecific wing patterns (Jiggins et al. 2001; Jiggins 2008). Assortative mate preference based on color pattern also limits hybridization between H. erato and H. himera (Jiggins et al. 1997; McMillan et al. 1997; Merrill et al. 2014), as well as between H. cydno and H. pachinus (Kronforst et al. 2006b, 2007), pairs of closely related species that are otherwise interfertile. Hence, divergent mimicry phenotypes contribute to premating reproductive isolation as well. Perhaps surprisingly, the association between mimicry and mate choice extends even further because crosses between H. cydno and H. melpomene, as well as crosses between H. cydno and H. pachinus, reveal that mate preference is genetically linked to the dominant wing color cue distinguishing the hybridizing species: red vs. black in cydno/melpomene crosses (Merrill et al. 2011b) and white vs. yellow in cydno/pachinus (Kronforst et al. 2006b). This genetic linkage between preference and wing color has likely contributed to the recent Heliconius radiation because it facilitates the co-evolution of mimicry and mate preference and also maintains their association despite on-going hybridization among species.
Genetic Basis of Wing Patterning in Heliconius
Given that Heliconius wing patterns play such a central role in mediating critical aspects of their biology, as well as the fact that they stand out as adaptive traits that also influence speciation, it is not surprising that the genetic basis of Heliconius wing patterning has been the subject of considerable research. Here we briefly outline this work, starting with the original investigations of basic Mendelian genetics, all the way up to the latest discoveries that have identified the specific genes, and even the exact mutations in some cases, that control Heliconius wing-pattern diversity.
Mendelian genetics
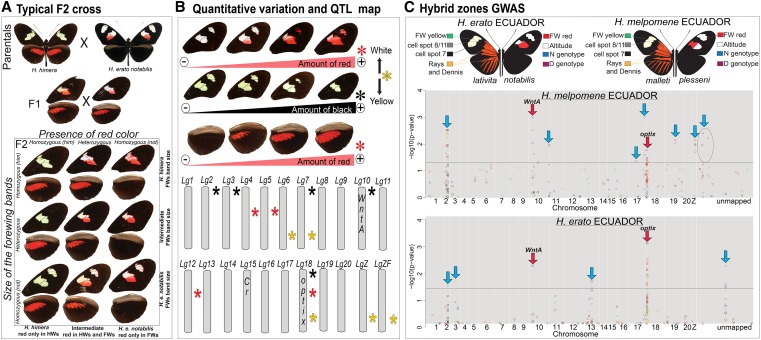
Crossing experiments with Heliconius butterflies date back to at least 1954 (Beebe 1955), which is actually quite recent given that similar experiments in swallowtail butterflies began as early as 1902 (Leigh and Poulton 1909). A recent example of a Heliconius crossing experiment (Papa et al. 2013) is shown in Figure 3. The earliest work on Heliconius mimicry genetics (Beebe 1955; Turner and Crane 1962; Sheppard 1963; Emsley 1964; Turner 1971) culminated in the expansive treatment of Sheppard et al. (1985). This dense, >170-page paper provided a comprehensive summary of wing-pattern segregation based on 68 crosses (encompassing 1978 hybrid offspring) among eight color pattern races of H. melpomene and 69 crosses (1325 offspring) among eight races of H. erato. This early work revealed that much of the wing-pattern variation in both H. melpomene and H. erato is controlled by large-effect Mendelian loci that switch portions of the wing from one color to another. Sheppard et al. (1985) also identified a relatively large number of distinct loci in each species—11 loci in H. melpomene and 15 in H. erato —and showed that, despite some instances of linkage, many of the Mendelian switch loci appeared to be unlinked.
Figure 3.
Mapping color-pattern variation in Heliconius: Mendelian segregation, quantitative variation, and genome-wide association. (A) An F2 mapping family from a cross between sister species H. himera and H. erato notabilis shows Mendelian segregation of black (Sd = WntA) and red (D = optix) wing-scale distributions (Papa et al. 2013). (B) Quantitative wing-color variation and linkage group distribution of QTL for black, red, and yellow/white color-pattern variation (stars) in the same mapping family. Stars indicate the presence of at least one locus modulating black (black star), red (red star), or yellow/white (yellow star) pattern variation. (C) Genome-wide association study of wing-color-pattern variation in H. melpomene and H. erato Ecuadorian hybrid zones (Nadeau et al. 2014). The different colors of points represent individual SNPs associated with distinct pattern elements shown in the wing images above. Points above the lines represent significant associations. Red arrows indicate the positions of optix (D locus) and WntA (Sd locus). Blue arrows indicate the positions of putatively undescribed color-pattern loci, many of which are not shared between H. melpomene and H. erato.
In synthesizing this previous work on Heliconius mimicry genetics and in incorporating newer observations (Nijhout and Wray 1988; Mallet 1989; Nijhout et al. 1990), Nijhout (1991) identified a total of 22 distinct mimicry loci in H. melpomene and 17 in H. erato. Similar work in H. cydno showed that this species had some unique mimicry loci but shared others with H. melpomene (Linares 1996, 1997; Merchan et al. 2005). Importantly, Nijhout et al. (1990)tied color-pattern variation in Heliconius back to the highly conserved elements of the nymphalid ground plan. In contrast to previous work, which viewed Heliconius wings as a black background overlaid with color-pattern elements, inferences of homology offered by the nymphalid ground plan revealed that on Heliconius wings black and red regions are frequently the pattern with white and yellow as the background. Gilbert (2003) expanded on this pattern vs. background distinction by developing a unifying model of wing patterning in Heliconius. This model, which was based on decades of intra- and interspecific crossing experiments, combined with fundamental correlations among scale ultrastructure and pigmentation (Gilbert et al. 1988), characterized all observed wing-pattern variation in terms of three distinct elements: light colored (white or yellow) “windows,” melanic or red “shutters” that overlay windows, and melanic “walls” that border the wing and are generally invariant. Gilbert’s model also separated wing position from patterning locus, which are separable units that previously had been confounded, thereby disentangling allelic effects from epistasis.
Comparative genetic mapping
A long-term goal of research on Heliconius mimicry has been both to determine the genetic basis of adaptation and to infer the extent to which it is conserved over evolutionary time. The earliest crossing experiments in Heliconius characterized the very discrete genetic basis of wing patterning, but they were incapable of addressing the question of homology between species or even among different subspecies in many cases. This led to inflation in locus nomenclature, with presumably homologous mimicry loci being given different names in different species and subspecies (hence Nijhout’s list of 39 loci in H. melpomene and H. erato alone). Given that hybridization is relatively common among Heliconius species, an initial step in tracing homology over evolutionary time came in the form of interspecific crossing experiments. Jiggins and McMillan (1997) analyzed wing-pattern segregation in crosses between H. erato and H. himera, Naisbit et al. (2003) in crosses between H. melpomene and H. cydno, and Gilbert (2003) among H. melpomene, H. cydno, and H. pachinus. In combination with the results from prior work, these three studies revealed that the same factors responsible for subspecific variation within H. erato, and within H. melpomene, also appeared to be responsible for the wing-pattern differences among closely related species. However, sister species, like subspecies, generally display divergent wing patterns, so the results largely reinforced the notion that across various young divergence times the same switch loci seem to control diversification. What about convergence? To address the genetic basis of convergence required comparisons between the two major Heliconius subclades because the majority of mimicry occurs between these two lineages. This, however, presented an obstacle because species from these two clades cannot interbreed. Comparative genetic mapping provided an initial step forward.
Genetic linkage maps with homologous markers offered the first glimpse of possible mimicry gene homology among convergent taxa. Beginning with Jiggins et al. (2005), Tobler et al. (2005), and Kapan et al. (2006), complete genetic linkage maps were published for the co-mimics H. erato and H. melpomene. While built on backbones of anonymous amplified fragment length polymorphism (AFLP) markers, which generally cannot be compared across species or even different mapping studies, the genetic maps of both species included an assortment of gene-based markers, allozymes, and microsatellite markers that could serve as anchors across taxa. These first studies were significant because they localized major mimicry switch loci to specific portions of chromosomes. The first of these loci to be mapped were H. erato D (red patterning), Sd (forewing melanin shuttering), and Cr (hind-wing melanin shuttering), and H. melpomene Yb and Sb (linked loci controlling hind-wing melanin shuttering) (Table 1). Kronforst et al. (2006a) showed that multiple mimicry loci with similar phenotypic effects mapped to homologous chromosomes in the H. melpomene and H. erato lineages. Specifically, they generated a genetic map using interspecific crosses between H. cydno and H. pachinus, close relatives of H. melpomene, and used it to localize the positions of three Mendelian mimicry loci: Yb, Ac (forewing melanin shuttering), and G (red wing spots). By using some of the same anchor loci from previous H. erato maps, Kronforst et al. (2006a) were able to subsequently show that Yb and Cr mapped to similar positions on homologous chromosomes, as did Ac and Sd and G and D. Joron et al. (2006b) explored this at a much finer scale and with an added twist, showing that H. melpomene Yb and H. erato Cr colocalized to within 1 cM of one another (1% recombination) and that this was precisely the same location as the single Mendelian “supergene” that controls all wing-pattern variation in H. numata (Table 1). In H. melpomene, red-wing patterning is thought to be controlled by tightly linked but separate loci, the B and D loci. Using a similar approach of comparative fine-mapping, Baxter et al. (2008) showed that H. melpomene B/D mapped to the same genomic location as H. erato D. It turns out this is the same location as the H. cydno/pachinus G locus (Chamberlain et al. 2011).
