Abstract
Background: Determining the etiology of biliary strictures is challenging, and the sensitivities of the current tests to diagnose them are low. Protein biomarkers in bile, in combination with other tests, may improve sensitivity in diagnosing biliary strictures.
Objective: To analyse the differential abundance of proteins in benign and malignant biliary strictures through proteomic analysis of bile.
Methods: In this prospective, cross-sectional study, bile was aspirated in 24 patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) including six patients with primary sclerosing cholangitis (PSC), three with cholangiocarcinoma (CCA), ten with pancreatic cancer, and five with benign biliary conditions. Liquid chromatography/mass spectrometry was used to examine the bile for differential abundance of protein biomarkers. The relative abundance of various proteins was compared in the malignant vs. benign groups and in CCA vs. PSC.
Results: The majority of the proteins identified in bile were similar to those of the plasma (plasma proteins) and certain proteins were differentially expressed among the different groups (CCA, pancreatic cancer, PSC or benign). A total of 18 proteins were identified as being more abundant in the malignant group (CCA and pancreatic cancer) than in the benign strictures group, including myeloperoxidase, complement C3, inter-alpha-trypsin inhibitor heavy chain H4, apolipoprotein B-100, and kininogen-1 isoform 2. A total of 30 proteins were identified to be less abundant in the malignant group than in the benign group, including trefoil factor 2, superoxide dismutase [Cu-Zn], kallikrein-1, carboxypeptidase B and trefoil factor 1.
Conclusions: Protein biomarkers in bile may differentiate malignant from benign biliary strictures. Larger studies are warranted to validate these observations.
Keywords: pancreatic cancer, proteomics, bile, cholangiocarcinoma
Introduction
Biliary strictures can be due to benign or malignant causes. Cholangiocarcinoma (CCA) and pancreatic cancer account for the majority of malignant biliary strictures, and are often associated with grave prognosis at the time of diagnosis [1, 2]. Detecting malignancies at an earlier stage is of paramount importance for effective management. Diagnosing malignant strictures at an early stage remains very challenging, especially when there is no image evidence of a mass lesion (indeterminate biliary stricture).
Endoscopic retrograde cholangiopancreatography (ERCP) with brush cytology and endoscopic ultrasound (EUS) with fine-needle aspiration (FNA) are the usual modalities for diagnosing biliary strictures. EUS-FNA is highly sensitive and superior to ERCP cytology in diagnosing malignant biliary strictures associated with pancreatic mass lesions, yet its sensitivity is similar to brush cytology for diagnosing indeterminate strictures in the setting of CCA [3]. Also, the sensitivities of diagnosing malignant lesions through ERCP brushings in the published studies is low [4–6]. Although the addition of Fluorescence in situ hybridization (FISH) to cytology improves the sensitivity rates of ERCP brushings [7–9], the combined sensitivity is still low in detecting malignant strictures. Lower diagnostic sensitivities of the current techniques to detect malignant strictures warrant additional methodologies to improve the diagnosis.
Use of proteomics to detect biomarkers in bile may hold promise in aiding differentiation of malignant from benign biliary strictures [10]. Previous studies have attempted to study bile proteomics to identify peptides that will differentiate malignant from benign biliary strictures [11–15]. Most of the studies are limited because of lack of co-existing clinical information and a small sample size. The main aim of our study was to analyse the differential abundance of proteins in benign vs. malignant biliary strictures.
Methods
Patients
The Cleveland Clinic biliary fluid database is a prospectively maintained database of bile obtained by direct aspiration from the common bile duct in patients referred to our center for ERCP. We established this database in 2012 and included all patients in our center who had bile aspirated prior to contrast injection at the time of ERCP. The study was approved by the Cleveland Clinic Institutional Review Board and registered with the National Institute of Health (NIH) clinical trials registry. (NCT01565460) The patients included in our study were recruited between September 2012 and November 2012 and had a minimum of 1 year of clinical follow-up. Informed consent was obtained from each patient included in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the institution's human research committee.
Inclusion and exclusion criteria
The inclusion criteria were ability to give informed consent and age >18 years. Patients who had acute cholangitis were not included in our biliary fluid database. The diagnosis of pancreatic cancer and CCA was based on tissue diagnosis, either at surgery or on fine needle aspiration on subsequent endoscopic ultrasound on follow-up. Tissue diagnosis was established, based on histology in all patients with cancer in our cohort.
Biliary fluid sampling procedure
At the time of ERCP, once we had cannulated the common bile duct, approximately 1 to 5 mL of bile was aspirated through the sphincterotome. We transported these bile samples to the laboratory on ice; they were then frozen at –80°C until use.
