Abstract
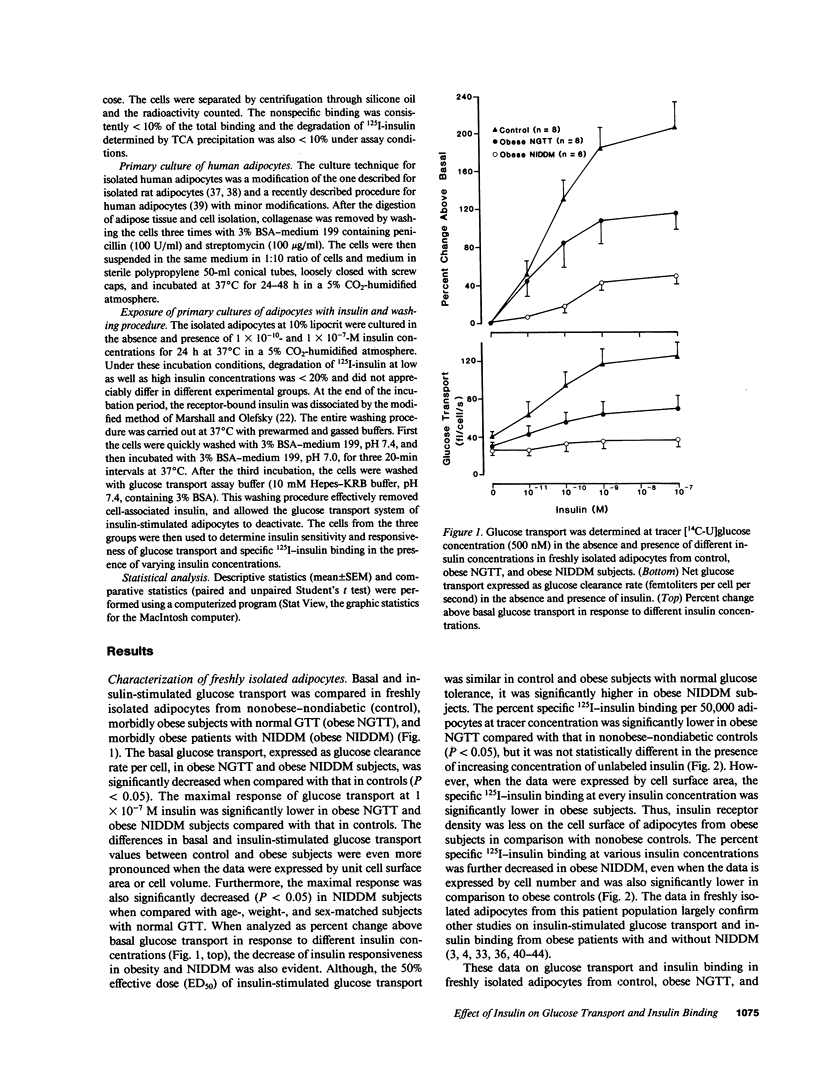
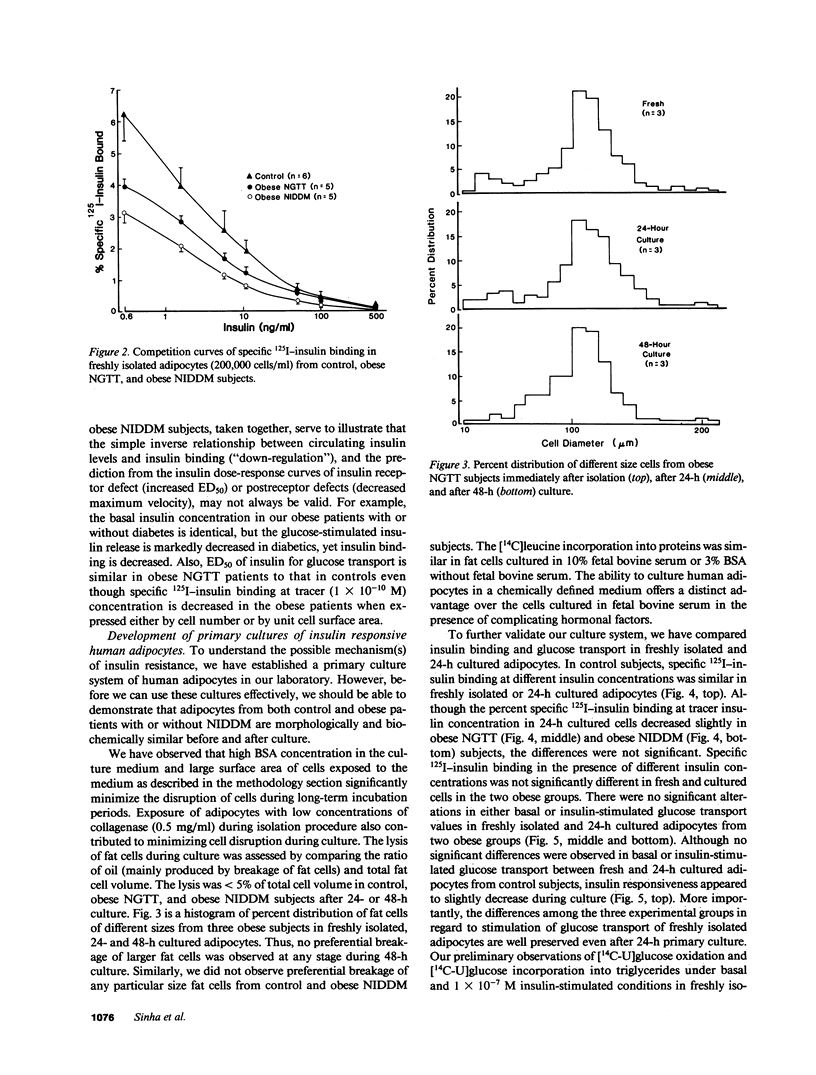
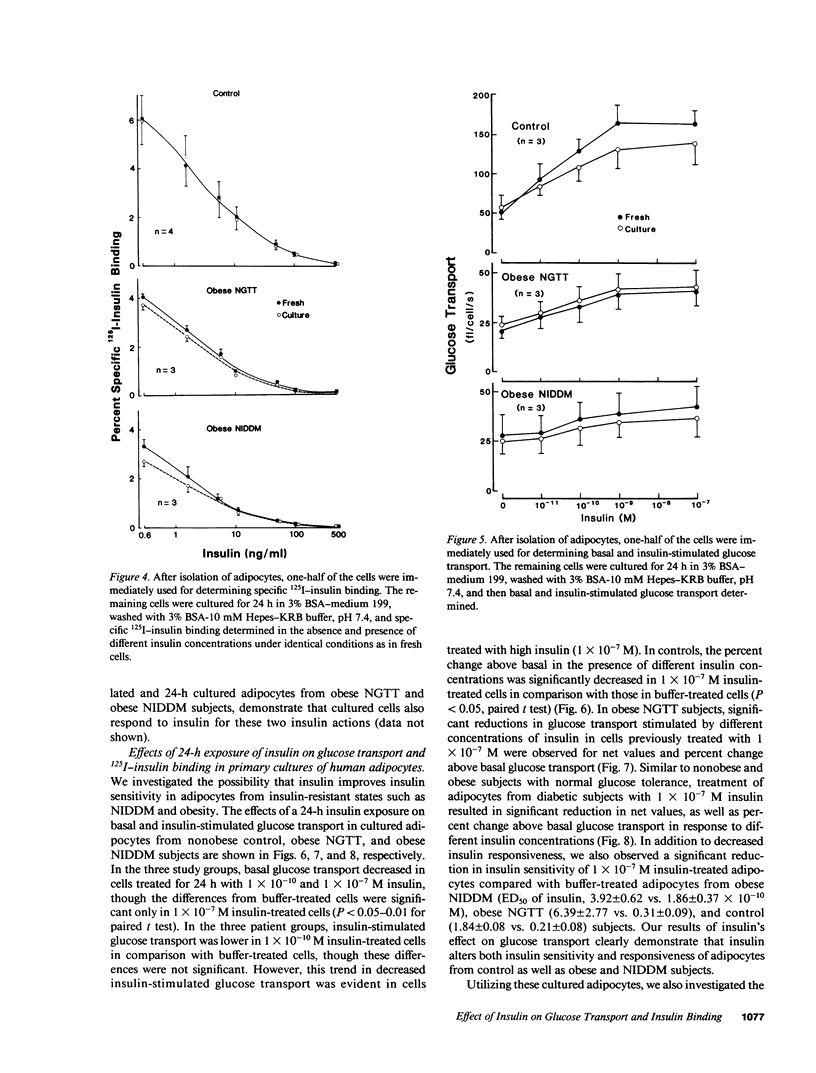
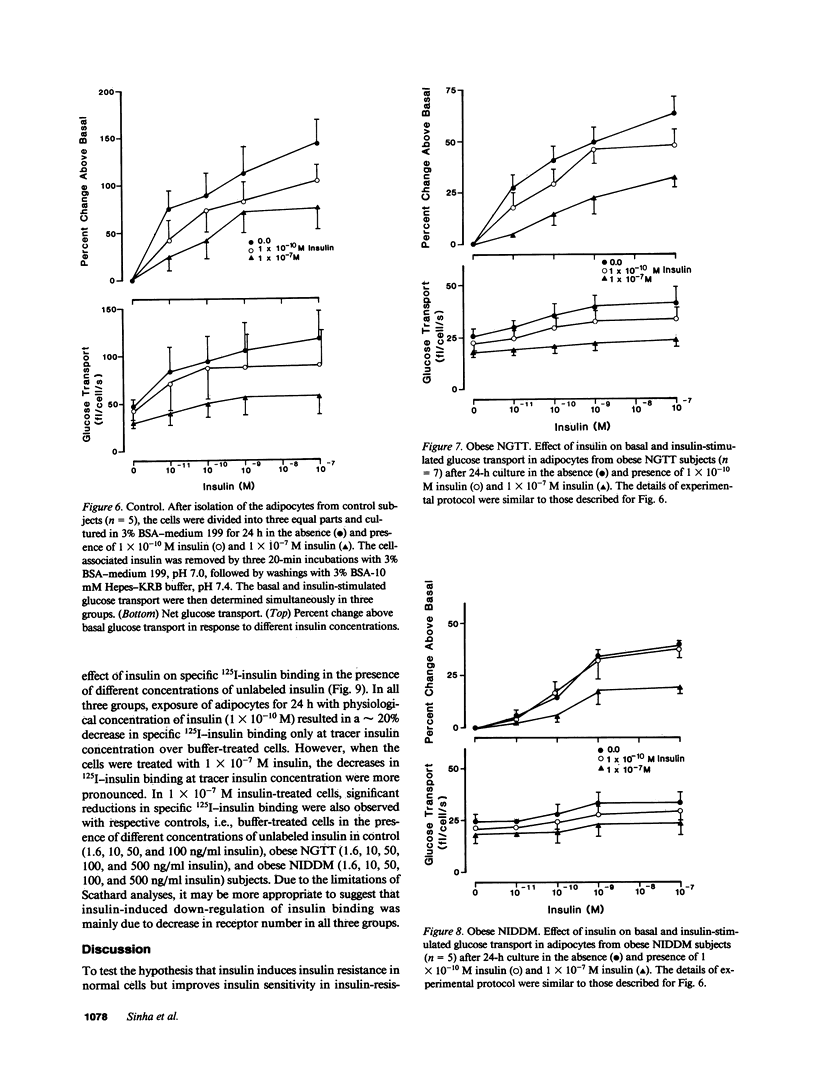
We have tested the hypothesis that in vitro exposure of insulin-resistant adipocytes with insulin results in improved insulin action. A primary culture system of adipocytes from obese subjects with or without non-insulin-dependent diabetes mellitus (NIDDM) and nonobese control subjects has been developed. The adipocytes when cultured in serum-free medium do not lose their original characteristics in regard to insulin binding and glucose transport. The adipocytes from three groups were incubated with insulin (0, 10(-10) M, and 10(-7) M) for 24 h at 37 degrees C, receptor-bound insulin was dissociated, and basal and insulin (1 X 10(-11)-10(-7) M)-stimulated glucose transport and 125I-insulin binding were determined. The 24-h insulin exposure of adipocytes from control subjects decreased basal and insulin-stimulated glucose transport. The effects of 1 X 10(-7) M insulin were more pronounced than 1 X 10(-10) M insulin. Similarly, insulin exposure decreased insulin sensitivity and responsiveness of cultured adipocytes from obese and NIDDM patients. The insulin-induced reduction in insulin sensitivity and responsiveness for glucose transport in three groups were due to alterations at insulin binding and postbinding levels. In conclusion, insulin induces insulin resistance in control adipocytes and further worsens the insulin resistance of adipocytes from obese and NIDDM subjects. For insulin to improve the insulin resistance of adipocytes from NIDDM patients, either more prolonged in vitro insulin exposure and/or other hormonal factors might be required.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amatruda J. M., Chang C. L. The regulation of lipid synthesis in primary cultures of hepatocytes from nonketotic streptozotocin diabetic rats. Metabolism. 1983 Mar;32(3):224–229. doi: 10.1016/0026-0495(83)90186-5. [DOI] [PubMed] [Google Scholar]
- Amatruda J. M., Newmeyer H. W., Chang C. L. Insulin-induced alterations in insulin binding and insulin action in primary cultures of rat hepatocytes. Diabetes. 1982 Feb;31(2):145–148. doi: 10.2337/diab.31.2.145. [DOI] [PubMed] [Google Scholar]
