Abstract
The identity of a given cell type is determined by the expression of a set of genes sharing common cis-regulatory motifs and being regulated by shared transcription factors. Here, we identify cis and trans regulatory elements that drive gene expression in the bilateral sensory neuron ASJ, located in the head of the nematode Caenorhabditis elegans. For this purpose, we have dissected the promoters of the only two genes so far reported to be exclusively expressed in ASJ, trx-1 and ssu-1. We hereby identify the ASJ motif, a functional cis-regulatory bipartite promoter region composed of two individual 6 bp elements separated by a 3 bp linker. The first element is a 6 bp CG-rich sequence that presumably binds the Sp family member zinc-finger transcription factor SPTF-1. Interestingly, within the C. elegans nervous system SPTF-1 is also found to be expressed only in ASJ neurons where it regulates expression of other genes in these neurons and ASJ cell fate. The second element of the bipartite motif is a 6 bp AT-rich sequence that is predicted to potentially bind a transcription factor of the homeobox family. Together, our findings identify a specific promoter signature and SPTF-1 as a transcription factor that functions as a terminal selector gene to regulate gene expression in C. elegans ASJ sensory neurons.
Keywords: Caenorhabditis elegans, ASJ neuron, promoter, thioredoxin, ASJ motif, SPTF-1 transcription factor
DURING development, spatiotemporal regulation is essential for the generation of the cell-type diversity found in multicellular organisms. The identity of any cell type is driven by the combinatorial interaction of specific trans-acting factors, mainly microRNAs and transcription factors (TFs), with cis-regulatory elements present in noncoding DNA sequences within or near any given gene. This physical interaction is necessary for the activation and maintenance of gene transcription (Davidson 2006; Levine 2010; Bulger and Groudine 2011). However, the identification of cis- and trans-acting elements is complex as TFs bind to short and degenerate sequences that appear at high frequency in the genome. Furthermore, TFs sometimes regulate expression by interacting with other DNA-bound transcription factors and/or coregulators, instead of directly binding DNA. In this case, TFs are recruited to genomic locations lacking their binding site, further complicating the process of identification (Junion et al. 2012).
In multicellular organisms, individual cell types are defined by the expression of sets of genes that are coregulated by common cis-regulatory motifs, termed terminal differentiation genes (Morgan 1934; Davidson 2001; Etchberger et al. 2007; Flames and Hobert 2009). In turn, the genes that control these terminal differentiation genes usually code for postmitotically expressed transcription factors that directly bind to the common cis-regulatory motifs and are termed terminal selector genes (Hobert 2008).
Sequence comparisons of evolutionary divergent organisms have confirmed the conservation of gene-coding sequences throughout millions of years of evolution, as well as pointed out the presence of highly conserved noncoding DNA regions (Bejerano et al. 2004; Sandelin et al. 2004; Woolfe et al. 2005). Similar to mutations in coding sequences, variations in cis-regulatory regions can also generate phenotypic diversity (Wray 2007). Therefore, the identification of cis- and trans-regulatory elements and the study of the molecular mechanisms that control gene expression are essential in developmental biology.
The nematode Caenorhabditis elegans combines anatomical simplicity with relatively complex behaviors like sensory responses to touch, smell, taste, and temperature, as well as male mating, social behavior, learning, or memory (Rankin 2002; de Bono 2003). Although a large morphological and evolutionary distance exists between worms and humans, they share many conserved genes and most of the signaling pathways (Lai et al. 2000). The C. elegans nervous system is one of the most complex tissues in worms and is composed of 302 neurons in the adult hermaphrodite, which represents approximately one-third of its total number of somatic cells (White et al. 1986). The ability to monitor and manipulate gene expression in single neurons makes this nematode the ideal model to dissect the cis- and trans-regulation of neuron-specific gene expression.
Several cis-trans modules regulating gene expression in defined neuron types have been identified in C. elegans. For example, the CHE-1 terminal selector directly induces the expression of many terminal differentiation genes in the gustatory neurons ASE and controls the expression of various regulatory factors that trigger the asymmetric differentiation of the ASE neurons (Etchberger et al. 2007, 2009). Likewise, the LIM homeodomain transcription factor TTX-3 acts as a terminal selector to drive the terminal differentiation program of the serotonergic NSM neurons and the cholinergic interneurons AIY and AIA. The type of differentiation program controlled by TTX-3 in different neuron types is specified by a distinctive combination of additional transcription factors (Wenick and Hobert 2004; Zhang et al. 2014). Similar genetic studies on other neuronal types, including AWB (Nokes et al. 2009), AWC (Kim et al. 2010), and ciliated (Swoboda et al. 2000) and dopaminergic (Flames and Hobert 2009; Doitsidou et al. 2013) neurons have generated a unique understanding of the cis and trans mechanisms that initiate single neuronal identities and are responsible for cellular diversity and organism complexity.
C. elegans ASJ neurons are a bilaterally symmetric pair of ciliated sensory neurons located in the amphid, the main chemosensory organ of the nematode. ASJ controls entry and exit from the dauer stage (Bargmann and Horvitz 1991; Schackwitz et al. 1996; Cornils et al. 2011), an alternative developmental stage whereby the larva survives harsh conditions (Riddle et al. 1981). C. elegans ASJ neurons also play a role in the regulation of aging (Alcedo and Kenyon 2004), photoreception (Ward et al. 2008), and cold habituation (Ohta et al. 2014).
To date, only the genes trx-1 (thioredoxin-1) and ssu-1 (alcohol sulfotransferase-1) have been reported to be expressed exclusively in ASJ neurons (Carroll et al. 2006; Miranda-Vizuete et al. 2006). TRX-1 belongs to the thioredoxin family of redox proteins, playing a role in regulating worm longevity and dauer formation (Miranda-Vizuete et al. 2006; Fierro-Gonzalez et al. 2011a,b). SSU-1 is a sulfotransferase whose function is required for the volatile anesthetic sensitivity and uncoordinated movement phenotypes of unc-1 and unc-24 mutant worms (Carroll et al. 2006).
Here we dissect the promoters of C. elegans trx-1 and ssu-1 genes and identify cis- and trans-acting elements that drive expression in this pair of sensory neurons.
Materials and Methods
C. elegans strains and culture conditions
The standard methods used for culturing and maintenance of C. elegans were as previously described (Brenner 1974). The strains used in this work are the following: GJ1186, gjIs698 [Psptf-1::gfp; Pelt-2::mCherry]; GJ1401, gjEx856 [Pgpa-9::gfp; rol-6(su1006)]; GJ2296, gjIs698 [Psptf-1::gfp; Pelt-2::mCherry]; ofEx205 [Ptrx-1::trx-1::DsRed; lin-15ab(+)]; GR1455, mgIs40 [Pdaf-28::gfp]; OE3007, lin-15 (n765ts) X; ofEx1 [Ptrx-1::gfp; lin-15ab(+)]; VZ126, lin-15 (n765ts) X; vzEx29 [Pssu-1::gfp; rol-6(su1006)]; VZ250, sptf-2(tm1130) II; vzEx87 [Ptrx-1(-300-100)::gfp; Punc-122::gfp; VZ260, lin-15(n765ts) X; ofEx205 [Ptrx-1::trx-1::DsRed; lin-15ab(+)]; vzEx95 [Ptrx-1[tandem 3x(-200, -173)]::gfp; rol-6(su1006)]; VZ289, rhIs13 [Punc-119::unc-119::gfp; dpy-20(+)] nre-1(hd20) lin-15b(hd126) X; vzEx80 [Ptrx-1(-342,-84)::gfp; rol-6(su1006)]; VZ382, rrf-3(pk1426) II; vzEx81 [Pssu-1(-330,0)::gfp; rol-6(su1006)]; VZ383, tax-2(p691) I; rrf-3(pk1426) II; ofIs1 [Ptrx-1::trx-1::gfp; lin-15ab(+)] IV; VZ489, sptf-2(tm1130) II; vzEx81 [Pssu-1(-330,0)::gfp; rol-6(su1006)]; VZ504, sptf-3(gu85) I; ofEx1052 [Ptrx-1::trx-1::gfp; Punc-122::DsRed]; VZ509, sptf-3(gu85) I; vzEx81 [Pssu-1(-330,0)::gfp; rol-6(su1006)]; VZ577, sptf-1(tm784) I; tax-4(p678) III; ofIs1 [Ptrx-1::trx-1::gfp; lin-15ab(+)] IV; gjEx867 [Pelt-2::mCherry; sptf-1(+)]; VZ578, sptf-1(tm784) I; gjEx867 [Pelt-2::mCherry; sptf-1(+)]; mgIs40 [Pdaf-28::gfp]; VZ580, tax-2(p691) I; cog-1(sy607)/mnC1dpy-10(e128) unc-52(e444) II; ofIs1 [Ptrx-1::trx-1::gfp; lin-15ab(+)] IV; VZ582, sptf-1(tm784) I; gjEx867 [Pelt-2::mCherry; sptf-1(+)]; vzEx81 [Pssu-1(-330,0)::gfp; rol-6 (su1006)]; VZ583, sptf-1(tm784) I; gjEx867 [Pelt-2::mCherry; sptf-1(+)]; gjEx856 [Pgpa-9::gfp; rol-6(su1006)]; and VZ584, rrf-3(pk1426) II; gjIs698 [Psptf-1::gfp; Pelt-2::mCherry].