Measurement of protein/peptides in bile
Bile samples were fractionated on a sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel. Each sample was divided into three bands and analysed by liquid chromatograph mass spectrometer (LC-MS) or MS. To determine the protein content, dilutions were made to several samples in an attempt to try and equalize the overall amount of protein present in the SDS-PAGE gel. Several gels were run and the bands were cut from each gel. The protein bands were digested according to an in-gel digestion procedure. Briefly, the bands were cut from the gel and washed in 50% ethanol/ 5% acetic acid, alkylated with iodoacetamide and reduced with Dithiothreitol (DTT). All bands were completely digested ‘in-gel' using trypsin, by adding 5 μL trypsin (10 ng/μL) in 50 mmol/L ammonium bicarbonate and incubating overnight at room temperature. The peptides that were formed were extracted from the polyacrylamide in two aliquots of 30 μL 50% acetonitrile with 5% formic acid. These extracts were combined and evaporated to <10 μL in Speedvac (Thermo Fischer Scientific, San Jose, CA, USA) and then suspended in 1% acetic acid to make up a final volume of 30 μL for LC-MS analysis.
The LC-MS system was a Finnigan LTQ-Obitrap Elite hybrid mass spectrometer system. The HPLC column was a Dionex 15 cm × 75 μm. Acclaim Pepmap C18, 2 μm, 100 Å reversed phase capillary chromatography column. The extract was injected in 5 μL volumes and the peptides, eluted from the column by an acetonitrile/0.1% formic acid gradient at a flow rate of 0.25 μL/min, were introduced into the source of the mass spectrometer on-line. The microelectrospray ion source was operated at 2.5 kV. The digest was analysed using the data-dependent multitask capability of the instrument, acquiring full scan mass spectra, to determine peptide molecular weights and product ion spectra to determine amino acid sequence in successive instrument scans.
Database searching
Tandem mass spectra were extracted by Proteome Discoverer software, version 1.4.1.288. Charge state de-convolution and de-isotoping were not performed. All LC-MS/MS samples were analysed using Mascot (Matrix Science, London, UK; version 2.3.02), Sequest (Thermo Fisher Scientific, San Jose, CA, USA; version 1.4.0.288) and X! Tandem (The GPM, thegpm.org; version CYCLONE (2010.12.01.1)). Mascot, Sequest, and X! Tandem were set up to search the Human Reference Sequence database (33292 entries) assuming the digestion enzyme trypsin, fragment ion mass tolerance of 1.2 Da and a parent ion tolerance of 20 PPM. Carbamidomethyl of cysteine was specified as a fixed modification and oxidation of methionine was specified as a variable modifications.
Criteria for protein identification
Scaffold software (version Scaffold_4.3.2; Proteome Software Inc., Portland, Oregon, USA) was used to validate LC-MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability by the Peptide Prophet algorithm [16]. Protein identifications were accepted if they could be established at greater than 99.0% probability to achieve a false discovery rate (FDR) of less than 1.0% and contained at least two identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm [17]. Proteins that contained similar peptides and could not be differentiated based on LC-MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Comparison of malignant and benign biliary strictures
The relative abundance of the proteins was compared by using the label-free quantitative method, spectral counting [18]. This method utilizes a data-dependent analysis, which involves an initial mass scan followed by 20 LC-MS/MS scans on the most abundant peptides. The selection of peptide ions for LC-MS/MS analysis was based on the abundance of the peptides and, therefore, the more abundant the protein in the sample the more often peptides from this protein were selected for LC-MS/MS analysis. The relative quantity of these proteins was determined by comparing the number of spectra (termed ‘spectral counts'), used to identify each protein. The numerical values used in the quantification correspond to the normalized spectral abundance factor (NSAF; SC/ΣSC*protein length) [19]. The error observed for the SC measurements is greater for less-abundant proteins than for more-abundant proteins. Because of this, different filtering criteria were used to determine whether proteins are differentially present, based on the overall abundance, as an NSAF ratio.
The schematic representation of the proteomics approach to identifying proteins in each bile sample is shown in Figure 1. The error rating of the label-free method used in this analysis is greater for less-abundant proteins and we therefore utilized different filtering criteria for proteins of varying abundance levels (Table 1) [20]. The relative abundances of the proteins in these samples was determined by spectral counts and different cut-off values and P-values. Based on the criteria, the relative abundances of 304, 425, and 459 proteins were compared in the CCA vs. PSC, and malignant (pancreatic cancer and CCA) vs. benign (PSC and benign samples) respectively as set out below.
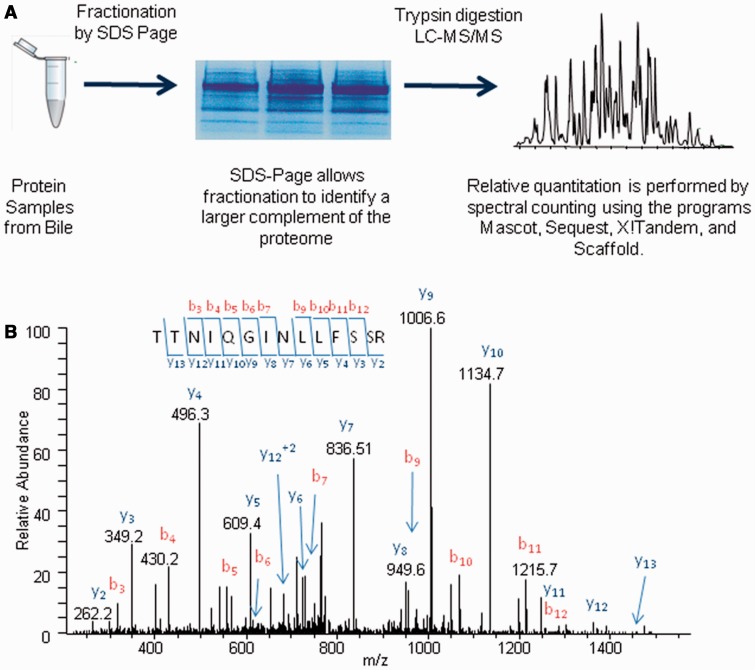
Figure 1.