- Arsenis G., Livingston J. N. Alterations in the tyrosine kinase activity of the insulin receptor produced by in vitro hyperinsulinemia. J Biol Chem. 1986 Jan 5;261(1):147–153. [PubMed] [Google Scholar]
- Blackard W. G., Guzelian P. S., Small M. E. Down regulation of insulin receptors in primary cultures of adult rat hepatocytes in monolayer. Endocrinology. 1978 Aug;103(2):548–553. doi: 10.1210/endo-103-2-548. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Howard B. V., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984 Oct;74(4):1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. K., Settle E. A., Van Rij A. M. Food intake patterns of gastric bypass patients. J Am Diet Assoc. 1982 May;80(5):437–443. [PubMed] [Google Scholar]
- Caro J. F., Amatruda J. M. Insulin receptors in hepatocytes: postreceptor events mediate down regulation. Science. 1980 Nov 28;210(4473):1029–1031. doi: 10.1126/science.7001632. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Siegel J. A., Olefsky J. M. Insulin-stimulated glucose transport in human adipocytes. Am J Physiol. 1979 Jun;236(6):E621–E625. doi: 10.1152/ajpendo.1979.236.6.E621. [DOI] [PubMed] [Google Scholar]
- Cigolini M., Smith U. Human adipose tissue in culture. VIII. Studies on the insulin-antagonistic effect of glucocorticoids. Metabolism. 1979 May;28(5):502–510. doi: 10.1016/0026-0495(79)90189-6. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin--receptor interactions in adipose tissue cells: direct measurement and properties. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1264–1268. doi: 10.1073/pnas.68.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Zarnowski M. J., Franzusoff A. J., Salans L. B. Alterations in glucose metabolism and its stimulation by insulin in isolated adipose cells during the development of genetic obesity in the Zucker fatty rat. Metabolism. 1978 Dec;27(12 Suppl 2):1930–1940. doi: 10.1016/s0026-0495(78)80010-9. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Richardson D. K., Becker S. G., Walters C. G., Gitomer W., Heinrich J. Insulin response in skeletal muscle and fat cells of the genetically obese Zucker rat. Metabolism. 1978 Dec;27(12 Suppl 2):1967–1981. doi: 10.1016/s0026-0495(78)80013-4. [DOI] [PubMed] [Google Scholar]
- Davidson M. B., Casanello-Ertl D. Insulin antagonism in cultured rat myoblasts secondary to chronic exposure to insulin. Horm Metab Res. 1979 Mar;11(3):207–209. doi: 10.1055/s-0028-1092708. [DOI] [PubMed] [Google Scholar]