GFP expression constructs and transgenesis
Ptrx-1(1kb)::gfp (Miranda-Vizuete et al. 2006) and Pssu-1(0.5kb)::gfp plasmids (Carroll et al. 2006) were used as the template for generating promoter deletion GFP reporters as PCR linear fragments to maximize expression efficiency (Etchberger and Hobert 2008). These constructs contained different promoter fragments fused to the gfp-coding sequence and the 3′ UTR region of the unc-54 gene. Mutagenesis reactions (deletions and substitutions) were performed using the QuickChange II XL Site Directed Mutagenesis Kit (Stratagene) and confirmed by sequencing. The 3× tandem-repeat fragment was synthesized de novo as two complementary 84 bp oligonucleotides, hybridized, and cloned into the HindIII and BamHI sites of the pPD95.77 vector and subsequently used as the basis for generating the corresponding linear fragment by PCR. The Ptrx-1::trx-1::DsRed construct was made by cloning the trx-1 promoter (1 kb) into the HindIII and BamHI sites of the pCJ102 plasmid (Winkelbauer et al. 2005). Primer sequences used for trx-1 and ssu-1 constructs can be provided upon request. The Psptf-1::gfp construct was generated by cloning a PCR fragment generated with primers forward AGCATGCTTGGGTAAACTGCTTGGGAAAGTGG and reverse AGGATCCGTGATTCTGGTTTTGCTGGCTCTGC into the SphI and BamHI sites upstream of the gfp-coding sequence of the vector pPD95.79. This construct includes an ∼3.5-kb upstream sequence and fuses the sptf-1 coding sequence at the end of the second exon to GFP. Transgenic worms were generated by DNA microinjection as previously described (Mello et al. 1991). The pRF4 [rol-6(su1006)] plasmid was used as injection marker at 50 ng/μl. All fragments were injected into N2 wild-type animals at varying concentrations ranging from 5 to 50 ng/μl.
Microscopy
GFP transgenic animals were mounted in a 5-μl drop of 10 mM levamisole (Sigma, St. Louis) on a 3% agarose pad covered with a 24- × 24-mm coverslip and scored on an Olympus BX61 fluorescence microscope. For GFP expression analysis in ASJ neurons, at least 50 worms per line/strain were analyzed under a ×20 objective. Cells expressing Psptf-1::gfp were identified based on their position within the animals by combining fluorescence and DIC microscopy using a Zeiss AxioImager Z1 based on colocalization with DsRed expressed in ASJ neurons from the trx-1 promoter and based on DiI fluorescent dye filling visualized using a Nikon Ti Eclipse microscope equipped with a Yokogawa CSU-X1 spinning disk unit, 491- and 561-nm lasers, and beam splitters.
Fluorescent dye-filling assays
Fluorescent dye filling was performed using 0.1 mg/ml DiI (Molecular Probes) as previously described (Perkins et al. 1986).
RNA interference
Feeding RNA interference (RNAi) was performed as previously described (Timmons et al. 2001). RNAi screening was performed in the VZ289, VZ382, and VZ383 strains.
Bioinformatics and database analysis
The initial motif-finding analysis in 70 ASJ-expressed genes was performed with the MEME tool (http://meme.sdsc.edu/meme/intro.html). Different lengths (up to 1 kb) of gene upstream regulatory sequences were analyzed. All sequence alignments were performed with the EMBOSS Water Nucleotide Alignment tool (http://www.ebi.ac.uk/Tools/psa/emboss_water/nucleotide.html). The ASJ motif logo shown in Figure 4F was generated with WebLogo (http://weblogo.threeplusone.com). For the identification of transcription factor(s) potentially binding to the ASJ motif, we used the following prediction programs and database resources: Promo 3.0 (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3); Alibaba 2.1 (http://labmom.com/link/alibaba_2_1_tf_binding_prediction); MatInspector (http://www.genomatix.de/online_help/help_matinspector/matinspector_help.html); and Jaspar (http://jaspar.genereg.net/).
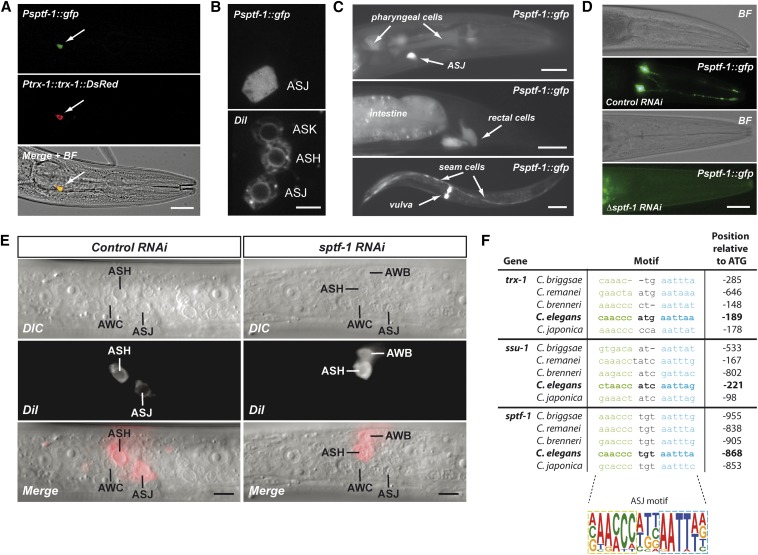
Figure 4.
SPTF-1 function in ASJ and conservation of the ASJ motif among C. elegans species. (A) Transgenic animals expressing GFP under control of the sptf-1 promoter and TRX-1::DsRed under control of the trx-1 promoter demonstrate ASJ colocalization (yellow color in the merged + bright-field image). Bar, 20 μm. (B, top) GFP expressed under control of the sptf-1 promoter is found exclusively in ASJ neurons. (Bottom) ASJ, ASH, and ASK neurons are stained with the fluorescent DiI dye. Bar, 5 μm. (C) GFP expressed under control of the sptf-1 promoter is found in other cell types such as pharyngeal cells, rectal cells, intestine, seam cells, and vulval cells. Bar, 20 μm (top and middle) and 100 μm (bottom). (D) RNAi downregulation of the endogenous sptf-1 gene in a rrf-3(pk1426) sensitized background demonstrates that SPTF-1 is autoregulated. Bar, 20 μm. (E) sptf-1 RNAi downregulation does not interfere with ASJ neurogenesis while it impacts the expression of genes required for DiI uptake. Bar, 10 μm. (F) Sequence alignment of the predicted ASJ motif found in the promoters of the trx-1, ssu-1 and sptf-1 genes of several Caenorhabditis species define the ASJ motif, which is composed of two well-conserved 6 bp elements separated by a 3 bp more divergent linker sequence.
