Summary
Despite resulting in a similar overall outcome, unlike antibodies directed against the DNABII protein, integration host factor (IHF), which induce catastrophic structural collapse of biofilms formed by nontypeable Haemophilus influenzae (NTHI), those directed against a recombinant soluble form of PilA [the majority subunit of Type IV pili (Tfp) produced by NTHI], mediated gradual ‘top-down’ dispersal of NTHI from biofilms. This dispersal occurred via a mechanism that was dependent upon expression of both PilA (and by inference, Tfp) and production of AI-2 quorum signaling molecules by LuxS. The addition of rsPilA to a biofilm-targeted therapeutic vaccine formulation comprised of IHF plus the powerful adjuvant dmLT, and delivered via a non-invasive transcutaneous immunization route, induced an immune response that targeted two important determinants essential for biofilm formation by NTHI. This resulted in significantly earlier eradication of NTHI from both planktonic and adherent populations in the middle ear, disruption of mucosal biofilms already resident within middle ears prior to immunization, and rapid resolution of signs of disease in an animal model of experimental otitis media. These data support continued development of this novel combinatorial immunization approach for resolution and/or prevention of multiple diseases of the respiratory tract caused by NTHI.
Keywords: transcutaneous immunization, AI-2, type IV pili, IHF, DNABII proteins
Introduction
The most common bacterial disease of childhood is otitis media (OM), an illness that is often chronic and recurrent in nature and difficult to treat with traditional antibiotics. The recalcitrance of OM is due to the ability of the predominant bacterium involved, nontypeable Haemophilus influenzae (NTHI), to establish biofilms within the middle ear (Post, 2001, Swords, 2012). Bacteria within biofilms are protected from both the host’s immune effectors and therapeutic interventions by the semipermeable barrier function, as well as other important qualities, of the extracellular polymeric substance (EPS) (Jones et al., 2013, Flemming & Wingender, 2010). In addition to an abundance of extracellular DNA (eDNA), it is shown that the EPS of biofilms formed by NTHI contain protein adhesins, enzymes, lipooligosaccharide and at least one DNABII binding protein (Jurcisek & Bakaletz, 2007, Gallaher et al., 2006, Murphy & Kirkham, 2002, Goodman et al., 2011). We have focused our efforts on two of these components, the DNABII binding protein, integration host actor (IHF) and one of the adhesins expressed by NTHI, the type IV pilus (Tfp), due to their established importance in biofilm formation, maintenance and structural stability in vitro and in vivo (Brockson et al., 2014, Bakaletz et al., 2005, Brandstetter et al., 2013, Goodman et al., 2011, Jurcisek et al., 2007).
In vitro, exposure of pre-formed NTHI biofilms to an arbitrary 1:50 dilution of antiserum against purified native IHF isolated from E. coli results in significant reductions in biomass and mean biofilm thickness, compared to treatment with naive serum (Goodman et al., 2011, Brockson et al., 2014). The mechanism for this outcome is the sequestration of IHF as it dissociates from eDNA, where it is localized at the vertices of each crossed strand of mesh-like eDNA within the biofilm and thus serves as a crucial structural constituent. Removal of available IHF results in destabilization with catastrophic collapse of the biofilm structure and, ultimately, release of the resident NTHI (Brockson et al., 2014). IHF-targeted resolution of established biofilms is also shown in vivo. Transcutaneous immunization (TCI) with E. coli IHF induces an effective compartmentalized immune response that rapidly resolves existing biofilms formed within the middle ears of chinchillas in an experimental model of NTHI-induced OM. We hypothesize that the predominant mechanism behind this observed disease resolution is likely due to the presence of IHF-specific antibodies within middle ear fluids that similarly facilitate collapse of the biofilm structure and exposure of NTHI to host immune effectors that are now capable of mediating its eradication (Goodman et al., 2011).
An additional biofilm-targeted approach to facilitate resolution of established NTHI biofilms focuses on NTHI Tfp, as expression of this adhesin is essential for NTHI adherence to respiratory epithelial cells, to maintain long-term colonization within the nasopharynx in an experimental model of OM and for twitching motility, critical functions for biofilm formation in vitro and in vivo (Jurcisek et al., 2007, Bakaletz et al., 2005, Carruthers et al., 2012). NTHI pilin protein is detected throughout immature and mature biofilms collected from the middle ears of chinchillas (Jurcisek et al., 2007) and TCI with a recombinant and soluble N-terminally truncated form of PilA, the majority subunit of Tfp (called rsPilA), shows significant efficacy as an immunogen to induce the formation of antibodies that resolve NTHI-induced OM with eradication of mucosal biofilms already established within the middle ears of chinchillas prior to immunization (Novotny et al., 2011, Novotny et al., 2013). In vivo, rsPilA-specific antibodies are detected within serum and middle ear fluids after TCI, and thus facilitated the observed disease resolution.
Whereas we now know substantially more about the mechanisms by which anti-IHF antibodies mediate structural collapse of an NTHI biofilm in vitro (Brockson et al., 2014), and by inference, likely contribute to their clearance in vivo, the exact mechanism(s) by which anti-rsPilA antibodies mediate NTHI Tfp-targeted biofilm resolution have not yet been defined. Thereby, herein we investigated these mechanism in vitro, and due to the nature of the disruption observed upon incubation of NTHI biofilms with anti-rsPilA, we examined the role of quorum signaling in this phenomenon. Our data revealed a mechanism for NTHI biofilm dispersal that involved both expression of Tfp and LuxS and in a manner that suggested tight co-regulation. As such, and given that immunization with IHF and rsPilA individually shows significant efficacy to induce resolution of NTHI biofilms in an experimental model of OM, we wondered whether addition of rsPilA to a therapeutic vaccine formulation that included IHF plus a powerful mucosal and systemic adjuvant might provide an added benefit. Toward that end, rsPilA plus IHF were administered by TCI to chinchillas with active NTHI-induced OM, with robust biofilms already present within the middle ears, and the resolution of established experimental OM was assessed. This immunization strategy resulted in a significant reduction in signs and severity of OM and significantly more rapid complete eradication of NTHI from both middle ear fluids and middle ear mucosal biofilms, compared to delivery of IHF alone.
Collectively, our data suggested that using a two-pronged biofilm-targeted approach against established NTHI biofilms by therapeutic immunization with both IHF and rsPilA to induce an immune response that results in disruption of NTHI biofilms via complimentary molecular mechanisms has considerable potential to resolve diseases of the respiratory tract due to NTHI.
Results
Inhibition of NTHI twitching motility by anti-rsPilA
As expression of functional Tfp by NTHI is needed for twitching motility and plays a major role in establishment of a biofilm both in vitro and in vivo (Bakaletz et al., 2005, Jurcisek et al., 2007, Carruthers et al., 2012), we wondered if one of the mechanisms for resolution of OM and eradication of middle ear mucosal biofilms following immunization with rsPilA was due to antibody mediated inhibition of twitching motility. To examine this in vitro, we developed a high throughput sub-agarose twitching motility assay wherein NTHI was inoculated under semi solid agarose so as to promote twitching motility. Twitching motility was observed as colony growth with a ‘fan-blade’ appearance that extended away from the inoculation site [Fig. 1A, row 1]. The parent strain exhibited this fan-blade growth when either saline diluent or naive rabbit serum was applied to the bottom of the well; however the application of anti-rsPilA inhibited formation of this fan-blade despite evidence of minimal growth at the site of inoculation. Generation of the fan-blade growth pattern required expression of PilA as an NTHI pilA mutant did not induce this pattern of growth under any condition tested [Fig. 1A, row 2]. Complementation of the pilA mutant restored the fan-blade growth phenotype which was again now inhibited by anti-rsPilA [Fig. 1A, row 3]. Measurement of the length of the fan-blade growth revealed that NTHI strains that expressed pilA (i.e. parent and ΔpilA/pPIL1 strains) and were inoculated into medium that contained anti-rsPilA were 80% and 77% shorter, respectively, compared to growth on medium with naive serum or diluent [Fig. 1B; P≤ 0.0001] These data demonstrated that antibody against rsPilA prevented NTHI twitching motility in vitro when generation of a fan-blade growth pattern was used as the readout.
Figure 1.
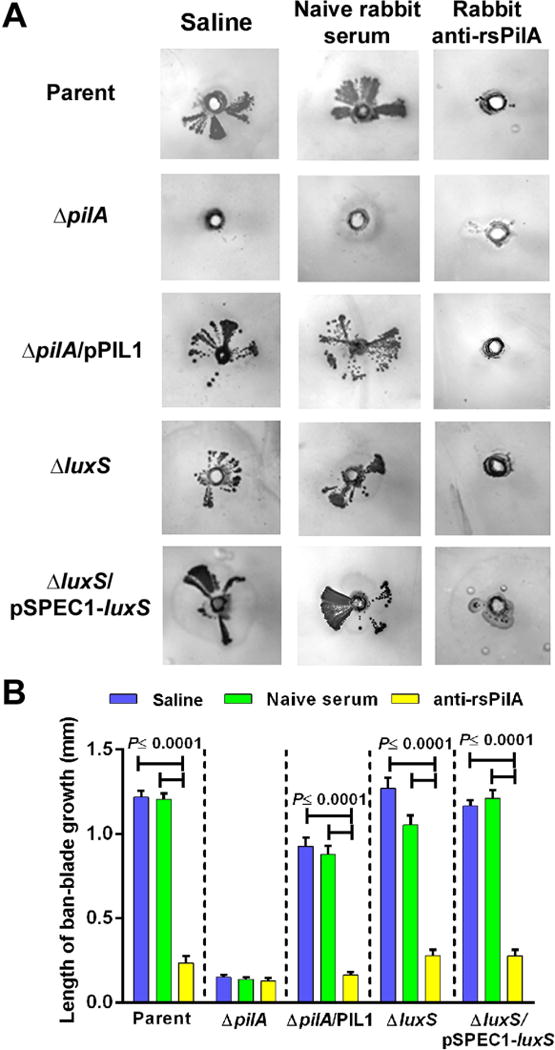
Sub-agarose twitching motility of NTHI strains. Images in (A) are representative from three independent assays and twitching motility is represented by ‘fan-blade’ growth of NTHI that extends out from a central inoculation site. Note inhibition of twitching motility by the parent strain, complemented pilA mutant, luxS mutant and complemented luxS mutant after inoculation into medium that contained rabbit anti-rsPilA but not by either naive rabbit serum or medium without serum. (B) Quantitation of twitching motility by measurement of the length of fan-blade growth showed significant reduction in bacterial outgrowth of pilA-expressing strains when inoculated into medium containing anti-rsPilA. Data are expressed as mean ± SEM, one-way ANOVA.
