Abstract
Background
HIV is associated with atherosclerosis and low HDL. With inflammation, HDL becomes dysfunctional. We previously showed that pro-inflammatory HDL has high HDL redox activity (HRA). In this study, we: 1) compare HRA in HIV-infected versus non-HIV-infected subjects and 2) relate HRA to indices of macrophage activation and cardiovascular disease risk.
Methods
102 HIV-infected subjects and 41 matched non-HIV controls without clinical cardiovascular disease underwent coronary CT angiography (CTA) and testing for immune/inflammatory biomarkers. The effect of purified HDL from each study subject on the oxidation rate of dihydrorhodamine-123(DOR) was normalized to the DOR of pooled HDL from healthy subjects. The normalized ratio DORsubject/DORpooled (nHRA) was used as a measure of HRA, with higher HRA suggesting dysfunctional HDL.
Results
HRA was higher in HIV-infected versus non-HIV subjects (1.4±0.01 versus 1.3±0.01, p=0.03). In multivariate modeling for HRA among all subjects, HIV status remained positively related to HRA (p=0.02), even after controlling for traditional cardiovascular risk factors, comorbid conditions, and immune activation. Among HIV-infected subjects, HRA correlated inversely with HDL (rho=−0.32, p=0.002) and log adiponectin (r=−0.28, p=0.006) and correlated positively with log sCD163 (r=0.24, p=0.02) - a monocyte/macrophage activation marker - and with percent non-calcified coronary atherosclerotic plaque (r=0.29, p=0.03). sCD163 remained significantly associated with HRA in multivariate modeling among HIV-infected subjects (p=0.03).
Conclusions
These data demonstrate increased HRA among HIV-infected subjects versus matched non-HIV subjects with comparable HDL levels. In HIV-infected subjects, HRA relates to macrophage activation and to non-calcified coronary atherosclerotic plaque, which may be rupture prone. Further studies are needed in HIV-infected patients to elucidate the interplay between immune activation, HDL function, and CVD risk.
Keywords: HIV, HDL, atherosclerosis, HDL oxidative potential
BACKGROUND
Relative to the general population, HIV-infected patients have a 50% increased risk of myocardial infarction (MI) above that which can be accounted for by traditional markers of cardiovascular disease (CVD) risk (1). Increasing evidence implicates immune activation as a contributor to heightened CVD risk in HIV (2): We previously showed that among ART-treated HIV-infected patients, macrophage activation relates to coronary atherosclerotic plaque inflammation (3) and vulnerability (4) and progression of subclinical atherosclerosis (5). In this study, we hypothesized that the interplay between macrophage activation and HDL function may be related to increased CVD risk in HIV.
HDL is generally accepted to have cardioprotective functions, participating in reverse cholesterol transport and exerting anti-inflammatory/anti-oxidant effects (6). However, HDL functionality is altered in inflammatory milieus such that this typically anti-inflammatory/anti-oxidant lipoprotein takes on pro-inflammatory and pro-oxidant properties (7) (8). Our study compares a functional property of HDL cholesterol - HDL redox activity (HRA) - in ART-treated HIV-infected subjects and non-HIV controls matched on traditional CVD risk factors. Our study also assesses, the relationship between HRA and both immune/inflammatory parameters and CVD risk markers. We have shown previously that increased HRA correlates with pro-inflammatory properties of HDL, the latter quantified using a physiologic cell-based assay (9) (10). Taken together, these data suggest that HRA is a measure of HDL dysfunction.
METHODS
Study participants
102 HIV-infected men and 41 non-HIV-infected men (ages 18–55) without known or clinically evident CVD were recruited from the Boston area to participate in the original study. non-HIV subjects were prospectively matched to HIV-infected subjects based on traditional CVD risk factors including age, gender, and body mass index (BMI). The clinical study was approved by Institutional Review Boards from the Massachusetts General Hospital (Partners Healthcare) and Massachusetts Institute of Technology, and all subjects gave informed consent. Data on the relationship between coronary atherosclerosis and circulating inflammatory biomarkers were previously published (11) (12) (4), but data on HRA in this group have not been reported.
Assessment of demographic, metabolic, immunologic/inflammatory and coronary atherosclerotic plaque parameters
Demographic data were assessed by history. Techniques for assessment of metabolic, immunologic/inflammatory, and coronary atherosclerotic plaque parameters are as per our previously published findings (11) (12) (4).