Results and Discussion
trx-1 gene promoter analysis identifies a bipartite motif necessary for gene expression in ASJ neurons
To date, ∼70 genes have been reported to be expressed in ASJ neurons (http://www.wormbase.org), whereby only the genes trx-1 and ssu-1 have been found to have an expression pattern restricted to this bilateral pair of neurons (Carroll et al. 2006; Miranda-Vizuete et al. 2006). To determine whether genes expressed in ASJ share a common regulatory motif, we first followed a bioinformatics approach. Alignment of the 70 promoters (each consisting of 1 kb upstream of the ATG codon of the respective ASJ-expressed gene) failed to identify any shared motif owing to the large sequence analyzed (data not shown). Next, we experimentally dissected the 1 kb upstream region of the trx-1 gene, which is sufficient to drive expression in ASJ neurons (Miranda-Vizuete et al. 2006) (Figure 1A). trx-1 promoter fragments of decreasing lengths were fused to the GFP-coding sequence, and GFP expression in ASJ neurons of transgenic animals was scored. As shown in Figure 1B, we identified a minimal region of 116 bp (positioned at −200 to −84 bp upstream of the trx-1a isoform ATG codon) that drives GFP expression in ASJ, thus containing the putative cis-regulatory motif responsible for ASJ expression (called “ASJ motif” hereafter).
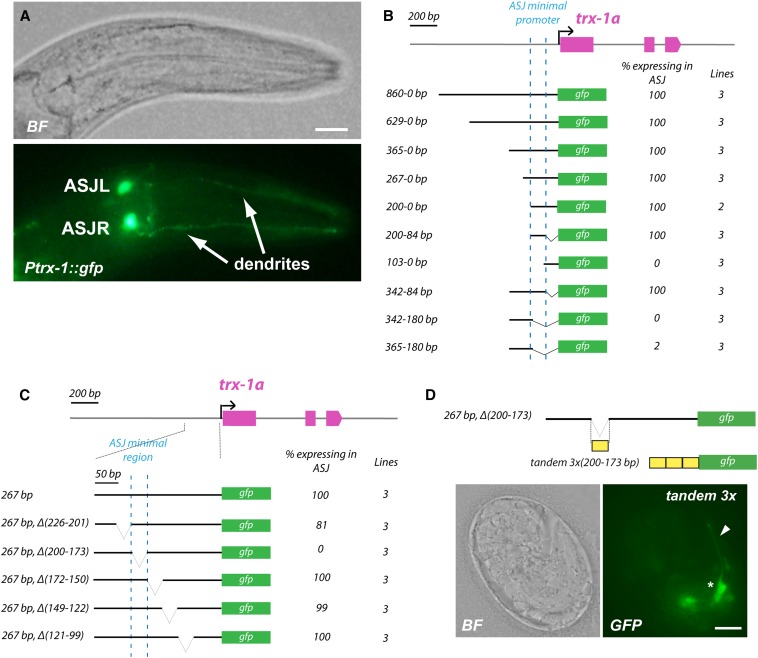
Figure 1.
Identification of the minimal trx-1 promoter region necessary for gene expression in ASJ neurons. (A) Bright-field (BF, top) and fluorescence (bottom) images of Ptrx-1(1kb)::gfp transgenic animals. ASJR (ASJ right) and ASJL (ASJ left) neurons are indicated. When expressed from the trx-1 promoter, GFP is found in the entire cell: in cilia, dendrites, cell bodies, and axons. Bar, 20 μm. (B) Identification of the minimal trx-1 promoter region that drives gene expression in ASJ neurons. Decreasing length fragments of the trx-1 promoter fused to the GFP-coding sequence have identified an ASJ minimal promoter (dotted blue line) located at position −200 to −84 upstream of the trx-1a ATG codon. (C) Serial deletions within the trx-1 minimal promoter region previously identified reveal the sequence −200 to −173 (dotted blue line) upstream of the trx-1a ATG codon as necessary for gene expression in ASJ neurons. For quantification, at least 50 animals per line were analyzed. (D) Transgenic animals expressing a 3× tandem construct of the sequence −200 to −173 (necessary for gene expression in ASJ) fused to GFP show fluorescence in head neurons with morphology and position consistent with being ASJ. The asterisk indicates the ASJ cell body, and the arrowhead points to the ASJ dendrite. Bar, 5 μm.
We then made 25 to 30 bp nonoverlapping deletions covering the 116 bp minimal region previously identified. As shown in Figure 1C, deletion of the region −200 to −173 bp upstream of the trx-1a ATG codon fully abolished expression in ASJ, whereas deletion of any of the other sequences within the minimal 116 bp region did not affect GFP expression in these neurons. These results indicate that the ASJ motif is located within the region from −200 to −173 bp of the trx-1a promoter.
To elucidate if this 28 bp sequence is sufficient to drive expression in ASJ neurons, we generated a 3×28 bp tandem repeat in an otherwise promoter-less GFP reporter. Transgenic embryos carrying this construct showed expression in a pair of head neurons with dendrites extending to the tip of the head, consistent with ASJ morphology and position (Figure 1D). However, once the embryo hatched, the number of animals with GFP-fluorescent ASJ neurons decreased dramatically. We could eventually identify very few L1 larvae with expression in ASJ, but none in L2–L4 larvae or adult animals. This is most likely due to the requirement of additional cis-regulatory regions in the surrounding promoter area necessary for post-embryonic maintenance of trx-1 expression, as has been reported for other genes with neuronal expression (Bertrand and Hobert 2009).
Finally, to unequivocally identify the cis-regulatory motif that controls trx-1 expression in C. elegans ASJ neurons, we performed a scanning substitution mutagenesis of the 28 bp sequence described above. As shown in Figure 2A, we identified a bipartite motif composed of two 6 bp elements (CAACCC and AATTAA), separated by a 3 bp linker, being absolutely required for ASJ expression. Hence, mutation of either of the two individual 6 bp elements resulted in the abolishment of GFP expression in ASJ neurons, while mutation of the 3 bp linker did not disrupt GFP expression (Figure 2A). Interestingly, a similar bipartite cis-regulatory motif has been described to regulate specific expression of genes in the AWB olfactory sensory neurons in C. elegans (Nokes et al. 2009).
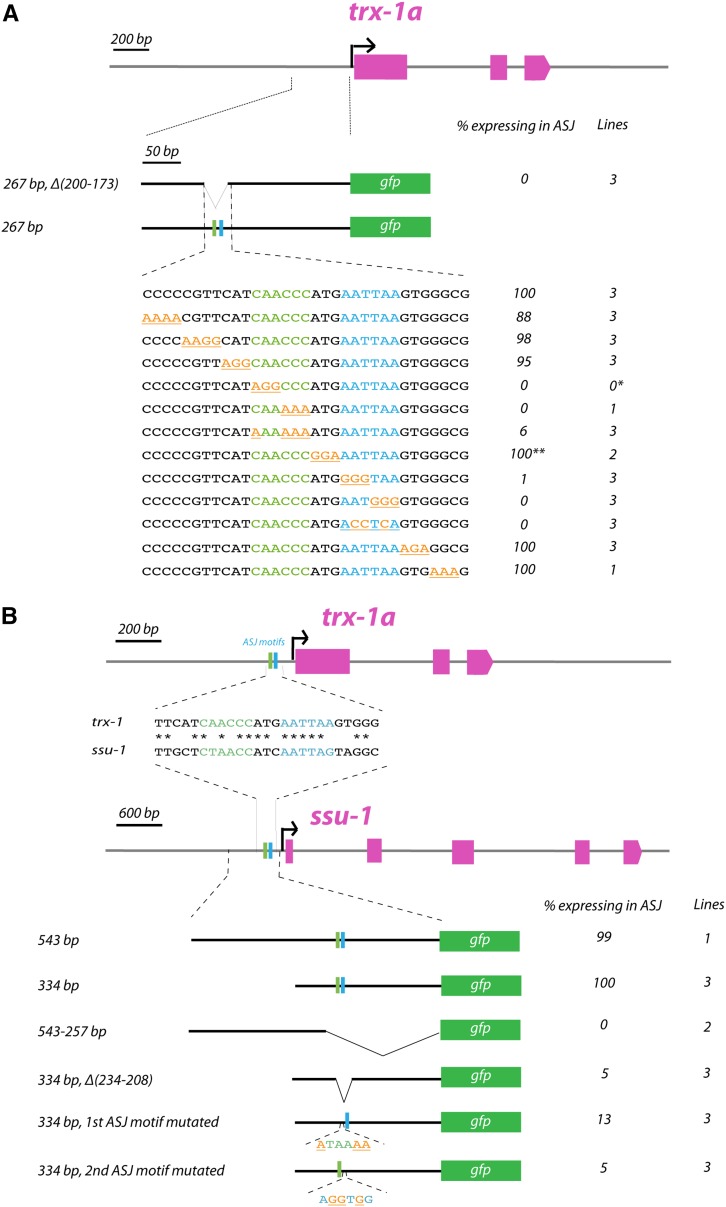
Figure 2.
