Abstract
Context
Deep brain stimulation (DBS) may be an effective intervention for treatment-resistant depression (TRD), but available data are limited.
Objective
To assess the efficacy and safety of subcallosal cingulate DBS in patients with TRD with either major depressive disorder (MDD) or bipolar II disorder (BP).
Design
Open-label trial with a sham lead-in phase.
Setting
Academic medical center.
Patients
Men and women aged 18 to 70 years with a moderate-to-severe major depressive episode after at least 4 adequate antidepressant treatments. Ten patients with MDD and 7 with BP were enrolled from a total of 323 patients screened.
Intervention
Deep brain stimulation electrodes were implanted bilaterally in the subcallosal cingulate white matter. Patients received single-blind sham stimulation for 4 weeks followed by active stimulation for 24 weeks. Patients then entered a single-blind discontinuation phase; this phase was stopped after the first 3 patients because of ethical concerns. Patients were evaluated for up to 2 years after the onset of active stimulation.
Main Outcome Measures
Change in depression severity and functioning over time, and response and remission rates after 24 weeks were the primary efficacy end points; secondary efficacy end points were 1 year and 2 years of active stimulation.
Results
A significant decrease in depression and increase in function were associated with chronic stimulation. Remission and response were seen in 3 patients (18%) and 7 (41%) after 24 weeks (n=17), 5 (36%) and 5 (36%) after 1 year (n=14), and 7 (58%) and 11 (92%) after 2 years (n=12) of active stimulation. No patient achieving remission experienced a spontaneous relapse. Efficacy was similar for patients with MDD and those with BP. Chronic DBS was safe and well tolerated, and no hypomanic or manic episodes occurred. A modest sham stimulation effect was found, likely due to a decrease in depression after the surgical intervention but prior to entering the sham phase.
Conclusions
The findings of this study support the long-term safety and antidepressant efficacy of subcallosal cingulate DBS for TRD and suggest equivalent safety and efficacy for TRD in patients with BP.
Treatment-resistant depression (TRD) has a prevalence in the United States of morethan1%and is a highly costly and disabling disorder.1,2 Deep brain stimulation (DBS) of various neuroanatomic targets has emerged as a potential treatment for TRD.3–6 An uncontrolled study3,4 demonstrated that DBS of the subcallosal cingulate (SCC) white matter was associated with antidepressant response rates of 60% and 55% after 6 and 12monthsof chronic DBS, respectively. Antidepressant efficacy was largely maintained, up to 6 years in some patients.7 Although the results were encouraging, this initial pilot study was limited by being an open-label study. Additionally, only 2 patients with TRD in the context of bipolar disorder were included, but neither received significant benefit from SCC DBS.
In addition to providing further data on the safety and long-term efficacy of SCC DBS for TRD, the current study was designed to address 2 additional questions. Is there an antidepressant effect associated with sham SCC DBS? Is SCC DBS safe and effective in patients with treatment-resistant bipolar depression? Patients with TRD in the context of either major depressive disorder (MDD) or bipolar II disorder (BP) were enrolled in a study of SCC DBS that included a 4-week singleblind, sham stimulation phase; a 24-week open-label, active stimulation phase; a single-blind discontinuation phase; and long-term (2 years) observation.
METHODS
STUDY OVERVIEW
Study phases included a screening/preoperative evaluation phase of at least 4 weeks; surgery; a 4-week, single-blind, sham stimulation phase; a 24-week open-label active stimulation phase; a single-blind discontinuation phase; and observational followup. All procedures were carried out at Emory University. Recruitment information was posted on Emory University’s Web sites, and a letter describing the study was sent to regional psychiatrists. Study procedures were approved by the Emory University Institutional Review Board and the US Food and Drug Administration under an Investigational Device Exemption (G060028 held by H.S.M.). The study was monitored by the Emory University Department of Psychiatry and Behavioral Sciences Data and Safety Monitoring Board. All patients gave written informed consent for participation.