Table 1. Summary of major Heliconius mimicry loci.
| Locus | Gene | Chromosome | Phenotype | H. melpomene | H. cydno | H. erato | H. himera | H. numata |
|---|---|---|---|---|---|---|---|---|
| D | optix | 18 | Red/orange at base of dorsal FW/HW | ✓ | NA | ✓ | ✓ | Modifier |
| B | optix | 18 | Red FW band | ✓ | NA | ✓(D) | ✓(D) | ? |
| R | optix | 18 | Red/orange HW rays | ✓ | NA | ✓(D) | ✓(D) | NA |
| G | optix | 18 | Red at base of ventral FW/HW | ✓ | ✓ | ✓(D) | ✓(D) | ? |
| Br | optix | 18 | Brown oval on ventral HW | NAa | ✓ | NA | NA | ? |
| Ac/Sd | WntA | 10 | Distribution of melanin across FW | ✓(Ac) | ✓ | ✓(Sd) | ✓(Sd) | Modifier |
| Fs/Ro | ? | 13 | Distribution of melanin in upper FW | ✓(Fs) | ?b | ✓(Ro) | ? | ? |
| Yb/Cr | ? | 15 | Distribution of melanin on FW and HW | ✓(Yb) | ✓(Yb) | ✓(Cr) | ✓(Cr) | Modifier |
| Sb | ? | 15 | Melanin along HW margin | ✓(Sb) | ✓(Sb) | ✓(Cr) | NA | ? |
| N | ? | 15 | Shape of FW band | ✓ | ✓ | ✓(Cr) | ? | ? |
| P | ? | 15 | H. numata mimicry supergene | NA | NA | NA | NA | ✓ |
| K | ? | 1 | White vs. yellow wing color | ✓ | ✓ | ? | ? | Modifier |
FW, forewing; HW, hind wing.
NA indicates that the phenotype does not exist in that species.
A question mark (?) indicates the mimicry locus is currently not known to influence wing pattern in that species.
These comparative genetic mapping experiments took the critical first steps toward positionally cloning Heliconius mimicry loci, and they also provided preliminary evidence of probable homology among co-mimetic species. Indeed, much of the wing-pattern variation across the genus was quickly tracing back to just a handful of discrete genomic intervals. Beyond that, this research was instrumental in developing genomic resources in Heliconius. As part of their genetic mapping efforts, research teams generated bacterial artificial chromosome libraries for H. melpomene and H. erato, using these to generate targeted reference sequences across focal mimicry intervals, as well as expressed sequence tag (EST) data for annotation and analyses of gene expression (Papanicolaou et al. 2005; Joron et al. 2006b; Kapan et al. 2006; Pringle et al. 2007; Baxter et al. 2008; Papa et al. 2008b; Reed et al. 2008; Ferguson and Jiggins 2009; Ferguson et al. 2010; Wu et al. 2010; Surridge et al. 2011; Hines et al. 2012). These genomic resources ultimately led to the identification of the first Heliconius mimicry genes and the beginning of the Heliconius Genome Project.
Molecular characterization of mimicry loci
Optix:
Comparative genetic mapping across H. erato, H. melpomene, and H. cydno/H. pachinus showed that various loci controlling red wing patterning (D, B/D, G) all mapped to the same genomic location, suggesting that species across the genus used the same gene or set of tightly linked genes to control red wing-pattern variation. The first surveys of population genetic variation among color-pattern races (Baxter et al. 2010; Counterman et al. 2010) or closely related species (Chamberlain et al. 2011) revealed striking signatures of genetic differentiation in this genomic interval, with a kinesin gene initially showing the strongest genotype–phenotype associations. This same gene also showed differential gene expression between divergent color-pattern phenotypes in both H. melpomene and H. erato (Baxter et al. 2010; Counterman et al. 2010).
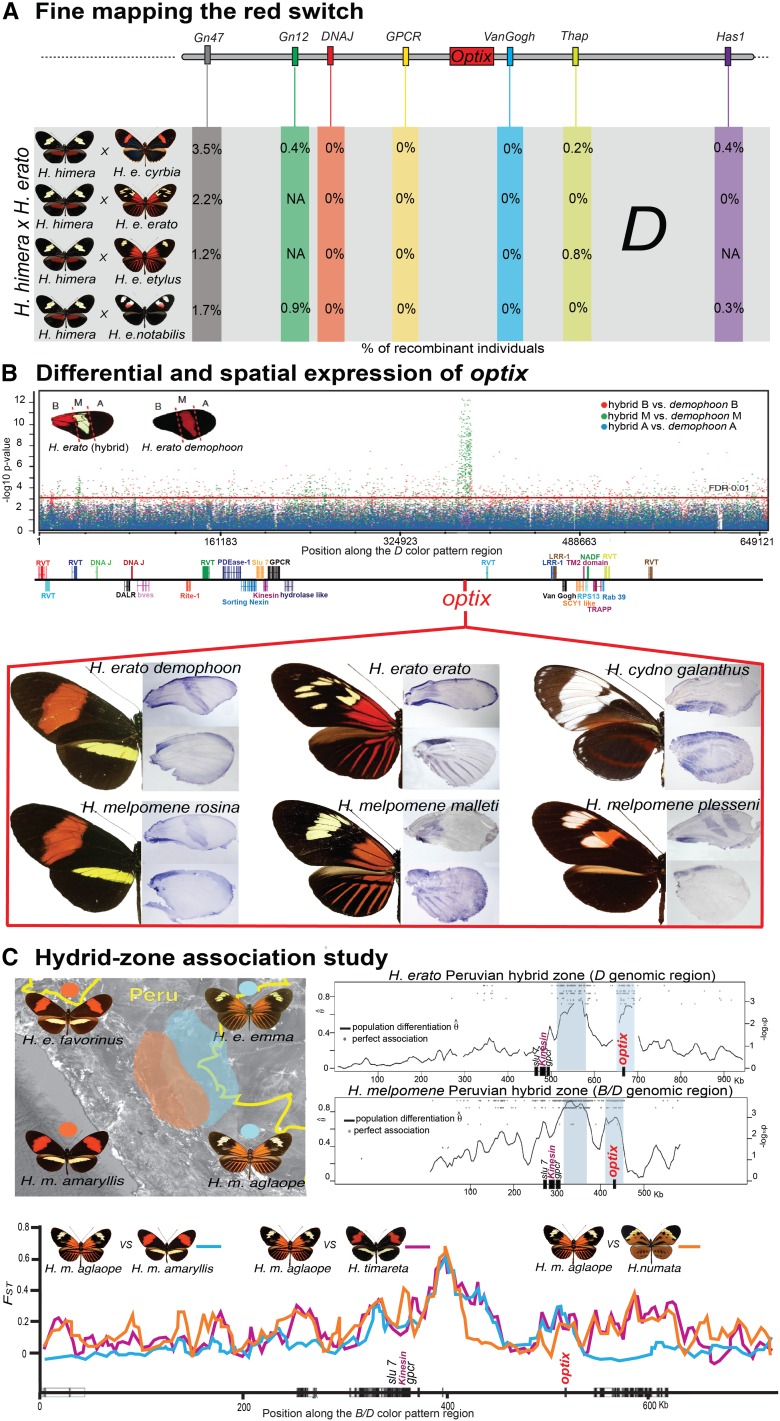
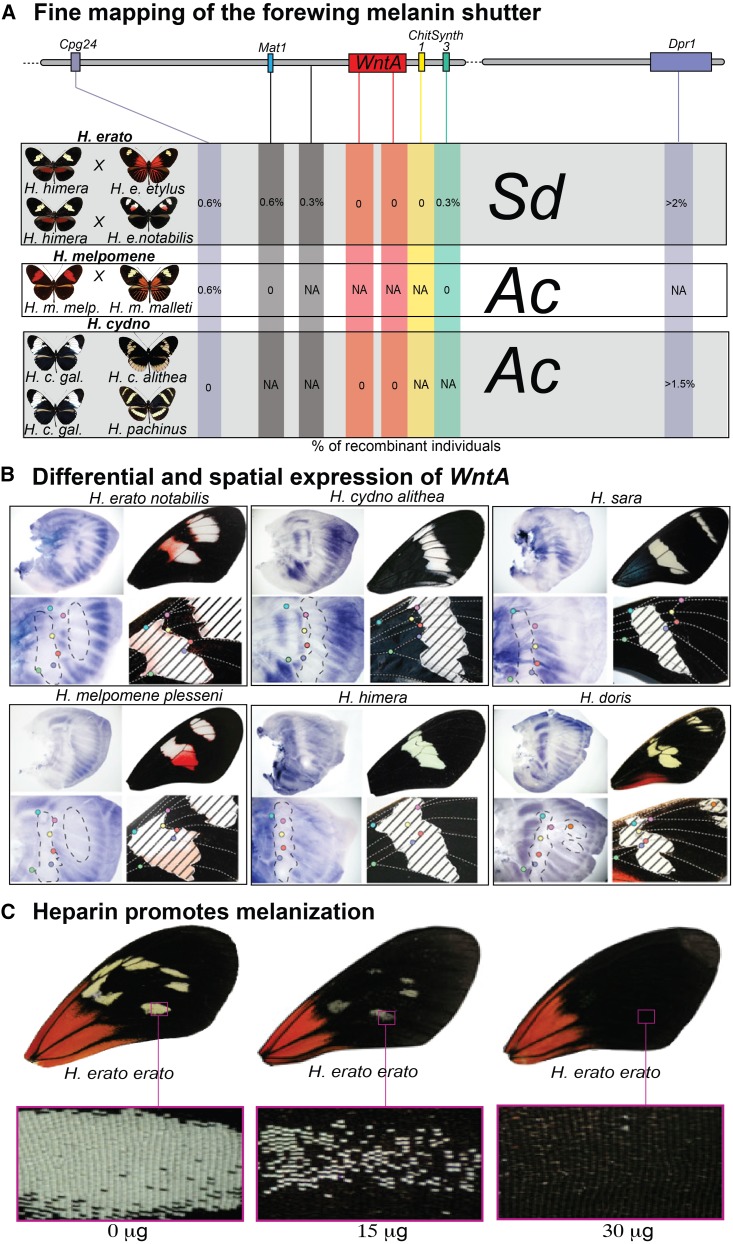
It turns out that the population genetics and expression variation associated with this kinesin gene are relatively small in comparison to the adjacent gene, optix. Using a tiling array, Reed et al. (2011) examined expression of the entire 700-kb D locus interval in H. erato, comparing segments of individual wings that would eventually be red, black, and yellow. This revealed a striking pattern of optix expression associated with red patterning, an expression pattern further corroborated via in situ hybridization (Figure 4) and immunohistochemistry (Martin et al. 2014). Across all Heliconius and other closely related species, optix appears to be the critical gene specifying red wing patterning. Outside of this clade, optix does not appear to control wing patterning although it does appear to play an ancient, conserved role in specifying wing-coupling scales on ventral forewings and dorsal hind wings (Reed et al. 2011; Martin et al. 2014). Red-pattern variation seems to be the product of regulatory variation upstream of the optix-coding sequence because there is little coding sequence variation in the gene among Heliconius butterflies (Reed et al. 2011) and SNP association tests (Supple et al. 2013), as well as genome scans comparing the strength of genetic differentiation among closely related species (Nadeau et al. 2012), consistently point to the noncoding region between optix and kinesin (Figure 4). Interestingly, Pardo-Diaz and Jiggins (2014) recently resurrected the link between kinesin and red wing patterning by showing that both optix and kinesin contribute to red pigmentation. Unlike optix, which is associated with all red patterning, the kinesin gene appears to be expressed only in the red forewing band of the postman phenotype.