Identification of the ASJ motif in promoters of the trx-1 and ssu-1 genes. (A) Scanning substitution mutagenesis (underlined in orange) within the sequence necessary for trx-1 expression in ASJ (−200 to −173 upstream of the trx-1a ATG codon) identifies a bipartite ASJ motif, composed of two individual 6 bp elements (in green and blue, respectively) separated by a 3 bp linker. For quantification, at least 50 animals per line have been analyzed. A single asterisk (*) indicates that we have been unable to get stably transmitting lines from this construct; however, 10 F1 transgenic animals were scored and no GFP expression in ASJ was observed. A double asterisk (**) indicates that these transgenic animals show several fluorescent head neurons including ASJ. (B) Comparison of the trx-1 ASJ motif and its homologous region in the ssu-1 promoter. The diagram below shows that the deletion or the mutation of the individual 6 bp elements of the ASJ motif in the ssu-1 promoter robustly decreases gene expression in ASJ, indicating that the ASJ motif is also functional in ssu-1. For quantification, at least 50 animals per line have been analyzed.
Together, these results indicate that the bipartite motif here identified is necessary and sufficient for trx-1 expression in ASJ neurons, although additional regulatory sequences are necessary for the (long-term) maintenance of expression in larvae and adult animals.
ssu-1 gene promoter analysis confirms the identity of the ASJ motif
To confirm that the identified ASJ motif was not specific to the trx-1 gene but conserved in another gene expressed in ASJ neurons, we performed a similar analysis of the ssu-1 promoter. ssu-1 together with trx-1 are the only two genes exclusively expressed in C. elegans ASJ neurons (Carroll et al. 2006; Miranda-Vizuete et al. 2006). A 500 bp promoter region upstream of the ssu-1 ATG codon has been shown to contain the cis-regulatory sequences required for ASJ expression (Carroll et al. 2006) (Figure 2B). Using sequential promoter deletions, we found a minimal region of 334 bp upstream of the ATG codon in the ssu-1 promoter that drives expression in ASJ neurons (Figure 2B). Sequence comparison of this 334 bp region of the ssu-1 promoter with trx-1 promoter sequences revealed an ssu-1 sequence stretch with substantial identity to the 28 bp minimal region identified in the trx-1 promoter containing the ASJ motif. This sequence stretch resides at −234 to −208 bp upstream of the ssu-1 ATG codon. Indeed, deletion of this region in the ssu-1 promoter or mutation of each individual element of the bipartite ASJ motif disrupted expression in ASJ (Figure 2B).
Collectively, these results suggest that the bipartite cis-regulatory elements identified in both the trx-1 and ssu-1 promoters define a novel ASJ motif responsible for gene expression in ASJ neurons in C. elegans.
RNAi screening identifies SPTF-1 as a regulator of ASJ expression
The bipartite ASJ motif found in the trx-1 and ssu-1 promoters is composed, on the one hand, of a sequence that resembles a CACCC-box and, on the other hand, of an AATTAA-related sequence (Figure 2, A and B). To identify the TFs binding to the ASJ motif, we first used the ASJ motif as bait in several different TF-binding prediction programs and databases available online (Materials and Methods). The strongest hits identified in these searches were members of the homeodomain and zinc-finger TF families. This is consistent with the fact that the AATTAA sequence resembles the core consensus sequence recognized by the majority of homeobox TFs (Svingen and Tonissen 2006) while many zinc-finger TFs are known to preferentially bind to GC-rich sites (Kaczynski et al. 2003).
Next, using Ptrx-1::gfp and Pssu-1::gfp reporter strains, we performed a feeding RNAi screen using the Ahringer TF RNAi sublibrary supplemented with individual homeodomain and zinc-finger TFs not represented in the Ahringer TF sublibrary but that appeared in the bioinformatics search described above (403 RNAi clones, Supporting Information, Table S1). Together, this combined sublibrary represents ∼40% of all predicted transcription factors in the nematode (Reece-Hoyes et al. 2005; Shaye and Greenwald 2011). In this screen we identified SPTF-1 (Specificity Protein Transcription Factor 1) as the only ASJ motif binding candidate, since sptf-1 RNAi fully abolished Ptrx-1::gfp and Pssu-1::gfp expression in ASJ neurons (Figure 3, A and B). Of note, ASJ labeling is abolished not only in adult animals but also in embryos and larvae, indicating that sptf-1 deficiency impairs ASJ initiation of gene expression, rather than ASJ gene expression maintenance.
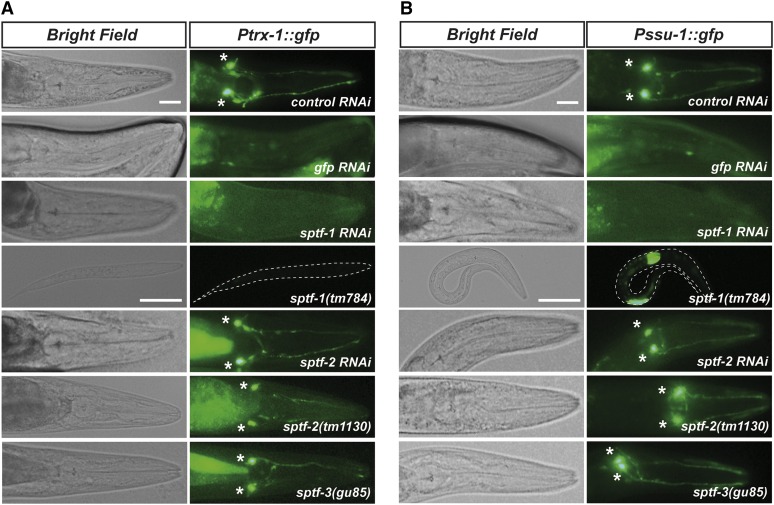
Figure 3.
The zinc-finger transcription factor SPTF-1 regulates gene expression in ASJ neurons. (A and B) sptf-1 mutation or RNAi downregulation abolishes trx-1 (A) or ssu-1 (B) promoter-dependent GFP expression in ASJ neurons. RNAi and/or mutant analysis of the other members of the Sp family, sptf-2 and sptf-3, demonstrate that these transcription factors are not involved in the regulation of either trx-1 or ssu-1 expression in ASJ. Asterisks indicate the ASJ cell bodies. The intestinal labeling of transgenic animals is nonspecific fluorescence due to the unc-54 3′ UTR present in the injected constructs (Boulin et al. 2006). Bar, 20 μm.
sptf-1 encodes a member of the Sp family TF that is composed of multiple zinc-finger-containing proteins that are important components of the eukaryotic cellular transcriptional machinery. Sp family TFs bind to GC-rich sites as the GC-box and CACCC-box (Suske 1999; Kaczynski et al. 2003). Interestingly, human Sp1 has been found to bind the human TRX1 promoter and this binding is, in turn, enhanced in a redox-dependent manner by Trx-1 (Bloomfield et al. 2003), suggesting that regulation of thioredoxin expression is evolutionarily conserved. Similarly, mammalian orthologs of C. elegans SSU-1 sulfotransferase have also been reported to be regulated by Sp1 (Hempel et al. 2004; Lee et al. 2005).
