Abstract
We previously reported that the functional deletion of p21, a cyclin-dependent kinase inhibitor, in mice attenuated renal cell senescence in streptozotocin (STZ)-induced type 1 diabetic mice. In the present study, we investigated the effect of iron chelation on renal cell senescence and inflammation in the type 1 diabetic kidney. STZ-treated mice showed increase in iron accumulation, tubular cell senescence and macrophage infiltration at week 28 in the kidney. Administering deferasirox, which removes only dietary iron, significantly attenuated iron accumulation in proximal tubules and the number of infiltrating F4/80-positive cells without effecting blood glucose, hematocrit or hemoglobin levels. In contrast however, deferasirox did not influence renal cell senescence. The lack of p21 decreased the renal tubular iron accumulation and did not change tubular cell senescence. Interestingly, the STZ-treated animals showed an increase in p16, another cyclin-dependent kinase inhibitor. The results suggest that type 1 diabetes increases renal tubular iron accumulation and macrophage infiltration through a p21-dependent mechanism, and that the chelation of dietary iron attenuates these responses.
Keywords: Diabetic nephropathy, Iron, P21, Proximal tubular cells, Cellular senescence
1. Introduction
Iron plays an important role in maintaining physiological home-ostasis in the body (for example in enzymatic reactions and oxygen transport). However, excess iron can lead to free radical damage via the Fenton reaction, resulting in tissue damage (Hentze et al., 2004). During the past decade, iron has been implicated in the pathogenesis of various cardiovascular diseases. For instance, iron deposition is associated with MCP1 release from macrophages and the development of atherosclerosis (Valenti et al., 2011). In addition, the increase in total iron stores was associated with an increased risk in the development of type 2 diabetes mellitus (Fernandez-Real et al., 2002a; Fernandez-Real et al., 2002b; Nankivell et al., 1994). Transferrin and iron can induce insulin resistance due to altered glucose transport in adipocytes through a mechanism independent of fatty acids (Green et al., 2006). A decrease in iron level by dietary iron restriction to the level that could induce anemia prevented the development of diabetic nephropathy in Otsuka Long-Evans Tokushima Fatty rats (Matsumoto et al., 2013) and db/db mice (Ikeda et al., 2013).
Cell senescence is characterized by irreversible growth arrest and is one of the fates of in vitro-cultured cells (Hayflick and Moorhead, 1961). P21, a cyclin-dependent kinase inhibitor, plays an important role in cell senescence and the decrease in innate the function of tubular cells, resulting in increased collagen expression, tumor necrosis factor α (TNF-α) secretion and apoptosis (Fan et al., 2011; Kitada et al., 2012). We recently reported that hyperglycemia causes kidney cell senescence through a p21-dependent pathway; this phenomenon was dramatically prevented by insulin-treatment in mice and sodium-glucose transporter 2 knockdown in proximal tubular cells (Kitada et al., 2014).
Interestingly, there is an increased iron level in the kidneys of older rats (Uchino et al., 1990). In the present study we therefore hypothesized that iron overload in the kidney induces cell senescence and inflammation via a p21-dependent mechanism. To address this hypothesis, we investigated the efficacy of deferasirox (DFX), an oral iron chelator, at a dosage that did not induce anemia, on cell senescence and macrophage infiltration in the kidney of streptozotocin (STZ)-induced type 1 diabetic mice. Furthermore, we compared its efficacy with the influence of p21-KO on the kidney in STZ-treated mice.
2. Materials and methods
2.1. Animals
All experimental procedures were performed according to the guidelines for the care and use of animals established by Kagawa University. Eight-week-old male C57BL/6J mice were purchased from CLEA (Tokyo, Japan). Eight-week-old male p21-KO mice on a C57BL/6J background were sourced from our breeding colony (Nishioka et al., 2014). We used Exjade® (DFX, 40 mg/kg/day, Novartis International AG, Basel, Switzerland) to selectively chelate orally taken iron. After measurement of baseline parameters, the wild-type mice were randomly divided into four groups; non-diabetic control with vehicle or DFX treatment and STZ-treated (100 mg/kg at day 1 and 50 mg/kg at day 2 and 3, i.p.) groups with vehicle or DFX treatment. Exjade® tablets were crushed to collect DFX, and dissolved in carboxymethyl cellulose. DFX was orally administrated (40 mg/kg/day) for 28 weeks. p21-KO mice were also randomly divided into two groups; non-diabetic control and STZ-treated groups.
In a separate experiment, mice received either FeCl4 (10 mg/kg, i.v. bolus, n=3) (Farrehi et al., 1998; Mussoni et al., 2001) or oleic acid-conjugated bovine serum albumin (BSA). BSA (low endotoxin, catalog # A9430, Sigma-Aldrich, St Louis, MO, USA) was conjugated with oleic acid (Sigma, catalog # O1008) in sterilized PBS at a 3:1 M ratio at 37 °C for 2.5 h, and then filtered through a 0.22 µm membrane. The prepared oleic acid-conjugated BSA was administered by i.p. injection (0.3 g / 20 g of body weight) once a day for 14 days (n=4) (Agrawal et al., 2014; Souma et al., 2011; Yamahara et al., 2013).