Figure 4.
The molecular basis of red wing patterning in Heliconius. (A) Genetic mapping of red wing color-pattern variation (percentage of recombinants at several genes is shown) across multiple H. erato × H. himera families points to a narrow genomic interval containing the transcription factor optix. (B) Comparison of gene expression between three forewing color-pattern sections of two H. erato morphs using a D-locus tiling array suggests optix as the gene regulating red patterning (Reed et al. 2011). Messenger RNA expression (in situ hybridization) of optix on pupal wing discs of different Heliconius species spatially prefigures adult red wing patterning (Reed et al. 2011). (C) Targeted analyses of SNP associations and genetic differentiation (FST) in H. erato and H. melpomene Peruvian hybrid zones (geographic distributions shown in the map) suggest two genomic regions, one centered on optix and another upstream of optix, strongly associated with red wing color-pattern variation (Supple et al. 2013). Patterns of genetic differentiation across the B/D interval between H. melpomene subspecies and closely related species also reveal enhanced divergence in these two genomic regions (bottom) (Nadeau et al. 2012).
WntA:
Similar to red patterning, melanic pattern variation on the forewing of various Heliconius species is Mendelian and has been shown to map to similar positions on homologous chromosomes (Kronforst et al. 2006a). Martin et al. (2012) showed that across H. erato, H. melpomene, and H. cydno, forewing melanic variation mapped to the gene WntA, and WntA expression on larval wing discs prefigured future adult melanin patterning (Figure 5). WntA, like wingless and other Wnt ligands, is an extracellular signaling molecule that presumably acts as a morphagen during wing-pattern specification. Heparin is known to potentiate Wnt signaling and Martin et al. (2012) showed that by injecting heparin sulfate into developing pupal wing discs, they were able to enhance melanization across the wing and produce allelic phenocopies (Figure 5). In line with the optix results, WntA shows little protein sequence change across all Heliconius species and color pattern forms, again implicating cis-regulatory changes in the evolution of novel wing-pattern phenotypes (Martin et al. 2012).
Figure 5.
The molecular basis of melanin patterning in Heliconius. (A) Genetic mapping of forewing melanin variation across different families of several Heliconius species (percentage of recombinants at several genes is shown) points to a narrow genomic interval containing the gene WntA (Martin et al. 2012). (B) Spatial expression (in situ hybridization) of WntA on larval wing discs prefigures adult melanin patterning and confirms the role of WntA in forewing black-scale variation (Martin et al. 2012). (C) Pupal injection of heparin sulfate, which is known to extend Wnt signaling, enhances wing melanization (Martin et al. 2012), further supporting the idea that WntA controls melanin patterning in Heliconius.
Recently, Gallant et al. (2014) stepped outside Heliconius on the butterfly phylogeny and found that WntA also controls a similar melanic shutter in Limenitis butterflies. Nonmimetic L. arthemis arthemis is black with white bands on both the fore- and hind wings while the subspecies L. arthemis astyanax mimics the toxic pipevine swallowtail, Battus philenor, and is all black. Using a combination of fine-mapping, in situ hybridization, RNA-seq, and heparin injections, Gallant et al. (2014) showed that the Mendelian melanin switch in Limenitis is also controlled by WntA. Furthermore, by sequencing and comparing 30 Limenitis genomes, Gallant et al. (2014) identified 173 SNPs and a 9-kb LINE upstream of the WntA-coding sequence that were perfectly associated with wing-pattern phenotype, again pointing to cis-regulatory variation. Parallel analysis of 45 Heliconius cydno genomes identified a single 1.8-kb indel upstream of WntA that was perfectly associated with wing pattern, the position of which overlapped the position of the LINE in Limenitis (Gallant et al. 2014). Surprisingly, it appears that similar phenotypes have originated independently in Heliconius and Limenitis butterflies from functionally similar mutations targeting the same region upstream of WntA.
P locus supergene in H. numata:
Few aspects related to mimicry genetics have received as much interest as supergene mimicry (Schwander et al. 2014; Thompson and Jiggins 2014). In a number of polymorphic species, the entire wing pattern is controlled by a single Mendelian locus, and this extreme genetic architecture has been dubbed a “supergene” (Fisher 1930; Clarke and Sheppard 1960; Charlesworth and Charlesworth 1975). There is a long history of work on supergenes in the context of both butterfly mimicry and self-incompatability loci in plants (Schwander et al. 2014; Thompson and Jiggins 2014). Clarke and Sheppard performed a large series of crossing experiments exploring supergene mimicry, primarily in Papilio swallowtail butterflies, and they envisioned supergenes as clusters of tightly linked loci brought together via interchromosomal translocation due to natural selection against maladaptive recombination (Clarke and Sheppard 1960; Thompson and Jiggins 2014). Charlesworth and Charlesworth (1975) explored the dynamics of supergene evolution using computer simulations and showed that the hypothesized translocation mechanism was unlikely because unlinked loci would not remain polymorphic in a population for very long. Rather, Charlesworth and Charlesworth (1975) proposed that distinct loci must be fairly tightly linked to begin with for natural selection to further reduce recombination and tighten them into a supergene.
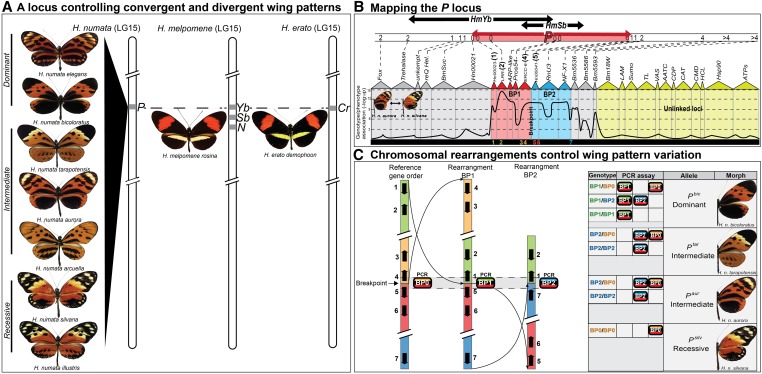
Heliconius generally do not exhibit supergene mimicry, but there is one highly polymorphic species, H. numata, which does (Brown and Benson 1974). In a series of detailed molecular investigations, Joron et al. (2006b, 2011) characterized the H. numata supergene, the P locus, and provided the very first window into this long-term evolutionary enigma (Figure 6). Comparative fine-mapping among H. numata and other Heliconius species showed that the P locus maps to the location of a mimicry locus that is conserved across the genus (Joron et al. 2006b). More so, in H. numata’s close relative H. melpomene, this region contains three tightly linked but separable mimicry loci: Yb, Sb, and N (Joron et al. 2006b). This appears to be precisely in line with the results of Charlesworth and Charlesworth (1975); the supergene in H. numata may have evolved by the tightening of linkage among previously linked loci. Joron et al. (2011) discovered that chromosomal rearrangements were ultimately responsible for enhanced linkage at the P locus with various color-pattern morphs being associated with different chromosomal inversions (Figure 6C). Which of the genes contained in these inversions ultimately contribute to color pattern remains a mystery, but the inference is that multiple distinct elements in this region contribute to the phenotype and the inversion polymorphism greatly reduces recombination among these elements.
Figure 6.
Characterizing the H. numata mimicry supergene. (A) A single Mendelian locus with multiple alleles (the P locus) controls wing-pattern diversity in H. numata. The P locus is positionally homologous to the Yb-Sb-N loci of H. melpomene and the Cr locus of H. erato (Joron et al. 2006b). (B) Fine-mapping and SNP associations narrow the P locus to a 400-kb interval spanning 31 genes and provide evidence of highly reduced recombination (red and blue areas) (Joron et al. 2011). Relative position of the genes (1–7) across the interval that were used to characterize genomic rearrangements is also shown. (C) Allelic variation at the P locus ultimately traces back to an inversion polymorphism with different wing-pattern morphs determined by distinct, nonrecombining haplotypes (Joron et al. 2011). PCR assay of the alternative breakpoints BP0, BP1, and BP2 (left) are perfectly associated with mimicry variation across four distinct morphs in eastern Peru (right).
Modifiers:
Much of the focus in the study of Heliconius mimicry has been on a handful of large-effect Mendelian switch loci. However, while certain major color-pattern elements segregate in a Mendelian fashion, overall color pattern is a complex trait ultimately controlled by allelic combinations at multiple unlinked large- and small-effect loci across the genome (Figure 3). This is apparent from multiple recent investigations that found widespread evidence for small-effect QTL and/or modifier loci influencing Heliconius wing-pattern mimicry (Baxter et al. 2009; Jones et al. 2012; Papa et al. 2013). Using a single cross between two H. melpomene races that differ in the size of their red forewing band, Baxter et al. (2009) found that at least 6 of 21 chromosomes influenced quantitative variation in this trait, including those now known to contain optix and WntA. Similarly, Jones et al. (2012) examined quantitative variation in crosses among H. numata morphs and found that 6 chromosomes, in addition to the supergene locus P, influenced color-pattern variation. In the case of H. numata, it is possible that many of these small-effect QTL will ultimately trace back to the major Mendelian switch loci present in other Heliconius species because 3 of the 6 chromosomes included those housing the K, B/D (optix), and Ac (WntA) loci. In the broadest survey of its kind, Papa et al. (2013) used a series of crosses between H. himera and different color-pattern races of H. erato to show that a number of the major switch loci previously thought to be distinct actually map to optix and WntA. Furthermore, Papa et al. (2013) showed that a substantial amount of variation not captured by these switch loci could be traced back to additional QTL scattered throughout the genome (Figure 3B). It is worth noting another source of complexity that has recently come to light: distinct but functionally similar color-pattern alleles in the same species, indicative of independent origins of the same phenotype or developmental drift over relatively short timescales (Maroja et al. 2012).