Resolution of established NTHI biofilms in vitro upon incubation with anti-rsPilA
Whereas both anti-IHF and anti-rsPilA can disrupt NTHI biofilms in vitro and in vivo and neither appear to induce significant bacterial cell death (as detected by LIVE-DEAD® staining), we did nonetheless observe that the mechanism(s) of biofilm disruption appeared to be distinct. For example, incubation of NTHI biofilms with antiserum directed against E. coli IHF results in catastrophic physical collapse of the biofilm with release of bacteria into the planktonic phase within approximately 6 hours of incubation and does not require direct contact between the antiserum and the biofilm (Brockson et al., 2014). Conversely, in unpublished early studies, we observed that incubation of biofilms with antibody against rsPilA, while significantly disruptive, did not appear to mediate catastrophic physical collapse of the biofilm despite the fact that bacteria were also ultimately released into the planktonic phase. To begin to understand this phenomenon better, here we established 24 h NTHI biofilms then incubated them with polyclonal rabbit serum for an additional 16 h prior to characterization by confocal microscopy with COMSTAT2 analysis. Compared to biofilms formed by the parent strain and maintained in medium, incubation with naive serum or anti-OMP P5 [another critical NTHI adhesin expressed in biofilms formed in vitro (Murphy & Kirkham, 2002)] did not substantially alter overall biofilm character [Fig. 2A, row 1] or biomass [Fig. 2B]. However, the biomass of biofilms treated with anti-rsPilA was reduced 82% compared to those incubated with naive serum (P≤0.0001). To determine whether the observed disruption was indeed Tfp-specific, biofilms formed by a nonpolar pilA mutant (which cannot express functional Tfp) were similarly incubated with these immune sera. As we showed previously (Jurcisek et al., 2007), biofilms formed by this mutant were less dense and shorter in height, compared to the parent strain, thus confirming the importance of Tfp in NTHI biofilm production. However, unlike biofilms established by the parent strain, incubation of the pilA mutant biofilm with anti-rsPilA did not induce any significant changes in structure or biomass [Fig. 2A, row 2 & Fig. 2B, respectively]. Complementation of the pilA mutant restored the ability of NTHI to form a robust biofilm, and further, restored our ability to significantly disrupt these biofilms via treatment with anti-rsPilA [70% reduction in biomass (P≤0.0001)] [Fig. 2A, row 3 & Fig. 2B, respectively]
Figure 2.
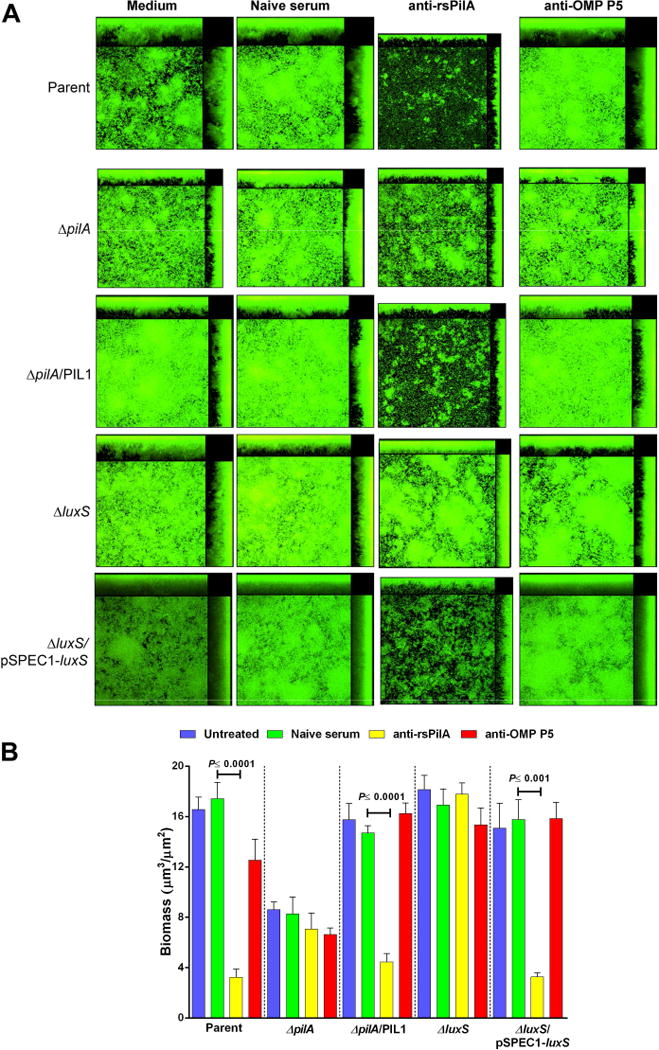
Disruption of established NTHI biofilms in vitro. Representative images of 24 h NTHI biofilms treated for 16 h with (A) medium or a 1:50 dilution of either naive rabbit serum, rabbit anti-rsPilA or rabbit anti-OMP P5. (B) Calculated mean biomass determined by COMSTAT2 analysis. Treatment of biofilms formed by the parent strain, complemented ΔpilA and complemented ΔluxS strains with anti-rsPilA resulted in significantly less biomass compared to exposure to naive serum which suggested a role for both Tfp and LuxS in biofilm disruption mediated by anti-rsPilA. Data in (B) are expressed as mean ± SEM, one-way ANOVA.
As stated above, the reduction in biomass after treatment with anti-rsPilA was not due to killing of the resident bacteria, as when stained with a viability stain, bacterial death was not observed. Interestingly, compared to prior work wherein catastrophic biofilm collapse was mediated by incubation of established biofilms with antiserum against IHF leaving only a monolayer of adherent bacteria (Goodman et al., 2011, Brockson et al., 2014), following treatment with anti-rsPilA, there was minimal residual biomass that nonetheless maintained traditional biofilm structure, including nominal towers and intervening water channels (see Fig. 2A, column 3). Thus, exposure of NTHI biofilms to anti-rsPilA appeared to mediate a ‘top-down’ form of disruption rather than the catastrophic collapse seen after incubation with anti-IHF. We therefore wondered if targeting the Tfp immunologically was inducing NTHI to perhaps disperse from the biofilm. If true, we hypothesized that this release might be the result of inter-bacterial communication.
To address our hypothesis, and with the knowledge that NTHI 86-028NP produces and responds to the quorum-sensing/signaling molecule, autoinducer-2 (AI-2) (Armbruster et al., 2011, Armbruster et al., 2009, Daines et al., 2005, Swords, 2012), we utilized an NTHI strain unable to synthesize this molecule due to insertion of a kanamycin resistance cassette within the luxS gene. We first confirmed that both ΔluxS and its complemented strain exhibited twitching motility in the sub-agarose twitching assay and observed fan-blade growth comparable to the parent on medium containing saline diluent or naive rabbit serum [Fig. 1A, rows 4 &5, Fig. 1B] Growth was inhibited 81% and 75%, respectively, on medium that incorporated anti-rsPilA, an outcome also similar to the parent strain. Therefore both the luxS mutant and its complemented strain exhibited twitching motility, likely due to the expression of functional Tfp.
To begin to determine whether the ability to quorum signal was related to the observed PilA-mediated release of NTHI from established biofilms, NTHI ΔluxS was next inoculated into chamber slides. The luxS mutant formed a biofilm comparable to that of the parent although with less characteristic towers and water channels; however, no significant reduction in biomass was induced upon incubation with any of the sera tested, including anti-rsPilA [Fig. 2A, row 4 & Fig. 2B, respectively]. Complementation of NTHI ΔluxS restored both a more characteristic biofilm architecture and the ability of anti-rsPilA to mediate significant biofilm disruption with a 79% reduction in biomass compared to incubation with naive serum (P≤0.001) [Fig. 2A, row 5 & Fig. 2B, respectively]. Incubation of the ΔluxS biofilm with naive serum or anti-OMP P5 had no effect, as expected. Collectively, these data showed that the inability of NTHI to express LuxS prevented biofilm disruption by anti-rsPilA, thus lending support to our hypothesis that a mechanism for the observed disruptive effect of anti-rsPilA on NTHI biofilms might involve quorum signaling.