Eligibility
Key inclusion criteria were (1) age 18 to 70 years; (2)MDDor BP identified via the Structured Clinical Interview for DSM-IV8 and confirmed by at least 2 of the 3 study psychiatrists (P.E.H., S.J.G., and D.W.); (3) current major depressive episode of at least 12 months’ duration and not responding to at least 4 adequate antidepressant treatments (scoring 3 or higher on the Antidepressant Treatment History Form9 and verified through medical records); (4) lifetime failure or intolerance of electroconvulsive therapy or inability to receive electroconvulsive therapy; (5) 17-itemHamilton Depression Rating Scale (HDRS)10 score of 20 or higher at screening; (6) preoperative HDRS score of 20 or higher averaged across 4 weeks preoperatively and 30% or less lower than the screening score; (7) Global Assessment of Functioning (GAF)11 of 50 or less; and (8) ability to provide informed consent.
Key exclusion criteria were (1) clinically significant medical or psychiatric comorbidity (including personality disorders as determined by a review of medical records, the Structured Clinical Interview-II,12 and clinical examination); (2) substance use disorder within the past 12 months; (3) active suicidal ideation with plan or intent, a suicide attempt within the past 6 months, or more than 2 suicide attempts within the past 2 years; (4) pregnancy or planning to become pregnant during the study; or (5) contraindication for DBS surgery or chronic stimulation.
Concomitant Treatments
Patients were allowed to continue taking current psychotropic medications with doses kept stable and no new psychotropic medications added from at least 4 weeks before surgery until the patient entered the observational phase. Any medication could be decreased or discontinued if intolerable adverse effects emerged with chronic DBS. Patients were encouraged to continue psychotherapy if this had been ongoing for at least 6 months. Other concomitant treatments were not allowed. Upon entry into the observational follow-up phase, changes in medications and psychotherapy were allowed.
Surgery
Using frame-based stereotactic neurosurgical techniques, the SCC target was selected in a manner consistent with a previous trial.3,4,13 The patients received local or general anesthesia, and the DBS quadripolar electrodes (Libra System, St. Jude Medical Neuromodulation) were bilaterally implanted using microelectrode mapping to confirm gray/white matter borders of the SCC genu. Details of surgical targeting are discussed in more detail elsewhere.3,13 Electrodes were approximately 1.4mmin diameter and consisted of one 3-mm-long active contact tip followed by three 1.5-mm contacts, each separated by 1.5 mm. Intraoperative testing of individual contacts was conducted in 12 of 17 patients, using parameters similar to those for chronic stimulation (130 Hz, 90-µs pulse width, 4–8mA, approximately 2 to 5 minutes of active stimulation at each contact). After electrode placement, an implantable pulse generator (IPG; Libra System, St Jude Medical Neuromodulation) was placed in the infraclavicular region, with the patient under general anesthesia, and connected to the DBS electrodes via subcutaneous extension wires. Postoperative magnetic resonance imaging (MRI) was performed to evaluate for intracranial hemorrhage. A high-resolution postoperative computed tomography scan was obtained to assist with visualization of the contact locations. Patients were discharged from the hospital within 3 days with the stimulator off (patients were aware that stimulation was off).
Single-blind Sham Lead-in
After surgery, patients entered a 4-week sham stimulation phase with weekly assessment. Patients were told that they were being randomized to receive either active stimulation or sham stimulation (ie, none) for 4 weeks, but all patients received sham stimulation. Because stimulation was not associated with any somatic sensation, patients were unable to determine whether stimulation was on or off. Eleven patients entered this phase at least 1 week after surgery, allowing for a formal postoperative clinical assessment prior to phase entry. For logistical reasons, the other 6 patients entered the sham stimulation phase within 2 to 3 days after the procedure, not allowing for a formal postoperative, presham phase HDRS assessment.
