Abstract
Among the numerous small molecules in the body, the very few aromatic ones include the estrogens and dopamine. In relation to cancer initiation, the estrogens should be considered as chemicals, not as hormones. Metabolism of estrogens is characterized by two major pathways. One is hydroxylation to form the 2- and 4-catechol estrogens, and the second is hydroxylation at the 16α position. In the catechol pathway, the metabolism involves further oxidation to semiquinones and quinones, including formation of the catechol estrogen-3,4-quinones, the major carcinogenic metabolites of estrogens. These electrophilic compounds react with DNA to form the depurinating adducts 4-OHE1(E2)-1-N3Ade and 4-OHE1(E2)-1-N7Gua. The apurinic sites obtained by this reaction generate the mutations that may lead to the initiation of cancer. Oxidation of catechol estrogens to their quinones is normally in homeostasis, which minimizes formation of the quinones and their reaction with DNA. When the homeostasis is disrupted, excessive amounts of catechol estrogen quinones are formed and the resulting increase in depurinating DNA adducts can lead to initiation of cancer. Substantial evidence demonstrates the mutagenicity of the estrogen metabolites and their ability to induce transformation of mouse and human breast epithelial cells, and tumors in laboratory animals. Furthermore, women at high risk for breast cancer or diagnosed with the disease, men with prostate cancer, and men with non-Hodgkin lymphoma all have relatively high levels of estrogen–DNA adducts, compared to matched control subjects. Specific antioxidants, such as N-acetylcysteine and resveratrol, can block the oxidation of catechol estrogens to their quinones and their reaction with DNA. As a result, the initiation of cancer can be prevented.
Keywords: Cancer etiology; Cancer prevention; Catechol estrogen-3,4-quinones; Depurinating estrogen-DNA adducts; Estrogen genotoxicity; Estrogen mutagenicity
1. Introduction
Aliphatic and heteroaromatic molecules are widely represented in the human body, whereas aromatic molecules are rarely present. Organic chemistry is divided into three major classes of compounds: aliphatic, aromatic and heteroaromatic. Aromatic chemistry is the chemistry of benzene and polycyclic aromatic hydrocarbons. Heteroaromatic chemicals contain in their aromatic rings one or more heteroatoms, such as nitrogen. The few aromatic biomolecules have only one benzene ring, and include the estrogen hormones and the neurotransmitter dopamine.
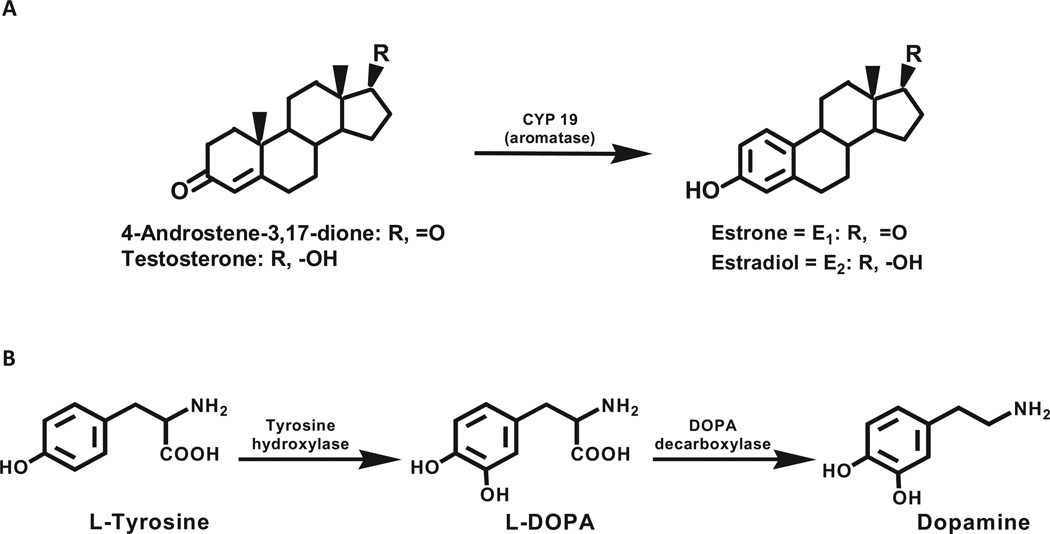
Conversion of testosterone to estradiol (E2) and androstenedione to estrone (E1) constitutes the biosynthesis of estrogens, catalyzed by the enzyme aromatase, cytochrome P450 (CYP)19 (Fig. 1A). Dopamine is biosynthesized by hydroxylation of the amino acid l-tyrosine to l-Dopa, catalyzed by tyrosine hydroxylase, and subsequent decarboxylation to dopamine, catalyzed by l-Dopa decarboxylase (Fig. 1B).
Fig. 1.
Biosynthesis of (A) estrogens and (B) dopamine.
The parent compound of aromatic chemistry is benzene, and it was found to induce leukemia a long time ago. The recognition that benzene is a human leukemogen required evaluation of large populations exposed to the chemical [1]. These data were obtained from Italian and Turkish workers in the shoemaking and printing industries, who had high incidences of acute myeloid leukemia [2]. More recently, the induction of non-Hodgkin lymphoma by benzene has been demonstrated [3,4]. Many polycyclic aromatic hydrocarbons (PAH) are carcinogenic, with potencies ranging from weak to very strong [5].
Metabolic activation of benzene and PAH to ultimate carcinogenic forms follows the principles of chemical carcinogenesis pioneered by James and Elizabeth Miller in the early 1960s [6,7], i.e., most chemical carcinogens (95%) are metabolically activated to electrophilic species that bind covalently to nucleophilic sites in DNA, forming predominantly DNA adducts of Ade and Gua. The other 5% are carcinogens that directly react with DNA without metabolic activation.
Most of the adducts of PAH are the depurinating adducts, which detach from DNA, leaving behind apurinic sites [8,9]. The apurinic sites can be erroneously repaired to give rise to mutations [10] that can initiate the cancer process. The sites of the depurinating adducts correlate with the sites of mutations in the Harvey (H)-ras oncogene [10]. The stable adducts, which remain in DNA unless removed by repair, are formed to a much smaller extent.
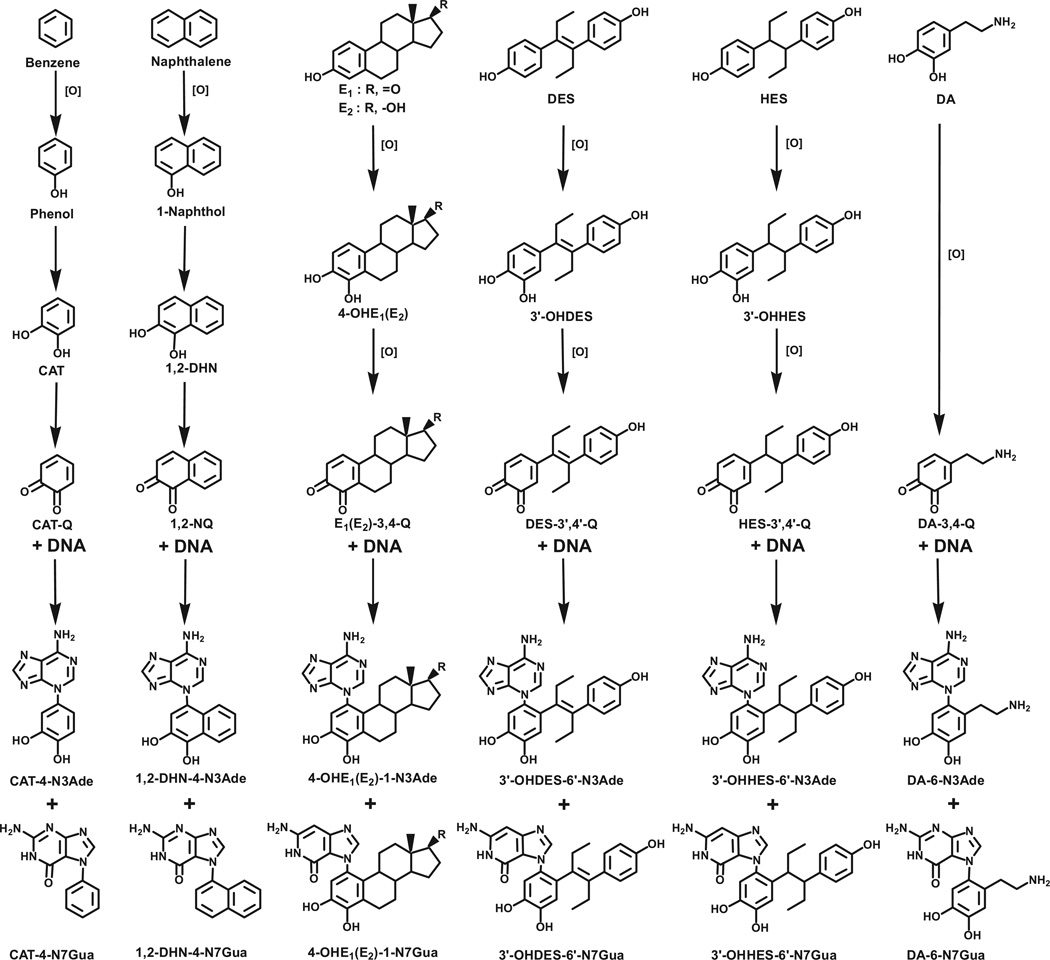
PAH have two major mechanisms of metabolic activation to form ultimate carcinogens: one is formation of radical cations, and the other is formation of bay-region diolepoxides [8,9]. A third mechanism of metabolic activation, which produces extremely weak ultimate carcinogens, generally involves compounds containing one or two benzene rings. In these compounds, activation occurs through formation of electrophilic catechol quinones, which react with DNA by Michael addition to form adducts. This mechanism of activation occurs with benzene [11,12], naphthalene [13,14], E1(E2) [15–17], diethylstilbestrol (DES) [18], hexestrol [19], and dopamine [11,12] (Fig. 2). In this mechanism, the benzene ring is enzymatically oxidized to form a phenol. A second hydroxylation leads to formation of a catechol, followed by a third oxidation to form the ultimate carcinogenic metabolite, an ortho-quinone (Fig. 2). The electrophilic ortho-quinone reacts with the purine bases of DNA to form N3Ade and N7Gua adducts (Fig. 2).
