Abstract
In 2012, the Joint Committee on Vaccination and Immunisation recommended that the National Immunisation Programme for influenza be extended to include healthy children/adolescents aged 2–17 years. In the UK, extension of this new immunisation programme began in 2013–2014 and targeted children aged 2 years and 3 years in primary care. Several implementation pilots were undertaken in primary schools across England, Scotland, Wales and Northern Ireland, as well as a single pilot in a secondary school in England. This article shares lessons learnt from experiences in England and Scotland to provide guidance for other countries considering the addition of childhood influenza vaccination into their national immunisation programmes. Recommendations are provided to help ensure effective preparation and management of new childhood influenza vaccination programmes in other countries. This article describes the processes utilised in England and Scotland for programme setup, workforce management, identification and care of contraindicated patients, collection of data on vaccine uptake, communication strategies, and education of parents and children.
Keywords: influenza, vaccine, vaccination, children, schools, pilot project, England, Scotland
Introduction
In 2012, the Joint Committee on Vaccination and Immunisation (JCVI) recommended that the national immunisation programme (NIP) for influenza be extended to include healthy children/adolescents aged 2–17 years to reduce the high paediatric burden of influenza by reducing the number of cases in children directly, and offering herd protection to others [1,2]. The JCVI advised that this extension would be best delivered through schools, though pre-school children would need to be vaccinated by general practitioners (GPs). The live attenuated influenza vaccine (LAIV) Fluenz† was selected as the vaccine of choice due to: its superior efficacy [3–6] in the 2–17-year age range versus the trivalent inactivated influenza vaccine (TIV); its comparable safety profile; the lack of an alternative equivalently effective vaccine for children [2]. Fluenz is not licensed for children younger than 2 years old [7], so the recommendations were made for children aged ≥2 years. This vaccine was provided free of charge to children through their GP or in the school pilot.
In the UK, extension of this programme began in October 2013–2014 by targeting children aged 2 years and 3 years in primary care, along with several implementation pilots in schools across England, Scotland, Wales and Northern Ireland for children aged 4–11 years. Successful implementation of a LAIV programme in NIPs in the first season required the support of a wide range of stakeholders. In addition, such programmes required expansion of arrangements for large-scale supply, storage and distribution, as well as advice on optimal management of the vaccination of children. Here, we share information and the lessons learnt from implementation pilots in England and Scotland to provide practical advice to other countries considering the addition of childhood influenza vaccination into their NIPs.
Overview of the influenza implementation programme in England and Scotland
Once rolled out completely, the UK influenza vaccination programme aimed to vaccinate 9 million school children in 6–8 weeks (during October to December for maximum efficacy). This task required substantial logistical preparation and management. The first phase of the programme comprised a national roll-out of the influenza vaccination for 2- and 3-year-olds in GP settings, and a series of pilots to test the feasibility of the programme on a smaller scale and in different healthcare settings to establish best practice for immunisation of school-aged children. In England, most pilots were based in primary schools, with one pilot based in a secondary school and one based in the community. In Scotland, most health boards delivered pilots in primary schools, with a small number including only those in the last 2 years of primary school (≈10–12 years of age).
Experience of programme implementation
Collaboration at the local level
Collaboration at the local level was undertaken between the services shown in Table 1. At the start of the project in June 2013, a project board was convened for the four countries undertaking the pilot programmes: England, Scotland, Wales, and Northern Ireland. The project board considered: vaccine supply; project management; surveillance of vaccine uptake; training; protocols. It also agreed on the design and schedule of the pilots. In Scotland, a governance structure was put in place to support the topics mentioned above.
Table 1.
Collaborators in implementation of the childhood immunisation programme during 2013–2014 in the UK.
|
It was decided that the pilot programmes would use several services to administer the vaccine, shown in Table 2 for England and Scotland.
Table 2.
Overview of pilot designs in 2013–2014 season in England and Scotland.
| Country | Age groups covered | Type of programmes utilised | Vaccinators |
|---|---|---|---|
| England | 2–3-year olds Primary schools mainly Single pilot in a secondary school |
General practices, schools, community centres | Immunisation team of the trust health, pharmacists, GPs, school nurses, self-administration/assisted administration |
| Scotland | 2–3-year olds Primary schools only |
General practices, schools, community centres | GPs, practice nurses, bank nurses, students and healthcare support workers |
GP, general practitioner.