Fluorometric biochemical cell-free assay for assessment of HDL Redox Activity (HRA)
HRA was assessed using a validated fluorometric biochemical cell-free assay (9) (13). This assay measures the effect of purified HDL from cryopreserved serum on the rate of oxidation of the fluorogenic probe dihydrorhodamine 123 (DHR) (9). In the performance of this assay, quadruplicates of 1.25 ug of HDL cholesterol were added to 96-well plates (polypropylene, flat bottom, black, Fisher Scientific). Hepes Buffered Solution was added to each well to a final volume of 150 μl, followed by addition of 25 μl of the working solution of 50 μM DHR, for a total volume of 175 μl (final DHR concentration of 7 μM). Immediately following DHR addition, the plate was protected from light and placed in a fluorescence plate reader. The fluorescence of each well was assessed at two minute intervals for an hour with a Synergy 2 Multi-Mode Microplate Reader (Biotek, VT), using a 485/538 nm excitation/emission filter pair with the photomultiplier sensitivity set at medium. The DHR oxidation rate (DOR) was calculated for each well as the slope for the linear regression of fluorescence intensity between 10 and 50 min, and this rate was expressed as fluorescence units per minute (FU minute−1). The mean rate of quadruplicates for the wells containing the HDL samples was recorded. The DOR of each sample was normalized to the DOR of HDL from pooled serum of healthy blood bank donors. The normalized ratio DORsubject/DORpooled (nHRA) was used as a measure of HRA, with higher HRA suggesting dysfunctional HDL.
Statistical analysis
Between-group comparisons were performed using the Student's t-test for normally distributed variables and the Wilcoxon rank sum test for non-normally distributed variables. Dichotomous parameters were compared between groups using chi-square test likelihood ratios. Univariate regression analyses were performed among all subjects and then separately in the HIV-infected and non-HIV groups to assess the relationship of demographic, cardiometabolic, and immunologic/inflammatory parameters to HRA. Non-normally distributed parameters were related to HRA using Spearman's rho or were log-transformed and related via the Pearson's correlation coefficient. Multivariate regression modeling for HRA was performed among all subjects and among HIV-infected subjects only. In the model among all subjects, independent variables included: a) relevant CVD risk parameters, including those which were significantly different between groups (e.g. diabetes, cigarette smoking, triglycerides, statin use, antihypertensive medicine use, hepatitis C, and sCD163 as a marker of systemic immune activation) b) parameters which were found to be related to HRA on univariate analysis in the overall group, the HIV-infected group, and/or the non-HIV infected group (age, BMI, HDL, and adiponectin, in addition to triglycerides and sCD163) and c) HIV status. In the model among HIV-infected subjects only, independent variables included only those variables related to HRA in the overall group and/or the HIV-infected group (BMI, triglycerides, HDL, sCD163, and adiponectin). JMP 9.0 (SAS Institute) was used to perform statistical analyses.
RESULTS
Characteristics of study participants
Study participants included 102 HIV-infected men and 41 non-HIV infected men prospectively recruited to match on age, gender, and BMI. As per Table 1, most HIV-infected subjects were receiving stable ART and were virologically suppressed. Between groups, there was no statistically significant difference in age, family history of premature heart disease, smoking, diabetes prevalence, BMI, and blood pressure. Levels of HDL cholesterol were not significantly different between groups (46 mg/dl (41, 52) versus 45 mg/dl (38, 55), non-HIV-infected versus HIV-infected, p=0.75). Levels of total and LDL cholesterol were also comparable between groups, whereas triglyceride levels were higher in the HIV-infected group (109 mg/dl (81,182) versus 80 mg/dl (64, 123), p=0.001).
Table 1.