2.2. Blood and urine chemistry
Blood glucose levels were measured daily in tail vein blood using a Glutest-Ace monitor (Sanwa Kagaku, Nagoya, Aichi, Japan). Hematocrit level was analyzed by the capillary centrifugal method. Hemoglobin level was measured using a commercially available kit following the manufacturer's instructions (Alfresa, Tokyo, Japan). Twenty-four-hour urine samples were collected using metabolic cages. Urinary albumin level was measured using a commercially available ELISA kit according to the manufacturer's instructions (Shibayagi, Gunma, Japan).
2.3. Histological analysis
Mice were killed by intraperitoneal injection of high-dose pentobarbital (300 mg/kg). Right kidneys were removed and fixed overnight in 15% formaldehyde at 4 °C. After defatting, the samples were cut into 3-μm-thick sections and stained with Perl's Prussian blue staining reagent. For analysis of cellular senescence, the samples were frozen in Tissue-Tek OCT compound (Sakura Finetek, AJ Alphen aan den Rijn, the Netherlands) and cut into 15-μm-thick sections with a cryostat, and stained with senescence associated-β-galactosidase (SABG) staining reagent.
2.4. Immunohistochemical staining
Paraffin-embedded kidney tissue samples were sectioned (3 μm) and deparaffinized. After antigen retrieval by heated citric acid, the tissue samples were incubated with the primary antibody (anti-F4/80, Abcam, Cambridge, UK) at 4 °C overnight. Antibody distribution was visualized using a streptavidin-biotin complex assay and a DAB substrate kit (DAKO, Glostrup, Denmark).
2.5. Real-time reverse transcription-PCR
mRNA expression in renal cortical tissues was analyzed quantitatively by real-time PCR using a LightCycler FastStart DNA Master SYBR Green I kit (Applied Biosystems, Foster City, CA, USA). The following hemoglobin-α (Hb-α) primers were used: sense 5-TGCTCTCTGGGGAAGACAAA-3′ and antisense 5′-GAGCCGTGGCTTACATCAAA-3′. The following ferroportin-1 primers were used: sense 5′-GGCTACGTCGAAAATGTGGC-3′ and antisense 5′-GGGGCTTCCAGGCATGAATA-3′. The following lip-ocalin-2 primers were used: sense 5′-GGCCAGTTCACTCTGG-GAAA-3′ and antisense 5′ -TGGCGAACTGGTTGTAGTCC-3′.
2.6. Statistical analysis
Results are expressed as means ± standard error of the mean. Statistical significance was assessed by one-way ANOVA followed by Tukey's multiple comparison test using a GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). Student's t tests were performed to compare the means in experiments with two individual groups. P-values of P<0.05 were considered statistically significant.
3. Results
3.1. General parameters
The average body weight of vehicle-treated STZ mice was smaller compared with non-diabetic mice after the 28-week experimental period (23.3 ± 0.7 vs. 29.3 ± 0.5g, n=10 and 9, respectively). DFX had no significant effect on body weight in STZ mice and non-diabetic mice (25.4 ± 0.7 and 27.8 ± 0.4 g, n=11 and 12, respectively). The body weight of p21-KO mice was greater than wild-type mice at baseline (26.4 ± 0.8 vs. 21.4 ± 0.2 g, n=17 and 42, respectively); however there was no significant difference in the gain in body weight between the counterparts of each strain at 28 weeks (8.0 ± 0.4 vs. 10.9 ± 1.0 g in vehicle-treated non-diabetic wild-type vs. p21-KO mice, and 2.0 ± 0.5 vs. 1.3 ± 0.8 g in vehicle-treated diabetic wild-type vs. p21-KO mice, n=10, 7, 9 and 10, respectively).
STZ groups exhibited significant elevation of blood glucose levels both in wild-type and p21-KO mice (Table 1). DFX had no effect on the increased blood glucose level in either group. STZ-induced diabetes did not affect either the hematocrit or blood hemoglobin levels. Similarly, dietary iron chelation by DFX treatment affected neither hematocrit nor blood hemoglobin level in the 28-week experimental period.
Table 1.