Overall, the current picture of Heliconius mimicry genetics may lend support to classic theoretical expectations. Punnett (1915), Nicholson (1927), and Turner (1977b, 1981) proposed a two-step model for the evolution of Müllerian mimicry whereby an initial large mutation would move a phenotype from one mimicry ring to another, after which additional smaller changes would refine mimetic resemblance. The combination of large-effect switch loci and small-effect modifiers that we see in the genetic control of Heliconius wing patterning is consistent with this model, but the order in which they occurred is an important aspect of the two-step model, which we currently know little about. It is interesting to note that, in a recent analysis of divergence across hybrid zones between color-pattern races of H. erato and H. melpomene, Nadeau et al. (2014) found strong divergence centered on large-effect mimicry loci in both species, but divergence associated with putative modifiers differed between species (Figure 3C). This result may indicate that large-effect loci are conserved among species but modifiers are not. In the end, we have come full circle in some respects in our view of Heliconius mimicry genetics; while originally believed to involve many switch loci, modern investigations have caused much of that variation to coalesce into a handful of loci but also have revealed the widespread action of unappreciated modifiers and quantitative variation.
The Molecules Matter
In an effort to characterize the mutational basis and evolutionary history of putatively adaptive phenotypic variation, evolutionary biologists are increasingly focusing on identifying the genes and causative molecular variation underlying traits of interest (Hoekstra and Coyne 2007; Nadeau and Jiggins 2010). Many of the major questions posed by the radiations and repeated instances of convergence seen in Heliconius ultimately rest on the identification and comparison of mimicry genes among species. For example, as noted above, biologists have long pondered the functional basis of supergenes and work on H. numata, and comparison to H. melpomene and H. erato, have provided the first insight into the molecular basis of supergene mimicry (Joron et al. 2006b, 2011). Similarly, our understanding of the molecular basis of mimicry in Heliconius provides genuine insight into a variety of additional evolutionary phenomena, including constraint and evolvability, the genetic basis of convergence, the potential of introgression to facilitate adaptation, the mechanisms of hybrid speciation in animals, and the process of ecological speciation.
Constraint vs. evolvability
Heliconius mimicry presents an enigma: the entire genus appears to use the same small number of large-effect switch loci, an apparent genetic constraint, yet this does not appear to constrain phenotype in any way as wing pattern is highly evolutionarily labile within and among species. In fact, there appears to be a virtually unlimited number of possible wing-pattern phenotypes available to them (Gilbert 2003). Potential evidence of genetic constraint appears at a deeper level, too, as the same narrow noncoding region upstream of the gene optix is most strongly associated with red wing patterning in both H. erato and H. melpomene (Supple et al. 2013). Furthermore, structural changes in the same or similarly located regulatory elements upstream of WntA appear to be responsible for melanic variation in both Heliconius and Limenitis butterflies (Gallant et al. 2014). Overall, the mutational targets available for color-pattern change appear to be narrow. While one might speculate if and how genetic or developmental constraints could limit evolutionary potential, Heliconius mimicry provides an example of apparently unlimited phenotypic potential operating over a constrained genetic system. This may suggest that genetic constraints are not imposed by mutational target size, and/or genetic constraints may not limit phenotypic evolution. Heliconius may circumvent apparent genetic constraints via the evolution of secondary modifiers or by modulating expression of the large-effect mimicry genes themselves. We currently have little information about the specific cis-regulatory elements that control Heliconius mimicry gene expression. However, it is likely that the architecture of these elements permits extensive phenotypic variation to emerge from each major switch locus.
Genetic basis of convergence
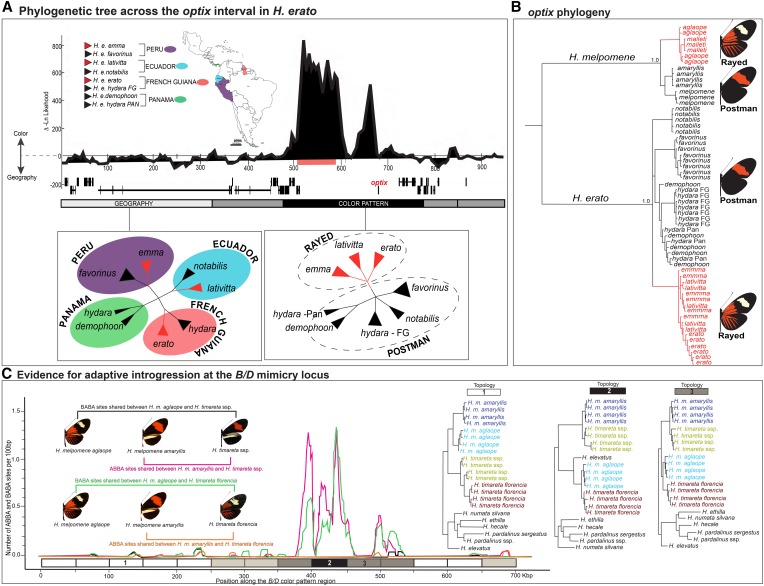
Examples of phenotypic convergence exist at many taxonomic levels in Heliconius, and having the genes responsible for mimicry in hand finally allows us to dissect the evolutionary history of convergent/parallel evolution in the context of mimicry. At the smallest taxonomic scale—within species—we see in both H. erato and H. melpomene a perplexing scenario whereby geographically disjunct populations display nearly identical color patterns, which has led to speculation that similar color patterns may have arisen multiple times within each species (Brower 1994). For example, the red and yellow banded postman phenotype occurs throughout Central America, a large section of Brazil, and in isolated patches of Peru and Colombia (Figure 2). Genetic markers not linked to mimicry loci, such as mitochondrial DNA (Brower 1994, 1996b) and nuclear markers (Flanagan et al. 2004; Quek et al. 2010), consistently revealed phylogeographic patterns in both H. erato and H. melpomene that grouped subspecies by geography rather than color pattern, further fueling speculation of possible intraspecific convergence. In sharp contrast, however, sequence variation at the optix gene itself reveals a very different story, clustering subspecies by color pattern (Figure 7A) and showing that each wing pattern, including the postman phenotype, originated a single time in each species (Hines et al. 2011; Supple et al. 2013). This is a striking case in which analysis of the causative gene offered unique insight into an evolutionary history that was not visible in the rest of the genome (Turner et al. 1979; Hines et al. 2011; Supple et al. 2013). It is possible that the histories of different genomic segments are decoupled due to gene flow among Heliconius subspecies and closely related species.
Figure 7.
Tracing the evolution of Heliconius mimicry. (A) Genetic variation across most of the genome clusters H. erato races by geography, but the genomic region around optix, which controls red wing patterning, groups races by phenotype (Supple et al. 2013). A similar phenomenon occurs in H. melpomene (Hines et al. 2011). (B) However, wing patterns shared between H. melpomene and H. erato are due to convergent evolution because there is no shared genetic variation between these two distantly related species. (C) Wing-pattern mimicry has been passed among closely related co-mimics H. melpomene, H. timareta, and H. elevatus by interspecific hybridization (Heliconius Genome Consortium 2012). Evidence for adaptive introgression includes an enrichment of shared alleles (ABBA and BABA sites) near optix and phylogenetic clustering among phenotypes across species boundaries (topology 2).
At the other end of the taxonomic continuum is mimicry among the most distantly related Heliconius lineages, such as between H. erato and H. melpomene. This appears to be genuine convergence because even though there is evidence that these species are using the same genes, and possibly even the same regulatory regions, to generate matching color patterns (Supple et al. 2013), analysis of sequence variation at those genes shows no shared variation between species, indicating independent origins of co-mimetic phenotypes (Figure 7B). Between these two taxonomic extremes are co-mimetic species from the same Heliconius subclade, such as H. melpomene and H. timareta. In one of the more recent discoveries related to Heliconius mimicry, we now know that these closely related co-mimics use not only the same genes but also the exact same sequence variation to generate convergent wing patterns, not because of constraint or convergent molecular evolution, but because of adaptive gene flow between species.
Adaptive introgression
Brower (1996a) described a new species of Heliconius, H. tristero, that had the color pattern of H. melpomene but grouped with H. cydno based on DNA sequence and other morphological data. This was unexpected because H. melpomene and H. cydno are closely related, partially interfertile species, and their divergent color patterns have been shown to play an important role in mediating reproductive isolation. Giraldo et al. (2008), Merot et al. (2013), and Nadeau et al. (2014) have since revealed this to be a general phenomenon: there are populations of the H. cydno relative H. timareta mimicking H. melpomene all along the western side of the Andes mountains. Because hybridization between H. melpomene and the H. cydno/timareta clade is widespread, interspecific gene flow could be the source of their shared warning patterns. Indeed, Gilbert (2003) showed that much Heliconius color diversity can be recreated by interspecific hybridization. Another example where this may have occurred is H. elevatus, a species in the silvaniform clade that mimics H. melpomene, because there is evidence of hybridization and gene flow between these lineages as well (Dasmahapatra et al. 2007; Mallet et al. 2007; Kronforst 2008). Recent genomic analyses, including de novo assembly of the H. melpomene genome and targeted resequencing of mimicry loci from a variety of taxa, paired with analyses of SNP allele sharing, sequence divergence, and phylogenetic patterns (Heliconius Genome Consortium 2012; Pardo-Diaz et al. 2012; Smith and Kronforst 2013), point to adaptive introgression of color-pattern mimicry among H. melpomene, H. timareta, and H. elevatus (Figure 7C). It is worth noting that mimicry involves more than wing pattern: it potentially involves many aspects of ecology and locomotion, and it remains to be seen whether genes that contribute to these aspects of mimicry have also moved between species.
