1. Introduction
Although earlier work has examined the relationship between the diagnosis of chronic posttraumatic stress disorder (PTSD) (American Psychiatric Association, 2004) and brain structure, studies have largely not taken into account the impact of early life trauma, which may also contribute to the reported structural abnormalities. Brain-imaging studies using the fear-conditioning model (LeDoux, 2000; Pitman et al., 2001) have found that structural alterations associated with PTSD are present in various regions of the limbic system. Specifically, a relative reduction in the volume of the hippocampus bilaterally (Gilbertson et al., 2002; Wignall et al., 2004; Smith, 2005; Carrion et al., 2007; Woon and Hedges, 2008) and reduced thickness and volume of the anterior cingulate cortex (ACC) have been observed (Rauch et al., 2003; Yamasue et al., 2003; Kitayama et al., 2006; Woodward et al., 2006; Dickie et al., 2013). Other studies have found a thinner cortex in dorsolateral areas (Geuze et al., 2008) and in the inferior frontal gyrus (Liu et al., 2012). These findings indicate that the alterations in thickness may encompass more regions than the hippocampus and cingulum. Additional regions of interest, potentially relevant to PTSD, are derived from studies of fear acquisition or fear conditioning. Studies of healthy adults have shown that the structural integrity of areas like the ventromedial prefrontal cortex (Milad et al., 2005) and insula (Hartley et al., 2011) is involved in the modulation of fear. Despite mounting evidence, one study by Landré and colleagues (Landre et al., 2010) failed to replicate findings of altered structural integrity in a sample of sexual assault victims, highlighting the need for further studies that may explain these discrepant findings.
One central question that has been raised by studies finding alterations in thickness and volume pertains to the direction of causality between brain integrity and PTSD. That is, do the observed differences in cortical integrity represent a pre-exposure risk factor for the development of PTSD, or do they represent the consequences of trauma exposure/PTSD? The findings of Gilbertson and colleagues, which showed a smaller volume even in a genetically identical but trauma-unexposed twin, strongly suggest that a smaller volume of the hippocampus would represent a pre-exposure risk factor to develop PTSD upon exposure to a traumatic event (Gilbertson et al., 2002). The authors specified that, while heredity is the most likely explanation for this smaller hippocampal volume, environmental influences might also have played a significant role. Emphasizing the role of environment, a study using the same sample of identical twins discordant for trauma exposure and PTSD diagnosis illustrated that the anatomical differences may be due more to a gradual decline in grey matter following the onset of PTSD (Kasai et al., 2008). While these twin studies provide important information about the potential direction of causality in very severe PTSD, neither one investigated specific elements of pre-deployment experiences, which also might have affected the volume of the structures of interest. Further, they did not investigate the potentially linear association between volumes and severity of symptoms. Thus, the question remains as to the impact of documented traumatic stress exposure during childhood on the linear association between brain structure and severity of PTSD symptoms following additional trauma exposure in adulthood.
While there is a wide spectrum of intensities of adverse events during childhood, it is possible to identify altered developmental trajectories. Magnetic resonance imaging (MRI) studies of healthy human adults (Buss et al., 2007) have shown that exposure to stressful events during critical periods of development may be associated with significantly smaller volumes in the limbic system during adulthood. Other studies (Cohen et al., 2006; Andersen et al., 2008; Dannlowski et al., 2012) specifically illustrated that exposure to traumatic events during childhood and adolescence had a negative impact on the volume of the hippocampus and the anterior cingulate cortex (ACC) in otherwise healthy adults. This did not extend to the amygdala. Reduced hippocampal volume due to childhood sexual abuse has also been shown in multiple studies (Stein et al., 1997; Bremner et al., 1999; Shin et al., 1999), summarized by a recent meta-analysis (Woon and Hedges, 2008). Studies investigating the impact of childhood trauma on the volume of the amygdala have presented mixed evidence. Lupien and colleagues (Lupien et al., 2011) have observed greater amygdala volume in 10 year-old children of mothers with major depression. However, studies conducted in adults with a history of childhood trauma have shown either no difference in amygdala volume (Woon and Hedges, 2008) or smaller amygdala (Kuo et al., 2012). In sum, the mixed findings for the amygdala may be partly due to the timing of measurement, with a progressive stress-induced decline in volume occurring throughout childhood and adolescence (Tottenham and Sheridan, 2009).
Despite the evidence provided in the previously mentioned studies of PTSD and childhood trauma, few studies have thoroughly investigated how trauma during development affects the relationship between brain structures and severity of symptoms as a consequence of re-exposure during adulthood. It must be noted that, while studies of childhood sexual abuse examined the consequences of PTSD acquired during childhood as a result of the abuse, and studies investigating early life trauma examined otherwise healthy adults, limited attention has been given to the impact of childhood trauma on the reaction to deployment-related trauma in military service members during adulthood. Moreover, the previously mentioned studies reported mostly main group differences, dichotomizing participants based on the diagnosis of PTSD. This may have limited the capacity to fully capture the association between brain volume and symptom severity. The current study examined the impact of exposure to trauma, as defined by exposure specifically to interpersonal traumatic events before 18 years of age, on the integrity of cortical thickness following exposure to military trauma during adulthood. Cortical thickness has been shown to be a reliable method of assessing cortical development as well as a sensitive measure for structural alterations due to major depression (Jarnum et al., 2011), schizophrenia and bipolar disorder (Rimol et al., 2010), aging (Salat et al., 2004) and Alzheimer's disease (Dickerson et al., 2009). We hypothesized that, because of the overlap between cortical regions affected by both early life trauma and PTSD, there would be a significant interaction between exposure to interpersonal traumatic events during childhood and current cortical thickness in the ACC such that the group reporting a history of childhood interpersonal trauma would show a negative relationship between thickness and the severity of current PTSD symptoms. A secondary aim was to examine the relationship between current symptoms of PTSD and volumetric measures of the amygdala and hippocampus. We hypothesized that individuals with pre-deployment trauma exposure would present smaller amygdala and hippocampal volumes compared with the control group.