Schematic representation of the proteomic approach used to identify and quantify proteins from bile samples. These experiments involve bile protein isolation, SDS-page fractionation, tryptic digestion, and LC-MS/MS analysis. The resulting data were searched with the programs Mascot, Sequest, and X!Tandem and the proteins were quantified by spectral counts (A). An example MS/MS spectrum from the doubly charged 782.4 Da peptide identified in the LC-MS/MS analysis of a cholangiocarcinoma sample. The amino acid sequence of this peptide was identified as TTNIQGINLLFSSR by the presence of several sequence specific N (b) and C-terminal (y) ions. This peptide was matched to the protein complement C4-A isoform 1, which was found to be more abundant in cancer than in control (B).
Table 1.
Criteria used to determine whether proteins are differentially present
| Abundance | SC range | NSAF ratio | P-value |
|---|---|---|---|
| Very low | 1.7–7 | 0.4 ≤ NSAF ratio ≥ 2.5 | ≤0.01 |
| Low | 8–19 | 0.5 ≤ NSAF ratio ≥ 2.0 | ≤0.01 |
| Medium | 20–79 | 0.5 ≤ NSAF ratio ≥ 2.0 | ≤0.05 |
| High | 80+ | 0.67 ≤ NSAF ratio ≥ 1.5 | ≤0.05 |
SC = spectral count; NSAF = normalized spectral abundance factor
Results
A total of 24 bile samples were analysed, including six patients with PSC, three with CCA, ten with pancreatic cancer, and five with benign biliary conditions. The patients were recruited from September 2012 to November 2012. Among the five patients with benign biliary conditions, three had sphincter stenosis, one had chronic pancreatitis, and one had benign stricture secondary to biliary stones. All the six patients with PSC had both intrahepatic and extrahepatic PSC. None of the patients had any dominant strictures. Of the three patients with CCA, one had hilar strictures, while the remaining two had distal biliary stricture. All three presented with indeterminate biliary strictures. All patients had a minimum follow-up of at least 1 year up to November 2013. The basic demographic information is summarized in Table 2.
Table 2.
Demographic and clinical characteristics of study cohort
| Variables | Benign biliary conditions (n = 5) | Cholangiocarcinoma (n = 3) | Pancreatic cancer (n = 10) | Primary sclerosing cholangitis (n = 6) |
|---|---|---|---|---|
| Age, mean (SD); years | 44.0 (13.7) | 78.0 (7.1) | 72.1 (8.2) | 59.8 (15.0) |
| Female/male (n) | 5/0 | 1/2 | 3/7 | 2/4 |
| Albumin, mean (SD); mg/dL | 3.3 (1.9) | 3.4 (0.7) | 3.3 (0.7) | 2.6 (2.0) |
| Bilirubin, mean (SD); mg/dL | 0.42 (0.41) | 1.3 (0.9) | 5.9 (6.4) | 1.1 (1.2) |
| AKP, mean (SD); U/L | 66.6 (37.0) | 161 (69.1) | 505.2 (296.7) | 224.3 (214.4) |
| AST, mean (SD); U/L | 26.8 (16.8) | 44.7 (23.1) | 94.1 (63.4) | 42.3 (39.7) |
| ALT, mean (SD); U/L | 28.2 (16.8) | 33.0 (4.9) | 136.7 (131.9) | 49.2 (45.8) |
| CA 19-9, mean (SD) | NA | 85.8 (61.7) | 2591.7 (1191.2) | 958 (817.3) |
AKP = alkaline phosphatase; AST = aspartate aminotransferase; ALT = alanine aminotransferase; NA = not available
Each of the 24 bile samples was subjected to SDS-PAGE fractionation, tryptic digestion and LC-MS/MS analysis for both protein identification and relative protein quantification. Each gel lane was cut into three areas and analysed by a 120-minute LC gradient. The LC-MS/MS experiments identified a total of 459 proteins by at least two peptides. The overall FDR for this analysis was less than 1%.
Malignant vs. benign strictures
A total of 459 proteins were quantified in the malignant and benign groups. The identification of proteins of different abundance was based on the criteria given in Table 1. Eighteen proteins (4%) were identified as more abundant in the cancer samples, including such proteins as MPO, complement C3, inter-alpha-trypsin inhibitor heavy chain H4, apolipoprotein B-100, and kininogen-1 isoform 2. Thirty proteins (7%) were identified as less abundant in cancer and included trefoil factor 2, superoxide dismutase [Cu-Zn], kallikrein-1, carboxypeptidase B and trefoil factor 1. The details were summarized in Tables 3 and 4.