Fig. 2.
Common mechanism of metabolic activation to form depurinating DNA adducts for benzene, naphthalene, estrone/estradiol, diethylstilbestrol, hexestrol and dopamine. 1,2-DHN, 1,2-dihydroxynaphthalene; 1,2-NQ, 1,2-naphthoquinone; HES, hexestrol; DA, dopamine.
2. Genotoxicity of estrogens
Exposure to estrogens has been epidemiologically associated with increased risk of breast cancer, and evidence of a dose–response relationship has been found [20,21]. Induction of prostate adenocarcinomas in 100% of Noble rats implanted with E2 plus testosterone, vs. 40% of rats treated only with testosterone, led to the hypothesis that E2 initiates and testosterone promotes the development of prostate tumors [22].
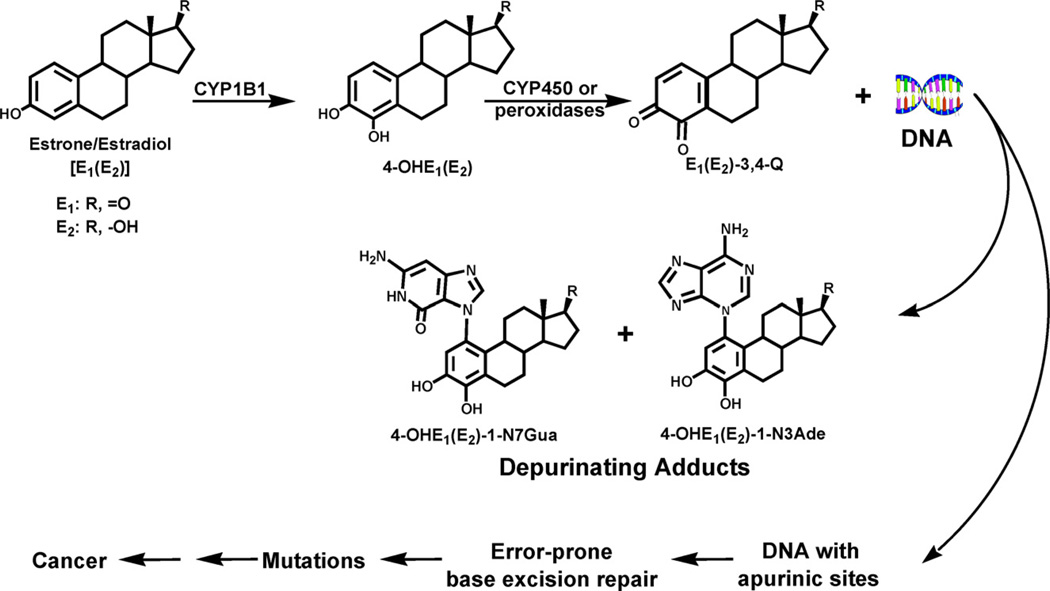
In relation to cancer initiation, estrogens should be considered as other chemicals, namely, their physicochemical and biochemical properties lead them to follow the principles of chemical carcinogenesis elucidated by the Millers [6,7], rather than considering them as hormones. Substantial evidence supports a genotoxicity paradigm for the initiation of cancer by endogenous estrogens. Specific oxidative metabolites of estrogens can react with DNA and generate the critical mutations that lead to the initiation of cancer (Fig. 3) [16,17,23–29]. Two major pathways of metabolism of estrogens are the formation of catechol estrogens, 2-hydroxy(OH)E1(E2) and 4-OHE1(E2), and the formation of 16α-OHE1(E2) [30]. If the catechol estrogens are not conjugated, they can lead through oxidation to semiquinones and quinones (Q, Figs. 3 and 4). Both the E1(E2)-2,3-Q and E1(E2)-3,4-Q react with DNA to form DNA adducts, but the 3,4-Q are more reactive with various nucleophilic groups of DNA than the 2,3-Q (Fig. 4) [16,17,23,26]. Depurination of the 4-OHE1(E2)-1-N3Ade and 4-OHE1(E2)-1-N7Gua adducts generates apurinic sites in the DNA. Error-prone repair of these apurinic sites may lead to specific mutations [27–29] that can initiate breast, prostate and other types of human cancer (Fig. 3) [31].
Fig. 3.
Major metabolic pathway in cancer initiation by estrogens.
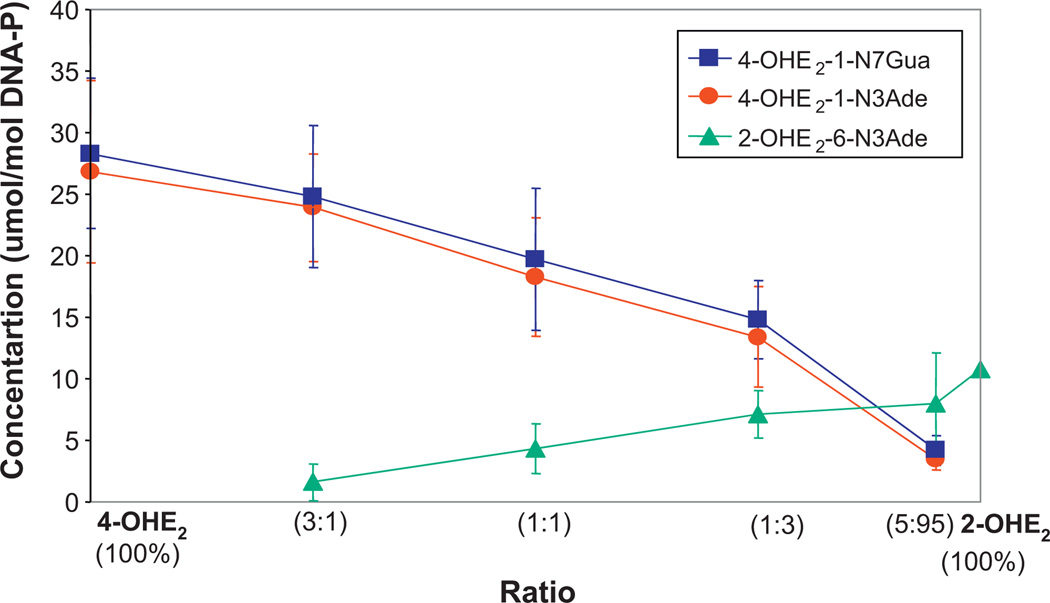
Fig. 4.
Depurinating adducts formed in the prostaglandin H synthase-catalyzed reaction of mixtures of 4-OHE2 and 2-OHE2 at different ratios after 10 h of incubation with DNA [23].
Carcinogenicity testing of the endogenous estrogens E1 and E2 and their catechols demonstrated that they induce cancer in hormone dependent and independent organs [32–36]. This paradigm suggests that specific critical mutations generate abnormal cell proliferation leading to cancer, rather than estrogen receptor-mediated cell proliferation giving rise to random cellular mutations. The specificity of these critical mutations arises from the intercalated complex between estrogens and DNA before conversion to a covalent bond between them, as demonstrated with DES [18].
3. Metabolism of estrogens
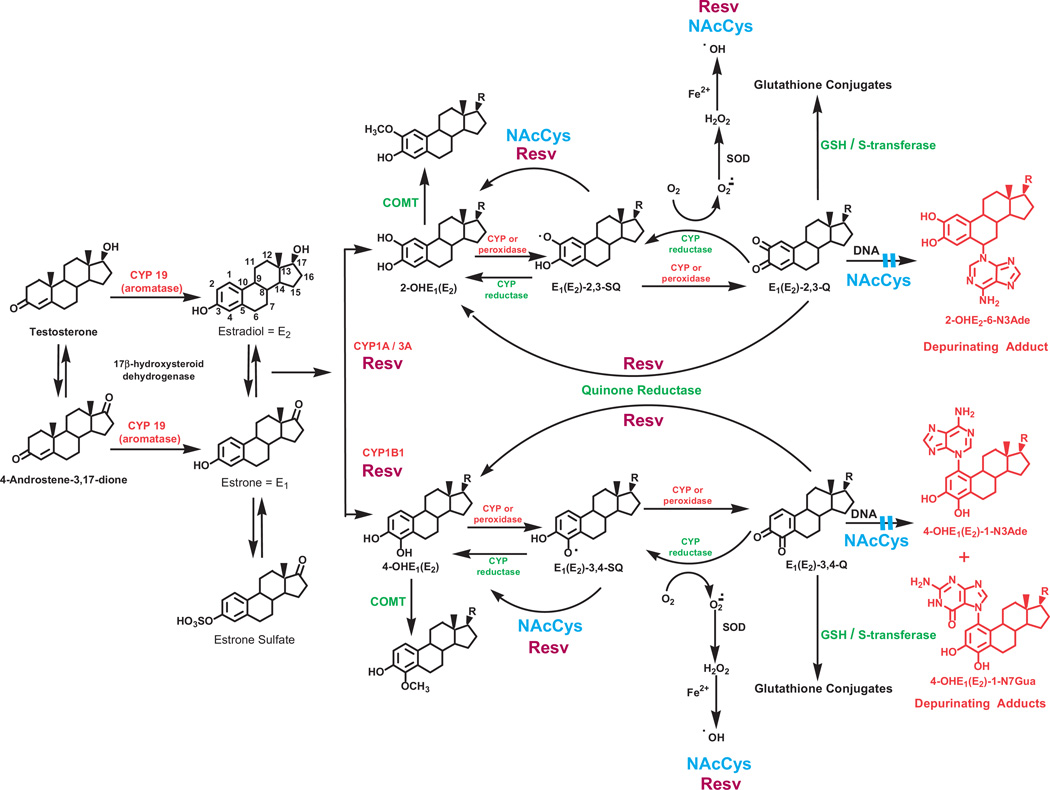
Metabolism of estrogens is characterized by a balanced homeostatic set of activating and deactivating pathways (Fig. 5). Aromatization of androstenedione and testosterone, catalyzed by aromatase (CYP19), yields E1 and E2, respectively. Excess estrogen is stored as E1 -sulfate. E1 and E2 are interconverted by 17β-hydroxysteroid dehydrogenase, and they are metabolized by hydroxylation at the 2- or 4-position to form 2-OHE1(E2) or 4-OHE1(E2). CYP1A1 preferentially hydroxylates E1 and E2 at the 2-position, whereas CYP1B1 almost exclusively catalyzes the formation of 4-OHE1(E2) [37–39]. The most common pathway of conjugation of catechol estrogens in extrahepatic tissues is O-methylation, catalyzed by catechol-O-methyltransferase (COMT) [40]. If the activity of COMT is low, competitive oxidation of the catechol estrogens to E1(E2)-2,3-Q and E1(E2)-3,4-Q by CYP or peroxidases can increase (Fig. 5).