To verify the role of sptf-1 in regulating ASJ-specific gene expression, we used sptf-1(tm784) mutants carrying a deletion that removes the sptf-1 third exon and causes an L1 larval arrest phenotype. As shown in Figure 3, A and B, sptf-1(tm784) arrested L1 larvae carrying the Ptrx-1::gfp or Pssu-1::gfp transgenes are devoid of ASJ fluorescence. Notably, a small number of sptf-1 L1 larvae displayed very weak GFP labeling in the ASJ neurons (Figure S1A), most likely due to the presence of maternal SPTF-1, similar to what has been reported for other members of the Sp family of TFs in both C. elegans (Ulm et al. 2011) and Drosophila (Wimmer et al. 1996). In other cases, arrested L1 larvae displayed GFP labeling in several neurons including ASJ, suggesting that sptf-1 deficiency causes an anomalous ectopic expression of the transgenes (Figure S1, B and C).
Importantly, the role of SPTF-1 in controlling gene expression in the ASJ neurons is not shared with other members of the Sp family, as neither sptf-2 RNAi (sptf-3 RNAi causes a highly penetrant embryonic lethal phenotype that precluded any analysis) nor sptf-2(tm1130) and sptf-3(gu85) mutants abolished Ptrx-1::gfp or Pssu-1::gfp expression in ASJ (Figure 3, A and B). The sptf-2(tm1130) mutant carries a deletion that results in a shorter protein lacking part of the zinc-finger domain and therefore presumably is a mutant deficient in DNA-binding activity. Similarly, (sptf-3gu85) is a hypomorphic mutant that lacks DNA-binding activity (Ulm et al. 2011). Therefore, we conclude that of three Sp family TFs in C. elegans, only SPTF-1 appears to be involved in the regulation of gene expression in ASJ neurons.
SPTF-1 displays characteristics of an ASJ terminal selector gene
If SPTF-1 is necessary for ASJ-specific gene expression, as a putative ASJ terminal selector gene, SPTF-1 should be expressed in these neurons, it should be autoregulated, and it should not interfere with the mitotic development of ASJ neurons because terminal selector genes code for postmitotically expressed TFs (Hobert 2010). Hence, we first aimed to determine the sptf-1 expression pattern in transgenic animals harboring an sptf-1 GFP reporter in which GFP is fused in-frame at the end of the second exon of sptf-1. Interestingly, among all the head neurons, SPTF-1 is exclusively expressed in ASJ neurons as shown both by colocalization with DsRed expressed in ASJ under the control of the trx-1 promoter (Figure 4A) and by fluorescent DiI staining and DIC microscopy (Figure 4, B and E). Consistent with its expression in ASJ neurons, we have identified a putative ASJ motif within the sptf-1 promoter region where both individual elements of the bipartite ASJ motif are relatively well conserved among Caenorhabditis species, while the 3 bp linker between them is more divergent (Figure 4F). In addition, SPTF-1 is also expressed in non-neuronal cells of the animal such as pharyngeal cells, rectal and intestinal cells, seam cells, and vulva epithelial cells (Figure 4C).
To analyze autoregulation of sptf-1 expression in ASJ, we knocked down sptf-1 using an RNAi construct directed at the last two sptf-1 exons, thus targeting only the endogenous sptf-1 gene but not the sptf-1 GFP reporter. This approach abolished GFP expression in ASJ (Figure 4D), although the penetrance of this RNAi was variable and some animals displayed weak fluorescence in one or both ASJ neurons.
Finally, L1 larvae fed with sptf-1 RNAi did not show DiI staining of the ASJ neurons although ASJ was present, as visualized by DIC microscopy (Figure 4E). This result demonstrates that sptf-1 is not required for ASJ neurogenesis but instead regulates the expression of genes required for DiI dye uptake into these neurons.
Collectively, these data indicate that the Sp transcription factor family member SPTF-1 is an ASJ neuron terminal selector gene, probably acting by binding to the CG-rich element of the bipartite ASJ motif in C. elegans. To the best of our knowledge, this is the first biological function assigned to C. elegans SPTF-1.
ASJ motif is found in the promoters of many ASJ neuron-expressed genes
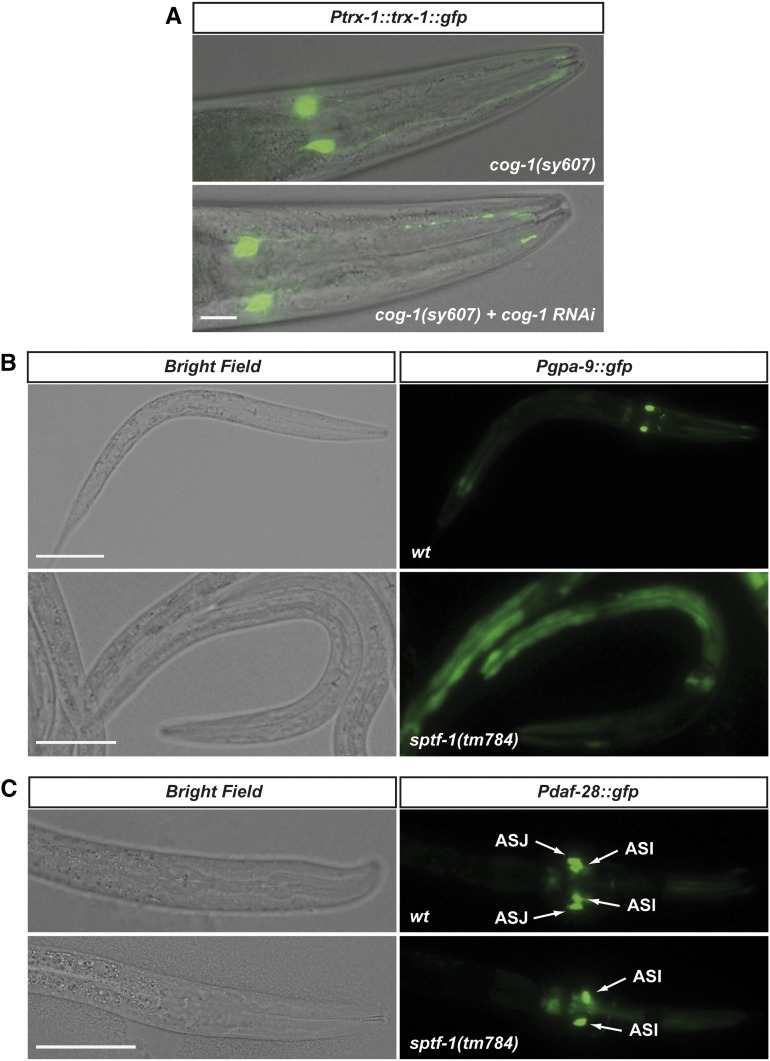
Next, we used the consensus ASJ motif from trx-1, ssu-1, and sptf-1 promoters (Figure 4F) to search for the respective ASJ motifs in the promoters of all genes reported to be expressed in ASJ neurons using the MEME Suite Software (http://meme.nbcr.net/meme/). As shown in Table 1, many of these ASJ-expressed genes have putative ASJ motifs within their promoters. Interestingly, among the genes with the highest score, we found cog-1, a homeobox transcription factor that has been implicated in C. elegans reproductive system development and ASE left/right asymmetry (Palmer et al. 2002; Chang et al. 2003). Given that the second element of the bipartite ASJ motif is an AT-rich sequence resembling a typical homeodomain-binding core (Berger et al. 2008), cog-1 appeared as a good candidate to bind this second element. However, neither cog-1 RNAi nor mutation suppressed ASJ labeling in a Ptrx-1::trx-1::gfp reporter strain, thus arguing against any regulatory role of this TF in ASJ-specific gene expression (Figure 5A).