Table 3.
Proteins identified as more abundant in cancer (malignant strictures) than in controls (primary sclerosing cholangitis and benign strictures)
| Proteins | Accession | MW (kDa) | Average SC |
NSAF ratio | P-value (t-test) | |
|---|---|---|---|---|---|---|
| Cancer | Control | |||||
| Fibrinogen beta chain isoform 1 pre-pro-protein | 70906435 | 56 | 138.8 | 52.1 | 2.2 | 0.01481 |
| C4b-binding protein alpha chain precursor | 4502503 | 67 | 37.3 | 11.5 | 2.3 | 0.04311 |
| Serum albumin pre-pro-protein | 4502027 | 69 | 986.0 | 332.8 | 2.4 | 0.00006 |
| Vitamin D-binding protein isoform 1 precursor | 32483410 | 53 | 51.9 | 19.2 | 2.4 | 0.03919 |
| Kininogen-1 isoform 2 precursor | 4504893 | 48 | 21.3 | 5.5 | 3.8 | 0.01109 |
| Alpha-2-macroglobulin precursor | 66932947 | 163 | 127.9 | 29.3 | 3.9 | 0.00618 |
| Haptoglobin isoform 1 pre-pro-protein | 4826762 | 45 | 83.8 | 18.0 | 4.2 | 0.00024 |
| Fibrinogen gamma chain isoform gamma-B precursor | 70906439 | 52 | 89.3 | 17.3 | 4.3 | 0.00133 |
| Complement C3 precursor | 115298678 | 187 | 174.7 | 35.6 | 4.3 | 0.00612 |
| Myeloperoxidase precursor | 4557759 | 84 | 70.2 | 11.0 | 5.3 | 0.02501 |
| Apolipoprotein A-I pre-pro-protein | 4557321 | 31 | 29.8 | 5.9 | 5.7 | 0.02170 |
| Fibrinogen alpha chain isoform alpha-E pre-pro-protein | 4503689 | 95 | 63.3 | 9.3 | 6.4 | 0.00031 |
| Alpha-2-HS-glycoprotein pre-pro-protein | 156523970 | 39 | 7.3 | 1.1 | 6.5 | 0.00173 |
| Ceruloplasmin precursor | 4557485 | 122 | 47.3 | 5.6 | 7.5 | 0.00114 |
| PREDICTED: complement C4-A isoform 1 | 341916194 | 193 | 32.1 | 4.5 | 7.7 | 0.01791 |
| Alpha-1B-glycoprotein precursor | 21071030 | 54 | 10.9 | 1.2 | 8.3 | 0.00468 |
| Inter-alpha-trypsin inhibitor heavy chain H4 isoform 1 precursor | 31542984 | 103 | 5.5 | 0.2 | 35.4 | 0.00484 |
| Apolipoprotein B-100 precursor | 105990532 | 516 | 49.3 | 1.0 | 55.7 | 0.02595 |
MW = molecular weight; SC = spectral count; NSAF = normalized spectral abundance factor
Table 4.
Proteins identified as less abundant in cancer (malignant strictures) than in controls (primary sclerosing cholangitis and benign strictures)
| Proteins | Accession | MW (kDa) | Average SC |
NSAF ratio | P-value (t-test) | |
|---|---|---|---|---|---|---|
| Cancer | Control | |||||
| Malate dehydrogenase, mitochondrial precursor | 21735621 | 36 | 0.0 | 3.5 | 0.0 | 0.00897 |
| Prostate stem cell antigen pre-pro-protein | 289547757 | 12 | 0.0 | 3.6 | 0.0 | 0.00926 |
| Enteropeptidase precursor | 223942069 | 113 | 0.0 | 2.9 | 0.0 | 0.00964 |
| Kallikrein-1 pre-pro-protein | 4504875 | 29 | 0.0 | 10.3 | 0.0 | 0.00052 |
| Chymotrypsinogen B2 precursor | 118498350 | 28 | 0.0 | 17.6 | 0.0 | 0.00086 |
| Colipase isoform 1 pre-pro-protein | 4502895 | 12 | 0.4 | 25.9 | 0.0 | 0.00009 |
| Chymotrypsin-like elastase family member 3A pre-pro-protein | 236460050 | 29 | 0.5 | 29.1 | 0.0 | 0.00015 |
| Phospholipase A2 precursor | 4505847 | 16 | 1.3 | 39.5 | 0.0 | 0.00018 |
| Superoxide dismutase [Cu-Zn] | 4507149 | 16 | 1.0 | 15.2 | 0.0 | 0.00005 |
| Lithostathine-1-alpha precursor | 29725633 | 19 | 1.6 | 30.3 | 0.1 | 0.00223 |
| Chymotrypsin-like elastase family member 3B pre-pro-protein | 6679625 | 29 | 2.4 | 48.7 | 0.1 | 0.00012 |
| Chymotrypsin-C pre-pro-protein | 62526043 | 29 | 0.9 | 18.6 | 0.1 | 0.00015 |
| Trypsin-1 pre-pro-protein | 4506145 | 27 | 3.1 | 41.2 | 0.1 | 0.00001 |