2. Methods
2.1. Participants and procedure
2.1.1. Recruitment
The first 108 service members who enrolled in the Veterans Affairs RR&D-supported Traumatic Brain Injury (TBI) Center for Excellence (CoE) at Veterans Affairs Boston Healthcare System: The Translational Research Center for Traumatic Brain Injury and Stress-Related Disorders (TRACTS), who had complete clinical data, and who completed the MRI were eligible for this study. Participants enrolled in the TRACTS CoE are recruited from the Boston Metropolitan area via a full-time recruitment specialist for the TRACTS who attends Yellow Ribbon Events, Task Force Meetings, and other events involving Army and Air National Guard, Marine and Marine Reserves, and Army and Army Reserve Units. Participants were excluded from the TRACTS study if they had a history of seizures, prior serious medical illness (e.g. cerebrovascular accident, myocardal infarction, and diabetes); current active suicidal and/or homicidal ideation, intent, or plan requiring immediate crisis intervention; current DSM-IV-TR diagnosis of bipolar disorder, schizophrenia or other psychotic disorder (except psychosis not otherwise specified due to trauma-related hallucinations); or cognitive disorder due to a general medical condition other than traumatic brain injury (TBI). Furthermore, with regards to the MRI acquisition, participants were excluded if they had any metal implant, shrapnel, aneurysm clip, pacemaker, or if they were pregnant. All procedures were approved by the Institutional Review Board (IRB) of the Veterans Affairs Boston Healthcare Center.
2.1.2. Clinical assessment
All participants underwent a complete psychological assessment by a doctoral level psychologist to determine if participants met diagnostic criteria for PTSD and TBI using standardized instruments, the Clinician-Administered PTSD Scale (CAPS) (Blake et al., 1995) and the Boston Assessment of TBI-Lifetime (BAT-L) (Fortier et al., 2013). Following this, the data from the assessment were reviewed by at least three doctoral level psychologists to achieve consensus diagnosis. Based on the reports from the Traumatic Life Events Questionnaire and the CAPS, two groups were formed. First, the Early Life Trauma (EL-Trauma+, N=43) group was composed of individuals who reported the occurrence of an interpersonal trauma (items 10-15 of the Traumatic Life Events Questionnaire: 10 family violence, items 11-12 physical punishment, items 13-15 sexual abuse) before the age of 18 coupled with an A2 (fear/helplessness/horror as defined by DSM-IV-TR) reaction. The age cut-off insured that the traumatic event would be pre-deployment. The control group (Control, N=65) was composed of individuals who reported no interpersonal trauma before the age of 18. While some participants of the control group did report exposure to traumatic events not of an interpersonal nature (e.g., natural disaster, motor-vehicle accidents, witness of robbery, criterion A2 of the DSMIV-TR), none were diagnosed with PTSD as a result of this pre-deployment event.
The psychological assessment consisted of the following:
Early Life Trauma exposure was assessed using the Traumatic Life Events Questionnaire (TLEQ), a 23-item self-report measure of 22 types of potentially traumatic events including natural disasters, exposure to warfare, robbery involving a weapon, physical abuse and being stalked. The TLEQ has good temporal stability, reliability, and validity (Kubany et al., 2000). In addition to exposure, age of exposure, number of exposures, and emotional reaction characterized by fear/horror/helplessness (DSM-IV diagnostic criteria A1 and A2 of PTSD) are recorded.
The Clinician Administered PTSD Scale (CAPS) (Blake et al., 1995) is the gold standard in PTSD assessment. The CAPS is a 30-item structured interview that corresponds to the DSM-IV criteria for PTSD. The CAPS was used to make a current (past month) and/or lifetime diagnosis of PTSD. To allow for a better characterization of the severity of symptoms, a global score was computed by summing the frequency and intensity of clusters of symptoms B (Flashback and Intrusive Memories), C (Avoidance and Emotional Numbing) and D (Hyperarousal). For the current study, two CAPS scores were collected, once for pre-deployment and once for the current PTSD severity (within the last month). Both current and lifetime versions of the CAPS are based on the TLEQ interview, and they asked the participant to report on the worst reactions to the main pre-deployment trauma that they had experienced and the worst events overall for current CAPS.
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/NP) is a semi-structured interview that includes modules designed to assess either the lifetime or current (past-month) experience of DSM-IV Axis I psychiatric disorders (http://www.scid4.org/psychometric/). All participants enrolled in the TRACTS receive the Structured Clinical Interview for DSM-IV/Non-patient version (SCID-I/NP) to determine eligibility and to characterize each participant's psychological history. The SCID-I/NP was used to identify participants with current alcohol/substance dependence and current mood disorder.
TBI exposure was assessed using the Boston Assessment of TBI-Lifetime (BAT-L) (Fortier et al., 2013; Fortier et al., in press), a measure developed at the TRACTS CoE, based on the diagnostic criteria of the Department of Defense, to capture the unique injuries sustained during OEF/OIF deployment (as well as injuries incurred during civilian experiences) with great detail, including the context and events occurring before, during, and after the injury. The diagnostic criteria encompass Mild, Moderate and Severe, depending on the duration of loss of consciousness, post-traumatic amnesia and altered state of consciousness. The BAT-L assesses TBI during three lifetime periods: pre-military, military, and post-military. TBI criteria are evaluated through open-ended questioning (to document alteration of mental status, posttraumatic amnesia, and loss of consciousness), and injuries are then graded (mild stage; moderate; severe). Initial interrater reliability and validity have been established for the BAT-L and are comparable to those for the Ohio State University TBI Identification Method (Corrigan and Bogner, 2007). For the purpose of this study, numbers of TBIs across the lifetime and military-related events were the variables retained as a measure for brain injuries.
Alcohol use was measured using the Lifetime Drinking History (Koenig et al., 2009). Combat exposure was measured using the Combat scale of the Deployment Risk and Resilience Inventory (Vogt et al., 2008).