Setup and workforce management
A programme of this scale required substantial logistical co-ordination at local and national levels. Pilots were set up in early–mid 2013 and this setup time was critical. Lack of sufficient time for setup was a key challenge in many pilot sites. The pilot programme and initial roll-out in general practice required substantial capacity in terms of healthcare providers and administrative support. Staffing levels varied between the school pilots depending on the healthcare providers used, but vaccinators as well as administrative support staff were essential. Health Immunisation Teams used a combination of qualified school nurses and other skilled staff (e.g., unqualified health support workers). Staffing in the general-practice programme relied on the existing general practice structure with a leading team that usually comprised a named physician, nurse, and administrative clerk responsible for the campaign, all of whom offered influenza vaccination and who were trained appropriately.
Community pharmacies also played an important part in the delivery of the vaccine in Cumbria [8], with ≈13,000 vaccines given in the community (of which >80% were given by community pharmacists). However, pharmacies were not used as part of the programme in Scotland for the pilot year, and are not being considered at the moment as part of the roll-out in Scotland.
Experiences garnered during the influenza season elucidated some key issues. For example, employing staff on temporary contracts was problematic due to training issues, lack of an experienced workforce, and obtaining the prerequisite checks (e.g., Disclosure and Barring Service). Self-administration, though well received by children, was not straightforward, because the children became over-excited, and a lack of structure and supervision hindered running of the programme.
Contracting with multiple pharmacy providers was time-consuming, and vaccine distribution to multiple providers introduced the potential for increased wastage.
Systems for data management
Data-management systems were an important component of the programme in general practice and the pilot studies. They enabled effective and efficient management of patient information and monitoring of immunisations. For example, in England, GPs could search the National Health Service (NHS) database to identify the children in the target group, and immunisation rates were reported every week to Public Health England. In Scotland, data for vaccine uptake were collected every week through remote extraction of information from GP vaccinations using an existing system modified to include the additional age groups targeted in the new programme.
In the pilot programmes in England, utilisation of an Internet-based system with ‘live’ data on uptake from individual pharmacies was extremely useful because it enabled timely monitoring and management of the project. In England, the Department of Health, NHS, and Public Health England utilise a system known as ImmForm to collect data on vaccine uptake within immunisation programmes, the incidence of influenza-like illnesses, and to provide vaccine-ordering facilities for the NHS [9]. In Scotland, data for vaccine uptake for school pilots were collected locally and sent to the NHS Board for provision to Health Protection Scotland by email and reported in weekly updates [10].
Managing wastage
Limiting the amount of wastage is important in any immunisation programme. General practice and pilot programmes took steps to avoid wastage where possible. GPs could order vaccines in stages, thereby avoiding wastage and optimising use of refrigerator storage, which is at a premium during the influenza season (when all manufacturers deliver their influenza vaccines to GP practices). In Scotland, vaccine wastage in the schools programme was monitored routinely as part of the weekly data on vaccine uptake. Also, vaccine-holding centres put systems in place to minimise wastage as much as possible.