Demographics
| Non-HIV-Infected Controls (n=41) | HIV-Infected Subjects (n=102) | P-value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 45.0 (41.5, 50.5) | 48.0 (41.8, 52.0) | 0.18 |
| Race | 0.19 | ||
| White | 61 | 63 | |
| Black | 17 | 22 | |
| Hispanic | 7 | 12 | |
| Asian | 7 | 1 | |
| Native American | 5 | 3 | |
| Family history of premature heart disease, % | 13 | 22 | 0.24 |
| Current smoker, % | 29 | 41 | 0.20 |
| Diabetes mellitus, % | 2 | 7 | 0.26 |
| Use of antihypertensive medications, % | 8 | 29 | 0.003 |
| Use of lipid-lowering medications, % | 13 | 27 | 0.05 |
| Hepatitis C infection, %, self-reported | 2 | 25 | 0.0003 |
| Metabolic Parameters | |||
| Body mass index, kg/m2 | 26.3 (23.5, 29.6) | 25.7 (23.7, 29.1) | 0.75 |
| Systolic blood pressure, mm Hg | 117 (110, 124) | 120 (112, 127) | 0.22 |
| Total cholesterol level, mg/dL | 177 (154, 201) | 173 (155, 201) | 0.94 |
| LDL cholesterol level, mg/dL | 110 ± 33 | 101 ± 31 | 0.15 |
| HDL cholesterol level, mg/dL | 46 (41, 52) | 45 (38, 55) | 0.75 |
| Triglyceride level, mg/dL | 80 (64, 123) | 109 (81, 182) | 0.001 |
| Adiponectin level, ng/mL | 4362 (3326, 7106) | 5454 (2521, 10125) | 0.50 |
| HIV Disease-Related Parameters | |||
| Duration of HIV infection, years | N/A | 13.8 ± 6.5 | N/A |
| Currently receiving antiretroviral therapy, % | N/A | 95 | N/A |
| Duration of antiretroviral therapy, years | N/A | 7.9 (3.1, 11.0) | N/A |
| Current PI treatment, % | N/A | 52 | N/A |
| Current NRTI treatment, % | N/A | 92 | N/A |
| Current NNRTI treatment, % | N/A | 47 | N/A |
| Current CD4+ count, cells/mm3 | N/A | 473 (303, 748) | N/A |
| Nadir CD4 count, cells/mm3, self-reported | N/A | 175 (57, 278) | N/A |
| HIV RNA level (viral load), copies/mL | N/A | <50 (<50 to <50) | N/A |
| Undetectable viral load (<50 copies/mL), % | N/A | 81 | N/A |
| Immunologic/Inflammatory Parameters | |||
| High-sensitivity C-reactive protein (CRP), mg/L | 1.3 (0.7, 3.3) | 1.6 (0.7, 3.8) | 0.49 |
| High-sensitivity interleukin-6 (IL-6), pg/mL | 0.6 (0.5, 1.0) | 0.9 (0.7, 1.5) | 0.01 |
| D-dimer, ng/mL | <220 (<220, 333) | <220 (<220, 322) | 0.93 |
| Lipopolysacharide (LPS), ng/mL | 0.07 (0.06, 0.1) | 0.1 (0.07, 0.1) | 0.0004 |
| Soluble CD 14 (cSD14), ng/mL | 211 (121, 374) | 305 (157, 440) | 0.08 |
| Soluble CD163 (sCD163), ng/mL | 765 (572, 1054) | 1063 (695, 1577) | 0.0007 |
LDL = low density lipoprotein; HDL = high density lipoprotein; PI = protease inhibitor; NRTI = nucleoside reverse transcriptase inhibitor; NNRTI = non-nucleoside reverse transcriptase inhibitor.
Comparison of HRA in HIV-infected group versus non-HIV group
HRA - a normalized value - was significantly higher in the HIV-infected group versus the non-HIV group (1.4 ± 0.01 versus 1.3 ± 0.01, p=0.03). HRA was not significantly different between HIV-infected subjects with detectable viral load and HIV-infected subjects with undetectable viral load (1.4 ± 0.02 versus 1.4 ± 0.01, p=0.68).
Univariate relationships between HRA and demographic, metabolic, immunologic/inflammatory, and CVD risk parameters
Among all subjects, HRA was inversely related to HDL levels (rho=−0.29, p=0.0007) and log adiponectin levels (r=−0.26, p=0.002). HRA was positively related to triglycerides (rho=0.18, p=0.04) and log sCD163 levels (r=0.22, p=0.009) among all subjects.
Among non-HIV controls, there was a statistically significant relationship between HRA and age (rho=0.31, p=0.05). Statistically significant relationships between HRA and other inflammatory/immune and cardiometabolic parameters were not detected among non-HIV controls.