General physiological parameters; postprandial blood glucose, hematocrit and hemoglobin level, at baseline and 28 weeks after STZ-treatment.
| Wild-type non-DM vehicle | Wild-type STZ vehicle | Wild-type non-DM DFX | Wild-type STZ DFX | p21-KO non-DM | p21-KO STZ | ||
|---|---|---|---|---|---|---|---|
| Blood glucose (mg/dL) | Baseline | 131±12 | 132±11 | 1 31±11 | 130±11 | 137±39 | 139±16 |
| 28 weeks | 139±13 | 570±26a | 108±11 | 534±26a | 107±6 | 547±19a | |
| Hematocrit (%) | Baseline | 43.6±1.8 | 43.0±1.5 | 43.1±1.6 | 44.6±1.7 | 46.7±1.7 | 45.8±1. 8 |
| 28 weeks | 45.4±0.8 | 46.1±1.2 | 46.2±0.9 | 48.8±0.7 | 44.8±0.5 | 46.8±1. 4 | |
| Hemoglobin (g/dL) | Baseline | 14.3±0.3 | 14.1±0.4 | 14.5±0.9 | 13±1.0 | 18.5±1.0 | 18.0±0.5 |
| 28 weeks | 12.9±0. | 13.8±0.5 | 13.2±0.3 | 13.5±0.4 | 13.5±0.5 | 13.9±0.4 |
P<0.05 vs. counterpart non-DM groups. Abbreviations: DFX, deferasirox; Non-DM, non-diabetes mellitus; STZ, streptozotocin.
3.2. Renal iron deposition
STZ-induced diabetes for 28 weeks increased iron deposition, especially in the renal cortical proximal tubules of wild-type mice (Fig. 1). Iron staining in the interstitial space was barely observed. DFX efficiently attenuated the increased iron deposition in the kidney of STZ-treated mice. However, STZ did not increase iron deposition in the kidney of p21-KO mice, although they had a similar blood glucose trend, indicating that both dietary iron and p21 contribute to iron deposition in the proximal tubules of diabetic kidney. We next investigated the mRNA expression levels of the iron transporters ferroportin-1, hemoglobin-α and lipocalin-2 in renal cortical tissues. The expression levels of mRNA of ferroportin-1 and hemoglobin-α were not altered by STZ or DFX. Notably, the mRNA expression of lipocalin-2, also known as neutrophil gelatinase-associated lipocalin (NGAL), was greater in the STZ-treated wild-type mice (1.8 ± 0.1 fold vs. non-diabetic control, n=11). In contrast, neither DFX nor p21-KO affected the increased lipocalin-2 level in the kidney (1.6 ± 0.2 and 1.8 ± 0.2 fold vs. non-diabetic control, n=10, respectively).
Fig. 1.
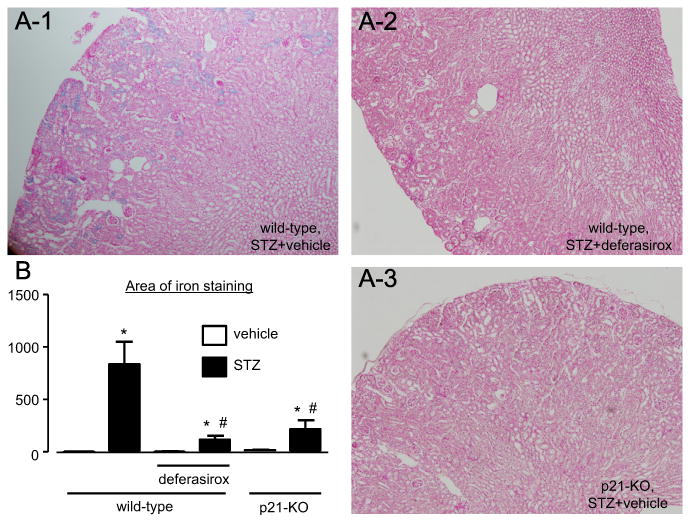
Iron deposition in the diabetic kidney (n=9–12). (A) STZ-induced diabetes increased the blue iron staining in the proximal tubules at 28 weeks. Eosin was used as a counterstain. (B) The staining area was dramatically smaller in the mice that received deferasirox and in the mice that lack p21. * P<0.05 vs. non-diabetic control mice, # P<0.05 vs. vehicle-treated diabetic wild-type mice.
FeCl4 was administered by i.v. bolus to estimate the origin of the accumulated iron in the proximal tubules. After the administration of FeCl4, iron staining was observed only in the peritubular capillaries in the kidney (Fig. 2) and the staining did not overlap with the red blood cells remaining in the capillaries. In addition, an albumin overload model that excreted 3-times more albumin in the urine than STZ-treated mice (118.0 ± 39.7 vs. 32.6 ± 9.0 μg/ day, P<0.05, n=6 and 4, respectively) did not show any iron staining in the tubules.
Fig. 2.