Open-label Stimulation Phase
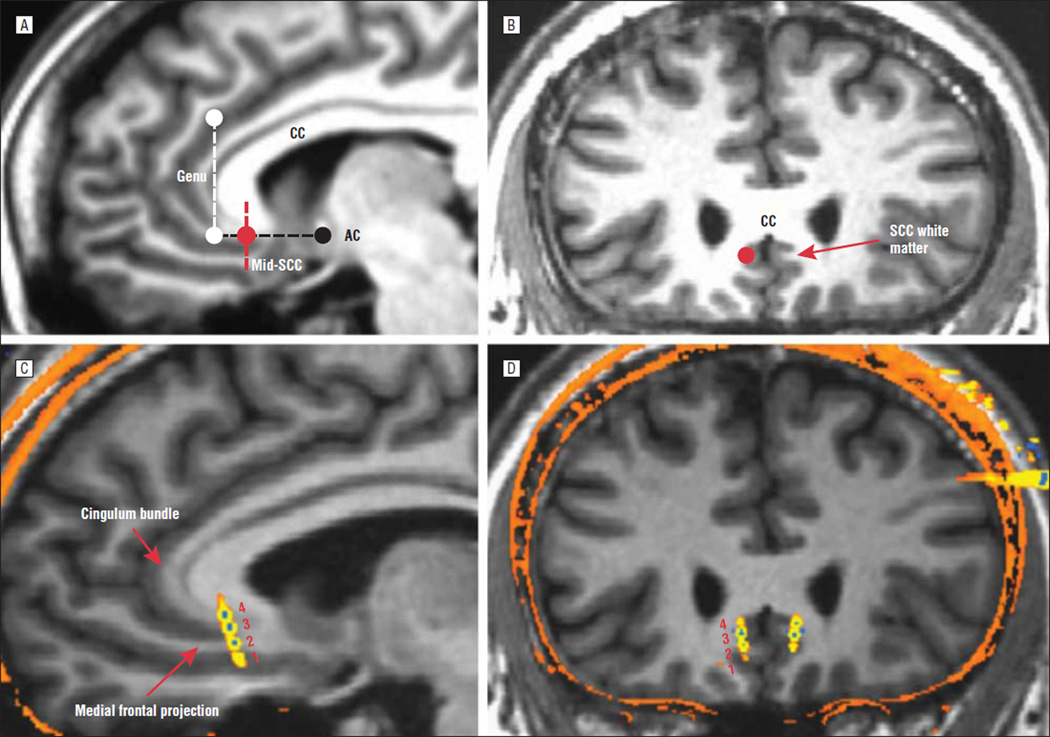
After 4 weeks of sham stimulation, all patients received open-label, active stimulation for 24 weeks, with evaluation every 1 to 2 weeks. The contact best situated in the SCC white matter on each side was selected on the basis of postoperative MRI and/or postoperative computed tomography merged with the preoperative MRI findings to best visualize contact locations. Contact location was chosen to stimulate white matter tracts projecting to various brain regions implicated in the pathophysiologic source of depression (based on the original rationale for this target for treating TRD; see Figure 1).3,4,13–15
Figure 1.
Surgical targeting. Preoperative magnetic resonance imaging (MRI) shows the sagittal (A) and coronal (B) views of the planned optimal subcallosal cingulate (SCC) white matter target (red circle). The dotted black line indicates the subcallosal plane of interest, parallel to the anterior-posterior commissural line; the dotted white line indicates the rostral limit of the subcallosal plane; and the dotted red line indicates the midsubcallosal plane. The red circle indicates demarcation of the SCC white matter target and surrounding gray matter (best seen in the coronal view [B]). C and D, Postoperative computed tomography scan merged with preoperative MRI showing a typical case with the deep brain stimulation electrodes in situ. Note that the contacts span the SCC gray and white matter in the vertical plane proximal to the split of the cingulum bundle and rostral medial frontal white matter tracts (C, red arrows, sagittal view). Contacts are numbered by convention (1–4 on the left, 5–8 on the right), inferior to superior. Contacts 2 and 3 are directly in the SCC white matter, and contacts 1 and 4 are in the inferior and superior gray matter, respectively. AC indicates anterior commissure; CC, corpus callosum.