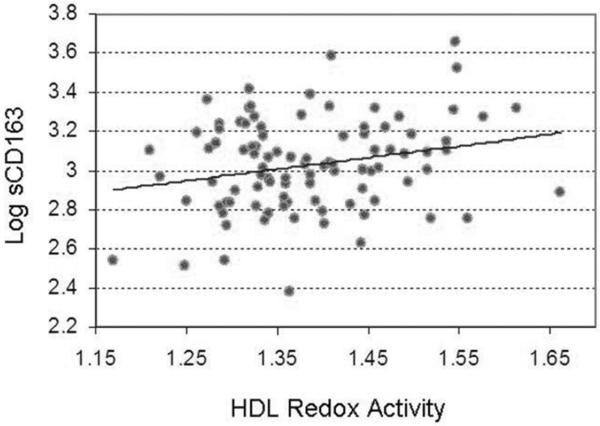
Among HIV-infected subjects, a significant inverse relationship was noted between HRA and HDL levels (rho=−0.32, p=0.002) and between HRA and log adiponectin levels (r=−0.28, p=0.006). Also among HIV-infected subjects, there was a significant positive relationship between HRA and levels of the monocyte/macrophage activation marker sCD163 (r=0.24, p=0.02) (Figure 1), but no statistically significant relationship between HRA and log levels of other circulating inflammatory/immunologic markers including high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), d-dimer, lipopolysaccharide (LPS), and sCD14. Moreover, there was a statistically significant positive relationship between HRA and the percentage of non-calcified coronary atherosclerotic plaque (r=0.29, p=0.03) among HIV-infected subjects, but no statistically significant relationship between HRA and the percentage of non-calcified coronary atherosclerotic plaque among all subjects (p=0.28) or among non-HIV controls (p=0.11). HRA was not significantly related to HIV-specific parameters including duration of HIV, duration of ART, ART class (data not shown), current CD4 count, nadir CD4 count, and log viral load (Table 2).
Figure 1.
Relationship between HDL Redox Activity (HRA) and log levels of the monocyte/macrophage activation marker soluble CD163 (sCD163) among HIV-infected subjects (n=102) (r=0.24, p=0.02).
Table 2.
Univariate Associations between HDL Redox Activity and Demographic, Metabolic, and Immunologic/inflammatory Parameters Among All Subjects, HIV-Infected Subjects, and Non-HIV-Infected Control Subjects
| All Subjects (n=143) | HIV-Infected Subjects (n=102) | Non-HIV-Infected Control Subjects (n=41) | ||||
|---|---|---|---|---|---|---|
| Demographic Parameters | ||||||
| Age, years | rho=0.13 | p=0.14 | rho=0.02 | p=0.88 | rho=0.31 | p=0.05* |
| Cigarette smoking, pack-years | rho=−0.07 | p=0.44 | rho=−0.08 | p=0.48 | rho=−0.29 | p=0.08 |
| Metabolic Parameters | ||||||
| Body mass index, kg/m2 | rho=0.17 | p=0.05* | rho=0.11 | p=0.30 | rho=0.30 | p=0.06 |
| Systolic blood pressure, mm Hg | rho=0.04 | p=0.64 | rho=−0.04 | p=0.67 | rho=0.15 | p=0.35 |
| Total cholesterol level, mg/dL | rho=−0.03 | p=0.75 | rho=−0.10 | p=0.35 | rho=0.17 | p=0.31 |
| HDL cholesterol level, mg/dL | rho=−0.29 | p=0.0007* | rho=−0.32 | p=0.002* | rho=−0.17 | p=0.29 |
| LDL cholesterol level, mg/dL | r=0.08 | p=0.39 | r=0.03 | p=0.74 | r=0.27 | p=0.09 |
| Triglyceride level, mg/dL | rho=0.18 | p=0.04* | rho=0.11 | p=0.29 | rho=0.20 | p=0.23 |
| Log adiponectin, ng/mL | r=−0.26 | p=0.002* | r=−0.28 | p=0.006* | r=−0.24 | p=0.14 |
| Immunologic/inflammatory Parameters | ||||||
| Log high-sensitivity C-reactive protein (CRP), mg/L | r=−0.10 | p=0.26 | r=−0.07 | p=0.50 | r=−0.21 | p=0.19 |
| Log high-sensitivity interleukin 6 (IL-6), pg/mL | r=−0.04 | p=0.66 | r=−0.03 | p=0.80 | r=−0.24 | p=0.20 |
| Log D-dimer, ng/mL | r=0.004 | p=0.97 | r=−0.004 | p=0.97 | r=−0.02 | p=0.93 |
| Log lipopolysacharide (LPS), ng/mL | r=0.02 | p=0.86 | r=−0.04 | p=0.72 | r=−0.03 | p=0.85 |