| Carboxypeptidase B pre-pro-protein | 54607080 | 47 | 5.2 | 79.5 | 0.1 | 0.00020 |
| Trypsin-2 pre-pro-protein | 4506147 | 26 | 2.3 | 31.7 | 0.1 | 0.00412 |
| Trefoil factor 1 precursor | 4507451 | 9 | 0.7 | 4.7 | 0.1 | 0.00868 |
| Carboxypeptidase A1 precursor | 4502997 | 47 | 2.7 | 32.5 | 0.1 | 0.00012 |
| Selenium-binding protein 1 | 16306550 | 52 | 2.8 | 19.1 | 0.1 | 0.00339 |
| Pro-activator polypeptide isoform a pre-pro-protein | 11386147 | 58 | 1.0 | 7.1 | 0.1 | 0.00016 |
| Junction plakoglobin | 12056468 | 82 | 1.7 | 13.5 | 0.1 | 0.00535 |
| Pancreatic secretory granule membrane major glycoprotein GP2 isoform 2 precursor | 119220571 | 59 | 4.5 | 33.7 | 0.1 | 0.00291 |
| Desmoplakin isoform I | 58530840 | 332 | 3.3 | 20.3 | 0.1 | 0.00322 |
| Serpin B6 | 41152086 | 43 | 8.6 | 40.7 | 0.2 | 0.00135 |
| Pancreatic alpha-amylase precursor | 4502085 | 58 | 8.9 | 59.9 | 0.2 | 0.02459 |
| Trefoil factor 2 precursor | 4885629 | 14 | 6.2 | 20.6 | 0.2 | 0.00049 |
| Non-secretory ribonuclease precursor | 4506549 | 18 | 3.6 | 9.7 | 0.3 | 0.00830 |
| Retinol-binding protein 4 precursor | 55743122 | 23 | 10.5 | 26.6 | 0.3 | 0.00201 |
| Hornerin | 57864582 | 282 | 7.8 | 18.7 | 0.4 | 0.00530 |
| Antithrombin- III precursor | 4502261 | 53 | 27.8 | 44.5 | 0.5 | 0.01112 |
MW = molecular weight; SC = spectral count; NSAF = normalized spectral abundance factor
PSC vs. CCA
A total of 304 proteins were quantified in the CCA and PSC groups. Fourteen proteins (6%) were identified as more abundant in cholangiocarcinoma, including such proteins as intercellular adhesion molecule 1, ceruloplasmin, haptoglobulin, complement c3, and -c4b. Nine proteins (3%) were identified as less abundant in cholangiocarcinoma, which included pro-activator polypeptide isoform a pre-pro-protein, carboxypeptidase B, chymotrypsin-like elastase family member 2A, chymotrypsin like elastase family member 3B, and chymotrypsin-C. The details are summarized in Tables 5 and 6.
Table 5.
Proteins identified as more abundant in cholangiocarcinoma than in primary sclerosing cholangitis
| Proteins | Accession | MW (kDa) | Average SC |
NSAF ratio | P-value (t-test) | |
|---|---|---|---|---|---|---|
| CCA | PSC | |||||
| Serum albumin | 4502027 | 69 | 1338.0 | 334.5 | 2.16 | 0.03297 |
| Alpha-2-macroglobulin precursor | 66932947 | 163 | 173.0 | 29.5 | 3.28 | 0.03422 |
| Haptoglobin isoform 1 pre-pro-protein | 4826762 | 45 | 108.7 | 14.7 | 3.79 | 0.02149 |
| Complement C3 precursor | 115298678 | 187 | 269.7 | 31.7 | 4.32 | 0.04534 |
| Ceruloplasmin precursor | 4557485 | 122 | 55.7 | 6.5 | 5.14 | 0.01608 |
| Apolipoprotein A-I pre-pro-protein | 4557321 | 31 | 40.3 | 1.7 | 13.81 | 0.02004 |
| Carbonic anhydrase 1 | 192447430 | 29 | 29.7 | 0.7 | 15.31 | 0.00834 |
| Peroxiredoxin-2 isoform a | 32189392 | 22 | 29.7 | 0.8 | 18.27 | 0.00005 |
| Hemoglobin subunit alpha | 4504345 | 15 | 271.7 | 3.7 | 26.52 | 0.01789 |
| Apolipoprotein B-100 precursor | 105990532 | 516 | 127.7 | 0.0 | PSC only | 0.01591 |
| Hemoglobin subunit delta | 4504351 | 16 | 26.7 | 0.0 | PSC only | 0.00001 |
| Complement C4-B pre-pro-protein | 178557739 | 193 | 52.3 | 0.0 | PSC only | 0.02317 |
| Intercellular adhesion molecule 1 precursor | 167466198 | 58 | 4.7 | 0.0 | PSC only | 0.00097 |
| Peptidyl-prolyl cis-trans isomerase A | 10863927 | 18 | 5.7 | 0.0 | PSC only | 0.00309 |
MW = molecular weight; SC = spectral count; NSAF = normalized spectral abundance factor.