Chronic, bilateral, continuous, monopolar (referential) stimulation was used (with the contact as anode and the implantable pulse generator as cathode). Initial stimulation parameters were 130 Hz, 91-µs pulse width, and 4-mA (mA) current. The following algorithm was then iteratively used: if no improvement occurred in 1 week (decrease in HDRS by ≤10% from the previous assessment), stimulation intensity was increased by 1mA (up to 8 mA). If there still was no improvement at 8 mA, the stimulation contact was changed. After the stimulation intensity was titrated up to at least 6 mA in first 3 patients, the algorithm was modified to initiate stimulation at 6 mA. If no improvement was observed after 4 weeks, the intensity was increased to 8 mA. After an additional 4 weeks without improvement, a contact change was made. No other parameter changes were allowed during the 24-week open stimulation phase. Stimulation parameters used in this study could not have exceeded the Food and Drug Administration’s maximum allowable charge density limit of 30 microcoulombs/cm.
Single-blind Discontinuation Phase
Single-blind discontinuation occurred after 24 weeks of active DBS. Patients were told they were being randomized to either active or sham stimulation, but all received sham stimulation. Full relapse of the depressive episode occurred across 2 weeks in all of the first 3 patients. Following stimulation reinitiation, depressive symptoms did not improve immediately, as expected from prior experience with stimulation cessation due to battery depletion3 (see the “Results” section for more details). This lack of initial improvement following reinitiation of previously beneficial stimulation led to significant distress and increased suicidal ideation in these patients. Because of patient safety concerns, this phase was eliminated for subsequent patients.
Observational Follow-up Phase
After the open stimulation (n=14) or discontinuation (n=3) phase, patients received open-label active stimulation and were evaluated monthly for 3 months, every 3 months for 9 months, and then every 6 months. Further changes in DBS parameters were allowed during this phase. Additionally, medication changes and psychotherapy were allowed at the discretion of the study team and the patients’ primary providers of psychiatric treatment.
Efficacy Measures
Efficacy measures included the HDRS, Beck Depression Inventory II (BDI-II),16 and GAF. For the HDRS and BDI, higher scores indicate increased depression severity. For the GAF, lower scores indicate increased symptom severity and/or dysfunction. A GAF score of 50 or lower indicates severe symptoms and/or psychosocial dysfunction, scores of 51 to 60 indicate moderate symptoms/ dysfunction, scores of 61 to 70 indicate mild symptoms/ dysfunction, and scores of 71 or above indicate absent or no more than transient symptoms and/or minimal dysfunction.
Safety Assessments
Prior to surgery, a neurosurgical evaluation, laboratory tests, and screening to ensure MRI safety were performed. At each study visit, patients were queried in detail about adverse events (AEs), and the Young Mania Rating Scale17 was administered. An AE was defined as an undesired change in physical or mental status, or in relevant laboratory measures, that warranted clinical assessment and/or intervention. A serious AE (SAE) was defined as an AE that resulted in death, permanent loss of biological function, and/or the need for or prolongation of hospitalization. The AEs/SAEs were further characterized by whether they were probably or definitely related to surgery, the DBS device, or stimulation.
Neuropsychological testing occurred at baseline and after 4 and 24 weeks of open-label active stimulation. The North American Adult Reading Test18 was given at baseline to provide a proxy of IQ. The neuropsychological battery included subtests from the Cambridge Neuropsychological Test Automated Battery (Cambridge Cognition Ltd).19 Cognitive domains tested included risk taking/decision making (Cambridge Gambling Task), set shifting (Intra-/Extra-Dimensional Shift Task), memory (short-term: Verbal Recognition Memory, and long-term semantic: Graded Naming Test), and executive functioning (Stockings of Cambridge).