| Log soluble CD14 (sCD14), ng/mL | r=0.10 | p=0.24 | r=0.04 | p=0.70 | r=0.17 | p=0.30 |
| Log soluble CD163 (CD163), ng/mL | r=0.22 | p=0.009* | r=0.24 | p=0.02* | r=0.03 | p=0.84 |
| Duration of HIV infection, years | N/A | N/A | r=0.10 | p=0.36 | N/A | N/A |
| Duration of antiretroviral therapy, years | N/A | N/A | rho=0.19 | p=0.14 | N/A | N/A |
| Current CD4 count, cells/mm3 | N/A | N/A | rho=−0.006 | p=0.96 | N/A | N/A |
| Nadir CD4 count, self-reported, cells/mm3 | N/A | N/A | rho=−0.009 | p=0.94 | N/A | N/A |
| Log HIV-RNA level (viral load) | N/A | N/A | r=0.18 | p=0.11 | N/A | N/A |
Multivariate regression modeling for HRA among all subjects and among HIV-infected subjects alone
Among all subjects, HIV status remained positively related to HRA in multivariate modeling controlling for CVD risk factors and parameters related to HRA on univariate analysis in the overall group, the HIV-infected group, and/or the non-HIV infected group (R2 for model 0.20, p=0.009; HIV estimate=0.02, standard error=0.01, p=0.02) (Table 3a). Among HIV-infected subjects only, sCD163 remained positively related to HRA controlling for variables related to HRA in the overall group and/or the HIV-infected group (R2 for model=0.16, p=0.007; sCD163 estimate 0.00003, standard error 0.00001, p=0.03) (Table 3b).
Table 3A.
Multivariate Model for HDL Redox Activity Among All Subjects Overall R2=0.20, p=0.009
| Parameter | Estimate | Standard Error | p value |
|---|---|---|---|
| HIV | 0.02 | 0.01 | 0.02* |
| Hepatitis C | 0.02 | 0.01 | 0.18 |
| sCD163 | 0.00002 | 0.00001 | 0.24 |
| Age | −0.0008 | 0.001 | 0.50 |
| Body mass index (BMI) | 0.002 | 0.002 | 0.36 |
| Diabetes mellitus (DM) | −0.003 | 0.02 | 0.86 |
| Current cigarette smoking | −0.01 | 0.009 | 0.09 |
| Triglycerides | −0.0001 | 0.00008 | 0.22 |
| HDL cholesterol | −0.001 | 0.0006 | 0.10 |
| Current statin use | 0.02 | 0.01 | 0.17 |
| Current antihypertensive medication use | −0.004 | 0.01 | 0.70 |
| Adiponectin | −0.000003 | 0.000002 | 0.15 |
DISCUSSION
Our data are the first to show increased HRA among chronically infected, ART-treated HIV-infected patients versus matched non-HIV subjects with comparable HDL levels. This observation suggests that HDL functional properties - specifically HRA - are not fully captured by simple measurement of HDL levels among HIV-infected patients. As discrepant lipid levels per se do not appear to account for the heightened CVD risk in HIV-infected patients (1), it is reasonable to further investigate the degree to which lipoprotein functional properties, such as HRA, contribute. Although the biological significance of high HRA has yet to be fully determined, high HRA is felt to represent a measure of HDL dysfunction (9). We previously demonstrated that higher HRA correlated significantly with “pro-inflammatory HDL”, or HDL with decreased ability to prevent LDL from inducing MCP-1 expression and monocyte migration into the subendothelial space of human aortic endothelial and smooth muscle cell co-cultures (9) (10). If HRA indeed reflects HDL dysfunction, HRA could have potential relevance to CVD risk in HIV.
