Abstract
Purpose
The purpose of this study was to measure the prevalence and configuration of dependent loops in urinary drainage systems in hospitalized, catheterized adults.
Subjects
The study sample was 141 patients with indwelling urinary catheters. Subjects were hospitalized at an academic health center in northern Florida.
Methods
We measured the prevalence of dependent loops in urine drainage systems and the incidence of urine-filled dependent loops over a 3 week period. We measured the heights of the crest (Hc), trough (Ht), and, when urine-filled dependent loops were present, the patient-side (Hp) and bag-side (Hb) menisci with a laser measurement system. All variables were measured in centimeters.
Results
The majority (85%) of observed urine drainage systems contained dependent loops in the drainage tubing and 93.8% of the dependent loops contained urine. Hc and Ht averaged 45.1 ± 11.1 and 27 ± 16.7 cm, respectively. Meniscus height difference (Hb − Hp) averaged 8.2 ± 5.8 and −12.2 ± 9.9 cm when Hp < Hb (65.3%) and Hp > Hb (32.7%), respectively.
Conclusions
We found that dependent loops are extremely common in urinary drainage systems among hospitalized patients despite manufacturer recommendations and nursing and hospital policies. Maintaining the urine drainage tubing free of dependent loops would require incorporation into nursing care priorities and workflow as inadvertent force on the tubing, e.g., patient movement or nurse contact can change tubing configuration and allow excess drainage tubing to re-form a dependent loop.
Introduction
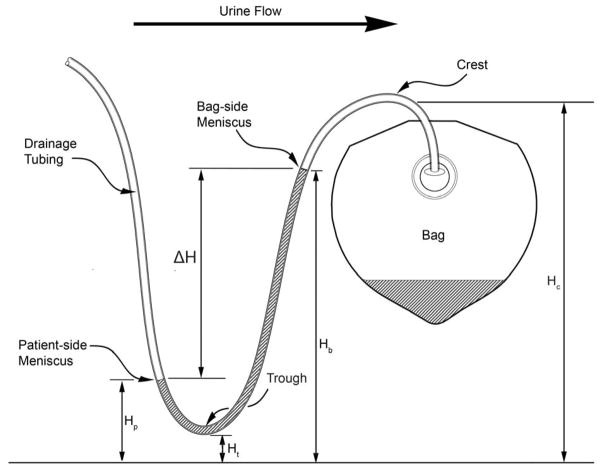
A dependent loop is formed by excess drainage tubing in a urine drainage system where urine or liquid can accumulate (Figure 1). Dependent loops trap drained urine and are suspected of impeding bladder drainage and increasing the residual volume of retained urine in the bladder1. Dependent loops have been associated with an odds ratio of 2.1 for developing catheter-associated urinary tract infection (CAUTI).2,3 Our hospital’s procedure manual, 4 CAUTI prevention guidelines from the Wound, Ostomy and Continence Nurses Society,5 and manufacturer instructions for the urine drainage system used at our hospital6 recommend avoiding dependent loops in urine drainage tubing.
FIGURE 1.
A stylized urine-filled dependent loop depicting the dimensions measured during the study.