Table 1. Identification of putative ASJ-motif sequences in the promoters of all C. elegans genes reported to be expressed in ASJ neurons.
| Gene | Promoter region analyzed (bp) | Motif | Strand | Position relative to ATG | Position P-value | E-value |
|---|---|---|---|---|---|---|
| trx-1 | 200 | CAACCCATGAATTAA | + | −189 | 9.1E-08 | 0.0013 |
| ssu-1 | 334 | CTAACCATCAATTAG | + | −221 | 1.3E-06 | 0.056 |
| sptf-1 | 1003 | CAACCCTGTAATTTA | + | −868 | 8.5E-07 | 0.12 |
| dyf-3 | 650 | CAAATCTGGAATTTT | − | −81 | 4.6E-06 | 0.41 |
| cog-1 | 2000 | CAAATCGGCAATTTG | + | −1487 | 4.0E-06 | 0.48 |
| AAAACCGGCAATTTG | + | −1431 | 5.9E-05 | |||
| CAAACCGGCAATTTG | + | −1387 | 1.7E-06 | |||
| daf-25 | 2000 | CAAACCGGCAATTTG | − | −1172 | 1.7E-06 | 0.48 |
| CAAATCGGTAATTGC | + | −986 | 3.2E-05 | |||
| osm-9 | 1600 | CAACTCTTTAATTTT | + | −37 | 4.0E-06 | 0.89 |
| mks-5 | 3946 | CAAACCGGCAAATTG | − | −3711 | 4.5E-05 | 0.94 |
| CAAACCGGCAATTTG | − | −3667 | 1.7E-06 | |||
| CAAATCGGCAATTTG | + | −3379 | 4.0E-06 | |||
| CAACACGGGAATTGT | − | −1224 | 4.9E-05 | |||
| C39D10.5 | 1005 | GAAACCGTTAATTAA | − | −240 | 7.4E-06 | 1 |
| srh-10 | 1005 | CAACCCGCCATTTAT | − | −861 | 1.7E-05 | 2.3 |
| ceh-13 | 1051 | CGAACCCCCAATCGA | − | −866 | 1.8E-05 | 2.6 |
| R102.2 | 700 | CTAAGCTTCAATTAA | + | −123 | 4.3E-05 | 4 |
| odr-8 | 1005 | CAACTCATTGATTGA | + | −483 | 3.6E-05 | 4.8 |
| ttx-7 | 524 | CAAATCACCATTTGT | − | −436 | 7.7E-05 | 5.3 |
| ins-6 | 1714 | AAACCCAGCAATTGA | − | −229 | 2.5E-05 | 5.7 |
| K07C11.10 | 2289 | TGACCCTCCAATTGG | + | −1958 | 5.9E-05 | 5.8 |
| CAATTCGCTAATTAG | + | −49 | 1.9E-05 | |||
| age1 | 900 | CAACTCCTCAATTCT | + | −494 | 6.3E-05 | 7.4 |
| ins-4 | 5716 | CTTATCCCTAATTGT | − | −4813 | 9.5E-05 | 8 |
| CAACACAGGAATTAA | − | −371 | 1.1E-05 | |||
| ins-3 | 5728 | CAACACAGGAATTAA | + | −5314 | 1.1E-05 | 8 |
| CTTATCCCTAATTGT | + | −871 | 9.5E-05 | |||
| tbx-2 | 1006 | CGAATCAGTAATAAG | + | −790 | 6.3E-05 | 8.2 |
| tub-1 | 2800 | GAAATCATCAATTAT | − | −2566 | 8.3E-05 | 10 |
| CGAACCTGGAATCAA | − | −2468 | 2.9E-05 | |||
| TAAATCTCTAATTAT | + | −1062 | 6.9E-05 | |||
| pnc-1 | 1001 | AAAACCTGCAATTTT | + | −340 | 8.5E-05 | 11 |
| K10D6.2 | 3450 | AAAACCCTTAATTGT | + | −3161 | 3.5E-05 | 11 |
| CTAACCTCGATTTTA | − | −2867 | 5.3E-05 | |||
| CGAACTTCTAATTAG | + | −1379 | 2.5E-05 | |||
| CAAATCGTTCATTTG | + | −931 | 7.7E-05 | |||
| dyf-2 | 3000 | ATAATCCTGAATTAA | + | −1920 | 8.9E-05 | 11 |
| CAAACCGGGAATGAT | + | −235 | 2.9E-05 | |||
| unc-103 | 5000 | CAAACAATGAATTGA | + | −4855 | 2.0E-05 | 13 |
| sra-39 | 2877 | CAAATTGTGAATTAA | + | −624 | 5.6E-05 | 19 |
| nphp-1 | 4820 | GGAATCACTAATTAA | − | −317 | 3.6E-05 | 21 |
| gpa-10 | 3000 | CGAACTCCGAATTTG | + | −1739 | 5.9E-05 | 21 |
| ins-1 | 4200 | CAAATATCTAATTAT | + | −2561 | 6.9E-05 | 23 |
| CAAAACTGCAATTAT | − | −1543 | 4.7E-05 | |||
| CTAATAATCAATTAA | − | −1266 | 8.3E-05 | |||
| add1 | 3100 | CAACTCATTATTTGT | + | −2288 | 6.9E-05 | 24 |
| nhr-67 | 5000 | CAAATCGACAATTAT | + | −2621 | 4.7E-05 | 26 |
| CTACCACTCAATTGA | + | −331 | 5.3E-05 | |||
| dyf-11 | 4101 | CAAATTCCCAATTTG | + | −2825 | 9.5E-05 | 36 |
| CAAATATGCAATTGA | + | −2597 | 9.5E-05 | |||
| CAATTCTTTAATTTT | − | −274 | 8.9E-05 | |||
| wrk-1 | 4642 | CAAATCATTTATTTT | + | −4469 | 8.3E-05 | 38 |
| gcy-27 | 1020 | 0 | 13 | |||
| K07C5.9 | 425 | 0 | 14 | |||
| arrd-4 | 1000 | 0 | 15 | |||
| gpa-1 | 1500 | 0 | 19 | |||
| npr-15 | 1010 | 0 | 20 | |||
| egap-5 | 1153 | 0 | 24 | |||
| daf-11 | 1020 | 0 | 25 | |||
| gpa-3 | 2000 | 0 | 26 | |||
| cng-2 | 2362 | 0 | 28 | |||
| ins-9 | 1100 | 0 | 29 | |||
| kin-29 | 830 | 0 | 34 | |||
| c34d4.1 | 1006 | 0 | 34 | |||
| odr-4 | 1026 | 0 | 35 | |||
| gpa-9 | 3000 | 0 | 35 | |||
| egl-43 | 1734 | 0 | 37 | |||
| tax-2 | 1982 | 0 | 40 | |||
| ncam-1 | 2069 | 0 | 41 | |||
| clhm-1 | 3000 | 0 | 42 | |||
| nlp-3 | 1024 | 0 | 42 | |||
| npr-5 | 1010 | 0 | 42 | |||
| nphp-4 | 4750 | 0 | 46 | |||
| F25B4.2 | 2875 | 0 | 47 | |||
| ins-32 | 454 | 0 | 47 | |||
| dpy-14 | 1006 | 0 | 48 | |||
| efn-2 | 2744 | 0 | 49 | |||
| arrd-17 | 1000 | 0 | 49 | |||
| Y55D5A.1 | 3419 | 0 | 54 | |||
| gpa-14 | 3000 | 0 | 56 | |||
| tax-4 | 1080 | 0 | 58 | |||
| sre-1 | 1006 | 0 | 61 | |||
| K10G6.4 | 2380 | 0 | 62 | |||
| c33A12.4 | 604 | 0 | 62 | |||
| tbb-4 | 3000 | 0 | 64 | |||
| gpc-1 | 4200 | 0 | 64 | |||
| jbts-14 | 3761 | 0 | 66 | |||
| itf-81 | 1457 | 0 | 67 | |||
| daf-28 | 3302 | 0 | 67 |
The E-value of a sequence is the expected number of sequences in a random database of the same size that would match the motifs as well as the sequence does.The position P-value of a match is the probability of a single random subsequence of the length of the motif scoring at least as well as the observed match. For each sequence, all motif occurrences with a position P-value <0.0001 are shown.