Table 6.
Proteins identified as less abundant in cholangiocarcinoma than in primary sclerosing cholangitis
| Proteins | Accession | MW (kDa) | Average SC |
NSA Fratio | P-value (t-test) | |
|---|---|---|---|---|---|---|
| CCA | PSC | |||||
| Pro-activator polypeptide isoform a pre-pro-protein | 11386147 | 58 | 0.0 | 8.0 | 0.00 | 0.00496 |
| Trypsin-1 pre-pro-protein | 4506145 | 27 | 0.0 | 38.7 | 0.00 | 0.01173 |
| Carboxypeptidase B pre-pro-protein | 54607080 | 47 | 0.0 | 70.2 | 0.00 | 0.02961 |
| Chymotrypsin-like elastase family member 2A pre-pro-protein | 15559207 | 29 | 0.0 | 35.5 | 0.00 | 0.03517 |
| Chymotrypsin-like elastase family member 3B pre-pro-protein | 6679625 | 29 | 0.0 | 43.0 | 0.00 | 0.03623 |
| Chymotrypsin-C pre-pro-protein | 62526043 | 29 | 0.0 | 21.2 | 0.00 | 0.04669 |
| Trefoil factor 2 precursor | 4885629 | 14 | 1.3 | 21.0 | 0.05 | 0.00235 |
| Antithrombin-III precursor | 4502261 | 53 | 10.7 | 43.3 | 0.18 | 0.03221 |
| IgG Fc-binding protein precursor | 154146262 | 572 | 73.7 | 180.2 | 0.33 | 0.00129 |
Discussion
Biliary tract and pancreatic malignancies are often diagnosed at advanced stages, with palliative measures being the major option for management of many cases. If diagnosed early, surgical resection or liver transplantation can offer increased survival. The sensitivities of various modalities for diagnosing malignancies in biliary strictures are currently low. We aimed to differentiate benign from malignant biliary strictures by analysing the bile for proteins using LC-MS/MS. We observed that the majority of the proteins identified in bile were similar to those of the plasma (plasma proteins) and certain proteins were differentially expressed among the different groups (CCA, pancreatic cancer, PSC, or benign). Some of those proteins, such as S100 A8 and S100 A9, which were significantly elevated, and TFF-2, which was significantly decreased in malignant strictures in our study, were found to be associated with tumorigenesis.
Serum CA 19-9 has been used as a routine diagnostic and prognostic tool for pancreatic cancer [21, 22]. Detecting malignancies at an earlier stage through lipidomics and proteomics seems promising, and these techniques are in the preliminary stages of being translated into clinical practice. We earlier reported the value of VEGF-1, oxidized phospholipids and volatile organic compounds in malignant biliary strictures and the results appear promising [23–25].
Tissue proteins are altered during the process of carcinogenesis, and this can occur during the earliest stages of malignancy [26]; these proteins can be detected in the extracellular fluid spaces. Detecting these changes in protein/peptide levels in the ECF can increase the diagnostic sensitivity, especially for early lesions. Since bile is in direct contact with biliary tract epithelial cells, malignant transformation of these cells is likely to affect expression of certain proteins in bile. Bile can be a representative of the products of molecular changes in biliary tract epithelia (novel proteins) as it is in direct contact with them. In an earlier study, proteomic analysis of bile fluid revealed that a larger percentage of proteins were cellular, from surrounding organs/tissues [10]. Many proteins associated with cancer, such as S100A9, MUC 1, NGAL, CEACAM6 and several other biomarkers [15, 27, 28] have been identified in bile in patients with malignant biliary strictures, further suggesting the importance of bile in proteomic analysis. Patients with PSC are at an increased risk of developing biliary tract malignancies [29–31], and multiple ERCPs are performed in those patients for relieving the obstruction in the ducts, as well as to rule out malignancies. Also, obtaining bile during routine ERCPs does not impart any major risk to the patients, beyond the baseline risks of the procedure.
We identified several proteins showing differential abundance. Comparing the overall benign (PSC and benign) and malignant lesions (pancreatic cancer and CCA), several proteins including myeloperoxidase, inter-alpha-trypsin inhibitor heavy chain H4, complement C3, ceruloplasmin, alpha-2-macroglobulin, apolipoprotein B-100 and kininogen-1 isoform 2 were found to be significantly elevated in patients with malignant biliary strictures. An earlier study created a model for identification of CCA using proteomic analysis based on 22 peptides, in which they found various proteins—including inter-alpha-trypsin inhibitor heavy chain H4, serum albumin, hemoglobin sub-units alpha and beta, and others—to be significantly elevated in malignant strictures caused by CCA [14]. We observed that similar proteins were also elevated in malignant strictures in our study. These proteins are normally present in bile; however their levels are elevated significantly in the setting of CCA. As in our study, the fact that the above-mentioned proteins were significantly elevated in malignant strictures could be indicative of the changes in protein catabolism/apoptosis taking place in the malignant cells. In another study involving analysis of bile in patients from pancreatic adenocarcinoma, several proteins were identified [10]; of these, proteins such as serum albumin, ceruloplasmin, alpha-2-macroglobulin, vitamin D-binding protein, apolipoprotein A-I were also found to be abundantly expressed in malignant strictures in our study. Our study further confirms the elevation of these above-mentioned proteins in malignant biliary strictures.