2.2. MRI acquisition and processing
All scans were performed in the Neuroimaging Research for Veterans (NeRVe) Center at the Boston VA Healthcare Center in Jamaica Plain, MA, using a Siemens 3T TIM Trio system with a 12-radiofrequency channels head coil. Two T1 structural MPRAGE scans were acquired [3D sequence, flip angle 7°, field of view 256 × 256, echo time = 3.32 ms, repetition time = 2530 ms, slice thickness = 1 mm]. Both scans were averaged to increase signal-to-noise ratio. At the time of acquisition, visual inspection of the quality of the images was performed, and scans with excessive movement artifact were re-acquired. Images were preprocessed using the recon-all tools provided in the FreeSurfer package (Dale et al., 1999; Fischl et al., 1999) version 5.1, and all images were processed using the same machine to avoid discrepant findings due to different versions of FreeSurfer or OSX (Gronenschild et al., 2012). Briefly, all images were corrected for motion artifacts and averaged. Following this, images were transformed using a 12 DoF affine Talairach transformation onto the Montreal Neurological Institute (MNI) 305 template. Signal-intensity correction and skull stripping were then applied, before automated segmentation of the white matter surface and reconstruction of the pial surface. A trained research assistant (E.L.) reviewed the integrity of the surface segmentation for each scan. Cortical thickness was extracted using the FreeSurfer suite (for detailed description, see Fischl and Dale, 2000; Salat et al., 2004; Leritz et al., 2011). Cortical thickness measures were mapped to the inflated surface, allowing the data to be visualized without interference from the cortical folding (Salat et al., 2004). Since thickness of the cortical mantle is not affected by intracranial volume (ICV), the thickness analyses were not ICV-corrected (Buckner et al., 2004). Maps were filtered using a surface-based smoothing kernel with a full-width at half-maximum (FWHM) Gaussian kernel of 20 mm and averaged across participants using a non-rigid high dimensional spherical averaging method to align cortical folding patterns (Fischl et al., 1999). Statistical comparisons of global data and surface maps were generated using a General Linear Model of the effects of early life trauma and clinical measures at each vertex along the cerebral surface.
The amygdala, hippocampus, and total intracranial volumes were computed using the tools in recon-all of the FreeSurfer package (Fischl et al., 2002; Buckner et al., 2004). Analyses were performed using the statistical software SPSS for Mac version 16.0.
2.3. Statistical analyses
Student's t-tests and Chi-square tests were used to compare groups on sociodemographic variables at a P-value of 0.05.
All statistical maps for thickness analyses were initially set at a P-value of 0.01. We performed General Linear Model analyses of the interaction between groups (EL-Trauma+ vs. Control) and current CAPS score on thickness across the whole cerebrum. The statistical design specified for FreeSurfer formally tested the interaction between group and PTSD severity. Because of its significant impact on cortical thickness, age was used as a covariate in all analyses. Further, we examined main effect of group on mean thickness, again controlling for age. Last, for clusters of significant interaction between current severity of PTSD symptoms and thickness, we extracted for each group the mean thickness and conducted partial correlations with CAPS score, controlling for age, using the software SPSS 16.0, in order to assess the exact level of association of all significant vertices.
Corrections for multiple comparisons for thickness maps were performed using a Monte-Carlo simulation with 5000 iterations at a cluster threshold of P < 0.05 and vertex-wise threshold of P < 0.01 (Hagler et al., 2006; Nichols, 2012).
For volumetric analyses, multiple analyses of covariance were performed using the software SPSS 16.0 for Macintosh computers with left and right hippocampus and amygdala volumes as dependent measures, group as independent factor and age, number of lifetime TBIs and ICV as covariates. Pearson's partial correlations were performed for both hemispheres on the amygdala and hippocampus with the clinical variables controlling for age. The Benjamini and Hochberg False Discovery Rate correction was used to control for multiple comparisons (Benjamini and Hochberg, 1995).
3. Results
3.1. Main effects of early life trauma on clinical variables
All sociodemographic data are summarized in Table 1. In the EL-Trauma+ group, three participants had a history of moderate TBI and one participant had a history of severe TBI, all being pre-deployment. These participants were not excluded from the analyses, since their inclusion did not change the effects reported below. T-tests revealed that the EL-Trauma+ group had greater current PTSD severity [t (106) = 2.13, P = 0.04], pre-deployment PTSD severity [t (88) = 4.46, P < 0.001], and number of TBIs across the lifetime [t (105) = 2.15, P = 0.03]. None of the other clinical variables or deployment variables (total duration, time since last deployment or combat exposure) differed between groups. Also, groups did not differ on the ratio of males and females [χ2 (1) = 2.72, P = 0.26], the number of individuals diagnosed with current alcohol/substance abuse [χ2 (1) = 1.34, P = 0.51], or the number of individuals diagnosed with current mood disorder [χ2 (1) = 1.07, P = 0.59]. No participant in the control group reported an onset of PTSD symptoms before the age of 18.
Table 1.
Sociodemographic information
| EL-Trauma + | Control | P | |
|---|---|---|---|
| Age (years) | 35.64 (1.32) | 33.35 (1.23) | 0.22 |
| range: 20-62 | range: 20-58 | ||
| CAPS Current | 53.09 (4.41) | 40.35 (3.90) | 0.04* |
| CAPS Pre-dep | 35.16 (3.71) | 13.55 (3.15) | 0.00*** |
| Dep Duration (months) | 13.46 (.948) | 13.25 (1.00) | 0.89 |
| Dep Time since (months) | 32.61 (3.24) | 26.36 (3-17) | 0.19 |
| Combat Exposure | 13.08 (1.89) | 14.80 (1.56) | 0.48 |
| LDH Total / KG | 1964.92 (383.97) | 1316.88 (170.36) | 0.08 |
| DASS Depression | 8.97 (1.43) | 8.00 (1.38) | 0.64 |
| DASS Anxiety | 7.64 (1.37) | 6.27 (1.21) | 0.46 |
| DASS Stress | 12.92 (9.80) | 12.34 (1.47) | 0.79 |
| Sex ratio (M:F) | 35:8 | 59:6 | 0.26 |
| Lifetime mTBIs | 1.70 (0.29) | 1.02 (0.18) | 0.03* |
| Military mTBIs | .59 (0.13) | .40 (0.09) | 0.21 |
Sociodemographic information; Dep = Deployment, measured in months; LDH Total / KG = Total alcohol during lifetime adjusted to weight.; mTBI = mild traumatic brain injury. Age is measured in years. Results show significant differences between groups on CAPS Current and Pre-deployment, as well as on number of TBIs experienced during lifetime.