Fig. 5.
Formation, metabolism and DNA adducts of estrogens. Activating enzymes and depurinating DNA adducts are in red and protective enzymes are in green. N-Acetylcysteine (NAcCys, shown in blue) and resveratrol (Resv, burgundy) indicate the various points where NAcCys and Resv could improve the balance of estrogen metabolism and minimize formation of depurinating estrogen–DNA adducts.
Oxidation of semiquinones to quinones can also be mediated by molecular oxygen. Reduction of estrogen quinones to semiquinones, catalyzed by CYP reductase, completes the redox cycle (Fig. 5). In this process, the molecular oxygen is reduced to superoxide anion radical, which is converted to H2O2, yielding hydroxyl radicals in the presence of Fe++. The hydroxyl radicals first generate lipid hydroperoxides, which can act as unregulated cofactors of CYP, leading to an abnormal increase in the oxidation of the catechol estrogens to their quinones. Thus, redox cycling can be a major contributor to the formation of E1(E2)-Q, which are the ultimate carcinogenic metabolites of estrogens.
The 4-OHE1(E2) have greater carcinogenic potency than the 2-OHE1(E2) [33–35], an effect that cannot be attributed to formation of hydroxyl radicals from redox cycling, because the 2-OHE1(E2) and 4-OHE1(E2) have the same redox potential [41,42]. Thus, the greater carcinogenic potency of 4-OHE1(E2) must be related to the much higher levels of depurinating DNA adducts formed by the 3,4-Q, compared to the 2,3-Q (Fig. 4) [23]. This is due to different mechanisms of adduction. The 3,4-Q react via a proton-assisted 1,4-Michael addition [43], where as the 2,3-Q rearrange to para-quinone methides, which react via a 1,6-Michael addition [44].
4. Imbalances in estrogen metabolism
The above paradigm of cancer initiation by estrogens hinges on disruption of homeostatic balance between activating and deactivating pathways of estrogen metabolism (Fig. 5). This homeostasis minimizes formation and reaction of the carcinogenic catechol estrogen quinones with DNA[45,46]. One factor that can help maintain estrogen homeostasis is the feedback inhibition exerted by methoxy estrogens on the expression of CYP1A1 and CYP1B1 [47], which helps regulate the levels of catechol estrogens.
A variety of endogenous and exogenous factors can disrupt estrogen homeostasis. These include diet, environment, lifestyle, aging and genetic factors. The first critical factor is elevation of estrogen levels by excessive synthesis of estrogens due to over-expression of CYP19 (aromatase) [48–50] and/or the presence of unregulated sulfatase that converts excess stored E1-sulfate to E1 [51,52]. Breast tissue can synthesize E2 in situ, suggesting that much more E2 is present in target tissues than would be predicted from plasma concentrations [48]. In fact, the E2 levels in breast tissue of postmenopausal women are similar to the levels in premenopausal women, although the plasma levels in postmenopausal women are 50–100 times lower [52,53].
A second critical factor unbalancing estrogen homeostasis may be the production of high levels of 4-OHE1(E2), due to overexpression of CYP1B1, which converts E1(E2) primarily to 4-OHE1(E2) (Fig. 5) [38,39,54,55]. High levels of 4-OHE1(E2) could result in more oxidation to E1(E2)-3,4-Q. Anadditional factor could be a lack or low level of COMT activity because of polymorphic variation [56]. Insufficient activity of this enzyme would be translated into low levels of methylation of 4-OHE1(E2), which could increase the competitive catalytic oxidation of 4-OHE1(E2) to E1(E2)-3,4-Q (Fig. 5).
Once the E1(E2)-3,4-Q are formed, they can be inactivated by conjugation with glutathione (GSH) or by reduction back to their catechols by quinone reductase (NQO1 and NQO2) (Fig. 5) [57,58], which can be induced by a variety of compounds [59]. Low cellular levels of GSH could be an additional factor resulting in higher levels of E1(E2)-3,4-Q. In addition, polymorphisms in NQO1 that decrease conversion of E1(E2)-3,4-Q to 4-OHE1(E2) [60] could also lead to higher levels of E1(E2)-3,4-Q. This unbalanced metabolism can result in excessive formation of the depurinating estrogen–DNA adducts, 4-OHE1(E2)-1-N3Ade and 4-OHE1(E2)-1-N7Gua (Fig. 5). The increased damage to DNA can generate mutations that initiate the series of events leading to breast, prostate and other human cancers.
5. Estrogen–DNA adducts in the etiology of human cancer
Our research has revealed that while most of us metabolize estrogens to products that are easily excreted from the body, others at risk for cancer have a metabolic pathway with increased levels of E1(E2)-3,4-Q that can react with DNA by proton-assisted 1,4-Michael addition [43] to form specific depurinating adducts (Figs. 3 and 5). These adducts are shed from DNA, and the resultant apurinic sites can be unfaithfully repaired to generate mutations leading to cancer [26,27,29,61]. The depurinating estrogen–DNA adducts travel out of cells and tissues and are excreted in urine, allowing their identification and quantification as biomarkers of risk of developing breast and other human cancers[25,45,46,62,63].
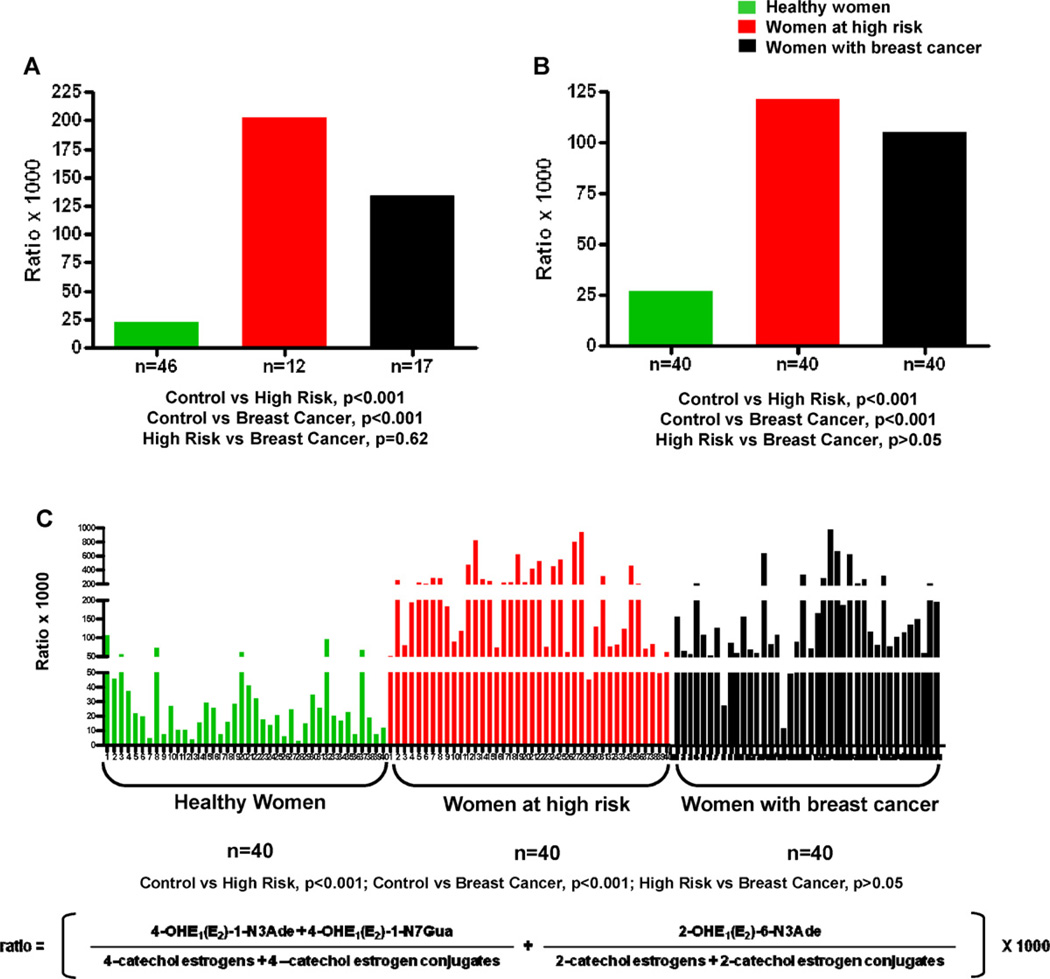
High levels of estrogen–DNA adducts have been seen in analyses of urine and serum from women that are at high risk of breast cancer or have the disease (Fig. 6) [45,46,64]. In these studies one urine and/or one serum sample was collected from women at normal risk for breast cancer, women at high risk for breast cancer (Gail Model score > 1.66% [65]) and women diagnosed with breast cancer. An aliquot of each sample was partially purified by solid phase extraction and 40 estrogen metabolites, conjugates and depurinating DNA adducts were analyzed by using ultraperformance liquid chromatography/tandem mass spectrometry (UPLC–MS/MS).
Fig. 6.
Depurinating estrogen–DNA adducts in (A) median level in urine of healthy women, high-risk women and women with breast cancer – first study [45]; (B) median level in urine of healthy women, high-risk women and women with breast cancer – second study [46]; and (C) serum of healthy women, high-risk women and women with breast cancer [64].
Risk of developing breast cancer is measured as the ratio of estrogen–DNA adducts to their respective estrogen metabolites and conjugates (Fig. 6), indicating the degree of imbalance in estrogen metabolism. In general, the ratio obtained for women at high risk or diagnosed with breast cancer derives from a high level of adducts and low levels of metabolites and conjugates. In some women, how-ever, the adduct level is not high, but the levels of metabolites and conjugates are very low, suggesting that a high proportion of the metabolites was converted to adducts. In this ratio, the 4-OHE1(E2) adducts predominate (97%), and the contribution of the 2-OHE1(E2) adducts is minimal (3%) [45,46,64].