Identification and care of contraindicated patients
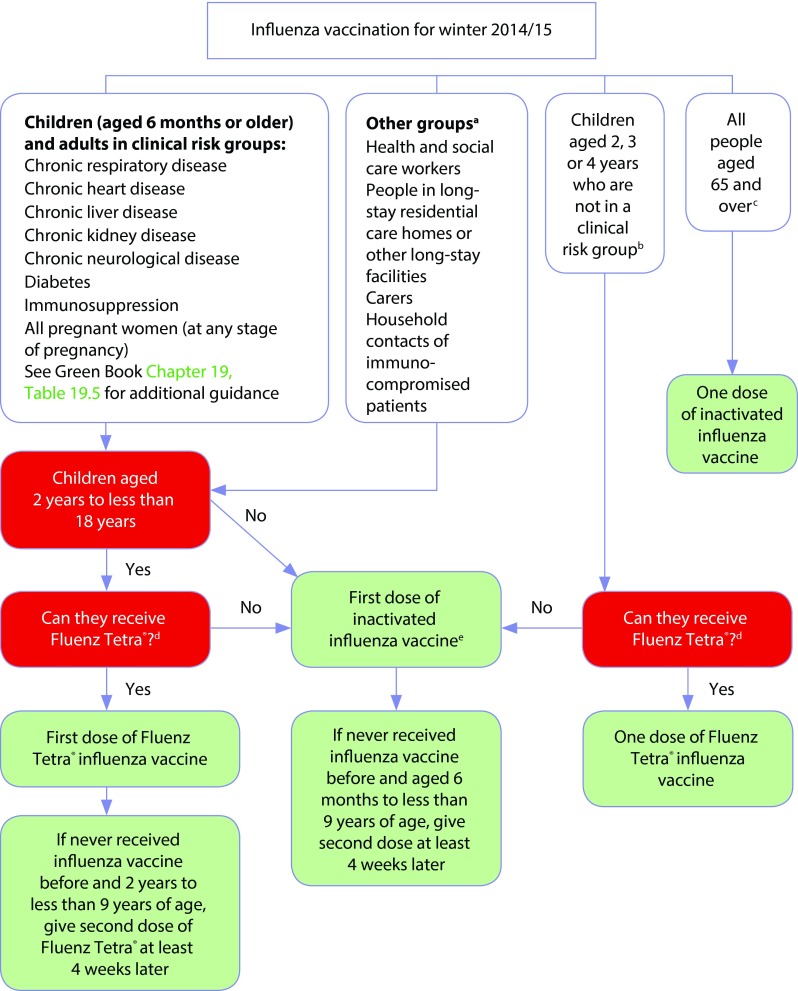
The LAIV utilised in the UK programme (Fluenz) is contraindicated in certain individuals, such as those with severe asthma [7,11]. Further details can be found in point ‘e’ of Figure 1.
Figure 1.
Algorithm for influenza vaccination for winter 2014–2015 from Influenza: the Green Book [1].
aFollow additional guidance from UK health departments; ball children aged 2, 3, or 4 years (but not ≥5 years) on or before 1 September 2014*; call those aged ≥65 years, including all those aged 65 years on or before 1 March 2015; dif quadrivalent inactivated vaccine is available, consider for children aged ≥3 years only. If quadrivalent is not available, offer a suitable trivalent inactivated influenza vaccine. See Table 19.6 in Influenza: the Green Book [1], which lists the vaccines that can be used in young children: some are not suitable for young children; ecannot receive if: aged <2 years; aged ≥18 years; have egg allergy; have a history of active wheezing at the time of vaccination (until ≥7 days after wheezing has stopped); taking oral corticosteroids or high-dose inhaled corticosteroids for asthma; have certain immunodeficiencies; pregnant. This chart should be read in conjunction with the contraindications and precautions sections as well as Table 19.6 in Influenza: the Green Book [1], which gives details about the age indications for influenza vaccines.
*Note: In addition to these age groups, the administrations of constituent countries will also offer influenza vaccination to children aged 5 years (Scotland), all primary school children (Scotland and Northern Ireland) and children in year 7 (Wales).
In England and Scotland, vaccine administration by healthcare practitioners was in accordance with written instructions known as Patient Group Directions (PGDs) and Patient Specific Directions (PSDs). PGDs and PSDs contain instructions for medication administration (e.g., eligible patients, a description of those excluded from treatment, details of the administration process, relevant warnings).
Guideline definitions were employed to determine specific contraindications. For example, ‘severe asthma’ was defined according to SIGN guidelines set by the British Thoracic Society in children aged 5–12 years [1,12]. In the GP programme, physicians and nurses checked for contraindications in the NHS computer file of patients at the time of vaccination. In addition, parents and children were asked to confirm (in person or on the consent form) whether the child had contraindications. Healthcare providers in some school pilots had access to the NHS computer files of patients. Otherwise, relevant information was obtained from consent forms and discussions with parents and the young people themselves. If children had contraindications to the LAIV, alternative inactivated vaccines were available in some of the school pilots; in others, children were referred to their GP for an alternative vaccine.
Communication and education
Several initiatives were utilised in England and Scotland to encourage vaccine uptake in the GP setting: invitation letters containing information were sent to parents; special clinics for 2- and 3-year-olds were arranged; opportunistic vaccinations were given during other clinics/appointments; late-evening clinics were arranged; text messages via mobile telephones were sent to parents to remind them of clinics. Most Internet websites for practices also provided information on clinic times and how to obtain the vaccine. In addition, posters were distributed and interviews given on local radio stations. School-based pilots utilised local press and radio, newsletters, Internet websites for schools, text messaging on mobile telephones, and computer emails as part of their communication strategy. Educational resource packs were used in Scotland, and were well received. In England, school pilots held parent and school assemblies to encourage vaccine uptake, and engaged with community and faith groups.