Hybrid speciation
Hybrid speciation is generally thought to be rare in animals (Mallet 2007; Mavarez and Linares 2008), but the process is facilitated when traits that mediate reproductive isolation are influenced in a direct way by hybridization (Jiggins et al. 2008). Divergent wing patterns generate multiple forms of reproductive isolation in Heliconius, both prezygotic and postzygotic. Since hybridization can transport color patterns between species (Heliconius Genome Consortium 2012; Pardo-Diaz et al. 2012), or recombine between species to produce totally new phenotypes (Gilbert 2003), this provides a mechanism by which hybridization may contribute to the evolution of reproductive isolation and the origin of species. There are multiple suspected cases of this phenomenon in the genus, the best documented of which is Heliconius heurippa (Salazar et al. 2005, 2008, 2010; Mavarez et al. 2006; Melo et al. 2009). H. heurippa has an intermediate, nonmimetic wing pattern that can be recreated by interbreeding red-banded H. melpomene and a yellow-banded species, either H. cydno or H. timareta (Mavarez et al. 2006). Furthermore, in mate choice trials, H. heurippa individuals prefer to approach, court, and mate with individuals that share their recombined wing pattern, as opposed to those of the parental species (Mavarez et al. 2006). Amazingly, experimentally recreated H. heurippa, produced by interbreeding H. melpomene and H. cydno, show similar assortative mate preference for the H. heurippa wing pattern (Melo et al. 2009). Detailed molecular genetic characterization further supports the hybrid origin of H. heurippa, showing that this species has a genome largely derived from the H. cydno/H. timareta clade but with a contribution from H. melpomene near the optix and kinesin genes that generate its red forewing band (Salazar et al. 2008, 2010).
H. elevatus provides a second likely example of potential hybrid speciation. Genomic analysis places H. elevatus as a very recently diverged taxon nested within another species, H. pardalinus, but it appears to have recently acquired its entire wing pattern from H. melpomene (Heliconius Genome Consortium 2012). Such rapid, recent divergence associated with mimicry introgression is suggestive of a hybrid speciation scenario although more work needs to be done to clarify the details. Currently, we do not know how many times color-pattern alleles have moved between species or how many times this may have contributed to speciation. However, based on everything we do know about Heliconius, it could be quite common.
Ecological speciation
Ecological speciation is a phenomenon by which divergent ecological selection contributes to the evolution of reproductive isolation between populations, eventually leading to the origin of species. Owing to wing patterning and divergence for mimicry, Heliconius is likely to provide multiple examples of ecological speciation in action (Jiggins 2008). Recently, genome sequencing, combined with our detailed understanding of the genetic basis of wing patterning, has permitted a new take on the question of ecological speciation in Heliconius, again finding that color patterns appear to drive divergence between species. Using various genome-wide sequencing approaches, from restriction site associated DNA (RAD) markers to full-genome resequencing, Nadeau et al. (2012, 2013), Kronforst et al. (2013), and Martin et al. (2013) examined patterns of divergence and gene flow among H. melpomene, H. cydno, and related species. Despite focusing on different taxa, geographic locations, and analytical methods, these studies all converged on a combined role for gene flow and selection in the speciation process. Notably, this work also found that the most recently diverged Heliconius taxa—subspecies, incipient species, and sister species—show the most pronounced divergence at color-pattern loci. This is consistent with the hypothesis that divergence for mimicry generates early reproductive isolation that eventually results in speciation. It is perhaps remarkable that lessons learned from decades of detailed behavioral and field studies are today born out in comparisons of the > 100 Heliconius genome sequences thus far analyzed.
Conclusions
The study of butterfly mimicry has been interwoven with the theory of natural selection since its origin >150 years ago (Darwin and Wallace 1858). Identifying the molecules behind mimicry is now allowing us to test some long-standing questions about general evolutionary principles, and the answers so far are surprising. From here, research focused on Heliconius mimicry genetics is likely to progress in a variety of directions, moving both deeper into mechanistic details and expanding out in a more comparative context. At a functional level, we still lack an understanding of the molecular, cellular, and developmental mechanisms that link mimicry genes to mimetic wing patterns. At a comparative level, the surface has just been scratched, but the initial results are fascinating, revealing for example, that optix has only very recently been co-opted to a role in color patterning in Heliconius and relatives (Monteiro 2012; Martin et al. 2014) while WntA appears to have a much older, more fundamental role in butterfly wing patterning (Martin and Reed 2014). In addition, a notable strength of the Heliconius system, provided by a long history of excellent field work, is that we know so much about the amazing behavior, ecology, and biogeography of the many diverse species in the genus. Perhaps the most exciting future prospect is to take this newly acquired genetic and genomic information back to the field to explore age-old questions in a classic system using totally new techniques.
Acknowledgments
We thank the long and venerable line of Heliconius researchers whose discoveries have made it the wonderful and tractable research system it is today and the Heliconius Genome Consortium, in particular Jim Mallet, Chris Jiggins, and Owen McMillan, for pushing modern, comparative genomics work forward. It is notable that much of the Heliconius biology that we review here, including chemical defense and subsequent warning coloration and mimicry, traces back to their host plant associations and peculiar pollen-feeding behavior. This review is dedicated to Larry Gilbert, whose discovery of pollen feeding in Heliconius was published 43 years ago.
Footnotes
Communicating editor: M. Turelli
Literature Cited
- Bates H. W., 1862. Contributions to an insect fauna of the Amazon Valley. Lepidoptera: Heliconidae. Trans. Linn. Soc. Lond. 23: 495–566. [Google Scholar]
- Bates H. W., 1864. The Naturalist on the River Amazons. A Record of Adventures, Habits of Animals, Sketches of Brazilian and Indian life, and Aspects of Nature Under the Equator, During Eleven Years of Travel. J. Murray, London. [Google Scholar]
- Baxter S. W., Papa R., Chamberlain N., Humphray S. J., Joron M., et al. , 2008. Convergent evolution in the genetic basis of Mullerian mimicry in Heliconius butterflies. Genetics 180: 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter S. W., Johnston S. E., Jiggins C. D., 2009. Butterfly speciation and the distribution of gene effect sizes fixed during adaptation. Heredity 102: 57–65. [DOI] [PubMed] [Google Scholar]
- Baxter S. W., Nadeau N. J., Maroja L. S., Wilkinson P., Counterman B. A., et al. , 2010. Genomic hotspots for adaptation: the population genetics of Müllerian mimicry in the Heliconius melpomene clade. PLoS Genet. 6: e1000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe W., 1955. Polymorphism in reared broods of Heliconius butterflies from Surinam and Trinidad. Zoologica 40: 139–143. [Google Scholar]
- Beldade P., Brakefield P. M., 2002. The genetics and evo-devo of butterfly wing patterns. Nat. Rev. Genet. 3: 442–452. [DOI] [PubMed] [Google Scholar]
- Beldade P., McMillan W. O., Papanicolaou A., 2008. Butterfly genomics eclosing. Heredity 100: 150–157. [DOI] [PubMed] [Google Scholar]
- Beltran M., Jiggins C. D., Brower A. V. Z., Bermingham E., Mallet J., 2007. Do pollen feeding, pupal-mating and larval gregariousness have a single origin in Heliconius butterflies? Inferences from multilocus DNA sequence data. Biol. J. Linn. Soc. Lond. 92: 221–239. [Google Scholar]
- Bennett D. C., Lamoreux M. L., 2003. The color loci of mice: a genetic century. Pigment Cell Res. 16: 333–344. [DOI] [PubMed] [Google Scholar]
- Benson W. W., 1972. Natural selection for Müllerian mimicry in Heliconius erato in Costa Rica. Science 176: 936–939. [DOI] [PubMed] [Google Scholar]
- Benson W. W., Brown K. S., Gilbert L. E., 1975. Coevolution of plants and herbivores: passion flower butterflies. Evolution 29: 659–680. [DOI] [PubMed] [Google Scholar]
- Boggs C. L., Smiley J. T., Gilbert L. E., 1981. Patterns of pollen exploitation by Heliconius butterflies. Oecologia 48: 284–289. [DOI] [PubMed] [Google Scholar]
- Briscoe A. D., Bybee S. M., Bernard G. D., Yuan F., Sison-Mangus M. P., et al. , 2010. Positive selection of a duplicated UV-sensitive visual pigment coincides with wing pigment evolution in Heliconius butterflies. Proc. Natl. Acad. Sci. USA 107: 3628–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower A. V. Z., 1994. Rapid morphological radiation and convergence among races of the butterfly Heliconius erato inferred from patterns of mitochondrial DNA evolution. Proc. Natl. Acad. Sci. USA 91: 6491–6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower A. V. Z., 1996a A new mimetic species of Heliconius (Lepidoptera: Nymphalidae), from southeastern Colombia, as revealed by cladistic analysis of mitochondrial DNA sequences. Zool. J. Linn. Soc. 116: 317–332. [Google Scholar]
- Brower A. V. Z., 1996b Parallel race formation and the evolution of mimicry in Heliconius butterflies: a phylogenetic hypothesis from mitochondrial DNA sequences. Evolution 50: 195–221. [DOI] [PubMed] [Google Scholar]
- Brown K. S., 1981. The biology of Heliconius and related genera. Annu. Rev. Entomol. 26: 427–456. [Google Scholar]
- Brown K. S., Benson W. W., 1974. Adaptive polymorphism associated with multiple Müllerian mimicry in Heliconius numata (Lepid. Nymph.). Biotropica 6: 205–228. [Google Scholar]