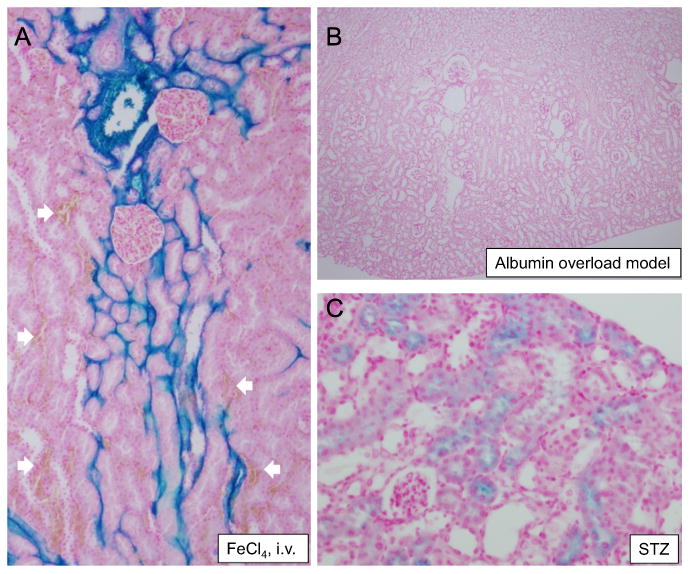
Iron staining in the diabetic mice at week 28 was not dependent on free iron, red blood cells or the uptake of filtered iron-bound plasma protein due to damage of the glomerular filtration barrier. (A) Only capillaries were stained after the intravenous injection of FeCl4. There were some clots in the section (white arrows), which did not respond to the iron staining. (B) The mice that received fatty acid-conjugated albumin did not show iron staining, although the mice excreted greater than 3-times more urinary albumin. (C) STZ-induced diabetes showed positive staining for iron in proximal tubules. Eosin was used as a counterstain.
3.3. Cell senescence in the kidney
STZ-induced diabetes increased the number of senescent renal cells, as determined by SABG staining, and the expression level of p16 mRNA in the kidney (Fig. 3). Neither DFX nor the lack of p21 attenuated the level of cell senescence. Next, we measured the expression level of p16, another cyclin dependent kinase inhibitor, which arrests the cell cycle independently of the p21-dependent pathway. As shown in Fig. 3, the level of p16 mRNA in STZ-treated groups was significantly higher than in the non-diabetic controls.
Fig. 3.
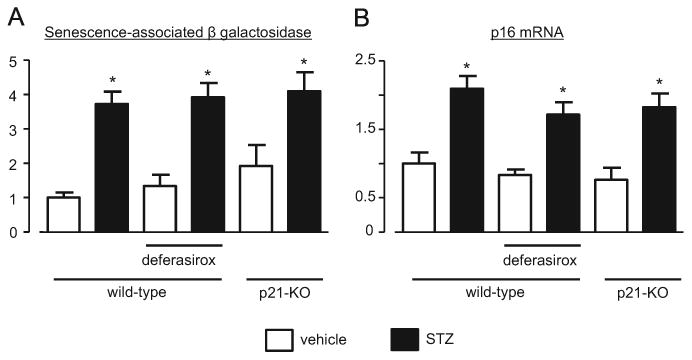
STZ-induced diabetes induced kidney cell senescence at week 28, independently of iron chelation and p21. Senescence-associated β galactosidase staining (A) and p16 mRNA level (B) were increased in the diabetic groups. No treatment induced a significant effect on these changes. * P<0.05 vs. non-diabetic control mice.
3.4. Macrophage infiltration of the diabetic kidney
We investigated the effect of iron chelation on macrophage infiltration by immunohistochemical analysis using an anti-F4/80 antibody. As shown in Fig. 4, STZ-induced diabetes increased the number of F4/80-positive cells in the kidney of vehicle-treated wild-type mice. DFX treatment reduced the number of F4/80-positive cells in diabetic wild-type mice. The lack of p21 itself also reduced the numbers of F4/80-positive cells compared with diabetic wild-type mice. STZ-induced diabetes did not significantly change macrophage infiltration in p21-KO mice. We next investigated the co-localization of macrophages and iron deposition with Perl's Prussian blue stain with F4/80 co-staining. As shown in Fig. 5, F4/80-positive macrophages were not always adjacent to the proximal tubules showing iron deposition. These results indicate that iron deposition in the proximal tubules might not directly induce macrophage infiltration to the surrounding interstitial spaces.
Fig. 4.
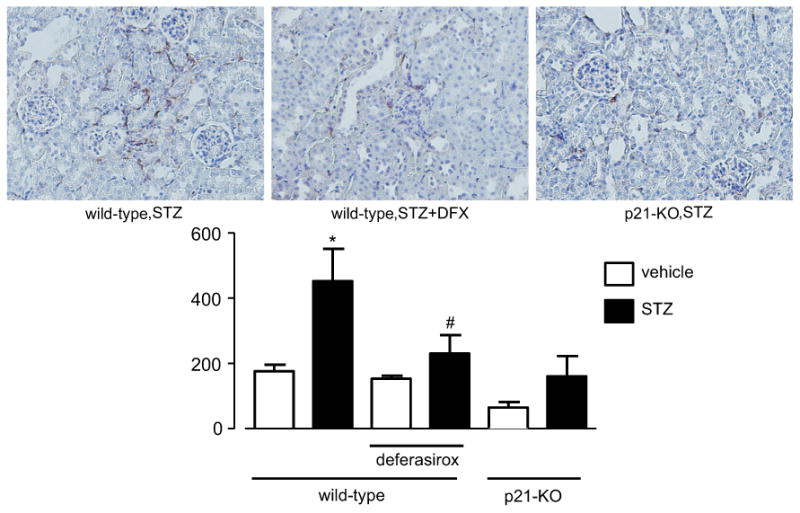
Iron chelation suppressed macrophage infiltration into the diabetic kidney. STZ-induced diabetes for 28 weeks accelerated the macrophage infiltration into the renal interstitial spaces. Deferasirox attenuated this macrophage infiltration. The lack of p21 resulted in a decrease in macrophage infiltration. Diabetes tended to increase the macrophage infiltration in p21-KO mice, but the difference was not statistically significant. * P < 0.05 vs. non-diabetic control mice, # P < 0.05 vs. vehicle-treated diabetic wild-type mice.