Urinary tract infections are the most frequent, accounting for 60% of device-associated nosocomial infections in clinical settings7. The overwhelming majority of hospital acquired urinary tract infections (80%–90%) are caused by urinary catheters. The Centers for Medicare and Medicaid Services have classified hospital-acquired CAUTI as a “never event” for which hospitals will not receive additional reimbursement to cover the cost of therapy. A never event is generally defined as one that should not occur in the course of ordinary evidence-based care.8
Our hospital policy includes surveillance for CAUTI as part of routine quality metrics using the standard methodology for determination of CAUTI as prescribed by the National Health Safety Network.9 Rates are calculated as occurrences per 1,000 catheter days and are reported for every inpatient unit. Intensive care units have the highest catheter use rates. In 2012, NHSN reported that over 80% of patients in general adult medical surgical intensive care units and 88% of patients in neurosurgical intensive care units have an indwelling urinary catheter7, which is consistent with historical utilization data from our own institution. Our internal data also reveal CAUTI rates above the National Health Safety Network 25th percentile. Medical/surgical units have an average of 4 to 7 patients per day with an indwelling catheter. Current national and local strategies to decrease the incidence of CAUTI have focused primarily on decreasing the length of time patients have a catheter.10
Avoidance of urine-filled dependent loops, as a factor that can potentially increase the likelihood of developing a urinary tract infection, is not consistently recommended for prevention of CAUTI10. Our institution has adopted avoidance of dependent loops based on current best evidence, but this has been challenging to implement as every change in patient position alters tubing position. Beyond removal of the urinary catheter as soon as clinically indicated, routine urinary catheter care to decrease CAUTI has focused on catheter securement to prevent movement up and down the urethra, placement of the urinary drainage bag below the level of the bladder, and basic perineal hygiene. These care strategies, along with early catheter removal, have been somewhat successful in decreasing CAUTI at our hospital (an overall 30% drop in the rate from 3.91/1,000 catheter days in fiscal year (FY) 2010 to 2.73/1,000 catheter days in FY 2011) but we achieved no further gains in reduction of the CAUTI incidence in FY 2012.
Based on these data, we elected to explore additional strategies to further reduce CAUTI incidence using our prevention bundle. One of the strategies we explored was the presence of urine filled dependent loops in the drainage systems of patients with indwelling urinary catheters. As noted earlier in this article, a literature search found inadequate evidence to determine a causative link between urine-filled dependent loops and an increased risk of CAUTI. Before investing the time and effort to examining whether there is a causal relationship between dependent loops and CAUTI, we elected to determine the prevalence of dependent loops among patients with indwelling urinary catheters, which was the goal of this clinical study.
Methods
We conducted a prevalence study over 2 consecutive days and completed one additional study day 2 weeks later. All data were collected in a large academic health center in the southeastern United States. The study sample comprised all patients 18 years or older who had an indwelling catheter in place and were in an adult intensive care unit, intermediate care unit, or a medical/surgical unit and were identified from a hospital electronic database. Research nurses notified the charge and patient nurses that data and measurements would be collected, but the catheters would not be manipulated. If the nurse indicated the patient was alert or had family present, the research nurse approached the patient or family, explained the study procedure, and elicited verbal consent to collect the measurements. If the patient or family declined to participate, the patient was not included in the study. If the patient was not alert and the family was not present, only the patient data that did not require any physical contact was collected (height measurement for specified locations in the urinary drainage system). No protected health information was collected. No patient was identifiable once the measurements were obtained. Measuring prevalence requires sampling of “what is” from a population describing the proportion of the population having a condition. As we were not collecting PHI, the study methodology was planned to include all patients during the study period having a urinary catheter where the tubing configuration could be assessed. Some of these patients were cognitively unable to consent for the study and no family were present. To more completely represent the population of interest, we did not wish to eliminate these patients from the study. Research procedures were reviewed and approved by the University of Florida Institutional Review Board.