- Brown K. S., Sheppard P. M., Turner J. R. G., 1974. Quaternary refugia in tropical America: evidence from race formation in Heliconius butterflies. Proc. R. Soc. Lond. B Biol. Sci. 187: 369–378. [Google Scholar]
- Brown K. S., Trigo J. R., Francini R. B., Barros de Morais A. B., Motta P. C., 1991. Aposematic insects on toxic host plants: coevolution, colonization, and chemical emancipation, pp. 375–402 in Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions, edited by P. P. W., T. M. Lewinsohn, G. W. Fernandes. and W. W. Benson. John Wiley & Sons, New York. [Google Scholar]
- Caro T., 2005. The adaptive significance of coloration in mammals. Bioscience 55: 125–136. [Google Scholar]
- Chamberlain N. L., Hill R. I., Kapan D. D., Gilbert L. E., Kronforst M. R., 2009. Polymorphic butterfly reveals the missing link in ecological speciation. Science 326: 847–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain N. L., Hill R. I., Baxter S. W., Jiggins C. D., Kronforst M. R., 2011. Comparative population genetics of a mimicry locus among hybridizing Heliconius butterfly species. Heredity 107: 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth D., Charlesworth B., 1975. Theoretical genetics of Batesian mimicry II. Evolution of supergenes. J. Theor. Biol. 55: 305–324. [DOI] [PubMed] [Google Scholar]
- Clarke C. A., Sheppard P. M., 1960. Super-genes and mimicry. Heredity 14: 175–185. [Google Scholar]
- Cott H. B., 1940. Adaptive Colouration in Animals. Methuen and Co., London. [Google Scholar]
- Counterman B. A., Araujo-Perez F., Hines H. M., Baxter S. W., Morrison C. M., et al. , 2010. Genomic hotspots for adaptation: the population genetics of Müllerian mimicry in Heliconius erato. PLoS Genet. 6: e1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthill J. H., Charleston M., 2012. Phylogenetic codivergence supports coevolution of mimetic Heliconius butterflies. PLoS ONE 7: e36464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. R., 1863. [Review of] Contributions to an insect fauna of the Amazon Valley. By Henry Walter Bates, Esq. Transact. Linnean Soc. Vol. XXIII. 1862, p. 495. Nat. Hist. Rev. 3: 219–224. [Google Scholar]
- Darwin C. R., Wallace A. R., 1858. On the tendency of species to form varieties; and on the perpetuation of varieties and species by natural means of selection. Zool. J. Linn. Soc. 3: 46–50. [Google Scholar]
- Dasmahapatra K. K., Silva-Vasquez A., Chung J. W., Mallet J., 2007. Genetic analysis of a wild-caught hybrid between non-sister Heliconius butterfly species. Biol. Lett. 3: 660–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinert E. I., Longino J. T., Gilbert L. E., 1994. Mate competition in butterflies. Nature 370: 23–24. [Google Scholar]
- Dunlap-Pianka H., Boggs C. L., Gilbert L. E., 1977. Ovarian dynamics in Heliconiine butterflies: programmed senescence vs. eternal youth. Science 197: 487–490. [DOI] [PubMed] [Google Scholar]
- Eltringham H., 1916. On specific and mimetic relationships in the genus Heliconius. Trans. Entomol. Soc. London 1916: 101–148. [Google Scholar]
- Emsley M. G., 1964. The geographical distribution of the color-pattern components of Heliconius erato and Heliconius melpomene with genetical evidence for the systematic relationship between the two species. Zoologica NY 49: 245–286. [Google Scholar]
- Engler H. S., Spencer K. C., Gilbert L. E., 2000. Preventing cyanide release from leaves. Nature 406: 144–145. [DOI] [PubMed] [Google Scholar]
- Engler-Chaouat H. S., Gilbert L. E., 2007. De novo synthesis vs. sequestration: negatively correlated metabolic traits and the evolution of host plant specialization in cyanogenic butterflies. J. Chem. Ecol. 33: 25–42. [DOI] [PubMed] [Google Scholar]
- Estrada C., Gilbert L. E., 2010. Host plants and immatures as mate-searching cues in Heliconius butterflies. Anim. Behav. 80: 231–239. [Google Scholar]
- Estrada C., Jiggins C. D., 2002. Patterns of pollen feeding and habitat preference among Heliconius species. Ecol. Entomol. 27: 448–456. [Google Scholar]
- Estrada C., Yildizhan S., Schulz S., Gilbert L. E., 2010. Sex-specific chemical cues from immatures facilitate the evolution of mate guarding in Heliconius butterflies. Proc. Biol. Sci. 277: 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson L. C., Jiggins C. D., 2009. Shared and divergent expression domains on mimetic Heliconius wings. Evol. Dev. 11: 498–512. [DOI] [PubMed] [Google Scholar]
- Ferguson L., Lee S. F., Chamberlain N., Nadeau N., Joron M., et al. , 2010. Characterization of a hotspot for mimicry: assembly of a butterfly wing transcriptome to genomic sequence at the HmYb/Sb locus. Mol. Ecol. 19(Suppl. 1): 240–254. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. D., Briscoe A. D., Reed R. D., 2014. Warning signals are seductive: relative contributions of color and pattern to predator avoidance and mate attraction in Heliconius butterflies. Evolution 68: 3410–3420. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., 1930. The Genetical Theory of Natural Selection. Clarendon Press, Oxford. [Google Scholar]
- Flanagan N. S., Tobler A., Davison A., Pybus O. G., Kapan D. D., et al. , 2004. Historical demography of Müllerian mimicry in the neotropical Heliconius butterflies. Proc. Natl. Acad. Sci. USA 101: 9704–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J. R., Imhoff V. E., Martin A., Savage W. K., Chamberlain N. L., et al. , 2014. Ancient homology underlies adaptive mimetic diversity across butterflies. Nat. Commun. 5: 4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiradella H., 1991. Light and color on the wing: structural colors in butterflies and moths. Appl. Opt. 30: 3492–3500. [DOI] [PubMed] [Google Scholar]
- Gilbert L. E., 1971. Butterfly-plant coevolution: Has Passiflora adenopoda won the selectional race with Heliconiine butterflies? Science 172: 585–586. [DOI] [PubMed] [Google Scholar]
- Gilbert L. E., 1972. Pollen feeding and reproductive biology of Heliconius butterflies. Proc. Natl. Acad. Sci. USA 69: 1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L. E., 1975. Ecological consequences of a coevolved mutualism between butterflies and plants, pp. 210–240 in Coevolution of Animals and Plants, edited by Gilbert L. E., Raven P. R. University of Texas Press, Austin, TX. [Google Scholar]
- Gilbert L. E., 1976. Postmating female odor in Heliconius butterflies: A male-contributed antiaphrodisiac? Science 193: 419–420. [DOI] [PubMed] [Google Scholar]
- Gilbert L. E., 1991. Biodiversity of a Central American Heliconius community: pattern, process, and problems, pp. 403–427 in Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions, edited by P. W. Price, T. M. Lewinsohn, G. W. Fernandes, and W. W. Benson. John Wiley & Sons, New York. [Google Scholar]
- Gilbert L. E., 2003. Adaptive novelty through introgression in Heliconius wing patterns: evidence for a shared genetic “tool box” from synthetic hybrid zones and a theory of diversification, pp. 281–318 in Ecology and Evolution Taking Flight: Butterflies as Model Systems, edited by Boggs C. L., Watt W. B., Ehrlich P. R. University of Chicago Press, Chicago. [Google Scholar]
- Gilbert L. E., Forrest H. S., Schultz T. D., Harvey D. J., 1988. Correlations of ultrastructure and pigmentation suggest how genes control development of wing scales in Heliconius butterflies. J. Res. Lepid. 26: 141–160. [Google Scholar]
- Giraldo N., Salazar C., Jiggins C. D., Bermingham E., Linares M., 2008. Two sisters in the same dress: Heliconius cryptic species. BMC Evol. Biol. 8: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila M., Kaila L., Mutanen M., Pena C., Wahlberg N., 2012. Cretaceous origin and repeated tertiary diversification of the redefined butterflies. Proc. Biol. Sci. 279: 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heliconius Genome Consortium , 2012. Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. I., Gilbert L. E., Kronforst M. R., 2013. Cryptic genetic and wing pattern diversity in a mimetic Heliconius butterfly. Mol. Ecol. 22: 2760–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines H. M., Counterman B. A., Papa R., Albuquerque de Moura P., Cardoso M. Z., et al. , 2011. Wing patterning gene redefines the mimetic history of Heliconius butterflies. Proc. Natl. Acad. Sci. USA 108: 19666–19671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines H. M., Papa R., Ruiz M., Papanicolaou A., Wang C., et al. , 2012. Transcriptome analysis reveals novel patterning and pigmentation genes underlying Heliconius butterfly wing pattern variation. BMC Genomics 13: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra H. E., 2006. Genetics, development and evolution of adaptive pigmentation in vertebrates. Heredity 97: 222–234. [DOI] [PubMed] [Google Scholar]
- Hoekstra H. E., Coyne J. A., 2007. The locus of evolution: evo devo and the genetics of adaptation. Evolution 61: 995–1016. [DOI] [PubMed] [Google Scholar]
- Hubbard J. K., Uy J. A., Hauber M. E., Hoekstra H. E., Safran R. J., 2010. Vertebrate pigmentation: from underlying genes to adaptive function. Trends Genet. 26: 231–239. [DOI] [PubMed] [Google Scholar]
- Jiggins C. D., 2008. Ecological speciation in mimetic butterflies. Bioscience 58: 541–548. [Google Scholar]
- Jiggins C. D., McMillan W. O., 1997. The genetic basis of an adaptive radiation: warning colour in two Heliconius species. Proc. Biol. Sci. 264: 1167–1175. [Google Scholar]
- Jiggins C. D., McMillan W. O., King P., Mallet J., 1997. The maintenance of species differences across a Heliconius hybrid zone. Heredity 79: 495–505. [Google Scholar]
- Jiggins C. D., Naisbit R. E., Coe R. L., Mallet J., 2001. Reproductive isolation caused by colour pattern mimicry. Nature 411: 302–305. [DOI] [PubMed] [Google Scholar]
- Jiggins C. D., Estrada C., Rodrigues A., 2004. Mimicry and the evolution of premating isolation in Heliconius melpomene Linnaeus. J. Evol. Biol. 17: 680–691. [DOI] [PubMed] [Google Scholar]