Trefoil factor 2 plays an important role in maintaining the integrity of the gut, providing mucosal protection; it has also been found to activate CXCR4 chemokine receptors in epithelial and lymphocytic cancer cell lines, affecting their proliferation [32]. In our study, we found that trefoil factor 2 was significantly lower in the malignant biliary strictures, when compared to the benign ones. Down-regulation of TFF-2, suggestive of tumor invasiveness and metastasis, has been recently described in gastric cancers [33]. We also found that proteins including intercellular adhesion molecule 1, ceruloplasmin, haptoglobulin, complement c3, and -c4b were found to be significantly elevated in CCA when compared to patients with PSC. However, considering that only three patients in our study had CCA, the clinical significance of this observation warrants further validation from future studies. The S100 group of proteins, which are especially the markers of schwannomas, have been linked with many malignant conditions [34–36]. A significant elevation of S100 A9 in bile in patients has been demonstrated in CCA [27]. In our study, S100 A8, S100 A9 and a few other biomarkers were significantly elevated in pancreatic cancer, when compared with benign lesions and PSC.
Although the complex constitution of bile can limit proteomic analysis, recent improvements in technology have facilitated major advances in proteomic studies. Summarizing, proteomic analysis of bile reflects the cellular changes in the surrounding tissues/organs, which can be identified through differential expression of various peptides/protein markers. Our preliminary study, involving quantitative proteomic analysis of 24 patients with biliary strictures, shows that the protein biomarkers may differentiate malignant- from benign biliary strictures and may at an earlier stage detect malignancies (CCA) in PSC patients with indeterminate biliary strictures, and hence improve their prognoses. Future large-scale studies on proteomic analysis of bile, comparing benign and malignant strictures, will further enhance the results and its application in clinical practice.
Acknowledgements
We would like to thank the Clinical Research Unit members at the Cleveland Clinic who helped in processing the bile samples.
Funding
The study was supported by a research grant from the American College of Gastroenterology (ACG) (to U.N) and in part by the National Institutes of Health, National Center for Research Resources, CTSA, UL1TR 000439-06 Cleveland, Ohio. The Obitrap Elite Instrument was purchased from an NIH S10 shared instrument grant (1S10RR031537-01).
Conflict of interest statement: None declared.
References
- 1.Friman S. Cholangiocarcinoma: current treatment options. Scand J Surg 2011;100:30–4. [DOI] [PubMed] [Google Scholar]
- 2.Saif MW. Pancreatic neoplasm in 2011: an update. JOP 2011;12:316–21. [PubMed] [Google Scholar]
- 3.Weilert F, Bhat YM, Binmoeller KF, et al. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc 2014;80:97–104. [DOI] [PubMed] [Google Scholar]
- 4.Baron TH, Harewood GC, Rumalla A, et al. A prospective comparison of digital image analysis and routine cytology for the identification of malignancy in biliary tract strictures. Clin Gastroenterol Hepatol 2004;2:214–9. [DOI] [PubMed] [Google Scholar]
- 5.Parsi MA, Li A, Li CP, et al. DNA methylation alterations in endoscopic retrograde cholangiopancreatography brush samples of patients with suspected pancreaticobiliary disease. Clin Gastroenterol Hepatol 2008;6:1270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trikudanathan G, Navaneethan U, Njei B, et al. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:783–9. [DOI] [PubMed] [Google Scholar]
- 7.Smoczynski M, Jablonska A, Matyskiel A, et al. Routine brush cytology and fluorescence in situ hybridization for assessment of pancreatobiliary strictures. Gastrointest Endosc 2012;75:65–73. [DOI] [PubMed] [Google Scholar]
- 8.Moreno Luna LE, Kipp B, Halling KC, et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology 2006;131:1064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navaneethan U, Njei B, Venkatesh PG, et al. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:943–50.e3. [DOI] [PubMed] [Google Scholar]
- 10.Farina A, Dumonceau JM, Delhaye M, et al. A step further in the analysis of human bile proteome. J Proteome Res 2011;10:2047–63. [DOI] [PubMed] [Google Scholar]
- 11.Lukic N, Visentin R, Delhaye M, et al. An integrated approach for comparative proteomic analysis of human bile reveals overexpressed cancer-associated proteins in malignant biliary stenosis. Biochim Biophys Acta 2014;1844:1026–33. [DOI] [PubMed] [Google Scholar]
- 12.Barbhuiya MA, Sahasrabuddhe NA, Pinto SM, et al. Comprehensive proteomic analysis of human bile. Proteomics 2011;11:4443–53. [DOI] [PubMed] [Google Scholar]