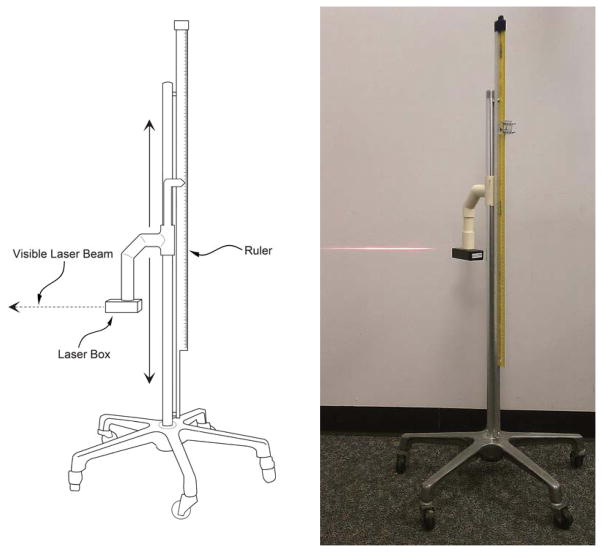
Research nurses examined patients with indwelling urinary catheters to determine the presence or absence of dependent loops in the urinary drainage tubing configuration and whether loops were fluid filled. All patients were in bed or on stretchers when assessed for the study. Data from experiments we have conducted with a simulated bladder model indicate that the heights of the menisci formed by the urine in the dependent loop may be relevant to urine drainage1, and we applied these findings to this study. We first identified whether dependent loops were present and fluid filled, followed by measurement of fluid meniscus height elevations using a laser leveling device (Figure 2). Specifically, research nurses obtained the following study measurements: 1) presence or absence of dependent loops, 2) height of the bed/surface, crest and trough of dependent loops, and, 3) if urine was present in the dependent loop, the patient-side and bag-side meniscus heights for each fluid meniscus. Current best practices require that urinary drainage tubing not rest on the floor, as contamination of collection tubing or drainage bag is associated with an increased risk of CAUTI due to migration of organisms up the tubing to the patient.10 We also recorded the number of cases where the urine drainage tubing was touching the floor and the type of unit (ICU vs medical-surgical unit) for each subject.
FIGURE 2.
The laser system we used for the measurement of heights without having to be in direct contact with the patient. The visible laser beam is projected on the wall in the photograph on the right.
Study Equipment and Measurement Procedures
Meniscus elevations are hard to read without parallax error, especially when the meniscus is close to the ground. We designed and built a laser height measurement system to facilitate and expedite contactless measurement to minimize inconvenience and the risk of patient infection from conducting the measurements (Figure 2). We adapted a laser leveling device (BDL220S; Black & Decker, Towson, MD; low power 2.2 mW, 633–670 nm, Class 2 laser product) commonly used to project a horizontal red line on a wall, e.g., to hang pictures level) and attached it on a slider that travels up and down the vertical shaft of a mobile IV pole. The horizontal visible beam from the laser leveling device was vertically adjusted (by sliding along the IV pole) until the visible beam impinges the meniscus or location whose elevation is desired. The laser leveling device can be aimed to impinge at any elevation from almost floor level (~1 cm) to 83 cm (2.7 ft). A Class 2 laser is similar to laser pointers used during class lectures and is safe during normal use15. A meter ruler (with mm graduations) is mounted parallel to the IV pole and vertically offset 35.5 cm higher than the laser leveling device so that the user did not have to stoop down to the floor to read the ruler in instances where the menisci levels are close to the floor (Figure 2).
To measure the difference in meniscus elevation, the laser beam was set to impinge the higher level meniscus and the elevation was read from the ruler and recorded into a mobile laptop computer running Excel (Microsoft, Redmond, WA). The visible beam was next aimed at the lower meniscus and the elevation was again read from the ruler and entered into the laptop.
The elevation of the crest (Figure 1) of the bag-side limb of the dependent loop was measured. The crest elevation determines the maximum bag-side meniscus height in the event of a urine-filled dependent loop that is never emptied. The proximity of the bag-side meniscus to the crest provides an indirect measure of the time elapsed since the last time the dependent loop was emptied. The height of the trough of the dependent loop was also measured and recorded, as well as the elevation of the bed. Subjects with a bed or tubing height outside of the measurement capabilities of the equipment (>83 cm high) were excluded from analysis.
The urine drainage systems used in patients were pre-connected closed-system Foley trays (all-silicone 400 series temperature-sensing, anti-microbial Foley catheter with hydrogel and Bacti-Guard silver alloy coating; bacteriostatic drainage tube and bag; REF 319516AM, CR Bard, Inc., Covington, GA). The specific catheter size was not included in the data collection for this study; however, our institution’s standard catheter size during the study period was 16 French. The standard size was subsequently changed to 14 French in 2012.