Significant differences (p < 0.001) in relative levels of estrogen–DNA adducts were observed when urine or serum samples from normal-risk women were compared to those from high-risk women or those with breast cancer (Fig. 6) [45,46,64]. These studies showed that unbalanced estrogen metabolism leading to elevation of estrogen–DNA adduct levels is associated with high risk of developing breast cancer.
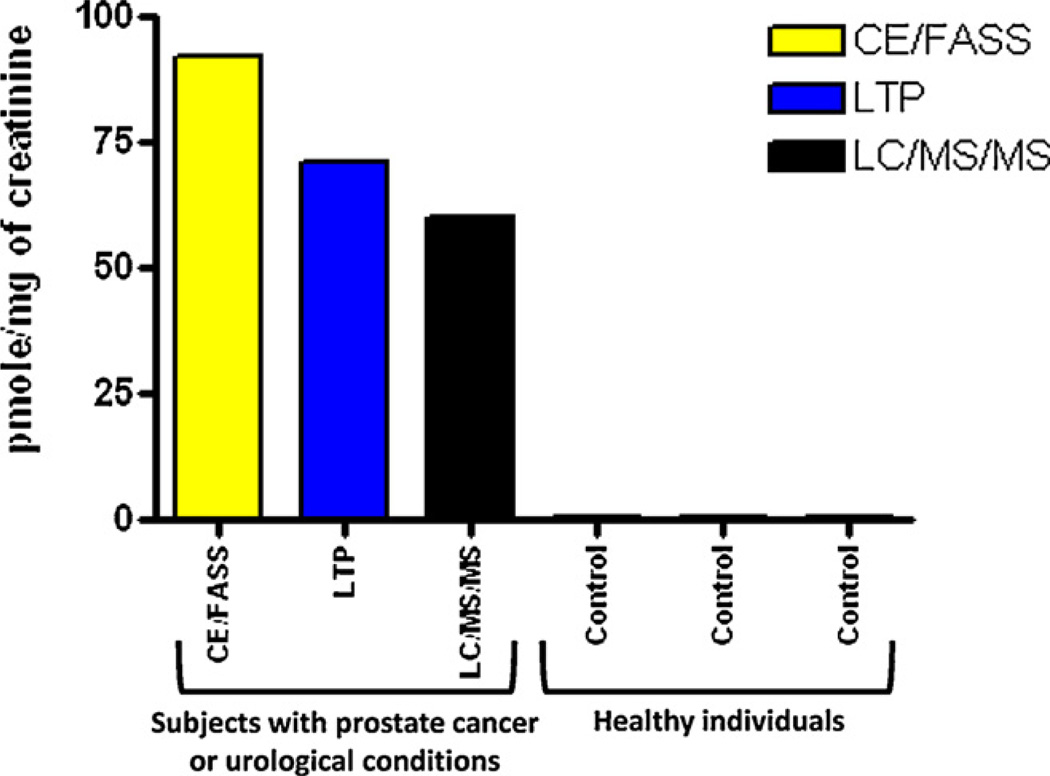
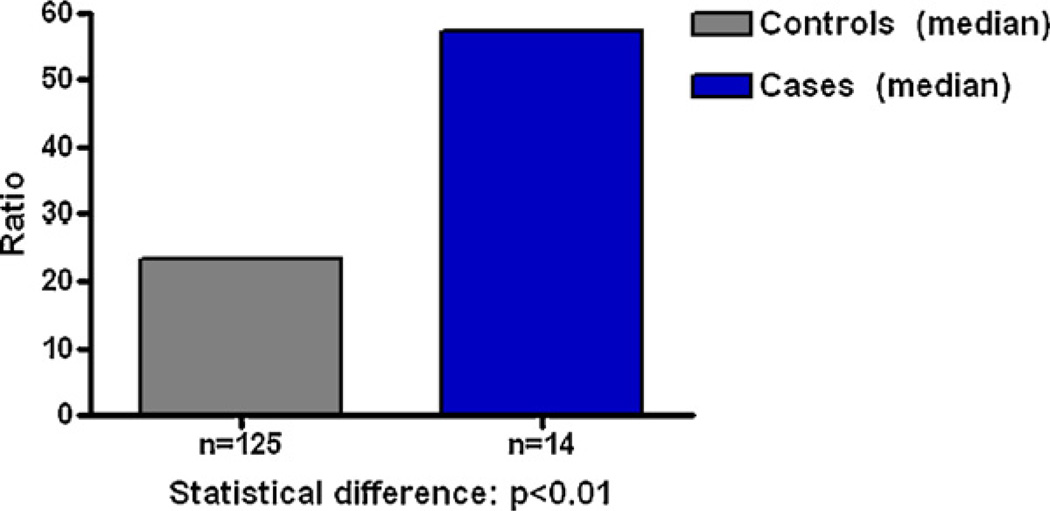
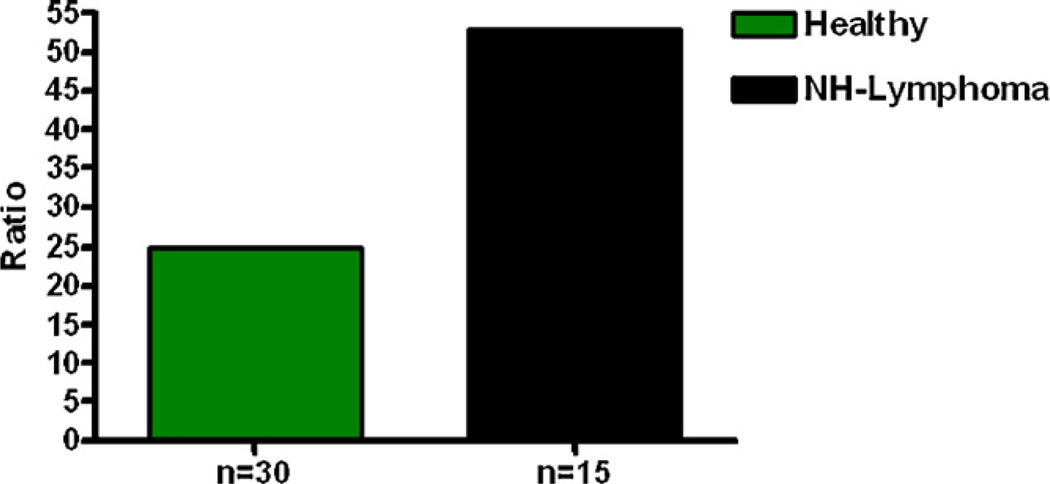
Analysis of urine samples from men with and without prostate cancer also showed that men with the disease have relatively high levels of estrogen–DNA adducts in their urine (Fig. 7) [25]. These results were confirmed in a second study of men with and without prostate cancer (Fig. 8) [62]. Men diagnosed with non-Hodgkin lymphoma also have relatively high levels of estrogen–DNA adducts (Fig. 9) [63]. Thus, formation of estrogen–DNA adducts is associated with these types of cancer and could play a critical role in their etiology.
Fig. 7.
Median level of the 4-OHE1-1-N3Ade adduct in human urine from men with prostate cancer or urological conditions and healthy men [25].
Fig. 8.
Average levels of estrogen–DNA adducts in urine samples from men with and without prostate cancer, p < 0.001 [62].
Fig. 9.
Median levels of depurinating estrogen–DNA adducts in urine of healthy control men and men with NHL. Healthy controls vs. NHL, p < 0.007 [63].
6. Mutagenicity of estrogens
The formation of depurinating estrogen–DNA adducts clearly indicates that E1(E2)-3,4-Q are, indeed, the predominant carcinogenic metabolites of estrogens. To determine that these quinones play the major role in carcinogenesis, we have investigated their mutagenic activity. Previously, investigators failed to detect estrogen-induced mutations in in vitro assays. These findings led to a denial of estrogen genotoxicity [66]. By determining the major pathways of metabolic activation of estrogens, namely, oxidation of catechol estrogens to semiquinones and quinones, and using more sensitive mutagenicity assays, we have succeeded to demonstrate that 4-OHE2 and E2-3,4-Q are mutagenic [27–29,61].
The mutagenicity of E2-3,4-Q was demonstrated by treating the dorsal skin of female SENCAR mice [27]. The mice were killed and the treated skin was excised to determine the levels of estrogen–DNA adducts and the H-ras mutations generated in the skin. Equal amounts of the depurinating 4-OHE2-1-N3Ade and 4-OHE2-1-N7Gua adducts were detected. The observed mutations, however, were A.T to G.C transitions. The same A.T to G.C mutations were predominantly obtained in the H-ras gene of mammary tissue of female ACI rats after treatment with E2-3,4-Q by intramammillary injection [29]. The high levels of mutations at A residues in the H-ras gene and only small numbers of mutations at G residues may be due to the rapid depurination of N3Ade adducts and much slower depurination of N7Gua adducts [23,67].
The genotoxicity of 4-OHE2 and E2-3,4-Q was also demonstrated in the Big Blue (BB) rat2 embryonic cell line, in which the cells contain approximately 60 copies of the λ-LIZ vector per cell [28]. Treatment of these cells six times with either compound generated statistically significant numbers of mutations. In contrast, no mutagenic activity was detected after treatment of the cells with 2-OHE2. The spectrum of mutations obtained with 4-OHE2 contained predominant mutations at A.T base pairs, presumably due to the rapid depurination of N3Ade adducts.
Mutagenicity was further studied in female BB rats, which have approximately 80 copies of the λ-LIZ vector per cell. The rats were implanted with E2, 4-OHE2 or both compounds, and, after 20 weeks, mutations in the cII gene in the mammary cells were analyzed. Only in the rats treated with 4-OHE2 (with or without E2) were A.T to G.C mutations detected [26]. These results demonstrated the mutagenicity of 4-OHE2 under conditions in which it could be metabolized to E2-3,4-Q. Thus, 4-OHE2 and E2-3,4-Q have been demonstrated to be mutagenic under appropriate assay conditions, whereas 2-OHE2 was not mutagenic in BB rat2 cells [28]. The spectrum of mutations was consistent with those expected from the DNA adducts derived from E2-3,4-Q, providing further support for the mutagenicity of estrogens and the hypothesis that estrogens are carcinogenic through their genotoxicity.