Tfp-targeted antibody mediated dispersal of NTHI from established biofilms in vitro
To further demonstrate the interrelatedness between expression of Tfp and quorum signaling via LuxS in biofilms disrupted by incubation with anti-rsPilA, NTHI biofilms were established in vitro and treated with antiserum as before. After 4, 6 or 8 h, the supernatants from biofilms formed by the parent strain, isogenic pilA and luxS mutants and their complemented strains were collected, the optical density (OD490) recorded and fluids plated to quantitate CFU NTHI. Incubation with naive serum resulted in no significant increase in OD490 or CFU NTHI/ ml supernatant at any time point for any strain tested [Fig. 3A & B]. Similarly, incubation with rabbit anti-OMP P5 mediated no increase in OD490 or bacterial concentration within supernatants for any strain tested, at any time point [Fig. 3C & D]. Conversely, six hours after application of anti-rsPilA serum on to biofilms formed by the parent strain, a significant 6.6-fold increase in optical density and 4.4-fold increase in bacterial concentration was shown in collected supernatants, compared to those retrieved at the 4 h time point (P≤0.01) [Fig. 3E & F]. These results were also significantly greater than the optical density or bacterial concentration within supernatants collected from biofilms incubated with either naive serum [Fig. 3A & B] or that directed against OMP P5 (P≤0.0001 for OD and P≤0.01 for NTHI concentration) [Fig. 3C & D]. The observed flux of bacteria into the supernatant was transient, as the OD490 of and bacterial concentration within supernatants collected after 8 h of incubation with anti-rsPilA was comparable to that seen at the 4 h time point. The observed dispersal was dependent upon ability of NTHI to express PilA (and by inference, Tfp), as treatment of biofilms formed by ΔpilA did not demonstrate release into the supernatant at any time point. Complementation of ΔpilA restored the dispersal activity with a significant 4.4-fold increase in optical density and 4.1-fold increase in CFU NTHI/ ml supernatant again shown after 6 h, compared to the 4 h time point (P≤0.001) [Fig. 3E & F], similar to that observed for the parent strain. Compared to biofilms treated with either naive serum or with anti-OMP P5, significantly more NTHI pilA/pPIL1 were released from biofilms incubated with anti-rsPilA when measured by optical density (P≤0.0001) and bacterial concentration (P≤0.01). This dispersal was also associated with expression of LuxS, as no change in bacterial density was observed in supernatants collected from biofilms formed by ΔluxS upon incubation with anti-rsPilA. Conversely, supernatants collected from biofilms formed by the complemented luxS strain exhibited a 5.9-fold increase in OD490 and 3.5-fold increase in CFU NTHI/ml 6 h after application of anti-rsPilA, a significant increase relative to the 4 h time point (P≤0.01). Again, compared to exposure to either naive serum or anti-OMP P5 at the 6 h time point, significantly more NTHI were detected in supernatants after incubation with anti-rsPilA as indicated by the increase in OD490 (P≤0.01) and bacterial concentration (P≤0.05). These data further supported the premise that anti-rsPilA-mediated biofilm disruption was a result of NTHI dispersal from the biofilm, and further that both expression of Tfp and quorum signaling via expression of LuxS was required to achieve this outcome.
Figure 3.

Release of biofilm-resident NTHI into the supernatant after incubation of established biofilms with anti-rsPilA. Optical density of culture supernatants (panels A, C, E) and CFU NTHI/ ml culture supernatants (panels B, D, F) collected from 16 h biofilms formed by NTHI 86-028NP, NTHI ΔpilA or its complemented pilA mutant (C’ΔpilA), NTHI ΔluxS or its complemented luxS mutant (C’ΔluxS) after 4 (green bars), 6 (yellow bars) and 8 h (blue bars) of treatment with a 1:50 dilution of (A & B) naive rabbit serum, (C & D) rabbit anti-OMP P5 or (E & F) rabbit anti-rsPilA. A significant increase in optical density and bacterial concentration was detected in supernatants collected 6 h after treatment of the parent strain, complemented ΔpilA and complemented ΔluxS strains with anti-rsPilA compared to naive serum and anti-OMP P5 thus supported our hypothesis that expression of both Tfp and LuxS are involved in biofilm disruption as mediated by anti-rsPilA. Data are expressed as mean ± SEM, two-way ANOVA. *P≤0.05, **P≤0.01, ***P≤0.001 compared to respective treatment at 4 or 8 h time points. +P≤0.05, ++ P≤0.01, ++++ P≤0.0001 compared to supernatants from biofilms exposed to naive serum or anti-OMP P5 at the 6 h timepoint.
Detection of AI-2 in supernatants collected from NTHI biofilms upon incubation with anti-rsPilA
To assess the relative quantity of the quorum signaling molecule AI-2, a product of the luxS gene, in NTHI biofilms exposed to antibody against rsPilA and to confirm the association of AI-2 signaling with NTHI biofilm dispersal, a V. harveyi bioluminescence assay was used. While validating experimental parameters, we observed nonspecific bioluminescence after incubation of V. harveyi with supernatants collected from biofilms treated with any of the polyclonal rabbit serum despite large differences in their relative ability to disrupt an NTHI biofilm (or not). We thereby applied these whole sera to Protein G columns to obtain IgG-enriched fractions and thus ensure that all bioactivity observed was predominantly due to antigen-specific antibody and not due to non-specific serum factors. Incubation of V. harveyi with column effluent fractions revealed that unidentified non-specific serum components did indeed induce light production by V. harveyi, whereas the IgG-enriched fractions did not (data not shown). Therefore, IgG-enriched naive rabbit serum, IgG-enriched rabbit anti-rsPilA or IgG-enriched rabbit anti-OMP P5 were applied to NTHI biofilms, the supernatants collected after 4, 6 and 8 h and bacteria were removed prior to incubation with V. harveyi.
As a positive control, the AI-2 precursor molecule (S)-4,5-Dihydroxy-2,3-pentanedione (DPD), was incubated with V. harveyi and induced robust bioluminescence [Fig. 4, red bar]. Relative light unit (RLU) values obtained following incubation of V. harveyi with supernatants collected from NTHI biofilms treated with IgG-enriched naive rabbit serum were subtracted from those obtained when V. harveyi was similarly incubated with IgG-enriched immune rabbit sera and these normalized values are shown in Fig. 4. Similar to trends shown in Fig. 3, supernatants collected from biofilms formed by the parent, complemented ΔpilA or complemented ΔluxS strains and treated with IgG-enriched anti-rsPilA induced significantly greater bioluminescence by V. harveyi at each time point (P≤0.05 compared to IgG-enriched naive serum). The observed bioluminescence was specific for supernatants collected from NTHI that expressed PilA and LuxS, as supernatants collected from biofilms formed by NTHI ΔpilA or NTHI ΔluxS after treatment with IgG-enriched anti-rsPilA did not result in light production by V. harveyi above that induced by IgG-enriched naive serum, indicating that AI-2 was not present. Maximal bioluminescence was induced by incubation of V. harveyi with supernatants collected from biofilms formed by the parent, complemented ΔpilA and complemented ΔluxS strains 6 h after application of IgG-enriched anti-rsPilA [Fig. 4, yellow bars]. This latter result was significantly greater than that observed with supernatants collected at the same time point from biofilms treated with IgG-enriched naive serum or IgG-enriched anti-OMP P5 (P≤0.05).
Figure 4.

Detection of AI-2 production by NTHI. Light production by V. harveyi after incubation with cell-free supernatants collected from 24 h NTHI biofilms treated for 4 h (green bars), 6 h (yellow bars) or 8 h (blue bars) with a 1:50 dilution of IgG-enriched naive rabbit serum, IgG-enriched rabbit anti-rsPilA or IgG-enriched rabbit anti-OMP P5. Positive control wells contained 0.2 μM DPD instead of supernatants (red bars). A significant increase in light production by V. harveyi was detected after incubation with NTHI biofilm supernatants collected from the parent strain, complemented ΔpilA and complemented ΔluxS strains treated with IgG-enriched rabbit anti-rsPilA, with maximal values shown 6 h after application of antibody and thus these data complement those shown in Fig. 3. Data are expressed as the fold increase in relative light units ± SEM, two-way ANOVA.
Exposure of biofilms formed by either the parental isolate, ΔpilA, ΔluxS or the complemented ΔpilA or ΔluxS strains to IgG-enriched anti-OMP P5 did not result in a significant increase in bioluminescence by V. harveyi over that induced by IgG-enriched naive serum except at the 6 h time point for the complemented ΔpilA strain (P≤0.01) . Thus, exposure of PilA- and LuxS-expressing NTHI to IgG-enriched anti-rsPilA induced production of an AI-2 quorum signaling molecule by biofilm-resident NTHI, with maximal production 6 h after incubation. These data correlated with the observed dispersal of NTHI from the biofilm 6 h after application of anti-rsPilA.
The V. harveyi utilized herein is a mutant strain that responds only to the autoinducer AI-2, suggesting a role for this molecule in the observed dispersal of bacteria. To further prove this premise, the AI-2 precursor molecule DPD was directly added to established NTHI biofilms. NTHI strains that expressed PilA (i.e. parent, ΔpilA/pPIL1, ΔluxS and ΔluxS /pSPEC1-luxS strains), exhibited a significant increase in CFU NTHI within supernatants collected from biofilms treated with DPD, compared to biofilms that received medium (P≤0.05) [Fig. 5]. In this assay system, the addition of exogenous autoinducer overcame the inability of NTHI to express luxS, as has been shown (Armbruster et al., 2009). In contrast, no change in bacterial concentration was detected in supernatants collected from biofilms formed by the pilA mutant. Collectively, this set of experiments showed that the expression of pilA and ability to synthesize the quorum signaling molecule AI-2 via LuxS was central to the observed increases in bioluminescence by V. harveyi and NTHI concentration in biofilm supernatants and thereby revealed a mechanism for PilA-targeted disruption of NTHI biofilms.
Figure 5.

AI-2-mediated quorum signaling induced dispersal of NTHI from established biofilms. CFU NTHI within supernatants collected from 16 h biofilms formed by NTHI 86-028NP, NTHI ΔpilA or its complemented pilA mutant (C’ΔpilA), NTHI ΔluxS or its complemented luxS mutant (C’ΔluxS) treated with medium only (blue bars) or 0.2 μM DPD (red bars). Note significant increase in CFU NTHI within supernatants of biofilms formed by pilA-expressing strains and exposed to DPD, compared to medium alone. Data are expressed as mean ± SEM, Student’s t-test.
Evidence for coordinated regulation of expression of Tfp and LuxS
To this point, we demonstrated that treatment of biofilms formed by wildtype NTHI (that express both PilA and LuxS) with antibodies against rsPilA induced NTHI to produce an AI-2 quorum signaling molecule and promoted dispersal of NTHI into the supernatant with concomitant biofilm disruption. To examine expression of PilA and LuxS simultaneously during biofilm formation and maturation in vitro, we generated NTHI reporter constructs wherein the promoter regions of each gene were used to drive expression of green fluorescent protein (GFP) and the relative fluorescence of each was measured at 3 h intervals for 42 h. The pilA promoter activity was strong upon inoculation of the chamberslide and this activity was maintained for the first 9 h in culture after which activity declined [Fig. 6A]. In contrast, luxS promoter activity was at an undetectable level during the initial 9 h of incubation but increased thereafter [Fig. 6B]. Over time, it became apparent that the activity of the pilA and luxS promoters fluctuated, exhibiting oppositional maximum peaks of activity at approximately 9 h intervals throughout the 42 h assay period that did not correlate with addition of fresh medium to maintain bacterial viability [Fig. 6C]. These data demonstrated that pilA and luxS promoter activity, and by inference, expression of PilA and LuxS, was important throughout establishment and maturation stages of NTHI biofilm formation in vitro. Moreover, the observed regular opposing fluctuations in promoter activity suggested that expression of PilA (and by inference, functional Tfp) and quorum signaling via LuxS are tightly co-regulated.