- DeFronzo R., Deibert D., Hendler R., Felig P., Soman V. Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes. J Clin Invest. 1979 May;63(5):939–946. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Donner C. C., Fraze E., Chen Y. D., Reaven G. M. Quantitation of insulin-stimulated glucose disposal in patients with non-insulin-dependent diabetes mellitus. Diabetes. 1985 Sep;34(9):831–835. doi: 10.2337/diab.34.9.831. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Konda T. S., Davis J. S., Standaert M. L., Pollet R. J., Cooper D. R. Insulin rapidly increases diacylglycerol by activating de novo phosphatidic acid synthesis. Science. 1987 May 1;236(4801):586–589. doi: 10.1126/science.3107122. [DOI] [PubMed] [Google Scholar]
- Firth R. G., Bell P. M., Rizza R. A. Effects of tolazamide and exogenous insulin on insulin action in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1986 May 15;314(20):1280–1286. doi: 10.1056/NEJM198605153142003. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Kashiwagi A., Verso M. A., Reaven G., Andrews J. Improvement in in vitro insulin action after one month of insulin therapy in obese noninsulin-dependent diabetics. Measurements of glucose transport and metabolism, insulin binding, and lipolysis in isolated adipocytes. J Clin Invest. 1983 Dec;72(6):1901–1909. doi: 10.1172/JCI111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Marshall S. Insulin induces progressive insulin resistance in cultured rat adipocytes. Sequential effects at receptor and multiple postreceptor sites. Diabetes. 1986 Mar;35(3):258–267. doi: 10.2337/diab.35.3.258. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Marshall S. Insulin receptor down-regulation is linked to an insulin-induced postreceptor defect in the glucose transport system in rat adipocytes. J Clin Invest. 1985 Jul;76(1):22–30. doi: 10.1172/JCI111950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H., Kimmerling G., Olefsky J. M., Reaven G. M. Demonstration of insulin resistance in untreated adult onset diabetic subjects with fasting hyperglycemia. J Clin Invest. 1975 Mar;55(3):454–461. doi: 10.1172/JCI107951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrick R. B. Morphological changes in the adipocyte during fat deposition and mobilization. Am J Physiol. 1967 Apr;212(4):777–782. doi: 10.1152/ajplegacy.1967.212.4.777. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Martin F. I., Melick R. A. Correlation between insulin receptor binding in isolated fat cells and insulin sensitivity in obese human subjects. J Clin Invest. 1976 Dec;58(6):1435–1441. doi: 10.1172/JCI108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J., Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976 Jul;5(2):299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- Jarett L., Seals J. R. Pyruvate dehydrogenase activation in adipocyte mitochondria by an insulin-generated mediator from muscle. Science. 1979 Dec 21;206(4425):1407–1408. doi: 10.1126/science.505013. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A., Verso M. A., Andrews J., Vasquez B., Reaven G., Foley J. E. In vitro insulin resistance of human adipocytes isolated from subjects with noninsulin-dependent diabetes mellitus. J Clin Invest. 1983 Oct;72(4):1246–1254. doi: 10.1172/JCI111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P. A., Marshall S., Eckel R. H. Regulation of lipoprotein lipase in primary cultures of isolated human adipocytes. J Clin Invest. 1985 Jan;75(1):199–208. doi: 10.1172/JCI111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M. Long-term regulation of adipocyte glucose transport capacity by circulating insulin in rats. J Clin Invest. 1978 Jul;62(1):73–81. doi: 10.1172/JCI109116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner J., Galasko G., Cheng K., DePaoli-Roach A. A., Huang L., Daggy P., Kellogg J. Generation by insulin of a chemical mediator that controls protein phosphorylation and dephosphorylation. Science. 1979 Dec 21;206(4425):1408–1410. doi: 10.1126/science.228395. [DOI] [PubMed] [Google Scholar]
- Livingston J. N., Purvis B. J., Lockwood D. H. Insulin-dependent regulation of the insulin-sensitivity of adipocytes. Nature. 1978 Jun 1;273(5661):394–396. doi: 10.1038/273394a0. [DOI] [PubMed] [Google Scholar]
- Lönnroth P., DiGirolamo M., Smith U. Influence of ambient glucose and insulin concentrations on adipocyte insulin binding. Metabolism. 1983 Jun;32(6):609–614. doi: 10.1016/0026-0495(83)90032-x. [DOI] [PubMed] [Google Scholar]
- Lönnroth P., Digirolamo M., Krotkiewski M., Smith U. Insulin binding and responsiveness in fat cells from patients with reduced glucose tolerance and type II diabetes. Diabetes. 1983 Aug;32(8):748–754. doi: 10.2337/diab.32.8.748. [DOI] [PubMed] [Google Scholar]