7. Transformation of human breast epithelial cells by estrogens
Studies with cultured human breast epithelial cells have provided further evidence for the initiation of cancer by formation of estrogen–DNA adducts. The MCF-10F cell line is an immortalized, nontransformed estrogen receptor-α-negative cell line. Treatment of these cells with E2 or 4-OHE2 generates the depurinating estrogen–DNA adducts [54,68,69]. At doses of 0.007–3.5 nM, treatment with E2 or 4-OHE2 leads to transformation of the cells as detected by their ability to form colonies in soft agar [70,71]. These cells are transformed by the estrogens even in the presence of the anti-estrogen tamoxifen or ICI-182,780 [72]. These results indicate that transformation occurs through the genotoxic effects of the estrogen metabolites. 2-OHE2 induces these changes to a much smaller extent. Implantation of estrogen-transformed MCF-10F cells, selected by their invasiveness, into severely compromised immunodeficient mice generates tumors [73].
These results demonstrate that estrogen receptor-α-negative human breast epithelial cells are transformed by the genotoxic effects of estrogen metabolites, supporting the hypothesis that formation of specific estrogen–DNA adducts is the critical event in the initiation of estrogen-induced cancer.
8. Carcinogenicity of estrogens in animal models
The carcinogenicity of natural and synthetic estrogens was demonstrated by the induction of kidney tumors in Syrian golden hamsters by implantation of E1, E2, DES, or hexestrol [32]. The 4-OHE1(E2) were carcinogenic in the hamsters, but the 2-OHE1(E2) were not [33,34]. The 4-OHE1(E2) were also carcinogenic in the CD-1 mouse uterus and the 2-OHE1(E2) had borderline carcinogenic activity [35]. The far greater carcinogenic activity of the 4-OHE1(E2) compared to the 2-OHE1(E2) can be understood from the much greater ability of E1(E2)-3,4-Q to form estrogen–DNA adducts compared to E1(E2)-2,3-Q [23].
Further important evidence for the role of estrogen genotoxicity in the initiation of cancer comes from studies of transgenic mice with estrogen receptor-α knocked out (ERKO/wnt-1 mice). The wnt-1 transgene in female ERKO/wnt-1 mice induced mammary tumors in 100% of the mice, despite the lack of estrogen receptor-α [74,75]. Both 4-OHE1(E2) and GSH conjugates formed by E1(E2)-3,4-Q were detected in the mammary tissue of these mice, but no methoxy estrogens were found [76], indicating that the estrogen metabolism was unbalanced toward excess activating pathways and limited deactivating pathways. When the mice were ovariectomized at 15 days of age to remove their major source of estrogens and implanted with E2, the E2-treated mice developed mammary tumors in a dose-dependent manner [77,78]. In addition, the mammary tumors developed even when the mice were implanted with both E2 and the anti-estrogen ICI-182,780 [79]. These results provide further strong evidence for tumor initiation by estrogen-induced genotoxic events.
9. Dopamine
In addition to the estrogens, the other significant aromatic biomolecule in the body is the neurotransmitter dopamine. Analogously to the catechol estrogens, dopamine can be easily oxidized to its quinone, dopamine quinone (DA-Q), by auto-oxidation, metal ion oxidation, or oxidation catalyzed by CYP or peroxidase [80,81].
At neutral pH, DA-Q undergoes intramolecular cyclization by 1,4-Michael addition, followed by oxidation to form leucochrome, and then aminochrome. Polymerization of the aminochrome leads to neuromelanin (Fig. 10). At lower pH, especially between pH 5 and 6, partial protonation of the amino group of dopamine slows down the intramolecular cyclization of DA-Q, rendering competitive the intermolecular reaction of the quinone with DNA to form depurinating N3Ade and N7Gua adducts that are analogous to the depurinating estrogen–DNA adducts (Figs. 2 and 10) [12,82].
Fig. 10.
Formation of neuromelanin from dopamine quinone at pH 7 and competitive formation of DA-6-N3Ade, DA-6-N7Gua and neuromelanin from dopamine quinone at pH 5–6.
Analogously to the depurinating estrogen–DNA adducts, the DA-6-N3Ade adduct depurinates from DNA instantaneously, whereas the DA-6-N7Gua adduct depurinates with a half-life of about 3 h [12]. This common feature is seen not only with the depurinating estrogen [15–17] and dopamine [12,82] adducts, but also with the depurinating adducts of benzene [11,12], naphthalene [13,14], DES [18] and hexestrol [19] (Fig. 2). This common feature may lead to the initiation of cancer or, for dopamine, neurodegenerative disease.
10. Prevention of cancer initiation
The paradigm of cancer initiation in the metabolism of estrogens is related to formation of catechol estrogens, with special emphasis on the 4-OHE1(E2). In the oxidative metabolism of estrogens, activating pathways can lead to formation of depurinating estrogen–DNA adducts and deactivating pathways lead to formation of estrogen metabolites and conjugates. This hypothesis has been supported by studies in which the levels of estrogen–DNA adducts in urine samples from women at normal risk for breast cancer are relatively low compared to the much higher levels in women at high risk for breast cancer or diagnosed with the disease (Fig. 6) [45,46]. In contrast, in women at normal risk for breast cancer, the levels of estrogen metabolites and conjugates are high compared to the relatively low levels of metabolites and conjugates in women at high risk or diagnosed with the disease (Fig. 6) [45,46].
The relative levels of estrogen metabolites, conjugates and depurinating DNA adducts can be related to the expression of five key estrogen-metabolizing enzymes in breast tissue [83]. The first activating enzyme is CYP19 (aromatase), which converts androgens to estrogens (Fig. 5). A case–control study of 2018 women found that women who consumed mushrooms in their diet had a 50% lower incidence of breast cancer than women who did not consume mushrooms. In addition, women consuming both mushrooms and green tea had a 90% lower incidence of breast cancer [84]. The mushrooms contain phytochemicals that inhibit aromatase activity. These effects have been studied in cell culture, and the anti-aromatase activity of the phytochemicals has been suggested to be responsible for the reduced incidence of breast cancer [85,86]. The second estrogen-activating enzyme is CYP1B1, which converts E1(E2) almost exclusively to 4-OHE1(E2) (Fig. 5). Further oxidation of 4-OHE1(E2) leads to E1(E2)-3,4-Q, the predominant metabolites in the initiation of cancer by estrogens.
Two protective phase II enzymes are COMT and quinone reductase. The former catalyzes the methylation of catechol estrogens, thereby preventing their conversion to semiquinones and quinones (Fig. 5), whereas the latter, NQO1 and NQO2, reduce catechol estrogen quinones back to catechol estrogens [57,58,87] (Fig. 5).
Breast tissue from women who do not have breast cancer has been found to have high levels of the protective enzymes COMT and NQO1, and low levels of expression of the activating enzymes CYP19 and CYP1B1 [83]. In contrast, non-tumor breast tissue from women diagnosed with breast cancer has high levels of the activating enzymes CYP19 and CYP1B1, with low levels of COMT and NQO1 [83]. An additional protective enzyme is glutathione-S-transferase (GST), which facilitates the reaction between catechol estrogen quinones and GSH (Fig. 5).
Homeostasis in estrogen metabolism minimizes reaction of the electrophilic catechol estrogen quinones with DNA, thereby reducing the amount of DNA damage and resulting risk of cancer initiation. This balance is naturally maintained by the enzyme COMT, which methylates catechol estrogens, impeding their further oxidation to semiquinones and quinones (Fig. 5) [54,69]. The ubiquitous antioxidant GSH, which reacts non-enzymatically with the catechol estrogen quinones or, more efficiently, with the catalytic activation of GST (Fig. 5), also helps maintain estrogen homeostasis. A third deactivating phase II enzyme is NQO1 (and NQO2) [57,58]. These enzymes limit the reaction of catechol estrogen quinones with DNA by reduction of the quinones to catechol estrogens [57,58,87].
Unbalanced estrogen homeostasis can be mitigated by the use of specific antioxidant compounds. N-Acetylcysteine (NAcCys) and resveratrol are particularly effective in inhibiting formation of estrogen–DNA adducts [88]. NAcCys is the acetyl derivative of the amino acid cysteine, which is one component in the antioxidant tripeptide GSH. Resveratrol, 3,5,4′-hydroxystilbene, is a natural antioxidant present in grapes and many other plants [89] and has anticarcinogenic effects in diverse in vitro and in vivo systems [90,91].
The human breast epithelial cell line MCF-10F (estrogen receptor-α-negative and aryl hydrocarbon receptor-positive) was used to study the effect of resveratrol [55,92] and NAcCys [93] on estrogen metabolism and formation of estrogen–DNA adducts. The combined antioxidant activity of NAcCys and resveratrol was also determined in MCF-10F cells [94]. In addition, the capacity of NAcCys to inhibit transformation of E6 mouse mammary cells [95] and resveratrol to inhibit transformation of MCF-10F cells [55] has been seen.
The antioxidant effect of NAcCys in reducing formation of estrogen–DNA adducts in MCF-10F cells treated with 4-OHE2 (Figs. 5 and 11) [93] is due to the reaction of NAcCys with E2-3,4-Q and the reduction of semiquinones to 4-OHE2 [88,96]. Resveratrol similarly reduces semiquinones to 4-OHE2 [55,88,92]. Furthermore, resveratrol induces NQO1, which catalyzes the reduction of E2-3,4-Q to 4-OHE2 [57], thereby limiting reaction of E2-3,4-Q with DNA. Another important action of resveratrol is to modulate the expression of CYP1B1 if it is overexpressed [55].