Figure 6.

Relative pilA or luxS promoter activity during biofilm formation and maturation by NTHI in vitro. Relative fluorescence of NTHI reporter constructs wherein the pilA promoter (panel A, red line) and luxS promoter (panel B, blue line) drive expression of GFP, as detected by Xenogen imaging, compared to the non-fluorescent parent strain (grey line). Data are expressed as the fold change in mean fluorescence ± SEM, compared to the parent strain. Note in panel C the regular cyclic and oppositional peaks of pilA and luxS promoter activity that occurred approximately every 9 h throughout the 42 h incubation period.
Detection of immunogen-specific antibody in middle ear fluids and serum recovered from chinchillas immunized transcutaneously with rsPilA + IHF
To understand implications of PilA- and IHF-targeted biofilm resolution in vivo, when administered as either an individual immunogen (IHF+dmLT) or with the addition of rsPilA to the formulation (IHF+rsPilA+dmLT), cohorts of chinchillas with active OM were immunized by transcutaneous immunization. Because antibody has a critical role in resolution of OM (Murphy & Yi, 1997), we assessed the relative quantity of immunogen-specific antibodies in serum and middle ear fluids in all cohorts at termination of the study. As all animals begin the study with ongoing robust NTHI-induced OM prior to immunization, titers obtained will reflect both a response to disease as well as that induced by TCI. Within serum obtained from the cohort immunized with the adjuvant dmLT only, there was minimal IHF- or rsPilA-specific antibody as expected (geometric mean titer [GMT] 57 against IHF, 95% confidence interval [CI] 31–104; GMT 63 against rsPilA CI 44–92). Animals immunized with IHF+dmLT yielded an IHF-specific serum antibody GMT of 254, CI 140–460 whereas those that received IHF+rsPilA+dmLT yielded an IHF-specific GMT of 226, CI 123–416. It was of note that the concentration of IHF in the vaccine formulation that also contained rsPilA (IHF+rsPilA+dmLT) was half that of the formulation to deliver IHF alone (IHF+dmLT) (5 µg and 10 µg, respectively) in order to maintain administration of 10 μg total protein per dose [in keeping with doses typically delivered to humans and as used in prior pre-clinical studies by our laboratory (Novotny et al., 2006, Bakaletz et al., 1999, Kennedy et al., 2000, Goodman et al., 2011, Novotny et al., 2011, Novotny et al., 2013)], yet comparable serum GMT versus IHF were detected. Specific to rsPilA, minimal specific serum antibody was detected in cohorts immunized with dmLT (GMT 63, CI 44–92) or IHF+dmLT (GMT 57, CI 38–84), however, as anticipated, in cohorts immunized with IHF+rsPilA+dmLT, the GMT for rsPilA-specific serum antibody was 453, CI 166–1233.
Within middle ear fluids, a similar concentration of IHF-specific antibody was detected in cohorts administered IHF+dmLT or IHF+rsPilA+dmLT (GMT 180, CI 104–311), whereas there was minimal reactivity in fluids collected after TCI with dmLT alone (GMT 22, CI 17–30). In cohorts immunized with IHF+rsPilA+dmLT, we detected rsPilA specific antibody (GMT 403, 190–855) whereas minimal rsPilA-specific antibody was detected in the cohorts immunized with either dmLT alone (GMT 28, CI 19–42) or with IHF+dmLT (GMT 22, CI 17–30), again as expected. Collectively, these data revealed that in animals with ongoing experimental NTHI-induced OM, we were nonetheless able to detect the influence of TCI on the production of immunogen-specific antibody both systemically and within middle ear fluids. These data also corroborated prior work which demonstrates that an immunogen-specific GMT of ≥160 in chinchilla middle ear fluids is associated with the ability to eradicate NTHI from the middle ear (Novotny et al., 2011).
Resolution of experimental NTHI-induced OM by transcutaneous immunization
Given that antibodies directed against both IHF and rsPilA mediated biofilm disruption in vitro and in vivo, yet appeared to do so via unique mechanisms, we were curious as to whether addition of rsPilA to a formulation of IHF plus dmLT would enhance the efficacy we’d seen when IHF was used as a singular immunogen in previous work (Goodman et al., 2011). Therefore, herein we examined the efficacy afforded by therapeutic TCI with rsPilA plus IHF in the resolution of established NTHI-induced OM compared to that attributable to immunization with IHF alone. Following induction of OM by direct challenge of the middle ear with NTHI and the formation of robust biofilms in the middle ears, we immunized animals by rubbing vaccine formulations on to the skin of the outer ear. To determine relative vaccine efficacy, we utilized an approach used clinically to determine signs and severity of OM in children. Thus, by video otoscopy, the tympanic membranes of each animal were visualized to determine whether TCI reduced signs of inflammation and/or induced clearance of fluids from the middle ear. Whereas throughout the study middle ear fluids were observed behind ≥95% of tympanic membranes in the cohort immunized with the adjuvant dmLT only [Fig. 7A], a significant increase in the proportion of middle ears that had cleared middle ear fluids was shown in the cohorts that received either IHF+dmLT (P≤0.05) or IHF+rsPilA+dmLT (P≤0.01). Moreover, one week after receipt of the second immunization, the cohort administered rsPilA plus IHF had resolved 53% of middle ear fluids, an outcome that was greater than the 30% resolution of fluids achieved by immunization with IHF alone [Fig. 7A]. Thus, by clinically-relevant measures, the addition of rsPilA to a therapeutic vaccine formulation of IHF+dmLT afforded an improved outcome relative to receipt of IHF alone.
Figure 7.
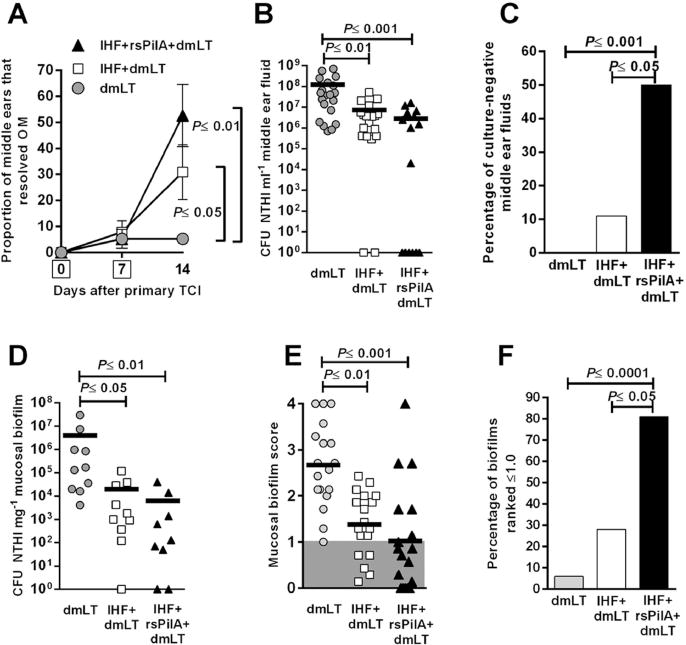
Transcutaneous immunization with IHF+dmLT or with the same formulation plus the addition of rsPilA resolved experimental NTHI-induced OM. (A) Resolution of middle ear fluids as determined by video otoscopy and tympanometry, boxes on days 0 and 7 in y-axis represent days of immunization. (B) Relative CFU NTHI in middle ear fluids and (C) percentage of culture-negative middle ear fluids for each cohort. (D) CFU NTHI per mg middle ear mucosal biofilm. (E) Mean NTHI mucosal biofilm scores based on blinded evaluation and ranked on a 0 to 4+ scale of relative residual biomass. Shaded region indicated middle ears in which <25% of the middle ear space was occluded with NTHI biofilm (F) Percentage of mucosal biofilms that were ranked ≤1.0 in each cohort (as shown in panel E), thus signifying that <25% of the middle ear space was occluded by a mucosal biofilm. A significant reduction in signs of OM, NTHI concentration in middle ear fluids, middle ear mucosal biofilms and relative amount of residual biofilm was achieved after receipt of IHF+dmLT or IHF+rsPilA+dmLT, compared to dmLT alone. Note that addition of rsPilA to a formulation of IHF+dmLT consistently induced an immune response that resulted in enhanced bacterial eradication and disease resolution. Data in panels A, C and F are expressed as percentage per cohort ± SEM, Mantel-Cox Log-rank test. Data in panels B, D and E are plotted as individual ears and the mean value is shown, one-way ANOVA.S
As an additional measure of vaccine-induced disease resolution, we also quantitated the relative bacterial load within middle ear fluids collected after receipt of both immunizing doses. As expected, the cohort administered dmLT alone exhibited a high concentration of NTHI in retrieved middle ear fluids (1.2 × 108 CFU ml−1) [Fig. 7B] (Novotny et al., 2013). A significant 16-fold fewer NTHI were cultured from fluids collected from the middle ears of chinchillas that received IHF+dmLT (P≤0.01) and an even greater 40-fold reduction was achieved after co-administration of IHF+rsPilA+dmLT (P≤0.001). Moreover, and whereas 11% (2/18) of middle ear fluids from animals administered IHF+dmLT were culture-negative [Fig. 7C], 50% (8/16) of middle ear fluids recovered from animals immunized with IHF+rsPilA+dmLT were culture-negative, a significant difference (P≤0.05). Thus, TCI with either IHF+dmLT or with IHF+rsPilA+dmLT induced eradication of the planktonic NTHI population from the middle ear; however, the addition of rsPilA to the formulation of IHF+dmLT resulted in greater bacterial clearance.