Among the EL-Trauma+ group, all participants were exposed to their first trauma between the ages 0 and 12 years. Table 2 summarizes the type of events and frequency of occurrence for each type of event in all participants of the EL-Trauma+ only.
Table 2.
Descriptive statistics of traumatic events in the EL-Trauma+ group only
| Frequency of Earliest trauma | N (43) | |
|---|---|---|
| >5 times | 27 | |
| 5 | 3 | |
| 4 | 2 | |
| 3 | 0 | |
| 2 | 3 | |
| 1 | 8 |
| Number of events on TLEQ 10-15 | N (43) | |
|---|---|---|
| 1 | 12 | |
| 2 | 24 | |
| 3 | 5 | |
| 4 | 1 | |
| 5 | 1 | |
| 6 | 0 |
| Type of event | N | |
|---|---|---|
| Family Violence | 29 | |
| Physical abuse | 34 | |
| Sexual Abuse <13 y.o. | 12 | |
| Sexual Abuse <13 y.o. (someone of same age) | 3 | |
| Sexual Abuse 13-18 years old | 4 | |
| Partner Violence | 2 |
TLEQ = Traumatic Life Events Questionnaire. “Number of events” refers to how many events the participants qualified for. Data shown is for the EL-Trauma+ group only.
3.2. Interaction between early life trauma and CAPS current score on cortical thickness
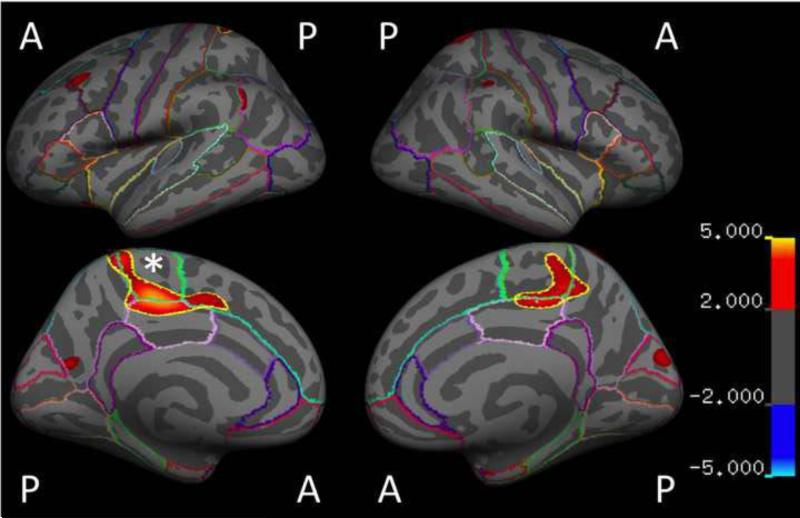
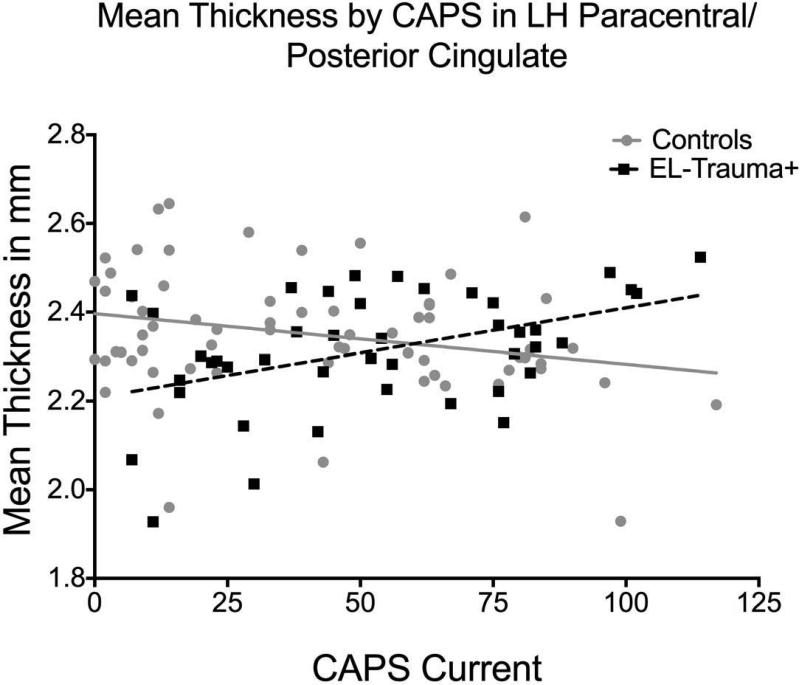
Examination of the interaction between Group and current PTSD severity revealed an opposing pattern of association with thickness (i.e., negative correlation between thickness and CAPS in Control but positive correlation in EL-Trauma+, P < 0.001) in a cluster located in the left paracentral and posterior cingulate gyrus [peak (x, y, z) = 28, -7, 40; Control r(64) = -0.383, P < 0.01; EL-Trauma+ r(42) = 0.416, P < 0.01]. This finding survived the Monte Carlo correction for multiple comparisons. A significant interaction was also observed for the volume of the left paracentral area [Fisher's Z = -3.09, P = 0.002, Control r(64) = -0.360, P = 0.004, EL-Trauma+ r(42) = 0.266, P = 0.089] and survived corrections for multiple comparisons. Additional clusters showing the same pattern in cortical thickness were detected at a P < 0.001 uncorrected level in the left precuneus [peak (x, y, z) = 27, -28, 61; Control r(64) = 0.148, P = 0.24; EL-Trauma+ r(42) = -0.082, P = 0.61], left rostral middle frontal gyrus [peak (x, y, z) =-6, 60, 28; Control r(64) = 0.023, P = 0.86; EL-Trauma+ r(42) = -0.185, P = 0.24], right superior parietal [peak (x, y, z) = -18, -40, 65] and right paracentral gyrus / posterior cingulate [peak (x, y, z) =-29, -29, 44; Control r(64) = -0.344, P < 0.001; EL-Trauma+ r(42) = 0.403, P < 0.001]. These clusters did not survive corrections for multiple comparisons. A significant interaction was also observed for the volume of the right posterior cingulate cortex [Fisher's Z = -2.79, P = 0.005, Control r(64) = -0.269, P = 0.033 EL-Trauma+ r(42) = 0.301, P = 0.052] and survived corrections for multiple comparisons. All results are summarized in Table 3 and Fig. 1. When analyses controlled for age, the results in most clusters increased in statistical strength, especially in the left paracentral/posterior cingulate and right superior parietal clusters. Controlling for total duration of deployment or number of lifetime TBIs did not affect the interaction. Fig. 2 illustrates the correlation between mean thickness of the peak cluster in the left hemisphere and the severity of symptoms.