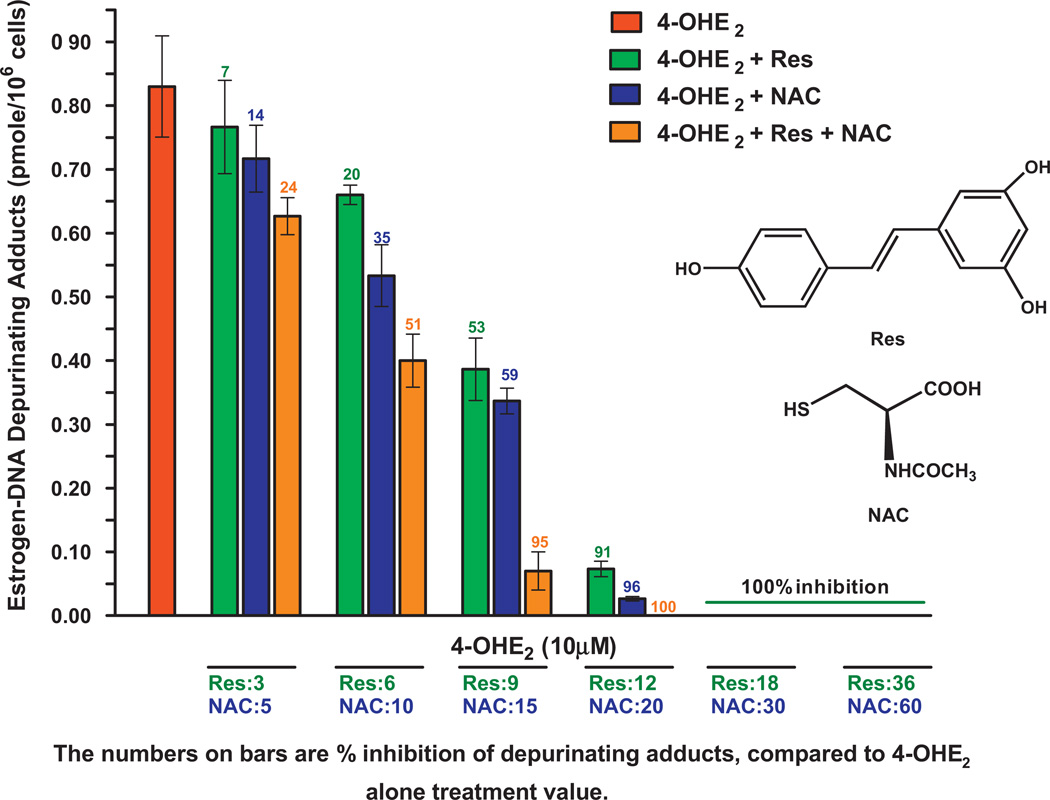
Fig. 11.
Effects of NAcCys, resveratrol, or NAcCys + resveratrol on the formation of depurinating estrogen–DNA adducts in MCF-10F cells treated with 4-OHE2. NAC, NAcCys; Res, resveratrol [94].
When NAcCys and resveratrol are mixed together, they have an additive effect in reducing formation of estrogen–DNA adducts in MCF-10F cells (Fig. 11) [94]. Low concentrations of NAcCys and resveratrol inhibit estrogen–DNA adduct formation similarly, but at higher doses, the effect of resveratrol is about 50% greater than that of NAcCys [94].
Use of these antioxidants is the logical preventive strategy to re-establish and/or maintain the homeostatic balance of estrogen metabolism, thereby reducing DNA damage, the resulting mutations and risk of initiating cancer.
11. Conclusions
Metabolism of estrogens is characterized by a homeostatic set of activating and deactivating pathways. The homeostasis minimizes formation of the catechol estrogen quinones, the ultimate carcinogenic metabolites of estrogens, and their reaction with DNA. When homeostasis is disrupted, excessive oxidation of catechol estrogens to semiquinones and quinones occurs. The quinones can react with DNA to form predominantly the depurinating adducts 4-OHE1(E2)-1-N3Ade and 4-OHE1(E2)-1-N7Gua. These adducts generate apurinic sites leading to the mutations that can initiate breast, prostate and other prevalent types of cancer. A similar mechanism of metabolic activation occurs for the carcinogens benzene, naphthalene, diethylstilbestrol and hexestrol (Fig. 2).
Substantial evidence for the genotoxicity of the endogenous estrogens has been obtained in studies conducted in vitro, in cell culture and in laboratory animals. In addition, women at high risk for breast cancer or diagnosed with the disease (Fig. 6), men with prostate cancer (Figs. 7 and 8), and men with non-Hodgkin lymphoma (Fig. 9) form relatively high levels of depurinating estrogen–DNA adducts compared to control subjects.
The initiating step of cancer by estrogens can be blocked by the antioxidants N-acetylcysteine and resveratrol (Fig. 11). By preventing the initiation of the disease, promotion, progression and development of cancer are eliminated. This strategy of prevention does not require knowledge of the genes involved or the complex series of events that occur after cancer initiation.
Acknowledgements
The progress in research on the etiology and prevention of cancer is due to the efforts, dedication, accomplishments and creativity of the fine scientists with whom we have worked over the years. We particularly acknowledge our long-term collaboration with Dr. D. Chakravarti of our research group. In addition we express our gratitude to Drs. N. Gaikwad, K.-M. Li, M. Saeed, S. Singh, D. Venugopal and M. Zahid, our graduate students F. Lu and L. Yang and our technical support P. Mailander and S. Higginbotham. Preparation of this article was supported by Prevention LLC. Core support at the Eppley Institute was supported by grant P30 36727 from the National Cancer Institute.
Abbreviations
- BB
Big Blue
- COMT
catechol-O-methyltransferase
- CYP
cytochrome P450
- CYP19
aromatase
- DA-Q
dopamine quinone
- DES
diethylstilbestrol
- E1
estrone
- E2
estradiol
- E1(E2)-Q
estrone(estradiol) quinone
- ERKO
estrogen receptor-αknocked out
- GSH
glutathione
- GST
glutathione-S-transferase
- H
Harvey
- NAcCys
N-acetylcysteine
- NQO1 and NQO2
NAD(P)H quinone oxidoreductase 1 and 2
- OHE1(E2)
hydroxyestrone(estradiol)
- PAH
polycyclic aromatic hydrocarbons
- UPLC-MS/MS
ultraperformance liquid chromatography/tandem mass spectrometry.
References
- 1.Benzene IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 29(1982):93–148. [PubMed] [Google Scholar]
- 2.Vigliani E, Forni A. Benzene and leukemia. Environ. Res. 1976;11:122. doi: 10.1016/0013-9351(76)90115-8. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor SR, Farmer PB, Lauder I. Benzene and non-Hodgkin’s lymphoma. J. Pathol. 1999;189:448–453. doi: 10.1002/(SICI)1096-9896(199912)189:4<448::AID-PATH458>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Smith MT, Jones RM, Smith AH. Benzene exposure and risk of non-Hodgkin lymphoma. Cancer Epidemiol. Biomarkers Prev. 2007;16:385–391. doi: 10.1158/1055-9965.EPI-06-1057. [DOI] [PubMed] [Google Scholar]
- 5.Polynuclear Aromatic Compounds. IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 1983;32 [Google Scholar]
- 6.Miller JA. Carcinogenesis by chemicals: an overview—G.H.A. Clowes memorial lecture. Cancer Res. 1970;30:559–576. [PubMed] [Google Scholar]
- 7.Miller EC, Miller JA. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer. 1981;47:2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Cavalieri EL, Rogan EG. The approach to understanding aromatic hydrocarbon carcinogenesis. The central role of radical cations in metabolic activation. Pharmacol. Ther. 1992;55:183–199. doi: 10.1016/0163-7258(92)90015-r. [DOI] [PubMed] [Google Scholar]
- 9.Cavalieri E, Rogan E. Mechanisms of tumor initiation by polycyclic aromatic hydrocarbons in mammals. In: Neilson AH, editor. The Handbook of Environmental Chemistry. 3J. Heidelberg: PAHs and Related Compounds, Springer-Verlag; 1998. pp. 81–117. [Google Scholar]
- 10.Chakravarti D, Pelling JC, Cavalieri EL, Rogan EG. Relating aromatic hydrocarbon-induced DNA adducts and c-Harvey-ras mutations in mouse skin papillomas: the role of apurinic sites. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10422–10426. doi: 10.1073/pnas.92.22.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalieri EL, Li K-M, Balu N, Saeed M, Devanesan P, Higginbotham S, Zhao J, Gross ML, Rogan E. Catechol ortho-quinones: the electrophilic compounds that form depurinating DNA adducts and could initiate cancer and other diseases. Carcinogenesis. 2002;23:1071–1077. doi: 10.1093/carcin/23.6.1071. [DOI] [PubMed] [Google Scholar]
- 12.Zahid M, Saeed M, Rogan EG, Cavalieri EL. Benzene and dopamine catechol quinones could initiate cancer or neurogenic disease. Free Radic. Biol. Med. 2010;48:318–324. doi: 10.1016/j.freeradbiomed.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saeed M, Higginbotham S, Rogan E, Cavalieri E. Formation of depurinating N3adenine and N7guanine adducts after reaction of 1,2-naphthoquinone or enzyme-activated 1,2-dihydroxynaphthalene with DNA. Implications for the mechanism of tumor initiation by naphthalene. Chem. Biol. Interact. 2007;165:175–188. doi: 10.1016/j.cbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Saeed M, Higginbotham S, Gaikwad N, Chakravarti D, Rogan E, Cavalieri E. Depurinating naphthalene-DNA adducts in mouse skin related to cancer initiation. Free Radic. Biol. Med. 2009;47:1075–1081. doi: 10.1016/j.freeradbiomed.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stack D, Byun J, Gross ML, Rogan EG, Cavalieri E. Molecular characteristics of catechol estrogen quinones in reactions with deoxyribonucleosides. Chem. Res. Toxicol. 1996;9:851–859. doi: 10.1021/tx960002q. [DOI] [PubMed] [Google Scholar]
- 16.Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, Ramanathan R, Cerny RL, Rogan EG. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li K-M, Todorovic R, Devanesan P, Higginbotham S, Köfeler H, Ramanathan R, Gross ML, Rogan EG, Cavalieri EL. Metabolism and DNA binding studies of 4-hydroxyestradiol and estradiol-3,4-quinone in vitro and in female ACI rat mammary gland in vivo. Carcinogenesis. 2004;25:289–297. doi: 10.1093/carcin/bgg191. [DOI] [PubMed] [Google Scholar]
- 18.Saeed M, Rogan E, Cavalieri E. Mechanism of metabolic activation and DNA adduct formation by the human carcinogen diethylstilbestrol: the defining link to natural estrogens. Int. J. Cancer. 2009;124:1276–1284. doi: 10.1002/ijc.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saeed M, Gunselman SJ, Higginbotham S, Rogan E, Cavalieri E. Formation of the depurinating N3adenine and N7guanine adducts by reaction of DNA with hexestrol-3′-4′-quinone or enzyme-activated 3′-hydroxyhexestrol. Implications for a unifying mechanism of tumor initiation by natural and synthetic estrogens. Steroids. 2005;70:37–45. doi: 10.1016/j.steroids.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Endogenous Hormones Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J. Cancer Natl. Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 21.Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, Secreto G, Amiano P, Bingham S, Boeing H, Bueno de Mesquita HB, Chang-Claude J, Clavel-Chapelon F, Fournier A, van Gils CH, Gonzalez CA, Gurrea AB, Critselis E, Khaw KT, Krogh V, Lahmann PH, Nagel G, Olsen A, Onland-Moret NC, Overvad K, Palli D, Panico S, Peeters P, Quiros JR, Roddam A, Thiebaut A, Tjonneland A, Chirlaque MD, Trichopoulou A, Trichopoulos D, Tumino R, Vineis P, Norat T, Ferrari P, Slimani N, Riboli E. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J. Natl. Cancer Inst. 2005;97:755–765. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 22.Bosland MC, Ford H, Horton L. Induction of a high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17β or diethylstilbestrol. Carcinogenesis. 1995;16:1311–1317. doi: 10.1093/carcin/16.6.1311. [DOI] [PubMed] [Google Scholar]