DATA ANALYSES
The primary outcome measure was the longitudinal change in HDRS over time. Rates of remission (defined as an HDRS score <8 at the end point) and response (defined as a ≥50% change in HDRS score from baseline) were also calculated. Baseline was defined as the average of the 4 weekly scores obtained before surgery. Patients who exited the study were counted as nonresponders. Because medications and psychotherapy were unchanged until 24 weeks of active DBS had been completed, this time point was chosen as the primary efficacy end point. Secondary efficacy end points were 1 and 2 years after the onset of active DBS.
Demographic and clinical variables were compared between the MDD and BP groups (and response/remission groups), using standard 2-group comparisons, ie, Poisson tests for the count data (eg, number of treatments), Wilcoxon rank sum tests for continuous measures, and χ2 tests for the nominal variables. Wilcoxon rank sum tests were used in place of t tests for all continuous measures because the majority of measures of interest had marked variance differences across groups. Linear mixed models based on all available data were used to determine changes in efficacy measures (HDRS, BDI, and GAF). All models were fit with a random intercept and time as a fixed repeated factor; this parameterization is equivalent to traditional repeated-measures analysis of variance but uses available rather than complete case data. Comparisons between specific times or groups (MDD vs BP) were performed using tests of the resulting model estimates.
RESULTS
PATIENTS
A total of 1091 individuals inquired about participation; 323 of these completed a telephone screen, 194 were asked to submit medical records, and 39 were screened in person. Seventeen patients underwent DBS surgery (MDD, 10; BP, 7). Demographic and clinical characteristics are provided in Table 1. Two patients had a history of binge eating disorder, 1 patient had a history of panic disorder (in remission), and 1 patient had generalized anxiety disorder (deemed by ≥2 of the 3 study psychiatrists [P.E.H., S.J.G., and D.W.] to not be clinically significant). No patient had a personality disorder diagnosed by DSM-IV criteria.
Table 1.
Demographic and Clinical Characteristics
| Characteristic | Total (n=17) |
MDD (n=10) |
BP (n=7) |
Statistical Valuea |
P Value |
|---|---|---|---|---|---|
| Female sex, No. (%) | 10 (59) | 7 (70) | 3 (43) | 1.25 | .26 |
| Age, mean (SD), y | 42.0 (8.9) | 40.0 (9.3) | 44.9 (8.1) | −1.08 | .28 |
| Years of education, mean (SD) | 16.4 (2.9) | 16.2 (2.7) | 16.7 (3.5) | −0.05 | .96 |
| Family history of mood disorder, No. (%) | 15 (88) | 9 (90) | 6 (86) | 0.07 | .79 |
| Age at onset, mean (SD), y | 19.9 (7.8) | 20.3 (5.6) | 19.4 (10.7) | −1.13 | .27 |
| Current episode duration, mean (SD), mo | 64.1 (53.7) | 91.2 (55.8) | 25.4 (8.1) | −3.03 | .001 |
| Lifetime No. of depressive episodes, mean (SD) | 6.9 (9.3) | 3.2 (2.5) | 12.4 (12.6) | 43.07 | .001 |
| Lifetime No. of hypomanic episodes, mean (SD) | 10.1 (17.6) | NA | 10.1 (17.6) | ||
| Prior psychiatric hospitalization, No. (%) | 14 (82) | 8 (80) | 6 (86) | 0.09 | .76 |
| Prior suicide attempt, No. (%) | 8 (47) | 5 (50) | 3 (43) | 0.08 | .77 |
| Prior or current psychotherapy, No. (%) | 17 (100) | 10 (100) | 7 (100) | ||
| Adequate medications, current episode, mean (SD), No. | 6.2 (2.7) | 5.8 (2.8) | 6.9 (2.5) | 0.74 | .39 |
| Total No. of treatments, lifetime, mean (SD) | 24.1 (10.6) | 22.0 (10.0) | 27.1 (11.5) | 4.50 | .03 |
| Prior ECT, failed or intolerant, No. (%) | 16 (94) | 9 (90) | 7 (100) | 0.74 | .39 |
| Unable to work, No. (%) | 14 (82) | 7 (70) | 7 (100) | 2.55 | .11 |
Abbreviations: BP, bipolar II disorder; ECT, electroconvulsive therapy; MDD, major depressive disorder; NA, not applicable.