Table 3.
Clusters of Significant effect
| Regions | Surface | Peak F value | p. |
|---|---|---|---|
| Early Life Trauma × CAPS Current | |||
| LH Paracentral/Posterior Cingulate | 860.60 | 3.424 | 0.0005† |
| LH Precuneus/Paracentral | 247.95 | 2.693 | 0.005 |
| LH Rostral Middle Frontal | 221.40 | 2.758 | 0.005 |
| LH Superior Frontal | 169.67 | 2.347 | 0.01 |
| RH Superior Parietal | 298.19 | 2.918 | 0.005 |
| RH Precuneus/Paracentral | 294.31 | 2.500 | 0.01 |
| Early Life Trauma × CAPS Current controlling AGE | |||
| LH Paracentral/Posterior Cingulate | 840.09 | 4.380 | 0.0001† |
| LH Caudal Middle Frontal | 208.42 | 2.710 | 0.005 |
| LH Insula/Precentral | 124.42 | 2.574 | 0.005 |
| LH Precuneus | 108.11 | 2.382 | 0.01 |
| LH Inferior Parietal | 106.84 | 2.142 | 0.01 |
| RH Superior Parietal | 388.92 | 3.346 | 0.001 |
| RH Posterior Cingulate/Paracentral | 979.89 | 2.888 | 0.005 |
| RH Cuneus | 149.21 | 2.473 | 0.01 |
All P-values reported are uncorrected for multiple comparisons using the Monte Carlo procedure.
Clusters that survived the Monte Carlo correction for multiple comparisons.
Fig. 1.
Maps of cortical areas where significant interactions between exposure to early life trauma and current severity of PTSD symptoms on thickness were detected. All results are shown at P < 0.001. Cluster designated with * survived correction for multiple comparisons.
Fig. 2.
Interaction of exposure to interpersonal early life trauma and mean thickness of the cluster in left paracentral/posterior cingulate cortex shows that the group exposed to early life trauma (EL-Trauma+, squares and dashed line) presents a positive relationship between current PTSD symptoms severity and cortical thickness, whereas the Controls (circles and full line) present a negative association (Control r (64)= -0.383, P = 0.002; EL-Trauma+ r (42)= 0.416, P = 0.006).
3.3 Main effects of early life trauma, lifetime TBIs and PTSD symptoms
General Linear Model analyses revealed a trend-level association (all P < 0.01 uncorrected) between thickness and severity of PTSD symptoms across all subjects in the left postcentral gyrus [peak (x, y, z) = -35, 5, 23] and entorhinal/fusiform cortex [peak (x, y, z) = -6, 13, -60], as well as in the right postcentral gyrus [peak (x, y, z) = 23, -31, 56], precuneus [peak (x, y, z) = 22, -55, 11] and middle/inferior temporal gyrus [peak (x, y, z) = 56, -15, -20]. When investigating the impact of childhood trauma exposure, analyses revealed a tendency for main group effects where the Control group showed on average greater thickness compared with the EL-Trauma+ group in bilateral posterior cingulate. In comparisons between groups of the mean thickness of the anterior portion of the cingulate gyrus, defined by the FreeSurfer atlas, no interaction between groups and hemisphere or main group differences emerged (all P > 0.40). Similarly, no volumetric differences were found between groups for the caudal and rostral regions of the anterior cingulate cortex.
The General Linear Model of the association between number of TBIs and thickness across all subjects revealed a cluster of trend-level negative associations in the right postcentral gyrus [peak (x, y, z) = 26, -37, 53, P < 0.01 uncorrected]. When the interaction between Group and number of lifetime TBIs on thickness was investigated, a trend-level interaction was detected in the right superior parietal cortex, where the Control group showed a negative association between thickness and number of TBIs, whereas the EL-Trauma+ group showed no association [peak (x, y, z) = 21, -83, 24, P < 0.01 uncorrected]. Similar findings were detected in the left isthmus cingulate [peak (x, y, z) = 25, -47, -10] and the superior parietal [peak (x, y, z) 10, -71, 36].
When the association between thickness and age of onset of trauma in the EL-Trauma+ group was investigated, a trend-level positive association was detected in the right pars triangularis of the inferior frontal gyrus [peak (x, y, z) = 19, 68, -23, P < 0.001 uncorrected]: later age of first trauma was associated with greater cortical thickness. No other association was detected in the right or left hemisphere.