- 23.Zahid M, Kohli E, Saeed M, Rogan E, Cavalieri E. The greater reactivity of estradiol-3,4-quinone versus estradiol-2,3-quinone with DNA in the formation of depurinating adducts. Implications for tumor-initiating activity. Chem. Res. Toxicol. 2006;19:164–172. doi: 10.1021/tx050229y. [DOI] [PubMed] [Google Scholar]
- 24.Markushin Y, Zhong W, Cavalieri EL, Rogan EG, Small GJ, Yeung ES, Jankowiak R. Spectral characterization of catechol estrogen quinone (CEQ)-derived DNA adducts and their identification in human breast tissue extract. Chem. Res. Toxicol. 2003;16:1107–1117. doi: 10.1021/tx0340854. [DOI] [PubMed] [Google Scholar]
- 25.Markushin Y, Gaikwad N, Zhang H, Kapke P, Rogan EG, Cavalieri EL, Trock BJ, Pavlovich C, Jankowiak R. Potential biomarker for early risk assessment of prostate cancer. Prostate. 2006;66:1565–1571. doi: 10.1002/pros.20484. [DOI] [PubMed] [Google Scholar]
- 26.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, Muti P, Rogan E, Russo J, Santen R, Sutter T. Catechol estrogen quinones as initiators of breast and other human cancers. Implications for biomarkers of susceptibility and cancer prevention. BBA - Rev. Cancer. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Chakravarti D, Mailander P, Li K-M, Higginbotham S, Zhang H, Gross ML, Cavalieri E, Rogan E. Evidence that a burst of DNA depurination in SENCAR mouse skin induces error-prone repair and forms mutations in the H-ras gene. Oncogene. 2001;20:7945–7953. doi: 10.1038/sj.onc.1204969. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Z, Kosinska W, Khmelnitsky M, Cavalieri EL, Rogan EG, Chakravarti D, Sacks P, Guttenplan JB. Mutagenic activity of 4-hydroxyestradiol, but not 2-hydroxyestradiol, in BB rat2 embryonic cells, and the mutational spectrum of 4-hydroxyestradiol. Chem. Res. Toxicol. 2006;19:475–479. doi: 10.1021/tx0502645. [DOI] [PubMed] [Google Scholar]
- 29.Mailander PC, Meza JL, Higginbotham S, Chakravarti D. Induction of A.T. to G.C. mutations by erroneous repair of depurinated DNA following estrogen treatment of the mammary gland of ACI rats. J. Steroid Biochem. 2006;101:204–215. doi: 10.1016/j.jsbmb.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;9:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Cavalieri EL, Rogan EG. Depurinating estrogen–DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010;6:75–91. doi: 10.2217/fon.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li JJ, Li SA, Klicka JK, Parsons JA, Lam LK. Relative carcinogenic activity of various synthetic and natural estrogens in the Syrian hamster kidney. Cancer Res. 1983;43:5200–5204. [PubMed] [Google Scholar]
- 33.Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J. Steroid Biochem. 1986;24:353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 34.Li JJ, Li SA. Estrogen carcinogenesis in Syrian hamster tissues: role of metabolism. Fed. Proc. 1987;46:1858–1863. [PubMed] [Google Scholar]
- 35.Newbold RR, Liehr JG. Induction of uterine adenocarcinoma in CD-1 mice by catechol estrogens. Cancer Res. 2000;60:235–237. [PubMed] [Google Scholar]
- 36.Santen RJ, Yue W, Bocchinfuso W, Korach K, Wang J-P, Rogan EG, Li Y, Cavalieri E, Russo J, Devanesan P, Verderame M. Estradiol-induced carcinogenesis via formation of genotoxic metabolites. In: Ingle JN, Dowsett M, editors. Advances in Endocrine Therapy of Breast Cance. New York: Marcel Dekker; 2004. pp. 163–177. [Google Scholar]
- 37.Spink DC, Hayes CL, Young NR, Christou M, Sutter TR, Jefcoate CR, Gierthy JF. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on estrogen metabolism in MCF-7 breast cancer cells: evidence for induction of a novel 17 beta-estradiol 4-hydroxylase. J. Steroid Biochem. Mol. Biol. 1994;51:251–258. doi: 10.1016/0960-0760(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 38.Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17|3-Estradiol hydroxylation catalyzed by human cytochrome p450 1b1. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spink DC, Spink BC, Cao JQ, DePasquale JA, Pentecost BT, Fasco MJ, Li Y, Sutter TR. Differential expression of CYP1a1 and CYP1b1 in human breast epithelial cells and breast tumor cells. Carcinogenesis. 1998;19:291–298. doi: 10.1093/carcin/19.2.291. [DOI] [PubMed] [Google Scholar]
- 40.Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol. Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 41.Mobley JA, Bhat AS, Brueggemeier RW. Measurement of oxidative DNA damage by catechol estrogens and analogues in vitro. Chem. Res. Toxicol. 1999;12:270–277. doi: 10.1021/tx980128i. [DOI] [PubMed] [Google Scholar]
- 42.Cavalieri E. Minisymposium on endogenous carcinogens: the catechol estrogen pathway. An introduction. Polycycl. Aromat. Compd. 1994;6:223–228. [Google Scholar]
- 43.Stack DE, Li G, Hill A, Hoffman N. Mechanistic insights into the Michael addition of deoxyguanosine to catechol estrogen-3,4-quinones. Chem. Res. Toxicol. 2008;21:1415–1425. doi: 10.1021/tx800071u. [DOI] [PubMed] [Google Scholar]
- 44.Bolton JL, Shen L. p-Quinone methides are the major decomposition products of catechol estrogen o-quinones. Carcinogenesis. 1998;17:925–929. doi: 10.1093/carcin/17.5.925. [DOI] [PubMed] [Google Scholar]
- 45.Gaikwad NW, Yang L, Muti P, Meza JL, Pruthi S, Ingle JN, Rogan EG, Cavalieri EL. The molecular etiology of breast cancer: evidence from biomarkers of risk. Int. J. Cancer. 2008;122:1949–1957. doi: 10.1002/ijc.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaikwad NW, Yang L, Pruthi S, Ingle JN, Sandhu N, Rogan E, Cavalieri E. Urine biomarkers of risk in the molecular etiology of breast cancer. Breast Cancer: Basic Clin. Res. 2009;3:1–8. doi: 10.4137/bcbcr.s2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dawling S, Roodi N, Parl FF. Methoxyestrogens exert feedback inhibition on cytochrome P450 1A1 and 1B1. Cancer Res. 2003;3:3127–3132. [PubMed] [Google Scholar]
- 48.Miller WR, O’Neill J. The importance of local synthesis of estrogen within the breast. Steroids. 1987;50:537–548. doi: 10.1016/0039-128x(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 49.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 50.Jefcoate CR, Liehr JG, Santen RJ, Sutter TR, Yager JD, Yue W, Santner SJ, Tekmal R, Demers L, Pauley R, Naftolin F, Mor G, Berstein L. Tissue-specific synthesis and oxidative metabolism of estrogens. In: Cavalieri E, Rogan E, editors. JNCI Monograph 27: Estrogens as Endogenous Carcinogens in the Breast and Prostate. Oxford Press; 2000. pp. 95–112. [DOI] [PubMed] [Google Scholar]
- 51.Santner SJ, Feil PD, Santen RJ. In situ estrogen production via the estrone sulfatase pathway in breast tumors: relative importance versus the aromatase pathway. J. Clin. Endocrinol. Metab. 1984;59:29–33. doi: 10.1210/jcem-59-1-29. [DOI] [PubMed] [Google Scholar]
- 52.Pasqualini JR, Chetrite G, Blacker C, Feinstein MC, Delalonde L, Talbi M, Maloche C. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J. Clin. Endocrinol. Metab. 1996;81:1460–1464. doi: 10.1210/jcem.81.4.8636351. [DOI] [PubMed] [Google Scholar]
- 53.Chetrite GS, Cortes-Prieto J, Philippe JC, Wright F, Pasqualini JR. Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J. Steroid Biochem. Mol. Biol. 2000;72:23–27. doi: 10.1016/s0960-0760(00)00040-6. [DOI] [PubMed] [Google Scholar]
- 54.Lu F, Zahid M, Saeed M, Cavalieri EL, Rogan EG. Estrogen metabolism and formation of estrogen–DNA adducts in estradiol-treated MCF-10F cells. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction and catechol-O-methyltransferase inhibition. J. Steroid Biochem. Mol. Biol. 2007;105:150–158. doi: 10.1016/j.jsbmb.2006.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu F, Zahid M, Wang C, Saeed M, Cavalieri EL, Rogan EG. Resveratrol prevents estrogen–DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev. Res. 2008;1:135–145. doi: 10.1158/1940-6207.CAPR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitrunen K, Hirvonen A. Molecular epidemiology of sporadic breast cancer. The role of polymorphic genes involved in oestrogen biosynthesis and metabolism. Mutat. Res. 2003;544:9–41. doi: 10.1016/s1383-5742(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 57.Gaikwad NW, Rogan EG, Cavalieri EL. Evidence from ESI-MS for NQO1-catalyzed reduction of estrogen ortho-quinones. Free Radic. Biol. Med. 2007;43:1289–1298. doi: 10.1016/j.freeradbiomed.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaikwad NW, Yang L, Rogan EG, Cavalieri EL. Evidence for NQO2-mediated reduction of the carcinogenic estrogen ortho-quinones. Free Radic. Biol. Med. 2009;46:253–262. doi: 10.1016/j.freeradbiomed.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talalay P, Dinkova-Kostova AT, Holtzclaw WD. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv. Enzyme Regul. 