Fig. 5.
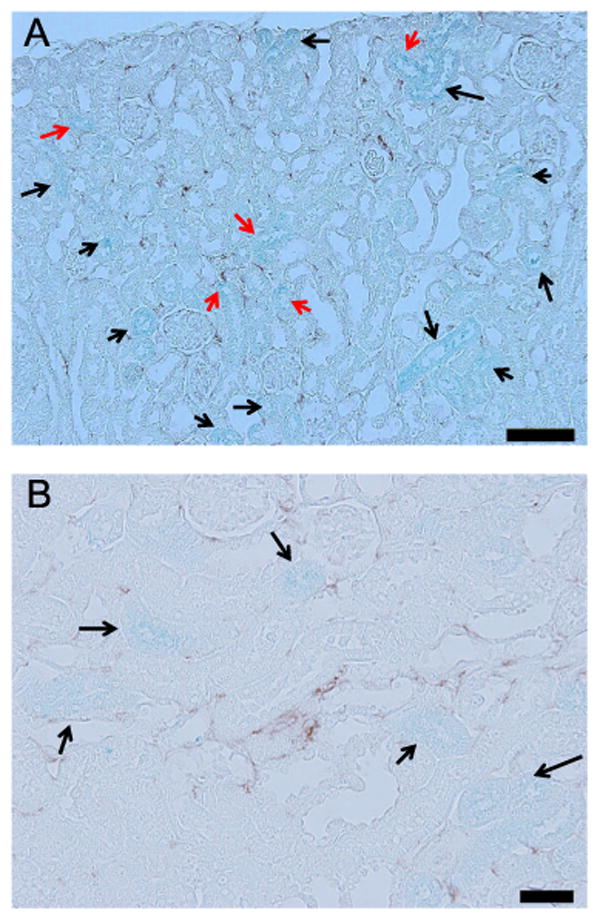
Two-dimensional colocalization between iron deposition (blue) and macro-phage infiltration (brown) at week 28 of STZ-induced diabetes. The red arrows indicate the iron-accumulated tubules that were surrounded by infiltrated macro-phages. The black arrows indicate the iron-accumulated tubules that were not surrounded by macrophages. The scale bar in the image represents 100 μm in A and 200 μm in B. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The present findings demonstrate that hyperglycemia increases both iron accumulation and cell senescence in proximal tubular cells, and macrophage infiltration in the kidney of STZ-induced type 1 diabetic mice. The inhibition of dietary iron absorption by DFX suppressed the increase in proximal tubular iron accumulation and macrophage infiltration into the interstitial space, but not cell senescence. This effect of DFX may be partially explained by a p21-dependent mechanism, because the lack of p21 gene expression also markedly attenuated both proximal tubular iron accumulation and macrophage infiltration in the kidney of STZ mice. These results suggest that inhibiting iron absorption only from the gastrointestinal system could prevent tissue iron overload and inflammation without inducing anemia or affecting blood glucose level in the type 1 diabetic kidney.
The mechanism by which p21 regulates iron accumulation in the proximal tubules of diabetic kidneys was unclear in the present study. Several recent studies show that the iron chelator deferoxamine or strict dietary iron restriction changes the expression level of iron transporters in extra-red blood cell tissues, such as liver and kidney (Gabrielsen et al., 2012; Ikeda et al., 2013; Ikeda et al., 2012; Matsumoto et al., 2013; Tajima et al., 2014). However, we did not find changes in expression levels of iron transporters, such as ferroportin-1, hemoglobin-α and lipocalin-2, after DFX treatment. We further examined the possibilities that 1) free iron and 2) heme-bound iron in red blood cells reacted during the staining process, or that 3) iron-bound plasma proteins were filtered from glomeruli and taken up by proximal tubules as a consequence of hyperfiltration in the diabetic kidney. These possibilities, however, are unlikely because 1) after the injection of FeCl4 iron staining was only demonstrated in peritubular capillaries, 2) coagulates did not show any iron staining, and 3) the albumin overload model that excreted 3-times more albumin in the urine than STZ-treated rats did not show any iron staining in the tubules. These results suggest that the accumulation of iron in diabetic proximal tubules may depend on the dysregulation of intracellular metabolism and clearance rather than increased uptake from extracellular spaces. Anderson et al. (2013) have reported that the dysregulation of hypoxia-inducible factor (HIF)-2 alpha, which regulates p21 transcription (Keith et al., 2012), led to tissue iron overload during beta-thalassemia. In addition, binding to iron is necessary for the function of prolyl hydroxylases that modifies HIFα and regulate its degradation (Nandal et al., 2011). Heme oxygenase-1 (HO-1), an enzyme that degrades heme into iron and biliverdin, is up-regulated in the diabetic kidney (Lee et al., 2009; Vallon et al., 2013), and may accelerate iron accumulation in the tubules, although no report has shown the induction of HO-1 by a p21-dependent mechanism. Taken together, it can be speculated that the changes in intracel-lular metabolism of iron containing enzymes, such as HIF/prolyl hydroxylases system or HO-1, may be dysregulated after the long time exposure to high glucose, resulting in the accumulation of iron in the tubular cells through a p21-dependent system.