- Jiggins C. D., Mavarez J., Beltran M., McMillan W. O., Johnston J. S., et al. , 2005. A genetic linkage map of the mimetic butterfly Heliconius melpomene. Genetics 171: 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiggins C. D., Salazar C., Linares M., Mavarez J., 2008. Review. Hybrid trait speciation and Heliconius butterflies. Philos. Trans. R. Soc. Lond. B 363: 3047–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. T., Salazar P. A., R. H. ffrench-Constant, C. D. Jiggins, and M. Joron, 2012. Evolution of a mimicry supergene from a multilocus architecture. Proc. Biol. Sci. 279: 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joron M., Jiggins C. D., Papanicolaou A., McMillan W. O., 2006a Heliconius wing patterns: an evo-devo model for understanding phenotypic diversity. Heredity 97: 157–167. [DOI] [PubMed] [Google Scholar]
- Joron M., Papa R., Beltran M., Chamberlain N., Mavarez J., et al. , 2006b A conserved supergene locus controls colour pattern diversity in Heliconius butterflies. PLoS Biol. 4: e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joron M., Frezal L., Jones R. T., Chamberlain N. L., Lee S. F., et al. , 2011. Chromosomal rearrangements maintain a polymorphic supergene controlling butterfly mimicry. Nature 477: 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapan D. D., 2001. Three-butterfly system provides a field test of Müllerian mimicry. Nature 409: 338–340. [DOI] [PubMed] [Google Scholar]
- Kapan D. D., Flanagan N. S., Tobler A., Papa R., Reed R. D., et al. , 2006. Localization of Müllerian mimicry genes on a dense linkage map of Heliconius erato. Genetics 173: 735–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak K. M., Wahlberg N., Neild A., Dasmahapatra K. K., Mallet J., et al. , 2015. Multilocus species trees show the recent adaptive radiation of the mimetic Heliconius butterflies. Syst. Biol. DOI: 10.1093/sysbio/syv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronforst M. R., 2008. Gene flow persists millions of years after speciation in Heliconius butterflies. BMC Evol. Biol. 8: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronforst M. R., Kapan D. D., Gilbert L. E., 2006a Parallel genetic architecture of parallel adaptive radiations in mimetic Heliconius butterflies. Genetics 174: 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronforst M. R., Young L. G., Kapan D. D., McNeely C., O’Neill R. J., et al. , 2006b Linkage of butterfly mate preference and wing color preference cue at the genomic location of wingless. Proc. Natl. Acad. Sci. USA 103: 6575–6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronforst M. R., Young L. G., Gilbert L. E., 2007. Reinforcement of mate preference among hybridizing Heliconius butterflies. J. Evol. Biol. 20: 278–285. [DOI] [PubMed] [Google Scholar]
- Kronforst M. R., Barsh G. S., Kopp A., Mallet J., Monteiro A., et al. , 2012. Unraveling the thread of nature’s tapestry: the genetics of diversity and convergence in animal pigmentation. Pigment Cell Melanoma Res. 25: 411–433. [DOI] [PubMed] [Google Scholar]
- Kronforst M. R., Hansen M. E., Crawford N. G., Gallant J. R., Zhang W., et al. , 2013. Hybridization reveals the evolving genomic architecture of speciation. Cell Reports 5: 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunte K., Zhang W., Tenger-Trolander A., Palmer D. H., Martin A., et al. , 2014. doublesex is a mimicry supergene. Nature 507: 229–232. [DOI] [PubMed] [Google Scholar]
- Leigh G. F., Poulton E. B., 1909. XX. Heredity in six families of Papilio dardanus, Brown, Subspecies cenea, Stoll., bred at Durban. Trans. Roy. Entomol. Soc. Lond. 56: 427–445. [Google Scholar]
- Linares M., 1996. The genetics of the mimetic coloration in the butterfly Heliconius cydno weymeri. J. Hered. 87: 142–149. [Google Scholar]
- Linares M., 1997. The ghost of mimicry past: laboratory reconstruction of an extinct butterfly ‘race’. Heredity 78: 628–635. [Google Scholar]
- Mallet J., 1986a Dispersal and gene flow in a butterfly with home range behavior: Heliconius erato (Lepidoptera, Nymphalidae). Oecologia 68: 210–217. [DOI] [PubMed] [Google Scholar]
- Mallet J., 1986b Gregarious roosting and home range in Heliconius butterflies. Natl. Geogr. Res. 2: 198–215. [Google Scholar]
- Mallet J., 1989. The genetics of warning colour in Peruvian hybrid zones of Heliconius erato and H. melpomene. Proc. R. Soc. Lond. B Biol. Sci. 236: 163–185. [Google Scholar]
- Mallet J., 1999. Causes and consequences of a lack of coevolution in Müllerian mimicry. Evol. Ecol. 13: 777–806. [Google Scholar]
- Mallet J., 2007. Hybrid speciation. Nature 446: 279–283. [DOI] [PubMed] [Google Scholar]
- Mallet J., 2010. Shift happens! Shifting balance and the evolution of diversity in warning colour and mimicry. Ecol. Entomol. 35: 90–104. [Google Scholar]
- Mallet J., Barton N. H., 1989. Strong natural selection in a warning-color hybrid zone. Evolution 43: 421–431. [DOI] [PubMed] [Google Scholar]
- Mallet J., Joron M., 1999. Evolution of diversity in warning color and mimicry: polymorphisms, shifting balance, and speciation. Annu. Rev. Ecol. Syst. 30: 201–233. [Google Scholar]
- Mallet J., Barton N., Lamas G., Santisteban J., Muedas M., et al. , 1990. Estimates of selection and gene flow from measures of cline width and linkage disequilibrium in Heliconius hybrid zones. Genetics 124: 921–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet J., Beltran M., Neukirchen W., Linares M., 2007. Natural hybridization in Heliconiine butterflies: the species boundary as a continuum. BMC Evol. Biol. 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manceau M., Domingues V. S., Linnen C. R., Rosenblum E. B., Hoekstra H. E., 2010. Convergence in pigmentation at multiple levels: mutations, genes and function. Phil. Trans. R. Soc. B 365: 2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroja L. S., Alschuler R., McMillan W. O., Jiggins C. D., 2012. Partial complementarity of the mimetic yellow bar phenotype in Heliconius butterflies. PLoS ONE 7: e48627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Reed R. D., 2014. Wnt signaling underlies evolution and development of the butterfly wing pattern symmetry systems. Dev. Biol. 395: 367–378. [DOI] [PubMed] [Google Scholar]
- Martin A., Papa R., Nadeau N. J., Hill R. I., Counterman B. A., et al. , 2012. Diversification of complex butterfly wing patterns by repeated regulatory evolution of a Wnt ligand. Proc. Natl. Acad. Sci. USA 109: 12632–12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., McCulloch K. J., Patel N. H., Briscoe A. D., Gilbert L. E., et al. , 2014. Multiple recent co-options of Optix associated with novel traits in adaptive butterfly wing radiations. Evodevo 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. H., Dasmahapatra K. K., Nadeau N. J., Salazar C., Walters J. R., et al. , 2013. Genome-wide evidence for speciation with gene flow in Heliconius butterflies. Genome Res. 23: 1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavarez J., Linares M., 2008. Homoploid hybrid speciation in animals. Mol. Ecol. 17: 4181–4185. [DOI] [PubMed] [Google Scholar]
- Mavarez J., Salazar C. A., Bermingham E., Salcedo C., Jiggins C. D., et al. , 2006. Speciation by hybridization in Heliconius butterflies. Nature 441: 868–871. [DOI] [PubMed] [Google Scholar]
- McMillan W. O., Jiggins C. D., Mallet J., 1997. What initiates speciation in passion-vine butterflies? Proc. Natl. Acad. Sci. USA 94: 8628–8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan W. O., Monteiro A., Kapan D. D., 2002. Development and evolution on the wing. Trends Ecol. Evol. 17: 125–133. [Google Scholar]
- Melo M. C., Salazar C., Jiggins C. D., Linares M., 2009. Assortative mating preferences among hybrids offers a route to hybrid speciation. Evolution 63: 1660–1665. [DOI] [PubMed] [Google Scholar]
- Merchan H. A., Jiggins C. D., Linares M., 2005. A narrow Heliconius cydno (Nymphalidae; Heliconiini) hybrid zone with differences in morph sex ratios. Biotropica 37: 119–128. [Google Scholar]
- Merot C., Mavarez J., Evin A., Dasmahapatra K. K., Mallet J., et al. , 2013. Genetic differentiation without mimicry shift in a pair of hybridizing Heliconius species (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. Lond. 109: 830–847. [Google Scholar]
- Merrill R. M., Gompert Z., Dembeck L. M., Kronforst M. R., McMillan W. O., et al. , 2011a Mate preference across the speciation continuum in a clade of mimetic butterflies. Evolution 65: 1489–1500. [DOI] [PubMed] [Google Scholar]
- Merrill R. M., Van Schooten B., Scott J. A., Jiggins C. D., 2011b Pervasive genetic associations between traits causing reproductive isolation in Heliconius butterflies. Proc. Biol. Sci. 278: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill R. M., Wallbank R. W., Bull V., Salazar P. C., Mallet J., et al. , 2012. Disruptive ecological selection on a mating cue. Proc. Biol. Sci. 279: 4907–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill R. M., Chia A., Nadeau N. J., 2014. Divergent warning patterns contribute to assortative mating between incipient Heliconius species. Ecol. Evol. 4: 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro A., 2012. Gene regulatory networks reused to build novel traits: co-option of an eye-related gene regulatory network in eye-like organs and red wing patches on insect wings is suggested by optix expression. BioEssays 34: 181–186. [DOI] [PubMed] [Google Scholar]
- Müller F., 1879. Ituna and Thyridia; a remarkable case of mimicry in butterflies. Trans. Entomol. Soc. London 1879: xx–xxix. [Google Scholar]
- Nadeau N. J., Jiggins C. D., 2010. A golden age for evolutionary genetics? Genomic studies of adaptation in natural populations. Trends Genet. 26: 484–492. [DOI] [PubMed] [Google Scholar]
- Nadeau N. J., Whibley A., Jones R. T., Davey J. W., Dasmahapatra K. K., et al. , 2012. Genomic islands of divergence in hybridizing Heliconius butterflies identified by large-scale targeted sequencing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367: 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau N. J., Martin S. H., Kozak K. M., Salazar C., Dasmahapatra K. K., et al. , 2013. Genome-wide patterns of divergence and gene flow across a butterfly radiation. Mol. Ecol. 22: 814–826. [DOI] [PubMed] [Google Scholar]