2003;43:121–134. doi: 10.1016/s0065-2571(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 60.Singh S, Zahid M, Saeed M, Gaikwad NW, Meza JL, Cavalieri EL, Rogan EG, Chakravarti D. NAD(P)H:Quinone oxidoreductase 1 arg139trp and pro187ser polymorphisms imbalance estrogen metabolism towards DNA adduct formation in human mammary epithelial cells. J. Steroid Biochem. Mol. Biol. 2009;117:56–66. doi: 10.1016/j.jsbmb.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandez SV, Russo IH, Russo J. Estradiol and its metabolites 4-hydroxyestradiol and 2-hydroxyestradiol induce mutations in human breast epithelial cells. Int. J. Cancer. 2006;118:1862–1888. doi: 10.1002/ijc.21590. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Gaikwad N, Meza J, Cavalieri E, Muti P, Trock B, Rogan E. Novel biomarkers for risk of prostate cancer. Results from a case-control study. Prostate. 2009;69:41–48. doi: 10.1002/pros.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaikwad N, Yang L, Weisenburger DD, Vose J, Beseler C, Rogan E, Cavalieri E. Urinary biomarkers suggest that estrogen–DNA adducts may play a role in the etiology of non-Hodgkin lymphoma. Biomarkers. 2009;14:502–512. doi: 10.3109/13547500903121715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pruthi S, Yang L, Sandhu NP, Ingle JN, Beseler CL, Suman VJ, Cavalieri EL, Rogan EG. Serum estrogen–DNA adducts as biomarkers for assessing breast cancer risk, unpublished results. doi: 10.1016/j.jsbmb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Protecting individualized probabilities of developing breast cancer for white females who are being examined annually. J. Natl. Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 66.Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr. Rev. 2000;21:40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- 67.Saeed M, Zahid M, Gunselman SJ, Rogan E, Cavalieri E. Slow loss of deoxyribose from the N7deoxyguanosine adducts of estradiol-3,4-quinone and hexestrol-3′,4′-quinone. Implications for mutagenic activity. Steroids. 2005;70:29–35. doi: 10.1016/j.steroids.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Saeed M, Rogan E, Fernandez SV, Sheriff F, Russo J, Cavalieri E. Formation of depurinating N3Adenine and N7Guanine adducts by MCF-10F cells cultured in the presence of 4-hydroxyestradiol. Int. J. Cancer. 2007;120:1821–1824. doi: 10.1002/ijc.22399. [DOI] [PubMed] [Google Scholar]
- 69.Zahid M, Saeed M, Lu F, Gaikwad N, Cavalieri EL, Rogan EG. Inhibition of catechol-O-methyltransferase increases estrogen–DNA adduct formation. Free Radic. Biol. Med. 2007;43:1534–1540. doi: 10.1016/j.freeradbiomed.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J. Steroid Biochem. Mol. Biol. 2003;87:1–25. doi: 10.1016/s0960-0760(03)00390-x. [DOI] [PubMed] [Google Scholar]
- 71.Russo J, Russo IH. Genotoxicity of steroidal estrogens. Trends Endocrinol. Metab. 2004;15:211–214. doi: 10.1016/j.tem.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Lareef MH, Garber J, Russo PA, Russo IH, Heulings R, Russo J. The estrogen antagonist ICI-182-780 does not inhibit the transformation phenotypes induced by 17-beta-estradiol and 4-OH estradiol in human breast epithelial cells. Int. J. Oncol. 2005;26:423–429. [PubMed] [Google Scholar]
- 73.Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, Garber J, Russo IH. 17β-Estradiol induces transformations and tumorigenesis in human breast epithelial cells. FASEB J. 2006;20:1622–1634. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- 74.Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J. Mammary Gland Biol. Neoplasia. 1997;2:323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- 75.Bocchinfuso WP, Hively WP, Couse JF, Varmus HE, Korach KS. A mouse mammary tumor virus-wnt-1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor-alpha. Cancer Res. 1999;59:1869–1876. [PubMed] [Google Scholar]
- 76.Devanesan P, Santen RJ, Bocchinfuso WP, Korach KS, Rogan EG, Cava-lieri EL. Catechol estrogen metabolites and conjugates in mammary tumors and hyperplastic tissue from estrogen receptor alpha knock out (ERKO)/Wnt 1 mice; implications for initiation of mammary tumors. Carcinogenesis. 2001;22:1573–1576. doi: 10.1093/carcin/22.9.1573. [DOI] [PubMed] [Google Scholar]
- 77.Yue W, Santen RJ, Wang JP, Li Y, Verderame MF, Bocchinfuso WP, Korach KS, Devanesan P, Todorovic R, Rogan EG, Cavalieri EL. Genotoxic metabolites of estradiol in breast: potential mechanism of estradiol induced carcinogenesis. J. Steroid Biochem. Mol. Biol. 2003;86:477–486. doi: 10.1016/s0960-0760(03)00377-7. [DOI] [PubMed] [Google Scholar]
- 78.Santen RJ, Yue W, Bocchinfuso W, et al. Estradiol-induced carcinogenesis via formation of genotoxic metabolites. In: Ingle JN, Dowsett M, editors. Advances in Endocrine Therapy of Breast Cancer. Marcel Dekker; 2003. pp. 163–177. [Google Scholar]
- 79.Santen R, Cavalieri E, Rogan E, Russo J, Guttenplan J, Ingle J, Yue W. Estrogen mediation of breast tumor formation involves estrogen receptor-dependent, as well as independent, genotoxic effects. Ann. NY Acad. Sci. 2009;1155:132–140. doi: 10.1111/j.1749-6632.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- 80.Kalyanaraman B, Felix CC, Sealy RC. Semiquinone anion radicals of cat-echol(amine)s, catechol estrogens, and their metal ion complexes. Environ. Health Perspect. 1985;64:185–194. doi: 10.1289/ehp.8564185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kalyanaraman B, Felix CC, Sealy RC. Peroxidatic oxidation of catecholamines: a kinetic electron spin resonance investigation using the spin stabilization approach. J. Biol. Chem. 1984;259:7584–7589. [PubMed] [Google Scholar]
- 82.Zahid M, Saeed M, Yang L, Rogan E, Cavalieri E. Proposed role of dopamine quinone–DNA adducts in the etiology of Parkinson’s disease, unpublished results. doi: 10.1002/iub.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh S, Chakravarti D, Edney JA, Hollins RR, Johnson PJ, West WW, Higginbotham SM, Cavalieri EL, Rogan EG. Relative imbalances in the expression of estrogen-metabolizing enzymes in the breast tissue of women with breast carcinoma. Oncol. Rep. 2005;14:1091–1096. [PubMed] [Google Scholar]
- 84.Zhang M, Huang J, Xie X, Holman CDJ. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. Int. J. Cancer. 2009;124:1404–1408. doi: 10.1002/ijc.24047. [DOI] [PubMed] [Google Scholar]
- 85.Grube BJ, Eng ET, Kao Y-C, Kwon A, Chen S. White button mushroom phytochemicals inhibit aromatase activity and breast cancer cell proliferation. J. Nutr. 2001;131:3288–3293. doi: 10.1093/jn/131.12.3288. [DOI] [PubMed] [Google Scholar]
- 86.Chen S, Oh S-R, Phung S, Hur G, Ye JJ, Kwok SL, Schrode GE, Belury M, Adams LS, Williams D. Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus) Cancer Res. 2006;66:12026–12034. doi: 10.1158/0008-5472.CAN-06-2206. [DOI] [PubMed] [Google Scholar]
- 87.Montano MM, Chaplin LJ, Deng H, et al. Protective roles of quinone reductase and tamoxifen against estrogen-induced mammary tumorigenesis. Oncogene. 2007;26:3587–3590. doi: 10.1038/sj.onc.1210144. [DOI] [PubMed] [Google Scholar]
- 88.Zahid M, Gaikwad N, Rogan EG, Cavalieri EL. Inhibition of depurinating estrogen–DNA adduct formation by natural compounds. Chem. Res. Toxicol. 2007;20:1947–1953. doi: 10.1021/tx700269s. [DOI] [PubMed] [Google Scholar]
- 89.Jang M, Cai L, Udeani GO. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 90.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 91.Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review) Int. J. Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- 92.Zahid M, Gaikwad N, Ali MF, Lu F, Saeed M, Yang L, Rogan EG, Cavalieri EL. Prevention of estrogen–DNA adducts in MCF-10F cellsby resveratrol. Free Radic. Biol. Med. 2008;45:136–145. doi: 10.1016/j.freeradbiomed.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zahid M, Saeed M, Ali MF, Rogan EG, Cavalieri EL. N-acetylcysteine blocks formation of cancer-initiating estrogen–DNA adducts in cells. Free Radic. Biol. Med. 2010;49:392–400. doi: 10.1016/j.freeradbiomed.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zahid M, Saeed M, Beseler C, Rogan EG, Cavalieri EL. Resveratrol and N-acetylcysteine block the cancer-initiating step in MCF-10F cells. Free Radic. Biol. Med. 2011;50:78–85. doi: 10.1016/j.freeradbiomed.2010.10.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Venugopal D, Zahid M, Mailander PC, Meza JL, Rogan EG, Cavalieri EL, Chakravarti D. Reduction of estrogen-induced transformation of mouse mammary epithelial cells by N-acetylcysteine. J. Steroid Biochem. Mol. Biol. 2008;109:22–30. doi: 10.1016/j.jsbmb.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Samuni AM, Chuang EY, Krishna MC, et al. Semiquinone radical intermediate in catecholic estrogen-mediated cytotoxicity and mutagenesis: chemoprevention strategies with antioxidants. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5390–5395. doi: 10.1073/pnas.0930078100. [DOI] [PMC free article] [PubMed] [Google Scholar]