Quantitation of NTHI that were either adherent to the mucosa or resident within middle ear mucosal biofilms, as determined by plating of tissue homogenates recovered one week after receipt of the second immunizing dose, revealed that TCI with IHF+dmLT resulted in a significant 2.3-log reduction in CFU NTHI, compared to receipt of dmLT alone (P≤0.05) [Fig. 7D]. A 2.8-log reduction was achieved by receipt of IHF+rsPilA+dmLT, compared to receipt of this adjuvant alone (P≤0.01). Thus, whereas via this measure, there was no significant difference between these latter two cohorts, co-administration of rsPilA and IHF induced a 0.5-log greater decrease in NTHI concentration in tissue homogenates relative to the cohort that received IHF alone. These data thus mirror the trend observed with clearance of NTHI from middle ear fluids and occurred despite the fact that the cohort that received both immunogens (IHF+rsPilA+dmLT) received only half the dose of IHF than was received by the cohort immunized with IHF+dmLT.
As an additional assessment of disease resolution, the relative quantity of biofilm remaining in the middle ear at the end of the study was determined. In this animal model of direct bacterial challenge of the middle ear, robust biofilms are established in the middle ear prior to immunization and earlier work demonstrates that 4 days after direct inoculation of the chinchilla middle ear, 83–100% of all middle ears develop a biofilm that occupies 75–100% of the middle ear space (Novotny et al., 2011). Blind ranking of each middle ear by means of an established 0 to 4+ scale (Goodman et al., 2011, Novotny et al., 2011, Novotny et al., 2013) to indicate the relative amount of middle ear space still occupied by a mucosal biofilm after immunization, revealed a significant reduction in the cohorts that had been immunized with either IHF+dmLT (P≤0.01) or IHF+rsPilA+dmLT (P≤0.001), compared to those immunized with dmLT alone [Fig. 7E]. Moreover, based on these data, whereas TCI with dmLT resulted in a mean score of 2.7, which indicated that ≥50–75% of the middle ear space remained filled with mucosal biofilm, the cohort immunized with IHF+dmLT received a mean score of 1.4, which thus indicated that ≥25–50% of the middle ear space contained mucosal biofilm, which was significantly less than that observed in the dmLT alone cohort (P≤0.01). These data were comparable to prior work thus validating the robustness of this animal model and measurement outcome (Goodman et al., 2011). Importantly, in the cohort immunized with IHF+rsPilA+dmLT, a mean score of 1.0 was obtained; which thus indicated that ≤25% of the middle ear space remained occupied by a mucosal biofilm after immunization. This result was also significantly greater than that obtained by immunization with dmLT alone (P≤0.001)
Whereas there was no significant difference between the cohort immunized with IHF+dmLT and that to which we’d added rsPilA to the formulation when mean counts of NTHI in either middle ear fluids or mucosal homogenates were assayed, we also determined how many ears received a score ≤1.0 (which indicated that ≤25% of the middle ear space was occluded by mucosal biofilm) at the termination of the study. We found that only 6% of ears received this score in the cohort immunized with dmLT alone. In the cohort immunized with IHF+dmLT, 28% of ears scored ≤1.0. However, in the cohort immunized with IHF+rsPilA+dmLT 81% of middle ears received a score ≤1.0 which indicated that this combinatorial and biofilm-targeted therapeutic immunization strategy resulted in a significant proportion of middle ears that had resolved ≥75% of established mucosal biofilms (P≤0.0001 compared to dmLT; P≤0.05 compared to IHF+dmLT).
Discussion
Biofilms play an important role in the pathogenesis of all NTHI-induced diseases of the respiratory tract (Post, 2001, Swords, 2012, Bakaletz, 2012, Murphy & Kirkham, 2002). Due to their innate resistance to clearance by antimicrobials and host immune effectors, mitigating these diseases will likely require a novel biofilm-targeted approach. However, as is characteristic of all biofilms, those formed by NTHI are highly recalcitrant to the activity of most antibodies, including those directed against an NTHI whole outer membrane preparation (author’s unpublished observations) or raised against the NTHI adhesin, OMP P5 as shown herein. To date, we’ve found that only antibodies directed against two proteins expressed by NTHI have demonstrated the ability to significantly disrupt or eradicate established NTHI biofilms in vitro and in vivo: rsPilA (Novotny et al., 2011) and at least one DNABII protein, IHF (Goodman et al., 2011, Brockson et al., 2014).
Based upon the observations made in vitro, whereas the outcome of treatment of established biofilms with antiserum against either rsPilA or IHF was similar overall, the mechanisms of disruption appeared to be unique. Thereby, we sought to gain a better understanding of the mechanisms for biofilm disruption mediated by anti-rsPilA as we had already unraveled much of this as related to the action of anti-IHF (Brockson et al., 2014). In vitro, exposure of NTHI biofilms to anti-IHF results in disruption of the biofilm due to the sequestration of IHF as it dissociates from eDNA where it serves as a critical structural component (Brockson et al., 2014). The result is catastrophic collapse of the biofilm structure with subsequent release of NTHI leaving only a monolayer of bacteria. Conversely, and as shown here, treatment of NTHI biofilms with antiserum against the majority subunit of NTHI Tfp (PilA) also resulted in significant biofilm disruption and reduction in biomass, however, nominal towers and water channels remained. This observation suggested a more gradual top-down disruption of the biofilm, but also with dispersal of NTHI into the planktonic phase. Moreover, we showed that biofilm disruption mediated by anti-rsPilA required expression of PilA and LuxS which suggested a role for both functional Tfp expression and LuxS-mediated quorum signaling in this outcome. To date, and through the efforts of multiple laboratories, an important role has been shown for Tfp, the DNABII protein – IHF and LuxS in terms of biofilm formation by NTHI both in vitro and in vivo (Bakaletz et al., 2005, Jurcisek et al., 2007, Carruthers et al., 2012, Goodman et al., 2011, Brockson et al., 2014, Gustave et al., 2013, Brandstetter et al., 2013, Armbruster et al., 2009, Armbruster et al., 2011, Daines et al., 2005), however to our knowledge, this is the first report of relatedness between Tfp- and LuxS-mediated events.
Since antibodies directed against either IHF or the majority subunit of Tfp (PilA) are able to significantly disrupt or eradicate biofilms formed by NTHI in vitro and in vivo, but appeared to do so via unique molecular mechanisms, these findings suggested to us the opportunity to develop a synergistic biofilm-targeted approach to disease prevention and/or resolution. As such, we decided to test the therapeutic efficacy afforded by immunization with a formulation of IHF+dmLT to which we added rsPilA. By co-immunization, we strived to induce the formation of antibodies against rsPilA that would inhibit adherence, twitching motility and biofilm formation by NTHI (Bakaletz et al., 2005, Carruthers et al., 2012, Jurcisek et al., 2007) as well as those against IHF which would induce catastrophic structural collapse of the biofilm with release into the planktonic phase of NTHI that show both greater sensitivity to the killing action of traditional antibiotics and sensitivity to immune-mediated clearance (Goodman et al., 2011, Gustave et al., 2013, Novotny et al., 2013, Brockson et al., 2014).
To determine if adding rsPilA to a formulation of IHF+dmLT would induce an immune response that had an additive effect, we used an experimental model wherein OM is established prior to immunization in order to determine whether induction of the formation of antibodies against both rsPilA and IHF would resolve active disease better than those against IHF alone. Chinchillas were immunized by TCI wherein vaccine formulations were applied to the skin on the inner surface of the ears of animals. This noninvasive immunization strategy exploits the immunocompetence of the skin by targeting the numerous cutaneous dendritic cells within the dermis to generate an effective, compartmentalized immune response, and is shown to be significantly efficacious when IHF and rsPilA are individually used as immunogens (Goodman et al., 2011, Novotny et al., 2011). To be consistent with amount of vaccine antigen typically delivered in pediatric vaccines and in concordance with all of our prior pre-clinical vaccine studies (Kennedy et al., 2000, Bakaletz et al., 1999, Novotny et al., 2006) the total protein delivered in any immunizing dose was 10 μg. As a result, the cohort co-administered rsPilA plus IHF received 5 μg of each protein whereas that immunized with IHF alone, received 10 µg of that immunogen.
By video otoscopy, to document signs and severity of OM, combined with quantitation of the relative bacterial loads within middle ear fluids and middle ear mucosal biofilms, we observed a significant reduction in all measures of relative disease severity after immunization with IHF+dmLT compared to TCI with adjuvant alone. This outcome was comparable to what we reported in earlier work (Goodman et al., 2011). However, the addition of rsPilA resulted in enhanced clearance of middle ear fluids and significantly greater eradication of NTHI from both any remaining middle ear fluids and mucosal biofilms. Despite receiving half as much IHF immunogen as the cohort immunized with IHF+dmLT, the addition of rsPilA to the formulation of IHF+ dmLT, induced enhanced disease resolution suggesting that there was no interference between these two immunogens and further, that there was an added benefit. These data thus support the premise that incorporation of multiple NTHI biofilm-targeted vaccine candidates within a single formulation could be greatly efficacious in development of a therapeutic vaccine, as well as potentially a more traditional preventative vaccine.