Figure 5.
COG-1 is not required for ASJ specification whereas SPTF-1 regulates ASJ expression of DAF-28 and GPA-9. (A) cog-1(sy607) homozygous animals express Ptrx-1::trx-1::gfp reporter in ASJ. cog-1 RNAi was used to exclude a possible effect of maternally contributed COG-1. Bar, 20 μm. (B and C) sptf-1 is required for ASJ expression of gpa-9 and daf-28 transcriptional GFP reporters in sptf-1(tm784) L1 larvae. Note that sptf-1 deficiency does not affect expression of daf-28 in ASI neurons. Bar, 20 μm.
To further experimentally validate sptf-1 as a terminal selector gene for ASJ-expressed genes other than trx-1, ssu-1, and sptf-1, we analyzed ASJ labeling in sptf-1 mutants expressing a Pgpa-9::gfp reporter expressed only in ASJ in the head (plus PVQ and PHB neurons in the tail) (Jansen et al. 1999) and a Pdaf-28::gfp reporter expressed in ASI and ASJ neurons (Li et al. 2003). In both cases, sptf-1(tm784) arrested L1 larvae were devoid of GFP expression in the ASJ neurons (Figure 5, B and C), further supporting sptf-1 as a ASJ terminal selector gene. As previously described for trx-1 and ssu-1 GFP reporters, we also found that the Pdaf-28::gfp transgene, in some cases, is ectopically expressed in several head neurons other than ASI and ASJ (Figure S1D).
A homeobox transcription factor may bind the second element of the bipartite ASJ motif
The second element of the bipartite ASJ motif is an AT-rich sequence, including a typical homeodomain-binding core (Berger et al. 2008). As mentioned above, our RNAi screening, which included all predicted homeobox TFs, did not result in any hit. This could either be due to the low efficiency of RNAi-mediated down-regulation, as neurons are usually quite refractory to feeding RNAi approaches (despite the use of an RNAi hypersensitive background, validated by the highly efficient sptf-1 RNAi) or due to redundancies among different homeobox TFs. We also used the modENCODE tool (http://www.modencode.org) to identify TFs that potentially bind the C. elegans trx-1 promoter. Based on this analysis, we selected homeobox TFs whose predicted binding sites resemble the ASJ motif. However, mutant alleles of the five candidates identified, mab-5, zag-1, ceh-14, ceh-26, and ceh-30, failed to abolish Ptrx-1::gfp expression in ASJ neurons (data not shown). Together, these data suggest that several homeobox TFs might redundantly bind to this second element of the bipartite ASJ motif, as has been reported for other homeobox and T-box TFs (Good et al. 2004; Furuya et al. 2005). Alternatively, it is possible that this AT-rich element is necessary for sptf-1-dependent ASJ expression but does not require binding of any transcription factor, as described for the GC-box-binding MIG1 zinc finger (Lundin et al. 1994).
Conclusions
In summary, we report here the identification of the cis-regulatory promoter motif responsible for gene expression in the ASJ neurons in C. elegans. This ASJ motif is bipartite and composed of CG-rich and AT-rich sequence elements separated by a divergent linker sequence. Given this sequence composition, we propose that one or more still-to-be-identified homeobox TF(s) might bind the AT-rich element of the bipartite ASJ motif. In turn, we have identified the Sp family member SPTF-1 as a transcription factor required for ASJ-specific gene expression, most likely by binding to the CG-rich element of the bipartite motif. Among all C. elegans neurons, SPTF-1 is expressed only in ASJ neurons where it functions as a terminal selector gene.
Supplementary Material
Acknowledgments
Some C. elegans strains were provided by the CGC, which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440), and by the Japanese National Bioresource Project, which is funded by the Japanese Ministry of Education, Culture, Sport, Science and Technology. We thank Nuria Flames for advice and support and María Jesús Rodríguez-Palero and Francisco José Naranjo-Galindo for excellent technical assistance. This work was financed by grants to A.M.-V. from the Junta de Andalucía (Projects P07-CVI-02697 and P08-CVI-03629). Work in the laboratory of P.S. was supported by grants from the Swedish Research Council and the Torsten Söderberg Foundation. E.K. was supported by a grant from the European Union FP6 Marie Curie Research Training Network “EUrythron” MRTN-CT-2004-005499.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.176172/-/DC1.
Communicating editor: P. Sengupta
Literature Cited
- Alcedo J., Kenyon C., 2004. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41: 45–55. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Horvitz H. R., 1991. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science 251: 1243–1246. [DOI] [PubMed] [Google Scholar]
- Bejerano G., Pheasant M., Makunin I., Stephen S., Kent W. J., et al. , 2004. Ultraconserved elements in the human genome. Science 304: 1321–1325. [DOI] [PubMed] [Google Scholar]
- Berger M. F., Badis G., Gehrke A. R., Talukder S., Philippakis A. A., et al. , 2008. Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell 133: 1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand V., Hobert O., 2009. Linking asymmetric cell division to the terminal differentiation program of postmitotic neurons in C. elegans. Dev. Cell 16: 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield K. L., Osborne S. A., Kennedy D. D., Clarke F. M., Tonissen K. F., 2003. Thioredoxin-mediated redox control of the transcription factor Sp1 and regulation of the thioredoxin gene promoter. Gene 319: 107–116. [DOI] [PubMed] [Google Scholar]
- Boulin T., Etchberger J. F., Hobert O., 2006. Reporter gene fusions (April 5, 2006), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.106.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger M., Groudine M., 2011. Functional and mechanistic diversity of distal transcription enhancers. Cell 144: 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll B. T., Dubyak G. R., Sedensky M. M., Morgan P. G., 2006. Sulfated signal from ASJ sensory neurons modulates stomatin-dependent coordination in Caenorhabditis elegans. J. Biol. Chem. 281: 35989–35996. [DOI] [PubMed] [Google Scholar]
- Chang S., Johnston R. J., Jr, Hobert O., 2003. A transcriptional regulatory cascade that controls left/right asymmetry in chemosensory neurons of C. elegans. Genes Dev. 17: 2123–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornils A., Gloeck M., Chen Z., Zhang Y., Alcedo J., 2011. Specific insulin-like peptides encode sensory information to regulate distinct developmental processes. Development 138: 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. H., 2001. Genomic Regulatory Systems. Academic Press, The Netherlands. [Google Scholar]
- Davidson E. H., 2006. The Regulatory Genome: Gene Regulatory Networks in Development and Evolution. Academic Press, The Netherlands. [Google Scholar]
- de Bono M., 2003. Molecular approaches to aggregation behavior and social attachment. J. Neurobiol. 54: 78–92. [DOI] [PubMed] [Google Scholar]
- Doitsidou M., Flames N., Topalidou I., Abe N., Felton T., et al. , 2013. A combinatorial regulatory signature controls terminal differentiation of the dopaminergic nervous system in C. elegans. Genes Dev. 27: 1391–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchberger J. F., Hobert O., 2008. Vector-free DNA constructs improve transgene expression in C. elegans. Nat. Methods 5: 3. [DOI] [PubMed] [Google Scholar]
- Etchberger J. F., Lorch A., Sleumer M. C., Zapf R., Jones S. J., et al. , 2007. The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev. 21: 1653–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchberger J. F., Flowers E. B., Poole R. J., Bashllari E., Hobert O., 2009. Cis-regulatory mechanisms of left/right asymmetric neuron-subtype specification in C. elegans. Development 136: 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro-Gonzalez J. C., Cornils A., Alcedo J., Miranda-Vizuete A., Swoboda P., 2011a The thioredoxin TRX-1 modulates the function of the insulin-like neuropeptide DAF-28 during dauer formation in Caenorhabditis elegans. PLoS ONE 6: e16561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro-Gonzalez J. C., Gonzalez-Barrios M., Miranda-Vizuete A., Swoboda P., 2011b The thioredoxin TRX-1 regulates adult lifespan extension induced by dietary restriction in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 406: 478–482. [DOI] [PubMed] [Google Scholar]