Results
Of 141 hospitalized patients who were observed, valid data sets were collected on 133 patients. A dependent loop was present in 113 of 133 (85%) of the urine drainage systems. One hundred six of the 113 (93.8%) drainage systems with dependent loops contained urine at the time of observation (Table 1). The percentage of urine drainage systems with dependent loops varied between the 15 different hospital locations where the data were collected, ranging from 100% to 86.1%.
TABLE 1.
Prevalence of Urine in Urinary Drainage Tubing Dependent Loop
| Urine Drainage Systems Observed | Systems with Dependent Loops | No Urine in Dependent Loop | Urine in Dependent Loop | % Dependent Loops with Urine | |
|---|---|---|---|---|---|
| ICU | 95 | 79 | 7 | 72 | 91.14% |
| IMC | 9 | 9 | 9 | 100% | |
| MS | 29 | 25 | 25 | 100% | |
| Total | 133 | 113 | 7 | 106 | 93.81% |
ICU, intensive care unit; IMC, intermediate medical care; MS, medical/surgical
Of 106 urine drainage systems with trapped urine noted in the dependent loops, 4 systems had heights exceeding 83 cm, the upper range of the laser measurement system, and another 4 systems had incomplete data sets, leaving 98 urine drainage systems with urine-filled dependent loops and complete data sets. Of these 98 systems, the bag-side meniscus height (Hb) was higher, the same, and lower than the patient-side meniscus (Hp) in 64, 2, and 32 patients, respectively. The difference in meniscus height (ΔH = Hb − Hp) was 8.2 ± 5.8 cm (range 0.2–25.9 cm; median 7.6 cm) when Hb > Hp (65.3%) and −12.2 ± 9.9 cm (range −0.2 to −39 cm; median −8.8 cm) when Hb < Hp (32.7%; Table 2).
TABLE 2.
Characteristic Heights in Dependent Loops
| Hc | Ht | Bed Height | Hb | Hp | Hb − Hp (Hb > Hp) | Hb − Hp (Hp > Hb) | Hp = Hb | |
|---|---|---|---|---|---|---|---|---|
| Average (cm) | 45.13 | 27.77 | 71.94 | 39.52 | 38.38 | 8.22 | −12.24 | 0 |
| SD (cm) | 11.13 | 16.72 | 11.98 | 15.19 | 18.01 | 5.84 | 9.87 | 0 |
| Minimum (cm) | 25.2 | 0 | 47.5 | 25.2 | 2.9 | 0.2 | −0.2 | 0 |
| Maximum (cm) | 93.7 | 83 | 97 | 89 | 83 | 25.9 | −39 | 0 |
| Median (cm) | 43.1 | 27.3 | 72.62 | 38.1 | 36.8 | 7.55 | −8.8 | 0 |
| Percent of subjects | 65.3% | 32.7% | 2% | |||||
| N | 110 | 110 | 73 | 102 | 99 | 64 | 32 | 2 |
SD, standard deviation; Hc, crest height; Ht, trough height; Hb, bag-side meniscus height; Hp, patient-side meniscus height
The crest height (Hc) was 45.1 ± 11.1 cm (median 43.1 cm). The bottom of the loop touched the floor in 6 of 113 (5.3%) drainage systems with dependent loops. The height of the trough of the dependent loop (Ht) was 27 ± 16.7 cm. The bed height was 72.6 ± 11.98 cm in 73 subjects where this measurement was obtained.
Discussion
Existing urine drainage resources do not consistently address management of dependent loops in urine drainage systems5,12,13. For example, 2 online resources (Up To Date® and Mosby Skills®) do not mention dependent loops in urine drainage systems.12,13 A CAUTI Fact Sheet from the Wound, Ostomy and Continence Nurses Society recommends “Keep the collection device below the level of the bladder/tubing.”5 This recommendation does not explicitly mention or offer guidance concerning management of dependent loops.