3.4. Main rffects of early life trauma on amygdala and hippocampal volume
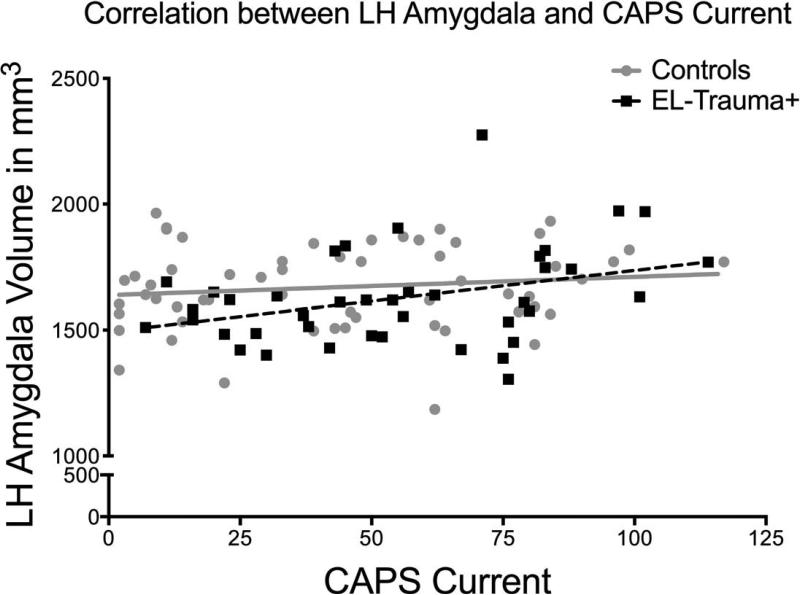
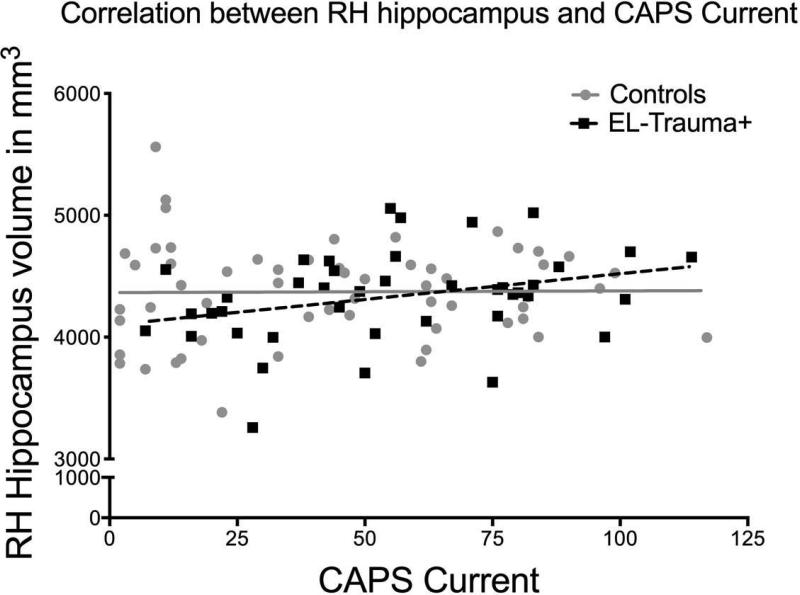
There was no significant main effect of group on the volumes of either the hippocampus or the amygdala. Similarly, formal testing of an interaction between PTSD severity and volumes using Fisher's z transformation did not reveal any significant interaction between group (EL-Trauma+/Control) and slopes. In the EL-Trauma+ group only, correlations revealed significant positive associations between current PTSD severity and the left amygdala [r (42) = 0.364, P = 0.019; right amygdale, P > 0.30] and a borderline effect in the right hippocampus [r (42) = 0.310, P = 0.049; left hippocampus, P > 0.08]. No correlation was detected in the Control group (see Fig. 3). Also, no correlations were detected for either group between volumes and severity of pre-deployment PTSD symptoms. Only the correlation between PTSD severity and volume of the right amygdala survived correction for multiple comparisons. There was a significant main effect of age on the right hippocampus [F (1, 104) = 6.33, P = 0.01]. Volumes of the bilateral hippocampus were also negatively correlated with age in the Control group only [left hemisphere: r (64) = -0.262, P = 0.049; right hemisphere: r (64) = -0.292, P = 0.028]. No such correlation with age was observed for the amygdala. Only the correlation in the right hemisphere survived correction for multiple comparisons. There was also a significant effect of number of TBIs on the right hippocampus [F (1, 104) = 5.24, P = 0.02], but no correlations in the left hippocampus or amygdala.
Fig. 3.
a (top) and 3b (bottom). Exploratory analyses revealed that correlations in the EL-Trauma+ group only showed associations between the severity of current PTSD symptoms and the volume of the left amygdala (top panel; r (42)= 0.364, P < 0.02) and right hippocampus (bottom panel; r (42)= 0.310, P < 0.05). The EL-Trauma+ group is represented by squares and dashed line, while the Control group is represented by circles and full line.
4. Discussion
The objective of the current study was to assess how a history of early life trauma would affect the relationship between cortical thickness and current severity of PTSD in OEF/OIF service members. Further, because these areas have been the focus of multiple studies of PTSD, we examined the potential impact of early life trauma on the relationship between the severity of current PTSD symptoms and the volume of the hippocampus and amygdala. Our analyses revealed that the thickness of the left posterior cingulate/paracentral area showed an opposite association with current severity of PTSD symptoms dependent on a history of trauma before the age of 18 years. Specifically, while subjects without a history of interpersonal trauma during childhood showed a negative association between thickness and current PTSD symptoms severity, the direction of association was positive in individuals with a positive history of childhood interpersonal trauma. This is a novel finding that illustrates how stressful events occurring during development may affect brain morphology and potentially impact individuals’ reactions to trauma.
Areas of the cingulum have been reported to be structurally and functionally different with respect to PTSD symptoms in a variety of studies. While structural alterations have been mostly observed in the more rostral areas (Rauch et al., 2003; Woodward et al., 2006; Dickie et al., 2013), a recent meta-analysis of functional studies (Hayes et al., 2012) has illustrated that areas more posterior in the cingulum (caudal to the genu of the corpus callosum extending to the supracallosal area) may be associated with greater activity in PTSD. The area of altered thickness in the current study was located in the midcingulate cortex (Vogt et al., 2003), or posterior cingulate parcellation of the Desikan-Killiany atlas of FreeSurfer (Desikan et al., 2006). Contrary to more anterior areas that share dense connections with the amygdala and hippocampus and that are actively involved in emotion regulation (Phan et al., 2002), this section of the cingulum bundle does not appear to be significantly associated with these subcortical structures or the emotional function supported by the more rostral portions. Rather, studies have linked this region with processing of pain stimuli and motor function (Vogt et al., 2003; Vogt, 2005; Beckmann et al., 2009; Shackman et al., 2011). This raises the question of why trauma exposure during development might affect the thickness of this specific area. However, considering the nature of the traumatic events experienced by our EL-Trauma+ participants (i.e., physical and sexual abuse, family violence), it is possible to speculate that areas processing pain may be very relevant targets for the impact of trauma. One recent study has found alterations in cortical thickness of the genital somatosensory field in women who had been exposed to childhood sexual abuse (Heim et al., 2013), suggesting an association between the development of the specific cortical areas and early life traumatic experiences. Further, the location of our finding also encompasses what has been described as the cingulate motor area (Vogt et al., 2003; Vogt, 2005). Thus, our findings suggest an impact of early life trauma on the basic development of motor functions as well as pain processing, possibly both at a physical and emotional level.