Statistical tests used were Z (Wilcoxon rank sum) for continuous variables, Wald χ2 (df = 1) for counts, and Pearson χ2 (df = 1) for binary variables.
Compared with patients with MDD, those with BP had a shorter duration of the current episode, larger number of lifetime depressive episodes, and more lifetime psychotropic medications. Patients were taking a mean (SD) of 3(2) psychotropic medications at the time of surgery. Thirteen patients were taking at least 1 antidepressant medication, and 11 were taking at least 1 augmentation medication. Four of the 7 patients with BP were taking mood stabilizers. Two patients (both with MDD) were taking no psychotropic medications.
Surgical placement of the DBS electrodes was adequate in all patients, and bilateral contacts best situated in the SCC white matter were selected for chronic stimulation, as described in the “Open-Label Stimulation Phase” subsection of the “Methods” section (this could have been any of the 4 contacts on each side, depending on electrode placement; see Figure 1 for an example). All patients completed the 4-week sham stimulation phase. Sixteen of 17 patients completed the 24-week active stimulation phase: 1 patient entered the observational phase at 21 weeks after explantation of the DBS system owing to infection. The system was reimplanted, and this patient contributed data to the 1- and 2-year analyses. Sixteen of 17 patients remain in the observational follow-up phase. Fourteen patients completed 1 year of active stimulation, and 11 patients have completed 2 years of active stimulation. One patient with MDD chose to exit the study because of lack of efficacy after 88 weeks of active stimulation. This patient had achieved a response at times during the study, but this was never maintained for more than 2 weeks, and this patient was not a responder at either the 24-week or 1-year time points.
ANTIDEPRESSANT EFFICACY
Significant improvement in all measures occurred, and there did not appear to be any large, clinically meaningful, or statistically significant differences between the MDD and BP groups (Table 2, Figure 2, and eFigure [http://www.archgenpsychiatry.com]). The HDRS scores decreased significantly from baseline to the end of the 4-week sham stimulation phase (estimate = −3.3 points, z = 2.41, P = .02, n=17 [10 MDD, 7 BP]). However, the difference from the postoperative stimulation-off time point to the end of the sham phase was not significant (estimate = −1.7 points, z = 1.08, P = .28, n = 11 [7 MDD, 4 BP]). Compared with the end of the sham phase, the decrease in HDRS scores after 4 weeks of active stimulation approached significance (estimate = −2.7 points, z = 1.92, P = .06, n = 17 [10 MDD, 7 BP]). At the end of the sham phase, patients were asked to guess whether they had received active vs sham stimulation during the prior 4 weeks. Three of 17 patients guessed that they had received active stimulation, and 14 of 17 patients guessed they had received sham stimulation. When asked to explain their guess, all patients stated that the guess was based on the perceived improvement in depression over the previous 4 weeks (none attributed the guess to a sensation that the stimulator was on).
Table 2.
Depression Severity and Function Over Time
| Mean (SE) | |||
|---|---|---|---|
| Study Phase | HDRS | BDI-II | GAF |
| Baseline (17 patients: 10 MDD, 7 BP) | 23.9 (0.7) | 38.4 (2.1) | 33.9 (1.7) |
| Postoperativea (11 patients: 7 MDD, 4 BP) | 21.5 (1.3) | 37.3 (3.1) | 33.2 (2.7) |
| 4-wk sham stimulation (17 patients: 10 MDD, 7 BP) | 20.5 (1.7) | 31.4 (3.0) | 36.9 (3.0) |
| 4-wk active stimulation (17 patients: 10 MDD, 7 BP) | 17.9 (0.9) | 31.0 (3.1) | 43.9 (3.4) |
| 24-wk active stimulation (16 patients: 10 MDD, 6 BP) | 13.1 (1.5) | 21.4 (3.3) | 60.8 (4.2) |
| 1-y active stimulation (14 patients: 9 MDD, 5 BP) | 13.6 (2.1) | 20.8 (3.9) | 62.2 (5.0) |
| 2-y active stimulation (11 patients: 8 MDD, 3 BP) | 7.3 (0.7) | 9.5 (1.8) | 78.7 (4.1) |
| P valueb | <.001 | <.001 | <.001 |
Abbreviations: BDI-II, Beck Depression Inventory II; BP, bipolar II disorder; GAF, Global Assessment of Function; HDRS, Hamilton Depression Rating Scale; MDD, major depressive disorder.