Additionally, our hospital’s Prevention of Urinary Tract Infections (UTI) Policy and Procedure document4 states: “Place the bags at the foot of the bed in order to avoid dependent loops and enhance visibility.” Again, there are no figures or photographs to depict a “dependent loop” to those unfamiliar with that term; furthermore, placing the bag at the foot of the bed does not in and of itself guarantee that there will be no dependent loop. Manufacturer instructions for use from Bard (C. R. Bard Inc. Covington GA) user instructions6 emphasize: “Important: Position drainage tube in a straight fashion from catheter to drainage bag”. We believe that this instruction may not be especially helpful if the reader is not aware that this instruction relates to dependent loops. The 85% prevalence of dependent loops in urine drainage systems of hospitalized patients found in this study clearly indicates poor adherence to policy or instruction or lack of awareness of such by hospital personnel.
Those catheter care protocols that do include instructions to the nurse to place the urine collection bag at the end of the patient bed to avoid dependent loops do nothing further to address the potential significance of a dependent loop or the relationship of a dependent loop to urine retention and the reported association with CAUTI. This observation may point to the need for education and training with respect to dependent loops.
Garcia and colleagues14 measured residual volumes in catheterized patients using transabdominal bladder volumetric ultrasonography with a Diagnostic Ultrasound 3.5 MHz transducer. In 75 intensive care and 75 general medical/surgical patients with urinary catheters, mean retained urine volumes were 96 mL (range 4–290 mL) and 136 mL (range 22–647 mL), respectively. Although the Garcia study did not measure the prevalence of dependent loops or meniscus height differences in urine-filled dependent loops, the authors postulated that the dependent loops may have contributed to the observed urine retention in catheterized patients. Bedside bladder ultrasonography is routinely used in non-catheterized patients, but not in catheterized patients. The Garcia findings suggest that if dependent loops cannot be avoided in routine clinical practice and if retained urine volume is equally undesirable in catheterized and non-catheterized patients, then bedside bladder ultrasonography may also be indicated for catheterized patients. Additional study is needed to determine relationships among optimum urinary drainage, management of dependent tubing, and positioning of the urinary drainage bag with respect to the indwelling urinary catheter.
Limitations
The data collected from this prevalence study are from a single institution. However, preliminary data from an online poll conducted at the time of writing in collaboration with the Anesthesia Patient Safety Foundation appear consistent with results of our prevalence study. There is currently insufficient evidence to conclude that the presence of dependent loops is associated with retained urine volume in catheterized bladders and an increased risk for CAUTI. The clinical relevance of our prevalence study is dependent on these findings. Therefore, additional research is urgently needed to determine whether the presence of dependent loops is associated with a statistically significant and clinically relevant increase in retained urine volume in the bladder of patients with indwelling urinary catheters and whether dependent loops are associated with an increased risk of CAUTI.
Conclusions
This prevalence study clearly demonstrated the high likelihood of a dependent loop in the urine drainage tubing of patients with indwelling urinary catheters. Dependent loops frequently contained urine, which may be associated with retained urine in patients with indwelling catheters and increase their risk for development of CAUTI. Further studies are urgently needed to describe the relationship between dependent loops and bladder volume in catheterized patients. Additional study is needed to find effective solutions for the prevention of dependent loop formation or mitigation of the undesired effects of dependent loops.
Supplementary Material
Acknowledgments
Supported in part by the National Institutes of Health (NIH) and the National Center for Research Resources (NCRR) CTSA grant (no. 1UL1RR029890).
The authors thank the UF Clinical Research Center and the UF Clinical and Translational Science Institute for supporting this study, and Teresa d’Angelo, Christel Gross, and Marlene Sarmiento for helping with data collection.