In our study, among all subjects and among HIV-infected subjects only, HRA was significantly associated with circulating levels of the monocyte/macrophage activation marker soluble CD163 (sCD163). Among HIV-infected subjects, sCD163 remained significantly associated with HRA even after controlling for other parameters associated with HRA on univariate analyses. One possible explanation for the relationship between sCD163 and HRA is that immune activation in HIV alters HDL structural/functional properties. Previous studies have demonstrated structural/functional (7) (8) changes to HDL circulating in experimental or naturally occurring infectious/inflammatory conditions (i.e. “acute-phase HDL”). Among HIV-infected patients, there have been studies relating HDL particle size to CVD risk (14) but no studies to our knowledge have related functional HDL changes to such risk. Moreover, among HIV-infected patients, there have been studies suggesting that new initiation of ART introduces salutory changes to HDL structure, possibly by dampening immune activation (15). Further studies are needed to elucidate the interplay between immune activation, ART, HDL structure/function, and CVD risk in HIV.
We also note a positive association between HRA - but not HDL levels - and percent non-calcified plaque among HIV-infected patients. Previous studies in non-HIV subjects with suspected CVD have shown that non-calcified plaque better predicts major adverse cardiac events versus calcified plaque (16). Our findings reinforce that HDL levels and HDL functional assessments may provide complementary information relevant to CVD risk in HIV. Indeed, in non-HIV cohorts with autoimmune diseases, acute-phase HDL has already been shown to track with CVD risk (17). We did not observe an association between HRA and percent non-calcified coronary plaque in the non-HIV-infected group, and this may have been due to the smaller sample size of this group and the lower frequency of plaque or to differences in mechanisms of atherogenesis between HIV-infected patients and non-HIV infected control subjects.
Limitations of this study include the cross-sectional design, which precludes conclusions about causality and about the biological significance of HRA. Moreover, the study was conducted among men from one demographic area, and further studies are needed to determine the generalizability of the results. Concerns regarding biochemical assessments of HDL function have previously been described (13) (18). Although the absolute difference in HRA between groups was small, this was statistically significant and was large relative to the SD of the assay. HRA in our subjects was normalized to HRA in a population mean of healthy blood bank donors, resulting in the low observed standard deviation of the assay. The statistically significant positive association of HRA to relevant immunological and cardiometabolic indices among all subjects and among HIV-infected subjects lends credence to the potential clinical relevance of the difference in HRA observed between HIV-infected subjects and controls. Strengths of the study include the relatively large size of the HIV-infected group, matching between groups on age, gender, and BMI, and extensive phenotypic data - including CT angiography data - available for novel comparisons with HRA. This study thus builds on a previous, smaller study by our group in a different, HIV-infected cohort, showing a relationship between HRA and waist circumference (19).
Our study shows increased HRA among chronically HIV-infected subjects versus matched non-HIV subjects with comparable HDL levels. Among all subjects, the relationship between HIV status and HRA holds even when controlling for traditional CVD risk factors and immune parameters. Moreover, the data show that HRA among HIV-infected subjects is significantly associated with macrophage activation and with a measure of CVD risk - percent non-calcified coronary plaque. This study suggests the need for future explorations of the biologic significance of HRA and the relationship between immune activation, HDL structure/function changes, and CVD risk in HIV. Of great interest would be the development of strategies to improve HDL structure and function while reducing CVD risk among ART-treated HIV-infected patients.
Table 3B.
Multivariate Model for HDL Redox Activity Among HIV-Infected Subjects Overall R2=0.16, p=0.007
| Parameter | Estimate | Standard Error | p value |
|---|---|---|---|
| sCD163 | 0.00003 | 0.00001 | 0.03 |
| HDL cholesterol | −0.001 | 0.0007 | 0.07 |
| Adiponectin | −0.000004 | 0.000002 | 0.09 |
| Body mass index (BMI) | 0.0007 | 0.002 | 0.74 |
| Triglycerides | −0.0001 | 0.00007 | 0.14 |
Acknowledgements
We would like to thank the study subjects for their participation and the nurses, nutritionists, and administrators in the MGH General Clinical Research Center for their collaboration.