The exact mechanism by which the stress and fear experienced during childhood would affect the development of the cortex is not yet fully understood. Some evidence emerging from studies on the hippocampal formation in rodents allow us to speculate on potential causes for the differences observed between our groups. Specifically, the main endocrine messenger responsible for the peripheral physiological stress response, corticotropic releasing factor (CRF), has been shown to induce cell loss in 10-day-old rat pups, which was not due to a reduction in newborn cells (Brunson et al., 2001). This cell loss is also accompanied by a reduction in dendritic arborization (Chen et al., 2004). However, the situation of the amygdala may be different, as was demonstrated by the study of Vyas and colleagues (Vyas et al., 2002), who showed an increase in dendritic arborization in the amygdala as a consequence of stress. This last finding indicates that the effects of stress and endocrine messengers may have region-specific results. Studies in humans have shown that early life trauma may increase the level of cerebrospinal fluid CRF in adulthood (Carpenter et al., 2004), which is consistent with the animal models of the effect of early life CRF on brain development. Another potential mechanism may involve direct action of cortisol, the end product of the hypothalamic-pituitary-adrenal axis, and which is increased in individuals exposed to early life trauma (Heim et al., 2002). Specifically, animal studies have shown that cortisol can affect the integrity of the mitochondrial membrane. This effect may trigger the activity of caspases, proteases essential for programmed neural death, and lead to increased levels of apoptosis. Critically, the impact of glucocorticoids has been shown in rats to be triggered by external stimulation, either by licking and grooming by the dam or by artificial tactile stimulation (Hellstrom et al., 2012). This finding further underscores the need for studies of the impact of parental care on hormonal levels and cortical development in children. In sum, these animal models may help shed light on how traumatic stress experienced at an early age can affect the course of brain development and its relationship with symptoms due to trauma exposure during adulthood. In sum, the animal models provide for two possible pathways by which stress in an early age may affect the natural development of cortical and subcortical regions, mostly with deleterious effects and/or over-pruning. However, the current models do not offer a clear explanation for the opposing patterns of association observed in our study.
An important consideration when studying the impact of early life trauma is to recognize that this impact serves a purpose. When considered from a public health perspective, childhood abuse is a toxic condition that leads to increased risks for mental and physical health disorders (van der Kolk, 2003). However, from an evolutionary biology perspective, the forces exerted by exposure to a traumatic environment help shape the cortex in order to cope and increase chances of survival until age of reproduction. As discussed by Zhang and associates (Zhang et al., 2006), it may be reductionist to consider these alterations as simply unhealthy. The results from the current study show one side of the effects of early life trauma, which are the association between altered regions of the brain and maladaptive and symptomatic reactions experienced upon further trauma exposure. This raises the question of the other possible behavioral effects associated with thinner gray matter. Potentially, greater capacity for threat detection and flight response may have been a necessary adaptation during these early years, though the adaptation becomes maladaptive in later life. Future functional brain imaging studies probing signal detection and attentional processes may be able to answer such questions.
The current study did suffer from some limitations. The absence of a healthy control group without any form of trauma exposure prevents us from fully characterizing the impact of early life trauma in the absence of exposure to trauma in adulthood. Also, our whole sample consisted of deployed personnel who were exposed to significant levels of stress for an extended period of time, most of whom presented symptoms of PTSD to some degree, in turn, limiting the generalization of our results to the civilian population. Finally, our EL-Trauma+ group was based on the presence of severe interpersonal trauma, which stands in contrast with other stressors that do not have an assaultive component (e.g. poverty/low socioeconomic status, emotional neglect, natural disasters). Perhaps, the effects observed in the current study are only present in the most severe of cases.
Our results with regards to clinical variables revealed significant group differences in both pre-deployment and current PTSD symptoms severity, despite the absence of difference in terms of duration of deployment, number of military-related TBIs, depression, anxiety and general stress levels. As proposed by the diathesis-stress model, it is possible that previous exposure to severe interpersonal trauma may have sensitized individuals to the impact of subsequent trauma exposure. Studies have previously shown that exposure to trauma or diagnosis of PTSD increases the probability of diagnosis upon further exposure (Breslau et al., 1998; Breslau, 2001). It is also possible that the number of TBIs experienced during the lifetime, which did differ between groups, may have contributed to this significant difference in current PTSD symptoms severity as well, through damaging areas associated with remission.
In sum, despite these limitations, the findings suggest a possibly altered pattern of brain development as a result of exposure to severe psychological trauma during childhood, which would modulate the relationship between neuroanatomy and severity of current symptoms of PTSD in adulthood. This underlies the importance, from both an experimental and clinical perspective, of assessing as much as possible the various environmental and psychological factors during critical periods of development that may affect the reaction in the face of trauma, even years later.
Acknowledgments
This research was support by the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research and Development Traumatic Brain Injury Center of Excellence (B6796-C).