Acceptance of the vaccine by parents and young people in schools and general practices
In general, the vaccine was well-accepted by children and parents. Parents brought their children for vaccination at general practices or gave permission for their children to be vaccinated in school pilots. Attitudes to vaccination in England were evaluated through questionnaires in school pilots and national attitudinal research, in addition to patient-satisfaction methods regarding immunisation services overall. Certificates and stickers for vaccinated children were popular in school-based pilots. In addition, healthcare providers found that acceptance from young people and involving them in the design of marketing campaigns (where possible) were important considerations.
In Scotland, research was commissioned by the Scottish Government to evaluate the acceptability and effectiveness of the new vaccine programme. This work showed that parents of 2- and 3-year-olds had very high awareness of the invitation letter and booklet, with virtually all readers rating it as ‘helpful’. The invitation letter from GPs was identified by parents as having a significant impact on their perception of the programme. Experience of the programme for parents whose children were vaccinated was overwhelmingly positive, and parents reported that they were likely to encourage use of the vaccine in the future. There was also good awareness of the nasal spray (LAIV) and use was high, with most parents considering the nasal vaccine better than the injection (TIV). In the primary-school pilots in Scotland, >80% of parents were happy that the vaccine was offered (especially those who had children with underlying medical conditions). However, fewer parents were satisfied with the programme in schools that piloted self-administration, and in smaller schools compared with other settings.
Use of porcine gelatine in the vaccine drew attention in the media. However, porcine gelatine has been certified as ‘acceptable’ by many multi-faith groups, and Public Health England has published advice from representatives of faith communities [13]. Some faith groups had similar concerns about use of porcine gelatine in the nasal spray in Scotland, and a statement was added to the Internet website of Immunisation Scotland to inform parents in response [14].
Results
The UK pilot programme demonstrated that several designs can be used for administration of school-based LAIV immunisations. Experiences from the school pilots and GP programme also provided insights into successful strategies as well as challenges that can be addressed in future programmes. Previously published data show that the UK inclusion of childhood influenza vaccination in school settings achieved an overall uptake of 52.5% in England, and 67.2% in Scotland (Table 3 contains uptake data for 2- and 3-year-olds) [8,15].
Table 3.
Vaccine uptake in general practice and pilot programmes during the 2013–2014 season.
Timely collaboration between the services shown in Table 1 was critical to successful roll-out of the programme, and engagement with local partnerships was crucial. School personnel and school nurses were found to play important parts in the delivery and management of school-based pilots, and studies have also highlighted the importance of their involvement [16]. School-based immunisation was well received by parents, as seen in previous programmes [17]. Similarly, in agreement with the finding that children have a preference for an intranasal vaccine [18], the vaccine was well-accepted by children in the UK programme, with positive feedback from attitudinal research in England and Scotland.
Discussion and recommendations
Uptake observed during the first season of the childhood immunisation programme in the UK compares favourably with that achieved in the USA (where LAIV and inactivated influenza vaccine have been used as part of universal childhood immunisation programmes for several years) [8]. In Canada, TIV uptake in infants aged 6–23 months was far lower (<9%) [19]. Consequently, UK uptake is very encouraging despite its use in a pilot year. The pilot programme demonstrates that a childhood immunisation programme, undertaken in schools, GP surgeries, and across different healthcare settings, is feasible and can achieve a good level of coverage. Experiences obtained from pilots are especially valuable as future roll-out of the programme expands to older age groups in subsequent years [20]. Here, we consider the key learnings from the experience in England and Scotland alongside recommendations from other studies of the implementation of immunisation programmes for children. A summary of our recommendations is shown in Table 4.
Table 4.
Key learning points from implementation in England and Scotland and future considerations.
|
Setup and planning
A successful immunisation programme requires thorough planning and adequate setup time. Compositions of local area teams/child health immunisation teams will vary depending on staff resources and local setups, so contact should be made during the planning stage as early as possible to establish the resources available. A project board/new governance structure should be established to plan the upcoming programme and, ideally, liaisons with schools should occur as early as possible to allow communication and planning with schools to be done in advance of school holidays. Relationships should be established early on and maintained through regular and clear communications. Previous school-based immunisation programmes have identified setup time as an essential factor to enable successful implementation [21]. Before introduction of school-based immunisation programmes, school visits should be undertaken to assess facilities and ensure that the requirements of the programme are understood. Assessment of the facilities can identify logistical/safety issues that need to be addressed before the programme starts. In addition, vaccinators should meet school staff and young people to promote the programme and share information. Few studies have evaluated the setup and logistics for delivery of a childhood influenza immunisation programme. However, in general, the key learning points of existing studies pertain to: effective planning; securing adequate staffing levels; education of parents and children; utilisation of reminder systems [22–25]. Finally, more flexible vaccination appointments at general practices should be made available during the half-term week, additional Saturday clinics, or midweek evening clinics to encourage vaccine uptake.