Contributor Information
Gale Danek, Administrative Director of Nursing Research, Shands Hospital at the University of Florida, Gainesville, Florida.
Nikolaus Gravenstein, The Jerome H. Modell, MD, Professor of Anesthesiology, Department of Anesthesiology, University of Florida College of Medicine, and Center for Safety, Simulation & Advanced Learning Technologies, University of Florida, Gainesville, Florida.
David E. Lizdas, Simulation Engineer, Department of Anesthesiology, University of Florida College of Medicine, and Center for Safety, Simulation & Advanced Learning Technologies, University of Florida, Gainesville, Florida.
Samsun Lampotang, Professor of Anesthesiology and Director, Center for Safety, Simulation & Advanced Learning Technologies, University of Florida College of Medicine, Gainesville, Florida.
References
- 1.Schwab WK, Lizdas DE, Gravenstein N, Lampotang S. Foley drainage tubing configuration affects bladder pressure: a bench model study. Urologic Nursing J. 2014;34(1):33–37. doi: 10.7257/1053-816X.2014.34.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Maki DG, Knasinski V, Tambyah PA. Risk factors for catheter-associated urinary tract infection (CAUTI): a prospective study showing the minimal impact of catheter care violations on the risk of CAUTI. Infect Control Hosp Epidemiol. 2000;21:165. [Google Scholar]
- 3.Maki DG, Tambyah PA. Engineering out the risk of infection in urinary catheters. Emerging Infect Dis. 2001;7:1–6. doi: 10.3201/eid0702.010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shands at the University of Florida Department of Nursing and Patient Services. Prevention of Urinary Tract Infections (UTI) Policy and Procedure, Policy Number GU-008. Oct, 2011. [Google Scholar]
- 5.Wound Ostomy and Continence Nurses Society. [Accessed on November 11, 2011];Catheter Associated Urinary Tract Infections (CAUTI): Fact Sheet. doi: 10.1097/01.WON.0000347656.94969.99. http://www.wocn.org/resource/resmgr/cauti_fact_sheet.pdf. [DOI] [PubMed]
- 6.CR Bard, Inc. Bard Infection Control Urinary Drainage Bag, Publication PK7608603 2/06. 2006. [Google Scholar]
- 7.Dudeck MA, Weiner LM, Allen-Bridson K, Malpiedi PJ, Peterson KD, Pollock DA, Sievert DM, Edwards JR. National Healthcare Safety Network (NHSN) report, data summary for 2012, Device-associated module. Am J Infect Control. 2013;41:1148–1166. doi: 10.1016/j.ajic.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services. [Accessed on November 18, 2011];Document SMDL #08-004. 2008 Jul 31; http://www.cms.gov/SMDL/downloads/SMD073108.pdf.
- 9.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA Healthcare Infection Control Practices Advisory Committee (HICPAC) Guideline for prevention of catheter-associated urinary tract infections. Centers for Disease Control and Prevention; 2009. [Accessed on November 2, 2011]. at http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf. [Google Scholar]
- 11.Hooton TM, Bradley SF, Cardenas DD, Colgon R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE Infectious Diseases Society of America. Diagnosis Prevention and Treatment of Catheter-Associated Urinary Tract Infection in Adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 12.Up To Date, Inc. [Accessed on November 17, 2011]; http://www.uptodate.com/contents/search.
- 13.Cohen K, Toppy SC, Jeter J, Owens VP, editors. Mosby’s Nursing Skills. Elsevier; [Accessed on December 1, 2011]. http://confidenceconnected.com/products/mosbys_nursing_skills/nurses/ [Google Scholar]
- 14.Garcia MM, Gulati S, Liepmann D, Stackhouse GB, Greene K, Stoller ML. Traditional Foley drainage systems: do they drain the bladder? J Urol. 2007;177:203–207. doi: 10.1016/j.juro.2006.08.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.