- Marshall S., Garvey W. T., Geller M. Primary culture of isolated adipocytes. A new model to study insulin receptor regulation and insulin action. J Biol Chem. 1984 May 25;259(10):6376–6384. [PubMed] [Google Scholar]
- Marshall S. Kinetics of insulin receptor biosynthesis and membrane insertion. Relationship to cellular function. Diabetes. 1983 Apr;32(4):319–325. doi: 10.2337/diab.32.4.319. [DOI] [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Effects of insulin incubation on insulin binding, glucose transport, and insulin degradation by isolated rat adipocytes. Evidence for hormone-induced desensitization at the receptor and postreceptor level. J Clin Invest. 1980 Oct;66(4):763–772. doi: 10.1172/JCI109914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misbin R. I., O'Leary J. P., Pulkkinen A. Insulin receptor binding in obesity: a reassessment. Science. 1979 Sep 7;205(4410):1003–1004. doi: 10.1126/science.472718. [DOI] [PubMed] [Google Scholar]
- Mott D. M., Howard B. V., Bennett P. H. Stoichiometric binding and regulation of insulin receptors on human diploid fibroblasts using physiologic insulin levels. J Biol Chem. 1979 Sep 25;254(18):8762–8767. [PubMed] [Google Scholar]
- Olefsky J. M. Decreased insulin binding to adipocytes and circulating monocytes from obese subjects. J Clin Invest. 1976 May;57(5):1165–1172. doi: 10.1172/JCI108384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Kolterman O. G., Scarlett J. A. Insulin action and resistance in obesity and noninsulin-dependent type II diabetes mellitus. Am J Physiol. 1982 Jul;243(1):E15–E30. doi: 10.1152/ajpendo.1982.243.1.E15. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Hjøllund E., Beck-Nielsen H., Lindskov H. O., Sonne O., Gliemann J. Insulin receptor binding and receptor-mediated insulin degradation in human adipocytes. Diabetologia. 1981 Jun;20(6):636–641. [PubMed] [Google Scholar]
- Pedersen O., Hjøllund E., Sørensen N. S. Insulin receptor binding and insulin action in human fat cells: effects of obesity and fasting. Metabolism. 1982 Sep;31(9):884–895. doi: 10.1016/0026-0495(82)90177-9. [DOI] [PubMed] [Google Scholar]