Data-management systems
Data-management tools enable timely monitoring and management of the project. Collection of electronic data on vaccine uptake for stakeholders should be utilised if available. For example, a system similar to the ImmForm system used by Public Health England to collect data on vaccine uptake and the incidence of influenza-like illnesses, and to provide vaccine-ordering facilities, would be valuable. If a system for collection of electronic data on vaccine uptake is lacking, use of a standard form or Microsoft Excel™ spreadsheet could be used (as was done in Scotland).
Managing wastage
Effective management of vaccines throughout the supply chain is essential to reduce wastage. Wastage can be reduced through careful planning for vaccine storage and ordering smaller numbers of vaccine doses frequently to optimise storage space. Furthermore, arrangements with schools should be secured well in advance of programme commencement, with robust systems in place for resolving issues relating to consent/contraindicated patients. In general, good control of programme logistics will reduce wastage. Ideally, the immunisation programme should begin as soon as the vaccine has been supplied.
Identification and care of contraindicated patients
Early identification of ‘high-risk’ children and prioritisation of schools for children with disabilities or complex health needs is important to ensure these individuals are included in the vaccination programme. National instructions on vaccine administration (PGDs/PSDs or equivalents) should be followed to ascertain which patients are suitable for vaccination. For example, a PGD template (as produced in Scotland [26]) could be adapted for use in local clinical governance arrangements in other countries. Vaccinators must be trained to identify contraindicated patients, and a system to provide contraindicated patients with an alternative vaccine should be in place. An algorithm for care of these patients should be developed as shown in Influenza: the Green Book (Figure 1) [1].
Communication and education
The patient’s perspective should be considered from the beginning. Children and parents should be involved in discussions when planning communication and education strategies. Prior testing of attitudes will be very useful for informing implementation and raising potential issues before programme commencement. Qualitative attitudinal research, before and after introduction of the programme, will also be valuable.
Parents and young people should be aware of the seriousness of influenza and its complications. Consistent and clear messages must be given to educate parents about the need for immunisation. A National Influenza Campaign that seeks to inform parents would be valuable to all stakeholders. A standard letter to parents from public health authorities is recommended to provide clear guidance and information to parents. This letter should include information on: the choice of vaccine, its respective indications, and the reason for its inclusion on the immunisation schedule. For young children, an equivalent letter from GPs may be more appropriate. After receipt of the standard letter, invitations for vaccination should be sent at the beginning of the academic year for school children, and parents of unvaccinated children should receive reminder letters where appropriate. Use of technology to facilitate easy dissemination of information should be utilised whenever possible. Optimisation of technology to encourage vaccine uptake has been utilised in other immunisation programmes [21,25].
Overall, effective education and communication are essential to raise awareness of the importance of immunisation, encourage vaccine uptake, and facilitate a well-organised programme. Educating parents and children is likely to improve parents’ willingness to provide consent for vaccination [16,22]. Education of healthcare workers is paramount for communication with patients and for adherence to protocols. Consequently, provision of educational resource packs to children, young people, parents, and healthcare workers in advance of each immunisation programme would be valuable, and has been identified as a key lesson from pilot studies. A checklist for communication and education is provided in Table 5.
Table 5.
Checklist for communication and education.
|
Conclusions
The UK implementation of childhood influenza vaccination for healthy children using LAIV during the 2013–2014 season demonstrated that school- and GP office-based influenza immunisation programmes for children and adolescents are feasible and can achieve a good rate of coverage in this age group. Given the high burden of paediatric influenza and the suitability of an intranasal LAIV, other national programmes similar to the UK implementation are likely to be utilised increasingly in the future. It is hoped that the recommendations outlined in this article may guide other countries looking to implement a childhood influenza immunisation programme using LAIV.
Acknowledgments
Editorial support was provided by Talya Underwood (Prime Medical Group, London UK), funded by AstraZeneca. The opinions, conclusions, and data interpretation in this article are the responsibility of the authors.