As to how this additive effect might have been achieved, we have investigated the mechanisms of action of antisera directed against either IHF or rsPilA in vitro in terms of their ability to disrupt an established NTHI biofilm. We showed earlier that anti-IHF induces catastrophic structural collapse of the biofilm matrix with release of resident bacteria via a mechanism that does not require direct contact and is active within 6 hours of exposure (Brockson et al., 2014). However, despite similarly mediating biofilm disruption, the mechanisms by which incubation with antibodies directed against PilA mediated this effect were heretofore unknown. Here, we showed that anti-rsPilA inhibited twitching motility in a soft agarose assay and induced disruption of biofilms formed by the wild type parental isolate but not those formed by a non-polar pilA mutant nor that formed by a luxS mutant. Complementation of either mutation resulted in restoration of our ability to demonstrate biofilm disruption upon incubation with anti-rsPilA and suggested that what appeared to be a top-down disruption of the biofilm might be tied to quorum signaling. Indeed release of NTHI from the biofilm into the planktonic phase as observed after 6 h incubation with anti-rsPilA was dependent upon ability to express both PilA (and by inference, Tfp) and LuxS. Further, this release was directly associated with the detection of AI-2 in the supernatants recovered from biofilms treated with IgG-enriched antiserum to rsPilA but not those treated with either IgG-enriched naive serum or serum directed against another NTHI adhesin, OMP P5. Using reporter constructs, we showed that both LuxS and PilA are expressed during biofilm formation and maturation however promoter activity for the genes that encoded these proteins was cyclic, with peak activity occurring approximately every 9 hours and in opposition. Collectively, our data suggested that expression of Tfp and LuxS is tightly co-regulated in NTHI and that both twitching motility and quorum signaling are essential to biofilm formation as well as dispersal as mediated by antibodies directed against an immunogen derived from the majority subunit of the type IV pilus of NTHI. We will follow up on these intriguing observations in future work.
By inducing an immune response against both IHF and rsPilA, we showed via use of a non-invasive immunization strategy that targeting two important determinants resulted in significantly earlier eradication of NTHI from both planktonic and adherent populations in the middle ear, disruption of mucosal biofilms already resident within middle ears, and rapid resolution of signs of disease in an animal model of experimental OM. These data support continued development of this novel combinatorial therapeutic immunization approach for resolution and/or prevention of diseases of the respiratory tract due to NTHI.
Experimental Procedures
Bacterial strains
Nontypeable Haemophilus influenzae (NTHI) strains used in this work are shown in Table 1. NTHI 86-028NP is a minimally passaged clinical isolate recovered from the nasopharynx of a child with chronic OM (Sirakova et al., 1994, Harrison et al., 2005). Deletion of pilA was performed to generate NTHI 86-028NP ΔpilA (Carruthers et al., 2012). NTHI 86-028NP ΔpilA/pPIL1 is the pilA mutant complemented with a plasmid to allow for expression of pilA (Carruthers et al., 2012). NTHI 86-028NP ΔluxS contains a kanamycin resistance cassette within the luxS gene (Armbruster et al., 2009). NTHI 86-028NP ΔluxS/pSPEC1-luxS is the luxS mutant complemented with a plasmid to permit expression of luxS. To construct the complemented ΔluxS strain, the luxS gene from NTHI 86-028NP was amplified by PCR using primers shown in Table 2, then ligated into pSPEC1 as a SphI to EcoRI fragment. The resulting plasmid, pSPEC1-luxS, was transformed into H. influenzae Rd and clones selected from overnight growth on chocolate agar plus 200 μg spectinomycin ml−1. The plasmid was isolated and transformed into NTHI 86-028NP ΔluxS as described (Mason et al., 2005) and clones selected from overnight growth on chocolate agar plus 200 μg spectinomycin ml−1 and 20 μg kanamycin ml−1.
Table 1.
Bacterial strains and plasmids used in this study
| Bacterial strains | Description | Source |
|---|---|---|
| NTHI 86-028NP | Isolated from the nasopharynx of a child with chronic OM | Sirakova et al., 1994 |
| NTHI 86-028NP ΔpilA | Nonpolar pilA mutant | Carruthers et al., 2012 |
| NTHI 86-028NP ΔpilA/pPIL1 | Complemented nonpolar pilA mutant | Carruthers et al., 2012 |
| NTHI 86-028NP ΔluxS | luxS mutant generated by insertion of kanamycin resistance cassette within luxS gene | Armbruster et al., 2009 |
| NTHI 86-028NP ΔluxS/pSPEC1-luxS | Complemented luxS mutant | This study |
| NTHI 86-028NP: pilA-GFP | NTHI 86-028NP in which expression of GFP is under control of the pilA promoter | This study |
| NTHI 86-028NP: luxS-GFP | NTHI 86-028NP in which expression of GFP is under control of the luxS promoter | This study |
| V.harveyi BB170 | Used in bioluminescence assay to detect production of AI-2 | ATCC |
| Plasmids | Description | Source |
|
| ||
| pSPEC1 | Haemophilus-E. coli shuttle vector pGZRS-39A wherein kanamycin resistance gene replaced by spectinomycin resistance gene from pSPECR | West et al., 1995 |
| pSPEC1-luxS | luxS gene from NTHI 86-028NP cloned into pSPEC1 vector | This study |
| pGFP | Promoterless derivative of Haemophilus-E. coli shuttle vector pGZRS-39A which incorporates gfpmut3 | Mason et al., 2003 |
| pGFP-PpilA | pilA promoter driving expression of gfpmut3 within pGFP | This study |
| pGFP-PluxS | luxS promoter driving expression of gfpmut3 within pGFP | This study |
Table 2.
Primers used in this study
| luxS gene | 5′GCGCGCATGCAATGAGCAAGAAAAAACCCCACAATAAAT 5′CCGGAATTCCGGCTATTTTAATAAGGAATTATCGAGTGACAAATCTTCAT |
| pilA promoter | 5′GAAGTGAGGTGACTGAATTTGCCGACAATC 5′GATAAACGCCCAAATCCACAGGATATTTCC |
| luxS promoter | 5′ACATGCATGCATGTAATGAGCAAGAAAAAACCCCACAATAAAT 5′CGGGATCCCGAAATTTTCCTTATAAATCAGTTGGTTAAGAAAT |
To approximate expression of NTHI pilA or luxS during biofilm formation in vitro, reporter constructs were generated wherein expression of green fluorescent protein (GFP) was under the control of the promoters for either pilA or luxS. The promoter for pilA was excised from plasmid pKMLN-02 (Jurcisek et al., 2007) by digestion with BamHI and SalI and gel purified. Plasmid pRSM2169 (Mason et al., 2005) was also digested with BamHI and SalI and gel purified. The products were ligated with T4 DNA ligase (Invitrogen) and the resulting plasmid, pGFP-PpilA, was used to transform Top 10 chemically competent E. coli cells (Invitrogen) and colonies selected after overnight growth on LB agar plus 20 μg kanamycin ml−1.
A luxS reporter was generated by PCR amplification of the luxS gene from NTHI 86-028NP genomic DNA using primers noted in Table 2, incorporating SphI and BamHI restriction sites, respectively. The plasmid pRSM2211 wherein GFP is driven by the promoter for NTHI outer membrane protein P2 (Mason et al., 2003) was digested with SphI and BamHI (NEB) to excise the promoter for P2, the plasmid backbone was gel purified and then ligated with the gel purified luxS promoter PCR product using T4 DNA ligase. Top 10 chemically competent E. coli cells were transformed with the resulting plasmid, pGFP-PluxS, and colonies selected from overnight growth on LB agar plus 20 μg kanamycin ml−1.
A colony with the correct plasmid construct for each reporter was inoculated in to LB broth plus 20 μg kanamycin ml−1 and plasmid expression induced by overnight incubation with 170 μg chloramphenicol ml−1. The plasmids were isolated, methylated with CpG methylase (NEB) and electroporated into NTHI 86-028NP (Mason et al., 2005). Colonies were selected by overnight growth on chocolate agar plus 20 μg kanamycin ml−1.
Sub-agarose twitching inhibition assay
To assess the ability of antibodies directed against Tfp to inhibit twitching motility in vitro, we developed a high-throughput sub-agarose assay. Polyclonal naive rabbit serum, rabbit anti-rsPilA, or rabbit anti-OMP P5 (generated at Spring Valley Labs) were diluted 1:2 in sterile 0.9% sodium chloride and a 5 μl volume applied to wells of sterile, non-tissue culture treated 24-well plates (Corning). Serum was then spread across the well bottom using a sterile pipette tip. As an additional negative control, 5 μl of the saline diluent was applied to a separate well. A 500 μl volume of soft agarose (0.3% agarose plus brain heart infusion broth supplemented with 2 μg ml−1 each of heme and β-NAD) (Sigma Aldrich) was then added to each well and allowed to set for 24 h at room temperature prior to inoculation. During assay development, NTHI was observed to twitch on the basolateral surface of the medium after piercing the agarose to deposit the inoculum at the center bottom of the well. Therefore, to assess twitching motility, or inhibition thereof, the semi-solid medium was removed from the microtiter plates after incubation and the bottom surface stained to reveal growth of NTHI.
To inoculate, a single isolated bacterial colony from a 20 h chocolate agar plate supplemented with appropriate antibiotic was suspended in 5 μl 0.9% sterile saline (to yield ~1.5 ×107 CFU NTHI ml−1). A volume of 0.4 μl of the bacterial slurry was then inoculated onto the bottom of the well by piercing though the soft agarose at the center of each well. Plates were incubated on a leveled surface for 24 h at 37°C, 5% CO2 in a humidified atmosphere. Following incubation, the top surface of the soft agarose (still in place within the 24-well plate) was gently swabbed with a sterile cotton swab dipped into 0.9% saline to remove any bacterial growth. To detect growth indicative of twitching motility, the soft agarose was removed from each well to expose the bottom surface of the plus. To remove any loosely adherent bacteria, soft agarose plugs were washed in sterile saline for 1 h with gentle rocking. Colony morphology was revealed by incubation with Bio-Safe Coomassie stain (Bio-Rad) followed by extensive washes in dH2O. Soft agarose plugs were examined using a dissecting microscope to determine NTHI colony morphology and images captured with AxioVision software (Zeiss). Experiments were performed three times and representative images shown. We additionally quantitated relative twitching motility of each strain using images collected at the same magnification. With AxioVision software, the length of bacterial growth extending from the inoculation site was measured in four directions for each image. The mean length ± SEM is reported.