We recently reported that hyperglycemia-induced p21 plays an important role in the development of tubular cell senescence in the early phase (at week 4) of type 1 diabetes (Kitada et al., 2014). However, in contrast, it appears that the acceleration of tubular cell senescence was independent of both iron overload- and p21-dependent mechanisms in the present study (at week 28). The increase in cell senescence in p21-KO mice might result from the compensative up-regulation of the other CDK inhibitors, such as p16, as a consequence of long time exposure to hyperglycemia (high glucose in the filtrate for the proximal tubules) in this study. The ineffectiveness of DFX, which did not affect blood glucose level, further supports the importance of glucose level on cell senescence in the diabetic kidney.
DFX prevented macrophage infiltration into the diabetic kidney. The iron-accumulated tubules might secrete cytokines that accelerate macrophage infiltration; however, the tubules that were surrounded by macrophages were not always positive for iron staining. It seems likely that macrophage infiltration occurs independently of iron accumulation in the proximal tubules. Another possibility regulating the DFX-inhibition of macrophage infiltration is that the inhibition of dietary iron absorption itself and/or the possible reduction of intra-macrophage iron suppressed macrophage sensitivity to inflammatory stimuli (Alcantara and Boldt, 2007; Persson et al., 2013; Sindrilaru et al., 2011; Valenti et al., 2011).
There are studies linking lower vitamin D levels and anemia risk (Perlstein et al., 2011). Since 1α-hydroxylase catalyzes 25-hydroxy vitamin D3 to calcitriol in the mitochondria of cortical proximal tubular cells, iron accumulation in these tubules might affect vitamin D activation in diabetes. Diabetes increases the secretion and the urinary excretion of megalin, a receptor for vitamin D binding protein that is necessary for the uptake of 25-hydroxy vitamin D3 into proximal tubular cells, possibly resulting in inefficient calcitriol production (Fowlkes et al., 2011). Furthermore, these changes were accompanied by anemia (Ogasawara et al., 2012). However, in the present study, we did not observe a consistent relationship between iron deposition and albuminuria, a consequence of reduced megalin level, among the groups (STZþvehicle vs. STZþDFX vs. albumin overload model). Thus, it is unlikely that iron chelation affected 25-hydroxy vitamin D uptake through the reduction of megalin level in the present study. An increase in FGF23 level, which decreases 1α-hydroxylase expression and activity, has also been reported in diabetes (Vervloet et al., 2014; Wolf, 2012), although there is no report connecting iron chelation and FGF23 activity yet.
DFX is reported to have nephrotoxic effects, partially due to its depletion of circulating iron (Diaz-Garcia et al., 2014). Nephrotoxi-city might not be relevant in this study as there were no changes in hematocrit and hemoglobin levels and DFX decreased, rather than increased, macrophage infiltration and inflammatory marker expression. This may be because our low dosage of deferasirox was at a sub-nephrotoxic level, in comparison with the dosage used in clinical applications, such as for thalassemia.
The precise mechanism by which the inhibition of iron reab-sorption in the gastrointestinal system reduced iron deposition in the proximal tubules of the kidney is unclear in the present study. We speculate that the restriction of iron supply may accelerate iron turnover with heme synthesis given a higher priority, rather than being taken up into the kidney (or the other extra-RBC organs, such as the liver).
In conclusion, the chelation of dietary iron attenuates renal iron accumulation and macrophage infiltration in the type 1 diabetic kidney. Furthermore, this phenomenon is, at least partially, dependent on p21 and independent of renal cell senescence.
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (23790299: to Daisuke Nakano).