Abbreviations:
- GP
general practitioner
- JCVI
Joint Committee on Vaccination and Immunisation
- LAIV
live attenuated influenza vaccine
- NIP
national immunisation programme
- NHS
National Health Service
- PGD
Patient Group Direction
- PSD
Patient Specific Direction
- TIV
trivalent inactivated influenza vaccine
Footnotes
Contributions
All authors were involved in drafting the manuscript. S White was a representative to the National Public Health England programme board and a member of Department of Health School Nursing Childhood Flu Working Party. AJ Reynolds was a representative of Health Protection Scotland (who are responsible for leading co-ordination of the planning and implementation of the Fluenz programme in Scotland). AJ Reynolds was also a Devolved Administration representative on the board of the National Public Health England programme. S Rajaram conceptualised the article with authors, and undertook the literature review to appraise evidence on implementation of immunisation programmes relevant to this article.
Potential conflicts of interest
The International Committee of Medical Journal Editors’ (ICMJE) Potential Conflicts of Interests forms for the authors are available for download at: http://www.drugsincontext.com/wp-content/uploads/2015/04/dic.212280-COI.pdf
G Kassianos has received honoraria from AstraZeneca for speaking at educational meetings and chairing advisory boards. S White has received honoraria from AstraZeneca for speaking at educational meetings and has worked as a consultant for AstraZeneca on resources for childhood influenza. AJ Reynolds has no conflicts of interest. S Rajaram is an employee of AstraZeneca.
Fluenz is a registered trademark belonging to AstraZeneca, UK.
Funding declaration
The LAIV is manufactured and supplied to the UK Department of Health by Medimmune, the biologicals arm of AstraZeneca.
George Kassianos and Sharon White received honoraria from AstraZeneca to present and discuss the UK implementation programme at a roundtable meeting sponsored by AstraZeneca at European Society for Paediatric Infectious Diseases (ESPID) 2014 in Dublin, Ireland. Arlene J. Reynolds provided expert opinion from Scotland without receiving honoraria.
References
- 1.Influenza: the Green Book, chapter 19. Available from: https://www.gov.uk/government/publications/influenza-the-green-book-chapter-19. Last accessed: 18 November 2014.
- 2.Joint Committee on Vaccination and Immunisation Minutes of the meeting held on Friday 13 April 2012. Available from: http://media.dh.gov.uk/network/261/files/2012/05/JCVI-minutes-13-April-2012-meeting.pdf. Last accessed: 11 September 2014.
- 3.Ashkenazi S, Vertruyen A, Aristegui J, Esposito S, McKeith DD, Klemola T, Biolek J, Kuhr J, Bujnowski T, Desgrandchamps D, Cheng SM, Skinner J, Gruber WC, Forrest BD. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006;25:870–9. doi: 10.1097/01.inf.0000237829.66310.85. [DOI] [PubMed] [Google Scholar]
- 4.Fleming DM, Crovari P, Wahn U, Klemola T, Schlesinger Y, Langussis A, Oymar K, Garcia ML, Krygier A, Costa H, Heininger U, Pregaldien JL, Cheng SM, Skinner J, Razmpour A, Saville M, Gruber WC, Forrest B. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006;25:860–9. doi: 10.1097/01.inf.0000237797.14283.cf. [DOI] [PubMed] [Google Scholar]
- 5.Belshe RB, Ambrose CS, Yi T. Safety and efficacy of live attenuated influenza vaccine in children 2–7 years of age. Vaccine. 2008;26(Suppl 4):D10–6. doi: 10.1016/j.vaccine.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 6.Rhorer J, Ambrose CS, Dickinson S, Hamilton H, Oleka NA, Malinoski FJ, Wittes J. Efficacy of live attenuated influenza vaccine in children: A meta-analysis of nine randomized clinical trials. Vaccine. 2009;27:1101–10. doi: 10.1016/j.vaccine.2008.11.093. [DOI] [PubMed] [Google Scholar]
- 7.Fluenz Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001101/WC500103709.pdf. Last accessed: 11 September 2014.
- 8.Pebody RG, Green HK, Andrews N, Zhao H, Boddington N, Bawa Z, Durnall H, Singh N, Sunderland A, Letley L, Ellis J, Elliot AJ, Donati M, Smith GE, de LS, Zambon M. Uptake and impact of a new live attenuated influenza vaccine programme in England: early results of a pilot in primary school-age children, 2013–14 influenza season. Euro Surveill. 2014;19:20823. doi: 10.2807/1560-7917.ES2014.19.22.20823. [DOI] [PubMed] [Google Scholar]
- 9. ImmForm. Available from: http://www.gov.uk/government/collections/immform. Last accessed: 16 October 2014.