Disruption of established biofilms in vitro
Biofilms formed by NTHI 86-028NP, ΔpilA, ΔpilA/pPIL1, ΔluxS and Δlux /pSPEC1-luxS were first established in 8-well chambered coverslips (Lab-Tek) for 24 h prior to treatment with an arbitrarily selected 1:50 dilution of antiserum for an additional 16 h as described (Jurcisek et al., 2011, Brockson et al., 2014). Polyclonal rabbit sera tested included naive rabbit serum and rabbit anti-NTHI OMP P5 as negative controls, and rabbit anti-rsPilA. Sera were not heat-inactived prior to use. Biofilms were stained with LIVE/DEAD® BacLight viability stain (Invitrogen) wherein live bacteria would appear green when viewed by confocal microscopy and dead bacteria would appear red. For all biofilm assays, duplicate wells were viewed on a Zeiss 510 Meta-laser scanning confocal microscope, images compiled with Zeiss Zen software and biomass values were calculated with COMSTAT2 software (Heydorn et al., 2000, Vorregaard, 2008). All biofilm assays were repeated a minimum of thee times, on separate days. Data represent mean ± SEM.
Assay to detect release of bacteria from a biofilm (dispersal) into the planktonic phase
To examine the hypothesis that antibody directed against NTHI Tfp induced dispersal of bacteria from established biofilms, an in vitro assay was developed. Biofilms formed by NTHI 86-028NP, ΔpilA, ΔpilA/pPIL1, ΔluxS and ΔluxS /pSPEC1-luxS strains were first established in 8-well chambered coverslips as described (Jurcisek et al., 2011). After 16 h, medium was aspirated and fresh medium containing an arbitrarily selected 1:50 dilution of the following polyclonal antisera were added: naive rabbit serum, rabbit anti-rsPilA, or rabbit anti-OMP P5 (generated at Spring Valley Laboratories). Sera were not heat-inactivated prior to use. Four, six and eight h after addition of sera, supernatants were collected, the optical density measured at 490 nm and then fluids were serially diluted and plated on to chocolate agar . This assay was repeated three times and the mean optical density ± SEM and mean CFU NTHI/ ml ± SEM reported.
Assay to demonstrate autoinducer-2 activity in NTHI biofilms upon incubation with anti-rsPilA
NTHI strain 86-028NP is shown to produce and respond to the quorum-sensing/signaling molecule autoinducer-2 (AI-2) (Armbruster et al., 2011, Daines et al., 2005). To determine whether dispersal of NTHI from biofilms after treatment with anti-rsPilA was dependent upon quorum signaling, we utilized a modified Vibrio harveyi reporter assay. The V. harveyi reporter strain BB170 (sensor 1−, sensor 2+; ATCC) was incubated in Marine broth at 30°C with shaking at 200 rpm for 16 h, then diluted 1:5000 into AB medium (Greenberg, 1979). Ninety microliters of the diluted V. harveyi cells were then added to wells of a 96-well, white microtiter plate (Corning). Supernatants from 16 h NTHI biofilms formed by NTHI 86-028NP, ΔpilA, ΔpilA/pPIL1, ΔluxS and ΔluxS /pSPEC1-luxS were collected 4, 6 and 8 h after addition of fresh medium or treatment. As during assay development, we observed that whole polyclonal rabbit serum nonspecifically induced light production by V. harveyi, we treated NTHI biofilms with a 1:50 dilution of IgG-enriched naive rabbit serum, IgG-enriched rabbit anti-rsPilA or IgG-enriched rabbit anti-OMP P5. IgG was enriched from polyclonal rabbit sera using a HiTrap Protein G column (GE Healthcare) per manufacturer’s instructions (Brockson et al., 2014). As the sera were collected from hyperimmune rabbits, the majority of IgG from rsPilA- and OMP P5-immunized rabbits was expected to be specific for the immunizing protein. Biofilm supernatants were then centrifuged at 16,000 x g for 5 min followed by filtration though a 0.2 μm syringe filter to remove bacteria and immediately frozen at −80°C until assayed. Ten microliters of the cell-free fluids were added to wells of microtiter plates containing V. harveyi and incubated at 30°C with shaking at 200 rpm in ambient air. Light production by V. harveyi was measured 4.5 h later using a Tecan M200PRO microplate reader. Positive control wells contained 10 μl of 0.2 μM (S)-4,5-Dihydroxy-2-3-pentadione (DPD; OMM Scientific Inc., Dallas, TX) instead of cell-free supernatants. The values for relative light production by V. harveyi after incubation in cell-free supernatants from biofilms treated with IgG-enriched naive serum were subtracted from values obtained with IgG-enriched anti-rsPilA or anti-OMP P5. Assays were repeated three times and the mean ± SEM reported.
To further confirm that quorum signaling via AI-2 molecule induced the PilA-mediated release of biofilm-resident NTHI, exogenous DPD (0.2 μM) was added to 16 h biofilms formed by NTHI 86-028NP, ΔpilA, ΔpilA/pPIL1, ΔluxS and ΔluxS /pSPEC1-luxS. After incubation at 37°C in a humidified atmosphere, static for 45 min, supernatants were collected, serially diluted and plated on to chocolate agar. Assays were repeated three times and the mean ± SEM reported.
Detection of pilA and luxS promoter activity during biofilm formation in vitro
As a surrogate to demonstrate the expression of PilA and LuxS during biofilm formation in vitro, we utilized NTHI reporter constructs that contained a plasmid wherein expression of green fluorescent protein (GFP) was under the control of the promoters for pilA or luxS. Biofilms were established as described in 8-well chambered coverslips. To maintain bacterial viability, the medium was replaced with fresh sBHI after 16 h and again at 24 h. Upon seeding the chamber sides and at 3 h intervals thereafter for 42 h, biofilms were imaged using a IVIS Spectrum optical imagine system (PerkinElmer, Waltham, MA) to detect GFP-specific fluorescence at the following parameters: excitation= 465 nm, emmision= 520 nm, 1 s exposure. The relative promoter activity at each time point was determined by measuring the fluorescent intensity within each chambered coverslip well using Living Image software (PerkinElmer). Each value was then compared to the non-fluorescent wildtype parent strain. The fold change in fluorescent intensity induced by the pilA and luxS promoters over that of the parent strain was plotted. Data represent mean ± SEM of three independent assays.
Immunogens and adjuvant
Purified E. coli IHF was a gift from the late Dr. Howard Nash (Rice et al., 1996). Recombinant soluble PilA, or rsPilA, is an N-terminally truncated protein representing a modified soluble form of mature PilA derived from NTHI 86-028NP (Novotny et al., 2009). A double mutant form of E. coli heat-labile enterotoxin, called LT(R192G-L211A) and abbreviated ‘dmLT’, wherein glycine is substituted for arginine at position 192 and alanine is substituted for lysine at position 211, served as the adjuvant (gift from Dr. John Clements, Tulane University). The amino acid substitutions render dmLT nontoxic while maintaining adjuvant properties (Norton et al., 2011).
Bacterial challenge and transcutaneous immunization regime
Forty-eight adult chinchillas (Chinchilla lanigera; 733 ± 19 g) were obtained from Rauscher’s Chinchilla Ranch (LaRue, Ohio) and rested 7 days. No evidence of middle ear disease was documented by video otoscopy and tympanometry prior to enrollment. All animals were challenged by transbullar inoculation with 1000 CFU NTHI 86-028NP per bulla to induce active experimental OM. Four days later, when signs of inflammation of the tympanic membrane (ear drum) and evidence of OM (middle ear fluid was visible behind the tympanic membrane) were present (Goodman et al., 2011, Novotny et al., 2013), animals were immunized by transcutaneous immunization (TCI) as described (Novotny et al., 2011, Novotny et al., 2013). Briefly, both pinnae, or external part of the ear, of each alert animal were hydrated for 5 min by placement of gauze soaked in sterile, pyrogen-free 0.9% sodium chloride (Hospira, Lake Forest, IL) on the inner surface, blotted with dry gauze and then 50 μl of each vaccine formulation was applied using a pipet. The pinnae were then folded in half and opposing surfaces gently rubbed together. A total protein content of 10 μg immunogen plus 10 μg adjuvant was administered by TCI. Formulations consisted 10 μg rsPilA admixed with 10 μg dmLT; 10 μg purified E. coli IHF plus 10 μg dmLT; 5 μg rsPilA plus 5 μg IHF (a total of 10 μg protein in total) admixed with 10 μg dmLT or 10 μg dmLT alone. Two doses were delivered one week apart.
ELISA to assess immunogen-specific antibody in serum and middle ear fluids
To determine the relative quantity of immunogen-specific antibody in serum and clarified middle ear fluids recovered at termination of the study, endpoint ELISA was performed. Individual samples were incubated in rsPilA- or IHF-coated wells (0.2 μg protein/well) for 1 h at 37°C and bound antibody was detected with HP-conjugated Protein A (Invitrogen). Color was developed with 3,3′,5,5′- tetramethylbenzidine (TMB; Pierce Biotechnology, Rockford, IL). Endpoint reciprocal titers were defined as the dilution that yielded an OD450nm value of 0.1 above control wells that were incubated without sample fluids. Assays were performed a minimum of three times and the geometric mean (GMT) with 95% confidence interval (CI) reported.
Eradication of NTHI from the middle ear
To assess efficacy of each vaccine formulation, video otoscopy and tympanometry were performed on all animals to detect signs of inflammation and severity of otitis media (Novotny et al., 2006). When middle ear fluids were observed behind the tympanic membrane, an ear was considered positive for OM. Additionally, upon sacrifice, bullae were dissected and middle ear fluids collected from all animals. To semi-quantitate the relative bacterial load within this planktonic population, fluids were serially diluted and plated on to chocolate agar. The mean CFU NTHI ml−1 middle ear fluid was determined for each cohort. Middle ear fluids were then clarified by centrifugation and the supernatants stored at −80°C until assessed for antibody titers by ELISA as described.