References
- Agrawal S, Guess AJ, Chanley MA, Smoyer WE. Albumin-induced podocyte injury and protection are associated with regulation of COX-2. Kidney Int. 2014;86:1150–1160. doi: 10.1038/ki.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara O, Boldt DH. Iron deprivation blocks multilineage haematopoietic differentiation by inhibiting induction of p21(WAF1/CIP1) Br J Haematol. 2007;137:252–261. doi: 10.1111/j.1365-2141.2007.06549.x. [DOI] [PubMed] [Google Scholar]
- Anderson ER, Taylor M, Xue X, Ramakrishnan SK, Martin A, Xie L, Bredell BX, Gardenghi S, Rivella S, Shah YM. Intestinal HIF2alpha promotes tissue-iron accumulation in disorders of iron overload with anemia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E4922–E4930. doi: 10.1073/pnas.1314197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Garcia JD, Gallegos-Villalobos A, Gonzalez-Espinoza L, Sanchez-Nino MD, Villarrubia J, Ortiz A. Deferasirox nephrotoxicity-the knowns and unknowns. Nat Rev Nephrol. 2014;10:574–586. doi: 10.1038/nrneph.2014.121. [DOI] [PubMed] [Google Scholar]
- Fan YY, Kohno M, Hitomi H, Kitada K, Fujisawa Y, Yatabe J, Yatabe M, Felder RA, Ohsaki H, Rafiq K, Sherajee SJ, Noma T, Nishiyama A, Nakano D. Aldosterone/Mineralocorticoid receptor stimulation induces cellular senescence in the kidney. Endocrinology. 2011;152:680–688. doi: 10.1210/en.2010-0829. [DOI] [PubMed] [Google Scholar]
- Farrehi PM, Ozaki CK, Carmeliet P, Fay WP. Regulation of arterial thrombolysis by plasminogen activator inhibitor-1 in mice. Circulation. 1998;97:1002–1008. doi: 10.1161/01.cir.97.10.1002. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002a;51:2348–2354. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Penarroja G, Castro A, Garcia-Bragado F, Lopez-Bermejo A, Ricart W. Blood letting in high-ferritin type 2 diabetes: effects on vascular reactivity. Diabetes Care. 2002b;25:2249–2255. doi: 10.2337/diacare.25.12.2249. [DOI] [PubMed] [Google Scholar]
- Fowlkes JL, Bunn RC, Cockrell GE, Clark LM, Wahl EC, Lumpkin CK, Thrailkill KM. Dysregulation of the intrarenal vitamin D endocytic pathway in a nephropathy-prone mouse model of type 1 diabetes. Exp Diabetes Res. 2011;2011:269378. doi: 10.1155/2011/269378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen JS, Gao Y, Simcox JA, Huang J, Thorup D, Jones D, Cooksey RC, Gabrielsen D, Adams TD, Hunt SC, Hopkins PN, Cefalu WT, McClain DA. Adipocyte iron regulates adiponectin and insulin sensitivity. J Clin Investig. 2012;122:3529–3540. doi: 10.1172/JCI44421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Basile R, Rumberger JM. Transferrin and iron induce insulin resistance of glucose transport in adipocytes. Metab : Clin Exp. 2006;55:1042–1045. doi: 10.1016/j.metabol.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Enomoto H, Tajima S, Izawa-Ishizawa Y, Kihira Y, Ishizawa K, Tomita S, Tsuchiya K, Tamaki T. Dietary iron restriction inhibits progression of diabetic nephropathy in db/db mice. Am J Physiol Ren Physiol. 2013;304:F1028–F1036. doi: 10.1152/ajprenal.00473.2012. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Tajima S, Izawa-Ishizawa Y, Kihira Y, Ishizawa K, Tomita S, Tsuchiya K, Tamaki T. Estrogen regulates hepcidin expression via GPR30-BMP6-dependent signaling in hepatocytes. PloS One. 2012;7:e40465. doi: 10.1371/journal.pone.0040465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada K, Nakano D, Hitomi H, Kobori H, Deguchi K, Mori H, Masaki T, Nishiyama A. Aldosterone induces p21-regulated apoptosis via increased synthesis and secretion of tumour necrosis factor-alpha in human proximal tubular cells. Clin Exp Pharmacol Physiol. 2012;39:858–863. doi: 10.1111/1440-1681.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada K, Nakano D, Ohsaki H, Hitomi H, Minamino T, Yatabe J, Felder RA, Mori H, Masaki T, Kobori H, Nishiyama A. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J Diabetes Complicat. 2014;28:604–611. doi: 10.1016/j.jdiacomp.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Han SH, Li JJ, Lee SH, Jung DS, Kwak SJ, Kim SH, Kim DK, Yoo TH, Kim JH, Chang SH, Han DS, Kang SW. Induction of heme oxygenase-1 protects against podocyte apoptosis under diabetic conditions. Kidney Int. 2009;76:838–848. doi: 10.1038/ki.2009.286. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Sasaki N, Tsujino T, Akahori H, Naito Y, Masuyama T. Iron restriction prevents diabetic nephropathy in Otsuka Long-Evans Tokushima fatty rat. Renal Fail. 2013;35:1156–1162. doi: 10.3109/0886022X.2013.819729. [DOI] [PubMed] [Google Scholar]