- 10.Health Protection Scotland Health Protection Scotland Seasonal Influenza Report 2015. Available at: http://www.hps.scot.nhs.uk/resp/seasonalInfluenza.aspx#report. Last accessed: 10 February 2015. [Google Scholar]
- 11.Fluenz Tetra Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002617/WC500158412.pdf. Last accessed: 11 September 2014.
- 12.British guideline on the management of asthma. Available from: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2014/. Last accessed: 17 November 2014.
- 13. GOV.UK letter. Vaccines and gelatine: PHE response. 14 A.D. Available at: https://www.gov.uk/government/news/vaccines-and-gelatine-phe-response. Last accessed: 18 November 2014.
- 14.Immunisation Scotland Immunisation Scotland Child Flu information. 2015. Available at: http://www.immunisationscotland.org.uk/vaccinesand-diseases/seasonalflu/childflu.aspx. Last accessed: 10 February 2015.
- 15. Surveillance of influenza and other respiratory viruses in the United Kingdom: Winter 2013–14. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/325203/Flu_annual_report_June_2014.pdf. Last accessed: 18 November 2014.
- 16.Boyer-Chuanroong L, Woodruff BA, Unti LM, Sumida YU. Immunizations from ground zero: lessons learned in urban middle schools. J Sch Health. 1997;67:269–72. doi: 10.1111/j.1746-1561.1997.tb03447.x. [DOI] [PubMed] [Google Scholar]
- 17.Middleman AB, Short MB, Doak JS. School-located influenza immunization programs: factors important to parents and students. Vaccine. 2012;30:4993–9. doi: 10.1016/j.vaccine.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Flood EM, Ryan KJ, Rousculp MD, Beusterien KM, Block SL, Hall MC, Mahadevia PJ. A survey of children’s preferences for influenza vaccine attributes. Vaccine. 2011;29:4334–40. doi: 10.1016/j.vaccine.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Campitelli MA, Inoue M, Calzavara AJ, Kwong JC, Guttmann A. Low rates of influenza immunization in young children under Ontario’s universal influenza immunization program. Pediatrics. 2012;129:e1421–30. doi: 10.1542/peds.2011-2441. [DOI] [PubMed] [Google Scholar]
- 20.Childhood flu immunisation programme: update and provisional roll-out schedule. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/331045/2014-15_update_and_roll-out_schedule_letter.pdf. Last accessed: 11 September 2014.
- 21.Watson M, Shaw D, Molchanoff L, McInnes C. Challenges, lessons learned and results following the implementation of a human papilloma virus school vaccination program in South Australia. Aust N Z J Public Health. 2009;33:365–70. doi: 10.1111/j.1753-6405.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- 22.Cooper Robbins SC, Ward K, Skinner SR. School-based vaccination: a systematic review of process evaluations. Vaccine. 2011;29:9588–99. doi: 10.1016/j.vaccine.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 23.Humiston SG, Szilagyi PG, Iwane MK, Schaffer SJ, Santoli J, Shone L, Barth R, McInerny T, Schwartz B. The feasibility of universal influenza vaccination for infants and toddlers. Arch Pediatr Adolesc Med. 2004;158:867–74. doi: 10.1001/archpedi.158.9.867. [DOI] [PubMed] [Google Scholar]
- 24.Humiston SG, Rosenthal SL. Challenges to vaccinating adolescents: vaccine implementation issues. Pediatr Infect Dis J. 2005;24(Suppl 6):S134–40. doi: 10.1097/01.inf.0000166161.12087.94. [DOI] [PubMed] [Google Scholar]
- 25.Jenlink CH, Kuehnert P, Mazyck D. Influenza vaccinations, fall 2009: model school-located vaccination clinics. J Sch Nurs. 2010;26(4 Suppl):7S–13S. doi: 10.1177/1059840510368960. [DOI] [PubMed] [Google Scholar]
- 26.Health Protection Scotland Health Protection Scotland Patient Group Direction template. 2015. Available at: http://www.documents.hps.scot.nhs.uk/respiratory/seasonal-influenza/pgd/pgd-fluenz-national-specimen.pdf. Last accessed: 10 February 2015.