- 13.Farid SG, Craven RA, Peng J, et al. Shotgun proteomics of human bile in hilar cholangiocarcinoma. Proteomics 2011;11:2134–8. [DOI] [PubMed] [Google Scholar]
- 14.Lankisch TO, Metzger J, Negm AA, et al. Bile proteomic profiles differentiate cholangiocarcinoma from primary sclerosing cholangitis and choledocholithiasis. Hepatology 2011;53:875–84. [DOI] [PubMed] [Google Scholar]
- 15.Farina A, Dumonceau JM, Frossard JL, et al. Proteomic analysis of human bile from malignant biliary stenosis induced by pancreatic cancer. J Proteome Res 2009;8:159–69. [DOI] [PubMed] [Google Scholar]
- 16.Keller A, Nesvizhskii AI, Kolker E, et al. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 2002;74:5383–92. [DOI] [PubMed] [Google Scholar]
- 17.Nesvizhskii AI, Keller A, Kolker E, et al. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 2003;75:4646–58. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Analytical chemistry 2004;76:4193–201. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Wen Z, Washburn MP, et al. Refinements to Label Free Proteome Quantitation: How to Deal with Peptides Shared by Multiple Proteins. Anal. Chem 2010;82:2272–81. [DOI] [PubMed] [Google Scholar]
- 20.Gokce E, Shuford CM, Franck WL, et al. Evaluation of normalization methods on gelc-ms/ms label-free spectral counting data to correct for variation during proteomic workflows. J Am Soc Mass Spectrom 2011;22:2199–208. [DOI] [PubMed] [Google Scholar]
- 21.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol 2012;3:105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parikh DA, Durbin-Johnson B, Urayama S. Utility of serum CA19-9 levels in the diagnosis of pancreatic ductal adenocarcinoma in an endoscopic ultrasound referral population. J Gastrointest Cancer 2014;45:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navaneethan U, Gutierrez NG, Jegadeesan R, et al. Vascular endothelial growth factor levels in bile distinguishes pancreatic cancer from other etiologies of biliary stricture: a pilot study. Dig Dis Sci 2013;58:2986–92. [DOI] [PubMed] [Google Scholar]
- 24.Navaneethan U, Gutierrez NG, Venkatesh PG, et al. Lipidomic profiling of bile in distinguishing benign from malignant biliary strictures: a single-blinded pilot study. Am J Gastroenterol 2014;109:895–902. [DOI] [PubMed] [Google Scholar]
- 25.Navaneethan U, Parsi MA, Gutierrez NG, et al. Volatile organic compounds in bile can diagnose malignant biliary strictures in the setting of pancreatic cancer: a preliminary observation. Gastrointest Endosc 2014. Jun 11, doi: 10.1016/j.gie.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Wulfkuhle JD, Liotta LA, Petricoin EF. Proteomic applications for the early detection of cancer. Nat Rev Cancer 2003;3:267–75. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Deng X, Zhan Q, et al. A prospective proteomic-based study for identifying potential biomarkers for the diagnosis of cholangiocarcinoma. J Gastrointest Surg 2013;17:1584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zabron AA, Horneffer-van der Sluis VM, Wadsworth CA, et al. Elevated levels of neutrophil gelatinase-associated lipocalin in bile from patients with malignant pancreatobiliary disease. Am J Gastroenterol 2011;106:1711–7. [DOI] [PubMed] [Google Scholar]
- 29.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu R, Cox K, Guthery SL, et al. Cholangiocarcinoma and high-grade dysplasia in young patients with primary sclerosing cholangitis. Dig Dis Sci 2014;59:2320–4. [DOI] [PubMed] [Google Scholar]
- 31.Burak K, Angulo P, Pasha TM, et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol 2004;99:523–6. [DOI] [PubMed] [Google Scholar]
- 32.Dubeykovskaya Z, Dubeykovskiy A, Solal-Cohen J, et al. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J Biol Chem 2009;284:3650–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang P, Yu G, Zhang Y, et al. Promoter hypermethylation and downregulation of trefoil factor 2 in human gastric cancer. Oncol Lett 2014;7:1525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Zhang DL, Jiao XL, et al. S100A4 regulates migration and invasion in hepatocellular carcinoma HepG2 cells via NF-κB-dependent MMP-9 signal. Eur Rev Med Pharmacol Sci 2013;17:2372–82. [PubMed] [Google Scholar]
- 35.Kaur J, Matta A, Kak I, et al. S100A7 overexpression is a predictive marker for high risk of malignant transformation in oral dysplasia. Int J Cancer 2014;134:1379–88. [DOI] [PubMed] [Google Scholar]
- 36.Xu C, Chen H, Wang X, et al. S100A14, a member of the EF-hand calcium-binding proteins, is overexpressed in breast cancer and acts as a modulator of HER2 signaling. J Biol Chem 2014;289:827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]