- Mussoni L, Sironi L, Tedeschi L, Calvio AM, Colli S, Tremoli E. Magnesium inhibits arterial thrombi after vascular injury in rat: in vivo impairment of coagulation. Thromb Haemost. 2001;86:1292–1295. [PubMed] [Google Scholar]
- Nandal A, Ruiz JC, Subramanian P, Ghimire-Rijal S, Sinnamon RA, Stemmler TL, Bruick RK, Philpott CC. Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metab. 2011;14:647–657. doi: 10.1016/j.cmet.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankivell BJ, Tay YC, Boadle RA, Harris DC. Lysosomal iron accumulation in diabetic nephropathy. Renal Fail. 1994;16:367–381. doi: 10.3109/08860229409044877. [DOI] [PubMed] [Google Scholar]
- Nishioka S, Nakano D, Kitada K, Sofue T, Ohsaki H, Moriwaki K, Hara T, Ohmori K, Kohno M, Nishiyama A. The cyclin-dependent kinase inhibitor p21 is essential for the beneficial effects of renal ischemic preconditioning on renal ischemia/reperfusion injury in mice. Kidney Int. 2014;85:871–879. doi: 10.1038/ki.2013.496. [DOI] [PubMed] [Google Scholar]
- Ogasawara S, Hosojima M, Kaseda R, Kabasawa H, Yamamoto-Kabasawa K, Kurosawa H, Sato H, Iino N, Takeda T, Suzuki Y, Narita I, Yamagata K, Tomino Y, Gejyo F, Hirayama Y, Sekine S, Saito A. Significance of urinary full-length and ectodomain forms of megalin in patients with type 2 diabetes. Diabetes Care. 2012;35:1112–1118. doi: 10.2337/dc11-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein TS, Pande R, Berliner N, Vanasse GJ. Prevalence of 25-hydroxyvitamin D deficiency in subgroups of elderly persons with anemia: association with anemia of inflammation. Blood. 2011;117:2800–2806. doi: 10.1182/blood-2010-09-309708. [DOI] [PubMed] [Google Scholar]
- Persson HL, Vainikka LK, Eriksson I, Wennerstrom U. TNF-alpha-stimulated macrophages protect A549 lung cells against iron and oxidation. Exp Toxicol Pathol. 2013;65:81–89. doi: 10.1016/j.etp.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macro-phage population induced by iron impairs wound healing in humans and mice. J Clin Investig. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souma T, Abe M, Moriguchi T, Takai J, Yanagisawa-Miyazawa N, Shibata E, Akiyama Y, Toyohara T, Suzuki T, Tanemoto M, Abe T, Sato H, Yamamoto M, Ito S. Luminal alkalinization attenuates proteinuria-induced oxida-tive damage in proximal tubular cells. J Am Soc Nephrol : JASN. 2011;22:635–648. doi: 10.1681/ASN.2009111130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima S, Ikeda Y, Enomoto H, Imao M, Horinouchi Y, Izawa-Ishizawa Y, Kihira Y, Miyamoto L, Ishizawa K, Tsuchiya K, Tamaki T. Angiotensin II alters the expression of duodenal iron transporters, hepatic hepcidin, and body iron distribution in mice. Eur J Nutr. 2014 doi: 10.1007/s00394-014-0749-1. http://dx.doi.org/10.1007/s00394-014-0749-1. [DOI] [PubMed]
- Uchino E, Tsuzuki T, Inoue K. The effects of age and sex on seven elements of Sprague-Dawley rat organs. Lab Anim. 1990;24:253–264. doi: 10.1258/002367790780866182. [DOI] [PubMed] [Google Scholar]
- Valenti L, Dongiovanni P, Motta BM, Swinkels DW, Bonara P, Rametta R, Burdick L, Frugoni C, Fracanzani AL, Fargion S. Serum hepcidin and macrophage iron correlate with MCP-1 release and vascular damage in patients with metabolic syndrome alterations. Arterioscler Thromb Vasc Biol. 2011;31:683–690. doi: 10.1161/ATVBAHA.110.214858. [DOI] [PubMed] [Google Scholar]
- Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Ren Physiol. 2013;304:F156–F167. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervloet MG, Massy ZA, Brandenburg VM, Mazzaferro S, Cozzolino M, Urena-Torres P, Bover J, Goldsmith D, CKD-MBD Working Group of ERA-EDTA Bone: a new endocrine organ at the heart of chronic kidney disease and mineral and bone disorders. Lancet Diabetes Endocrinol. 2014;2:427–436. doi: 10.1016/S2213-8587(14)70059-2. [DOI] [PubMed] [Google Scholar]
- Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82:737–747. doi: 10.1038/ki.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahara K, Kume S, Koya D, Tanaka Y, Morita Y, Chin-Kanasaki M, Araki H, Isshiki K, Araki S, Haneda M, Matsusaka T, Kashiwagi A, Maegawa H, Uzu T. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J Am Soc Nephrol : JASN. 2013;24:1769–1781. doi: 10.1681/ASN.2012111080. [DOI] [PMC free article] [PubMed] [Google Scholar]