- Pershadsingh H. A., McDonald J. M. Direct addition of insulin inhibits a high affinity Ca2+-ATPase in isolated adipocyte plasma membranes. Nature. 1979 Oct 11;281(5731):495–497. doi: 10.1038/281495a0. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Reaven G. M., Chen Y. I., Coulston A. M., Greenfield M. S., Hollenbeck C., Lardinois C., Liu G., Schwartz H. Insulin secretion and action in noninsulin-dependent diabetes mellitus. Is insulin resistance secondary to hypoinsulinemia? Am J Med. 1983 Nov 30;75(5B):85–93. doi: 10.1016/0002-9343(83)90258-9. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Mechanism and significance of insulin resistance in non-insulin-dependent diabetes mellitus. Diabetes. 1981 Dec;30(12):990–995. doi: 10.2337/diab.30.12.990. [DOI] [PubMed] [Google Scholar]
- Scarlett J. A., Gray R. S., Griffin J., Olefsky J. M., Kolterman O. G. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982 Jul-Aug;5(4):353–363. doi: 10.2337/diacare.5.4.353. [DOI] [PubMed] [Google Scholar]
- Scarlett J. A., Kolterman O. G., Ciaraldi T. P., Kao M., Olefsky J. M. Insulin treatment reverses the postreceptor defect in adipocyte 3-O-methylglucose transport in type II diabetes mellitus. J Clin Endocrinol Metab. 1983 Jun;56(6):1195–1201. doi: 10.1210/jcem-56-6-1195. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Hedo J. A., Cushman S. W. Insulin-induced internalization of the insulin receptor in the isolated rat adipose cell. Detection of both major receptor subunits following their biosynthetic labeling in culture. Diabetes. 1984 Jan;33(1):13–18. doi: 10.2337/diab.33.1.13. [DOI] [PubMed] [Google Scholar]
- Sinha M. K., Pories W. J., Flickinger E. G., Meelheim D., Caro J. F. Insulin-receptor kinase activity of adipose tissue from morbidly obese humans with and without NIDDM. Diabetes. 1987 May;36(5):620–625. doi: 10.2337/diab.36.5.620. [DOI] [PubMed] [Google Scholar]
- Trimble E. R., Weir G. C., Gjinovci A., Assimacopoulos-Jeannet F., Benzi R., Renold A. E. Increased insulin responsiveness in vivo and in vitro consequent to induced hyperinsulinemia in the rat. Diabetes. 1984 May;33(5):444–449. doi: 10.2337/diab.33.5.444. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Hirshman M., Pofcher E., Horton E. D., Mead P. M., Cushman S. W., Horton E. S. Regulation of glucose utilization in adipose cells and muscle after long-term experimental hyperinsulinemia in rats. J Clin Invest. 1985 Aug;76(2):460–469. doi: 10.1172/JCI111994. [DOI] [PMC free article] [PubMed] [Google Scholar]