- Nadeau N. J., Ruiz M., Salazar P., Counterman B., Medina J. A., et al. , 2014. Population genomics of parallel hybrid zones in the mimetic butterflies, H. melpomene and H. erato. Genome Res. 24: 1316–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrstedt, A., and R. H. Davis, 1981 The occurrence of the cyanoglucosides, linamarin and lotaustralin, in Acraea and Heliconius butterflies. Comp. Biochem. Physiol. 68B: 575–577. [Google Scholar]
- Nahrstedt A., Davis R. H., 1983. Occurrence, variation and biosynthesis of the cyanogenic glucosides linamarin and lotaustralin in species of the Heliconiini (Insecta, Lepidoptera). Comp. Biochem. Physiol. 75B: 65–73. [Google Scholar]
- Naisbit R. E., Jiggins C. D., Mallet J., 2001. Disruptive sexual selection against hybrids contributes to speciation between Heliconius cydno and Heliconius melpomene. Proc. Biol. Sci. 268: 1849–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbit R. E., Jiggins C. D., Linares M., Salazar C., Mallet J., 2002. Hybrid sterility, Haldane’s rule and speciation in Heliconius cydno and H. melpomene. Genetics 161: 1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbit R. E., Jiggins C. D., Mallet J., 2003. Mimicry: developmental genes that contribute to speciation. Evol. Dev. 5: 269–280. [DOI] [PubMed] [Google Scholar]
- Nicholson A. J., 1927. A new theory of mimicry in insects. Aust. Zool. 5: 10–104. [Google Scholar]
- Nijhout H. F., 1986. Pattern and pattern diversity on Lepidopteran wings. Bioscience 36: 527–533. [Google Scholar]
- Nijhout H. F., 1991. The Development and Evolution of Butterfly Wing Patterns. Smithsonian Institution Press, Washington, D.C. [Google Scholar]
- Nijhout H. F., Wray G. A., 1988. Homologies in the colour patterns of the genus Heliconius (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. Lond. 33: 345–365. [Google Scholar]
- Nijhout H. F., Wray G., Gilbert L. E., 1990. An analysis of the phenotypic effects of certain color pattern genes in Heliconius (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. Lond. 40: 357–372. [Google Scholar]
- Papa R., Martin A., Reed R. D., 2008a Genomic hotspots of adaptation in butterfly wing pattern evolution. Curr. Opin. Genet. Dev. 18: 559–564. [DOI] [PubMed] [Google Scholar]
- Papa R., Morrison C. M., Walters J. R., Counterman B. A., Chen R., et al. , 2008b Highly conserved gene order and numerous novel repetitive elements in genomic regions linked to wing pattern variation in Heliconius butterflies. BMC Genomics 9: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa R., Kapan D. D., Counterman B. A., Maldonado K., Lindstrom D. P., et al. , 2013. Multi-allelic major effect genes interact with minor effect QTLs to control adaptive color pattern variation in Heliconius erato. PLoS ONE 8: e57033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou A., Joron M., McMillan W. O., Blaxter M. L., Jiggins C. D., 2005. Genomic tools and cDNA derived markers for butterflies. Mol. Ecol. 14: 2883–2897. [DOI] [PubMed] [Google Scholar]
- Parchem R. J., Perry M. W., Patel N. H., 2007. Patterns on the insect wing. Curr. Opin. Genet. Dev. 17: 300–308. [DOI] [PubMed] [Google Scholar]
- Pardo-Diaz C., Jiggins C. D., 2014. Neighboring genes shaping a single adaptive mimetic trait. Evol. Dev. 16: 3–12. [DOI] [PubMed] [Google Scholar]
- Pardo-Diaz C., Salazar C., Baxter S. W., Merot C., Figueiredo-Ready W., et al. , 2012. Adaptive introgression across species boundaries in Heliconius butterflies. PLoS Genet. 8: e1002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penz C. M., 1999. Higher level phylogeny for the passion-vine butterflies (Nymphalidae, Heliconiinae) based on early stage and adult morphology. Zool. J. Linn. Soc. 127: 277–344. [Google Scholar]
- Pringle E. G., Baxter S. W., Webster C. L., Papanicolaou A., Lee S. F., et al. , 2007. Synteny and chromosome evolution in the Lepidoptera: evidence from mapping in Heliconius melpomene. Genetics 177: 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnett R. C., 1915. Mimicry in Butterflies. Cambridge University Press, London. [Google Scholar]
- Quek S. P., Counterman B. A., Albuquerque de Moura P., Cardoso M. Z., Marshall C. R., et al. , 2010. Dissecting comimetic radiations in Heliconius reveals divergent histories of convergent butterflies. Proc. Natl. Acad. Sci. USA 107: 7365–7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. D., McMillan W. O., Nagy L. M., 2008. Gene expression underlying adaptive variation in Heliconius wing patterns: non-modular regulation of overlapping cinnabar and vermilion prepatterns. Proc. Biol. Sci. 275: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. D., Papa R., Martin A., Hines H. M., Counterman B. A., et al. , 2011. optix drives the repeated convergent evolution of butterfly wing pattern mimicry. Science 333: 1137–1141. [DOI] [PubMed] [Google Scholar]
- Salazar C. A., Jiggins C. D., Arias C. F., Tobler A., Bermingham E., et al. , 2005. Hybrid incompatibility is consistent with a hybrid origin of Heliconius heurippa Hewitson from its close relatives, Heliconius cydno Doubleday and Heliconius melpomene Linnaeus. J. Evol. Biol. 18: 247–256. [DOI] [PubMed] [Google Scholar]
- Salazar C., Jiggins C. D., Taylor J. E., Kronforst M. R., Linares M., 2008. Gene flow and the genealogical history of Heliconius heurippa. BMC Evol. Biol. 8: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar C., Baxter S. W., Pardo-Diaz C., Wu G., Surridge A., et al. , 2010. Genetic evidence for hybrid trait speciation in Heliconius butterflies. PLoS Genet. 6: e1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S., Yildizhan S., Stritzke K., Estrada C., Gilbert L. E., 2007. Macrolides from the scent glands of the tropical butterflies Heliconius cydno and Heliconius pachinus. Org. Biomol. Chem. 5: 3434–3441. [DOI] [PubMed] [Google Scholar]
- Schulz S., Estrada C., Yildizhan S., Boppre M., Gilbert L. E., 2008. An antiaphrodisiac in Heliconius melpomene butterflies. J. Chem. Ecol. 34: 82–93. [DOI] [PubMed] [Google Scholar]
- Schwander T., Libbrecht R., Keller L., 2014. Supergenes and complex phenotypes. Curr. Biol. 24: R288–R294. [DOI] [PubMed] [Google Scholar]
- Sheppard P. M., 1963. Some genetic studies of Muellerian mimics in butterflies of the genus Heliconius. Zoologica 48: 145–154. [Google Scholar]
- Sheppard P. M., Turner J. R. G., Brown K. S., Benson W. W. and S. M. C., 1985. Genetics and the evolution of Muellerian mimicry in Heliconius butterflies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 308: 433–610. [Google Scholar]
- Smiley J., 1978. Plant chemistry and the evolution of host specificity: new evidence from Heliconius and Passiflora. Science 201: 745–747. [DOI] [PubMed] [Google Scholar]
- Smith J., Kronforst M. R., 2013. Do Heliconius butterfly species exchange mimicry alleles? Biol. Lett. 9: 20130503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supple M. A., Hines H. M., Dasmahapatra K. K., Lewis J. J., Nielsen D. M., et al. , 2013. Genomic architecture of adaptive color pattern divergence and convergence in Heliconius butterflies. Genome Res. 23: 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supple M., Papa R., Counterman B., McMillan W. O., 2014. The genomics of an adaptive radiation: insights across the Heliconius speciation continuum. Adv. Exp. Med. Biol. 781: 249–271. [DOI] [PubMed] [Google Scholar]
- Surridge A. K., Lopez-Gomollon S., Moxon S., Maroja L. S., Rathjen T., et al. , 2011. Characterisation and expression of microRNAs in developing wings of the neotropical butterfly Heliconius melpomene. BMC Genomics 12: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M. J., Jiggins C. D., 2014. Supergenes and their role in evolution. Heredity 113: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans M. J., Baxter S. W., Clark R., Heckel D. G., Vogel H., et al. , 2014. Comparative genomics of the mimicry switch in Papilio dardanus. Proc. Biol. Sci. 281: pii: 20140465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler A., Kapan D., Flanagan N. S., Gonzalez C., Peterson E., et al. , 2005. First-generation linkage map of the warningly colored butterfly Heliconius erato. Heredity 94: 408–417. [DOI] [PubMed] [Google Scholar]
- True J. R., 2003. Insect melanism: the molecules matter. Trends Ecol. Evol. 18: 640–647. [Google Scholar]
- Turner J. R. G., 1971. The genetics of some polymorphic forms of the butterflies Heliconius melpomene (Linnaeus) and H. erato (Linnaeus). II. The hybridization of subspecies from Surinam and Trinidad. Zoologica 56: 125–157. [Google Scholar]
- Turner J. R. G., 1976. Adaptive radiation and convergence in subdivisions of the butterfly genus Heliconius (Lepidoptera: Nymphalidae). Zool. J. Linn. Soc. 58: 297–308. [Google Scholar]
- Turner J. R. G., 1977a A Bibliography of Heliconius and the Related Genera. SUNY-Stony Brook, Stony Brook, NY. [Google Scholar]
- Turner J. R. G., 1977b Butterfly mimicry: the genetical evolution of an adaptation. Evol. Biol. 10: 163–206. [Google Scholar]
- Turner J. R. G., 1981. Adaptation and evolution in Heliconius: a defense of Neo Darwinism. Annu. Rev. Ecol. Syst. 12: 99–121. [Google Scholar]
- Turner J. R. G., Crane J., 1962. The genetics of some polymorphic forms of the butterflies Heliconius melpomene Linnaeus and H. erato Linnaeus, I: Major genes. Zoologica 47: 141–152. [Google Scholar]
- Turner J. R. G., Mallet J. L. B., 1996. Did forest islands drive the diversity of warningly coloured butterflies? Biotic drift and the shifting balance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351: 835–845. [Google Scholar]
- Turner J. R., Johnson M. S., Eanes W. F., 1979. Contrasted modes of evolution in the same genome: allozymes and adaptive change in Heliconius. Proc. Natl. Acad. Sci. USA 76: 1924–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. C., Joron M., Jiggins C. D., 2010. Signatures of selection in loci governing major colour patterns in Heliconius butterflies and related species. BMC Evol. Biol. 10: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F., Bernard G. D., Le J., Briscoe A. D., 2010. Contrasting modes of evolution of the visual pigments in Heliconius butterflies. Mol. Biol. Evol. 27: 2392–2405. [DOI] [PubMed] [Google Scholar]
- Zaccardi G., Kelber A., Sison-Mangus M. P., Briscoe A. D., 2006. Color discrimination in the red range with only one long-wavelength sensitive opsin. J. Exp. Biol. 209: 1944–1955. [DOI] [PubMed] [Google Scholar]