- Flames N., Hobert O., 2009. Gene regulatory logic of dopamine neuron differentiation. Nature 458: 885–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M., Qadota H., Chisholm A. D., Sugimoto A., 2005. The C. elegans eyes absent ortholog EYA-1 is required for tissue differentiation and plays partially redundant roles with PAX-6. Dev. Biol. 286: 452–463. [DOI] [PubMed] [Google Scholar]
- Good K., Ciosk R., Nance J., Neves A., Hill R. J., et al. , 2004. The T-box transcription factors TBX-37 and TBX-38 link GLP-1/Notch signaling to mesoderm induction in C. elegans embryos. Development 131: 1967–1978. [DOI] [PubMed] [Google Scholar]
- Hempel N., Wang H., LeCluyse E. L., McManus M. E., Negishi M., 2004. The human sulfotransferase SULT1A1 gene is regulated in a synergistic manner by Sp1 and GA binding protein. Mol. Pharmacol. 66: 1690–1701. [DOI] [PubMed] [Google Scholar]
- Hobert O., 2008. Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc. Natl. Acad. Sci. USA 105: 20067–20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O., 2010. Neurogenesis in the nematode Caenorhabditis elegans (October 4, 2010), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.12.2, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G., Thijssen K. L., Werner P., van der Horst M., Hazendonk E., et al. , 1999. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat. Genet. 21: 414–419. [DOI] [PubMed] [Google Scholar]
- Junion G., Spivakov M., Girardot C., Braun M., Gustafson E. H., et al. , 2012. A transcription factor collective defines cardiac cell fate and reflects lineage history. Cell 148: 473–486. [DOI] [PubMed] [Google Scholar]
- Kaczynski J., Cook T., Urrutia R., 2003. Sp1- and Kruppel-like transcription factors. Genome Biol. 4: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Kim R., Sengupta P., 2010. The HMX/NKX homeodomain protein MLS-2 specifies the identity of the AWC sensory neuron type via regulation of the ceh-36 Otx gene in C. elegans. Development 137: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. H., Chou C. Y., Ch’ang L. Y., Liu C. S., Lin W., 2000. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 10: 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. C., Higashi Y., Luu C., Shimizu C., Strott C. A., 2005. Sp1 elements in SULT2B1b promoter and 5′-untranslated region of mRNA: Sp1/Sp2 induction and augmentation by histone deacetylase inhibition. FEBS Lett. 579: 3639–3645. [DOI] [PubMed] [Google Scholar]
- Levine M., 2010. Transcriptional enhancers in animal development and evolution. Curr. Biol. 20: R754–R763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Kennedy S. G., Ruvkun G., 2003. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 17: 844–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin M., Nehlin J. O., Ronne H., 1994. Importance of a flanking AT-rich region in target site recognition by the GC box-binding zinc finger protein MIG1. Mol. Cell. Biol. 14: 1979–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Vizuete A., Fierro Gonzalez J. C., Gahmon G., Burghoorn J., Navas P., et al. , 2006. Lifespan decrease in a Caenorhabditis elegans mutant lacking TRX-1, a thioredoxin expressed in ASJ sensory neurons. FEBS Lett. 580: 484–490. [DOI] [PubMed] [Google Scholar]
- Morgan T. H., 1934. Embryology and Genetics. Columbia University Press, New York. [Google Scholar]
- Nokes E. B., Van Der Linden A. M., Winslow C., Mukhopadhyay S., Ma K., et al. , 2009. Cis-regulatory mechanisms of gene expression in an olfactory neuron type in Caenorhabditis elegans. Dev. Dyn. 238: 3080–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A., Ujisawa T., Sonoda S., Kuhara A., 2014. Light and pheromone-sensing neurons regulates cold habituation through insulin signalling in Caenorhabditis elegans. Nat. Commun. 5: 4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. E., Inoue T., Sherwood D. R., Jiang L. I., Sternberg P. W., 2002. Caenorhabditis elegans cog-1 locus encodes GTX/Nkx6.1 homeodomain proteins and regulates multiple aspects of reproductive system development. Dev. Biol. 252: 202–213. [DOI] [PubMed] [Google Scholar]
- Perkins L. A., Hedgecock E. M., Thomson J. N., Culotti J. G., 1986. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117: 456–487. [DOI] [PubMed] [Google Scholar]
- Rankin C. H., 2002. From gene to identified neuron to behaviour in Caenorhabditis elegans. Nat. Rev. Genet. 3: 622–630. [DOI] [PubMed] [Google Scholar]
- Reece-Hoyes J. S., Deplancke B., Shingles J., Grove C. A., Hope I. A., et al. , 2005. A compendium of Caenorhabditis elegans regulatory transcription factors: a resource for mapping transcription regulatory networks. Genome Biol. 6: R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle D. L., Swanson M. M., Albert P. S., 1981. Interacting genes in nematode dauer larva formation. Nature 290: 668–671. [DOI] [PubMed] [Google Scholar]
- Sandelin A., Bailey P., Bruce S., Engstrom P. G., Klos J. M., et al. , 2004. Arrays of ultraconserved non-coding regions span the loci of key developmental genes in vertebrate genomes. BMC Genomics 5: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schackwitz W. S., Inoue T., Thomas J. H., 1996. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron 17: 719–728. [DOI] [PubMed] [Google Scholar]
- Shaye D. D., Greenwald I., 2011. OrthoList: a compendium of C. elegans genes with human orthologs. PLoS ONE 6: e20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suske G., 1999. The Sp-family of transcription factors. Gene 238: 291–300. [DOI] [PubMed] [Google Scholar]
- Svingen T., Tonissen K. F., 2006. Hox transcription factors and their elusive mammalian gene targets. Heredity (Edinb) 97: 88–96. [DOI] [PubMed] [Google Scholar]
- Swoboda P., Adler H. T., Thomas J. H., 2000. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol. Cell 5: 411–421. [DOI] [PubMed] [Google Scholar]
- Timmons L., Court D. L., Fire A., 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263: 103–112. [DOI] [PubMed] [Google Scholar]
- Ulm E. A., Sleiman S. F., Chamberlin H. M., 2011. Developmental functions for the Caenorhabditis elegans Sp protein SPTF-3. Mech. Dev. 128: 428–441. [DOI] [PubMed] [Google Scholar]
- Ward A., Liu J., Feng Z., Xu X. Z., 2008. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat. Neurosci. 11: 916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenick A. S., Hobert O., 2004. Genomic cis-regulatory architecture and trans-acting regulators of a single interneuron-specific gene battery in C. elegans. Dev. Cell 6: 757–770. [DOI] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S., 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314: 1–340. [DOI] [PubMed] [Google Scholar]
- Wimmer E. A., Frommer G., Purnell B. A., Jackle H., 1996. buttonhead and D-Sp1: a novel Drosophila gene pair. Mech. Dev. 59: 53–62. [DOI] [PubMed] [Google Scholar]
- Winkelbauer M. E., Schafer J. C., Haycraft C. J., Swoboda P., Yoder B. K., 2005. The C. elegans homologs of nephrocystin-1 and nephrocystin-4 are cilia transition zone proteins involved in chemosensory perception. J. Cell Sci. 118: 5575–5587. [DOI] [PubMed] [Google Scholar]
- Woolfe A., Goodson M., Goode D. K., Snell P., McEwen G. K., et al. , 2005. Highly conserved non-coding sequences are associated with vertebrate development. PLoS Biol. 3: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray G. A., 2007. The evolutionary significance of cis-regulatory mutations. Nat. Rev. Genet. 8: 206–216. [DOI] [PubMed] [Google Scholar]
- Zhang F., Bhattacharya A., Nelson J. C., Abe N., Gordon P., et al. , 2014. The LIM and POU homeobox genes ttx-3 and unc-86 act as terminal selectors in distinct cholinergic and serotonergic neuron types. Development 141: 422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.