To assess the resolution of pre-existing middle ear mucosal biofilms induced by TCI, after collection of middle ear fluids, each bulla was washed gently with saline to remove loosely adherent bacterial biomass and images of the remaining adherent mucosal biofilm captured. Images of each middle ear mucosal biofilm were scored on a 0 to 4+ scale wherein 0= no mucosal biofilm, 1= biofilms fills ≤25% of middle ear space, 2= mucosal biofilm fills >25% to ≤50% middle ear space, 3= mucosal biofilm fills >50% to ≤75% of middle ear space and 4= >75% of middle ear space (Goodman et al., 2011, Novotny et al., 2013). Images were reviewed blindly by seven individuals and the mean score for each middle ear reported. Lastly, the mucosal biofilm was collected from the right bulla, homogenized in sterile saline and plated on to chocolate agar to determine the relative quantity of NTHI mg−1 biofilm. All animal experiments were performed under a protocol approved by the Institutional Animal Care and Use Committee at The Research Institute at Nationwide Children’s Hospital and in adherence to the NIH Guide for the Care and Use of Laboratory animals.
Statistical analyses
Statistical analyses were calculated with GraphPad Prism 6 (GraphPad Software, Inc.). Differences in the proportion of middle ears with OM were determined by Mantel-Cox log-rank test. Significance in CFU NTHI in middle ear fluids, mucosal biofilms and mucosal biofilm score, and differences in in vitro biofilm biomass were determined by One-way Analysis of Variance (ANOVA) with Dunn’s multiple comparisons test. Significance in length of NTHI twitching motility, NTHI biofilm dispersal and V. harveyi relative light units was calculated using a two-way ANOVA with Tukey’s multiple comparisons test. Significance in DPD-induced dispersal of NTHI from biofilms was determined by Student’s t-test. For all comparisions, a P-value of ≤0.05 was considered significant.
Acknowledgments
We thank Jennifer Neelans for manuscript preparation. We thank Dr. Ed Swords (Wake Forest University) for providing the NTHI ΔluxS strain. This study was funded by R01 DC003915 to LOB and R01 DC011818 to LOB and SDG, both grants from the NIH/NIDCD.
References
- Armbruster CE, Hong W, Pang B, Dew KE, Juneau RA, Byrd MS, Love CF, Kock ND, Swords WE. LuxS promotes biofilm maturation and persistence of nontypeable Haemophilus influenzae in vivo via modulation of lipooligosaccharides on the bacterial surface. Infect Immun. 2009;77:4081–4091. doi: 10.1128/IAI.00320-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster CE, Pang B, Murrah K, Juneau RA, Perez AC, Weimer KE, Swords WE. RbsB (NTHI_0632) mediates quorum signal uptake in nontypeable Haemophilus influenzae strain 86-028NP. Mol Microbiol. 2011;82:836–850. doi: 10.1111/j.1365-2958.2011.07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz LO. Bacterial biofilms in the upper airway – evidence for role in pathology and implications for treatment of otitis media. Paediatric respiratory reviews. 2012;13:154–159. doi: 10.1016/j.prrv.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz LO, Baker BD, Jurcisek JA, Harrison A, Novotny LA, Bookwalter JE, Mungur R, Munson RS., Jr Demonstration of Type IV pilus expression and a twitching phenotype by Haemophilus influenzae. Infect Immun. 2005;73:1635–1643. doi: 10.1128/IAI.73.3.1635-1643.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz LO, Kennedy BJ, Novotny LA, Duquesne G, Cohen J, Lobet Y. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect Immun. 1999;67:2746–2762. doi: 10.1128/iai.67.6.2746-2762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstetter KA, Jurcisek JA, Goodman SD, Bakaletz LO, Das S. Antibodies directed against integration host factor mediate biofilm clearance from Nasopore. The Laryngoscope. 2013;123:2626–2632. doi: 10.1002/lary.24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockson ME, Novotny LA, Mokrzan EM, Malhotra S, Jurcisek JA, Akbar R, Devaraj A, Goodman SD, Bakaletz LO. Evaluation of the kinetics and mechanism of action of anti-integration host factor-mediated disruption of bacterial biofilms. Mol Microbiol. 2014;93:1246–1258. doi: 10.1111/mmi.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers MD, Tracy EN, Dickson AC, Ganser KB, Munson RS, Jr, Bakaletz LO. Biological roles of nontypeable Haemophilus influenzae type IV pilus proteins encoded by the pil and com operons. J Bacteriol. 2012;194:1927–1933. doi: 10.1128/JB.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daines DA, Bothwell M, Furrer J, Unrath W, Nelson K, Jarisch J, Melrose N, Greiner L, Apicella M, Smith AL. Haemophilus influenzae luxS mutants form a biofilm and have increased virulence. Microbial pathogenesis. 2005;39:87–96. doi: 10.1016/j.micpath.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Gallaher TK, Wu S, Webster P, Aguilera R. Identification of biofilm proteins in non-typeable Haemophilus Influenzae. BMC microbiology. 2006;6:65. doi: 10.1186/1471-2180-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SD, Obergfell KP, Jurcisek JA, Novotny LA, Downey JS, Ayala EA, Tjokro N, Li B, Justice SS, Bakaletz LO. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal immunology. 2011;4:625–637. doi: 10.1038/mi.2011.27. [DOI] [PubMed] [Google Scholar]
- Greenberg EP, Hastings JW, Ulitzer S. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch Meicobiol. 1979;120:87–91. [Google Scholar]
- Gustave JE, Jurcisek JA, McCoy KS, Goodman SD, Bakaletz LO. Targeting bacterial integration host factor to disrupt biofilms associated with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2013;12:384–389. doi: 10.1016/j.jcf.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A, Dyer DW, Gillaspy A, Ray WC, Mungur R, Carson MB, Zhong H, Gipson J, Gipson M, Johnson LS, Lewis L, Bakaletz LO, Munson RS., Jr Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J Bacteriol. 2005;187:4627–4636. doi: 10.1128/JB.187.13.4627-4636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146(Pt 10):2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Jones EA, McGillivary G, Bakaletz LO. Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human beta defensin-3 and reduces its antimicrobial activity. Journal of innate immunity. 2013;5:24–38. doi: 10.1159/000339961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcisek JA, Bakaletz LO. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol. 2007;189:3868–3875. doi: 10.1128/JB.01935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcisek JA, Bookwalter JE, Baker BD, Fernandez S, Novotny LA, Munson RS, Jr, Bakaletz LO. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol Microbiol. 2007;65:1288–1299. doi: 10.1111/j.1365-2958.2007.05864.x. [DOI] [PubMed] [Google Scholar]
- Jurcisek JA, Dickson AC, Bruggeman ME, Bakaletz LO. In vitro biofilm formation in an 8-well chamber slide. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BJ, Novotny LA, Jurcisek JA, Lobet Y, Bakaletz LO. Passive transfer of antiserum specific for immunogens derived from a nontypeable Haemophilus influenzae adhesin and lipoprotein D prevents otitis media after heterologous challenge. Infect Immun. 2000;68:2756–2765. doi: 10.1128/iai.68.5.2756-2765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason KM, Munson RS, Jr, Bakaletz LO. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect Immun. 2003;71:3454–3462. doi: 10.1128/IAI.71.6.3454-3462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason KM, Munson RS, Jr, Bakaletz LO. A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infect Immun. 2005;73:599–608. doi: 10.1128/IAI.73.1.599-608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF, Kirkham C. Biofilm formation by nontypeable Haemophilus influenzae: strain variability, outer membrane antigen expression and role of pili. BMC microbiology. 2002;2:7. doi: 10.1186/1471-2180-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TF, Yi K. Mechanisms of recurrent otitis media: importance of the immune response to bacterial surface antigens. Annals of the New York Academy of Sciences. 1997;830:353–360. doi: 10.1111/j.1749-6632.1997.tb51907.x. [DOI] [PubMed] [Google Scholar]
- Norton EB, Lawson LB, Freytag LC, Clements JD. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clinical and vaccine immunology : CVI. 2011;18:546–551. doi: 10.1128/CVI.00538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny LA, Adams LD, Kang DR, Wiet GJ, Cai X, Sethi S, Murphy TF, Bakaletz LO. Epitope mapping immunodominant regions of the PilA protein of nontypeable Haemophilus influenzae (NTHI) to facilitate the design of two novel chimeric vaccine candidates. Vaccine. 2009;28:279–289. doi: 10.1016/j.vaccine.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny LA, Clements JD, Bakaletz LO. Transcutaneous immunization as preventative and therapeutic regimens to protect against experimental otitis media due to nontypeable Haemophilus influenzae. Mucosal immunology. 2011;4:456–467. doi: 10.1038/mi.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny LA, Clements JD, Bakaletz LO. Kinetic analysis and evaluation of the mechanisms involved in the resolution of experimental nontypeable Haemophilus influenzae-induced otitis media after transcutaneous immunization. Vaccine. 2013;31:3417–3426. doi: 10.1016/j.vaccine.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny LA, Jurcisek JA, Godfroid F, Poolman JT, Denoel PA, Bakaletz LO. Passive immunization with human anti-protein D antibodies induced by polysaccharide protein D conjugates protects chinchillas against otitis media after intranasal challenge with Haemophilus influenzae. Vaccine. 2006;24:4804–4811. doi: 10.1016/j.vaccine.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Post JC. Direct evidence of bacterial biofilms in otitis media. The Laryngoscope. 2001;111:2083–2094. doi: 10.1097/00005537-200112000-00001. [DOI] [PubMed] [Google Scholar]
- Rice PA, Yang S, Mizuuchi K, Nash HA. Crystal structure of an IHF-DNA complex: a protein-induced DNA U-turn. Cell. 1996;87:1295–1306. doi: 10.1016/s0092-8674(00)81824-3. [DOI] [PubMed] [Google Scholar]
- Sirakova T, Kolattukudy PE, Murwin D, Billy J, Leake E, Lim D, DeMaria T, Bakaletz L. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect Immun. 1994;62:2002–2020. doi: 10.1128/iai.62.5.2002-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swords WE. Nontypeable Haemophilus influenzae biofilms: role in chronic airway infections. Frontiers in cellular and infection microbiology. 2012;2:97. doi: 10.3389/fcimb.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorregaard M. Informatics and Mathematical Modeling. Kongens Lyngby, Denmark: Technical University of Denmark (DTU); 2008. COMSTAT2 – a modern 3D image analysis environment for biofilms. [Google Scholar]