We thank Wally Musto for his championship of our work among military personnel and his tireless recruitment efforts on our behalf, as well as Alexandra Kenna, Catherine Fortier, Ann Rasmusson, and Brad Brummett for the clinical assessments and diagnoses. We also thank Emily Lindemer for assistance in the processing of MRI data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text revision APA; Washington, DC.: 2004. [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. Journal of Neuropsychiatry and Clinical Neurosciences. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neurosciences. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of Royal Statistical Society Series B: Methodological. 1995;57:289–300. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The Development of a Clinician Administered PTSD Scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib L, Southwick S, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? Journal of Clinical Psychiatry. 2001;62:16–22. [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, Pruessner JC. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. Journal of Neurosciences. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004;29:777–784. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119:509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, Baram TZ. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, McFarlane A, Bryant R, Gordon E, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI Identification Method. Journal of Head Trauma and Rehabilitation. 2007;22:318–329. doi: 10.1097/01.HTR.0000300227.67748.77. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie EW, Brunet A, Akerib V, Armony JL. Anterior cingulate cortical thickness is a stable predictor of recovery from post-traumatic stress disorder. Psychological Medicine. 2013;43(3):645–653. doi: 10.1017/S0033291712001328. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fortier CB, Amick M, Grande L, McGlynn S, Kenna A, Morra L, Clark BA, Milberg WP, McGlinchey RE. The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) Semi-structured Interview: evidence of research utility and validity. The Journal of Head Trauma Rehabilitation. 2013;66:1373–1382. doi: 10.1097/HTR.0b013e3182865859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Amick M, Kenna A, Milberg WP, McGlinchey RE. Correspondence of the Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) Clinical Interview and the VA TBI Screening Instrument. The Journal of Head Trauma Rehabilitation, electronic publication 12 December, 2013. doi: 10.1097/HTR.0000000000000008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, Westenberg HG, Heinecke A, de Kloet CS, Goebel R, Vermetten E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008;41:675–681. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton M, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenschild EH, Habets P, Jacobs HI, Mengelers R, Rozendaal N, van Os J, Marcelis M. The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One. 2012;7:e38234. doi: 10.1371/journal.pone.0038234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr., Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cerebral Cortex. 2011;21:1954–1962. doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood and Anxiety Disorders. 2012;2(1):9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depression and Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Heim CM, Mayberg HS, Mletzko T, Nemeroff CB, Pruessner JC. Decreased cortical representation of genital somatosensory field after childhood sexual abuse. American Journal of Psychiatry. 2013;170:616–623. doi: 10.1176/appi.ajp.2013.12070950. [DOI] [PubMed] [Google Scholar]
- Hellstrom IC, Dhir SK, Diorio JC, Meaney MJ. Maternal licking regulates hippocampal glucocorticoid receptor transcription through a thyroid hormone-serotonin-NGFI-A signalling cascade. Philosophical Tansactions of the Royal Society of London. Series B, Biological Sciences. 2012;367:2495–2510. doi: 10.1098/rstb.2012.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnum H, Eskildsen SF, Steffensen EG, Lundbye-Christensen S, Simonsen CW, Thomsen IS, Frund ET, Theberge J, Larsson EM. Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatrica Scandinavia. 2011;124:435–446. doi: 10.1111/j.1600-0447.2011.01766.x. [DOI] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological Psychiatry. 2008;63:550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. Journal of Affective Disorders. 2006;90:171–174. doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig LB, Jacob T, Haber JR. Validity of the lifetime drinking history: a comparison of retrospective and prospective quantity-frequency measures. Journal of Studies on Alcohol and Drugs. 2009;70:296–303. doi: 10.15288/jsad.2009.70.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychological Assessment. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Kuo JR, Kaloupek DG, Woodward SH. Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Archives of General Psychiatry. 2012;69:1080–1086. doi: 10.1001/archgenpsychiatry.2012.73. [DOI] [PubMed] [Google Scholar]
- Landre L, Destrieux C, Baudry M, Barantin L, Cottier JP, Martineau J, Hommet C, Isingrini M, Belzung C, Gaillard P, Camus V, El Hage W. Preserved subcortical volumes and cortical thickness in women with sexual abuse-related PTSD. Psychiatry Research: Neuroimaging. 2010;183:181–186. doi: 10.1016/j.pscychresns.2010.01.015. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, Fischl B, McGlinchey RE, Milberg WP. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2011;54:2659–2671. doi: 10.1016/j.neuroimage.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li YJ, Luo EP, Lu HB, Yin H. Cortical thinning in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. PLoS One. 2012;7:e39025. doi: 10.1371/journal.pone.0039025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, Pruessner JC, Seguin JR. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE. Multiple testing corrections, nonparametric methods, and random field theory. Neuroimage. 2012;62:811–815. doi: 10.1016/j.neuroimage.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. Journal of Clinical Psychiatry. 2001;62:47–54. [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman R, Carson MA, McMullin K, Whalen PJ, Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvag R, Fennema-Notestine C, Hagler DJ, Jr., Pung CJ, Jennings RG, Haukvik UK, Lange E, Nakstad PH, Melle I, Andreassen OA, Dale AM, Agartz I. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biological Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Piman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. American Journal of Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Smith ME. Bilateral hippocampal volume reduction in adults with posttraumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG. Hippocampal volume in women victimized by childhood sexual abuse. Psychological Medicine. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk BA. The neurobiology of childhood trauma and abuse. Child and Adolescent Psychiatric Clinics of North America. 2003;12:293–317, ix. doi: 10.1016/s1056-4993(03)00003-8. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt DS, Proctor SP, King DW, King LA, Vasterling JJ. Validation of scales from the Deployment Risk and Resilience Inventory in a sample of Operation Iraqi Freedom veterans. Assessment. 2008;15:391–403. doi: 10.1177/1073191108316030. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wignall EL, Dickson JM, Vaughan P, Farrow TFD, Wilkinson ID, Hunter MD, Woodruff PWR. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biological Psychiatry. 2004;56:832–836. doi: 10.1016/j.biopsych.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decresed anterior cingulate volume in combat-related PTSD. Biological Psychiatry. 2006;59:582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in chldren and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18:729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N. Voxel-Based Analysis of MRI Reveals Anterior Cingulate Gray-Matter Volume reduction in posttraumatic stress disorder due to terrorism. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Bagot R, Parent C, Nesbitt C, Bredy TW, Caldji C, Fish E, Anisman H, Szyf M, Meaney MJ. Maternal programming of defensive responses through sustained effects on gene expression. Biological Psychology. 2006;73:72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]