Funding sources: This work was conducted with the support of a Medical Research Investigator Training (MeRIT) award from the Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8KL2TR000168-05) to Dr. Zanni. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health. This work was also conducted with research funding from Bristol Myers Squibb to Dr. Grinspoon and with the support of National Institutes of Health Grants RO1HL112661 to Dr. Fitzgerald, K23 HL092792 to Dr. Lo, K24 DK064545 and RO1HL095123 to Dr. Grinspoon, M01-RR-01066, M01-RR8-1066 and 1 UL1 RR 025758-01 to the Harvard Clinical and Translational Science Center from the National Center for Research Resources, and P30DK040561 to the Nutrition Obesity Research Center at Harvard. This work was also supported by the National Institute of Allergy and Infectious Diseases (AI068634 and AI056933), UCLA AIDS Institute, and the UCLA Center for AIDS Research (AI28697). Partial funding for laboratory work was also provided by the University of Washington's CVD and Metabolic Complications of HIV/AIDS Data Coordinating Center (5R01HL095126).
Clinical Trials Registration Number: NCT 00455793
Disclosures: Bristol Myers Squibb Inc provided research funding to Dr. Grinspoon for this investigator-initiated industry sponsored study. The processes of study design, data analysis, and manuscript preparation were not influenced by funding sources. A poster related to this study was presented by Dr. Zanni at the 2013 Endocrine Society Conference.
REFERENCES
- 1.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013 Apr 22;173(8):614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012 Jun;205(Suppl 3):S375–82. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012 Jul 25;308(4):379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, et al. Increased Coronary Atherosclerotic Plaque Vulnerability by Coronary Computed Tomography Angiography in HIV-Infected Men. AIDS. 2013 Jan 16; doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012 Nov 15;206(10):1558–67. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khovidhunkit W, Memon RA, Feingold KR, Grunfeld C. Infection and inflammation-induced proatherogenic changes of lipoproteins. J Infect Dis. 2000 Jun;181(Suppl 3):S462–72. doi: 10.1086/315611. [DOI] [PubMed] [Google Scholar]
- 7.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995 Dec;96(6):2758–67. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgantini C, Natali A, Boldrini B, Imaizumi S, Navab M, Fogelman AM, et al. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes. 2011 Oct;60(10):2617–23. doi: 10.2337/db11-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelesidis T, Currier JS, Huynh D, Meriwether D, Charles-Schoeman C, Reddy ST, et al. A biochemical fluorometric method for assessing the oxidative properties of HDL. J Lipid Res. 2011 Dec;52(12):2341–51. doi: 10.1194/jlr.D018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navab M, Imes SS, Hama SY, Hough GP, Ross LA, Bork RW, et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88(6):2039–46. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010 Jan 16;24(2):243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011 Oct 15;204(8):1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelesidis T, Yang OO, Currier JS, Navab K, Fogelman AM, Navab M. HIV-1 infected patients with suppressed plasma viremia on treatment have pro-inflammatory HDL. Lipids Health Dis. 2011;10:35. doi: 10.1186/1476-511X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duprez DA, Kuller LH, Tracy R, Otvos J, Cooper DA, Hoy J, et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 2009 Dec;207(2):524–9. doi: 10.1016/j.atherosclerosis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker JV, Neuhaus J, Duprez D, Cooper DA, Hoy J, Kuller L, et al. Inflammation predicts changes in high-density lipoprotein particles and apolipoprotein A1 following initiation of antiretroviral therapy. AIDS. 2011 Nov 13;25(17):2133–42. doi: 10.1097/QAD.0b013e32834be088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou ZH, Lu B, Gao Y, Jiang SL, Wang Y, Li W, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012 Oct;5(10):990–9. doi: 10.1016/j.jcmg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 17.McMahon M, Grossman J, FitzGerald J, Dahlin-Lee E, Wallace DJ, Thong BY, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006 Aug;54(8):2541–9. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 18.Kelesidis T, Reddy ST, Huynh D, Meriwether D, Fogelman AM, Navab M, et al. Effects of lipid-probe interactions in biochemical fluorometric methods that assess HDL redox activity. Lipids Health Dis. 2012;11:87. doi: 10.1186/1476-511X-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelesidis T, Yang OO, Kendall MA, Hodis HN, Currier JS. Dysfunctional HDL and progression of atherosclerosis in HIV-1-infected and -uninfected adults. Lipids Health Dis. 2013;12:23. doi: 10.1186/1476-511X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]