The postoperative time point reflects patients who had tests performed postoperatively but prior to entry into the sham-controlled phase (stimulation off).
Significance values are for the time effect of the linear mixed model.
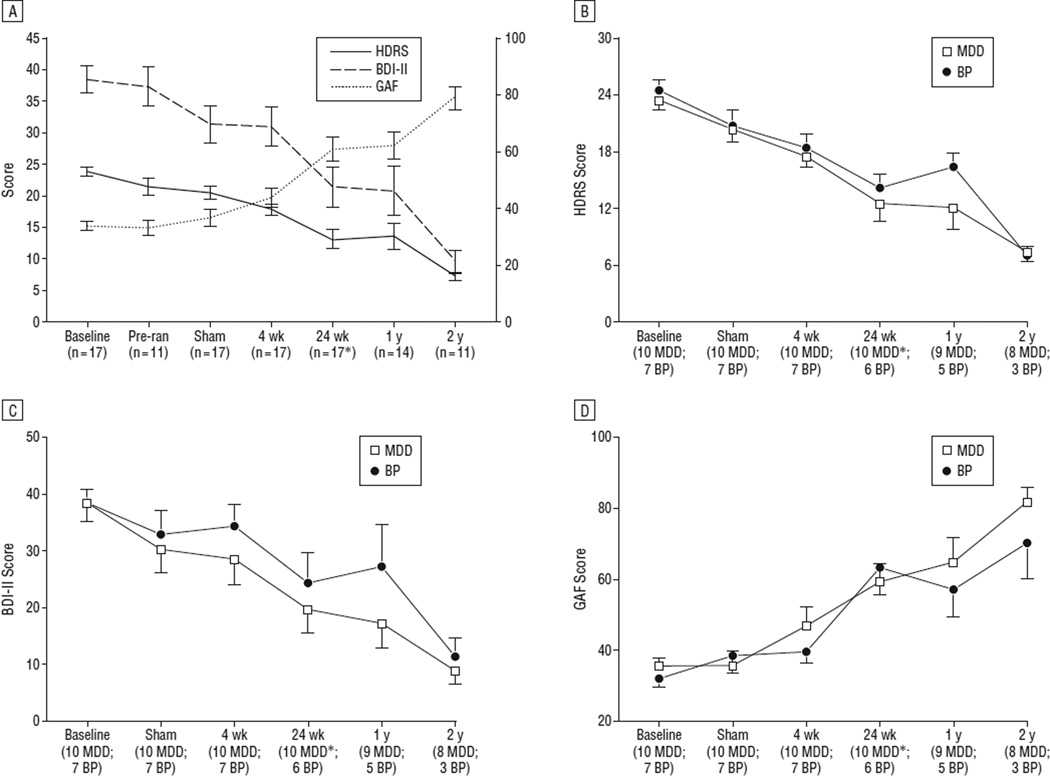
Figure 2.
Change in depression severity (left axis) and function (right axis) over time for the entire sample (A) and by diagnosis (B–D). Error bars represent standard error. *Twenty-four weeks was the primary end point, as this was the time point up to which medications and psychotherapy were maintained. After 24 weeks of active deep brain stimulation, medication changes and psychotherapy were allowed, as described in the text. BDI-II indicates Beck Depression Inventory II; BP, bipolar II disorder; GAF, Global Assessment of Functioning; HDRS, Hamilton Depression Rating Scale; MDD, major depressive disorder; and Pre-ran, at least 1 week after surgery before the 4-week sham stimulation phase.
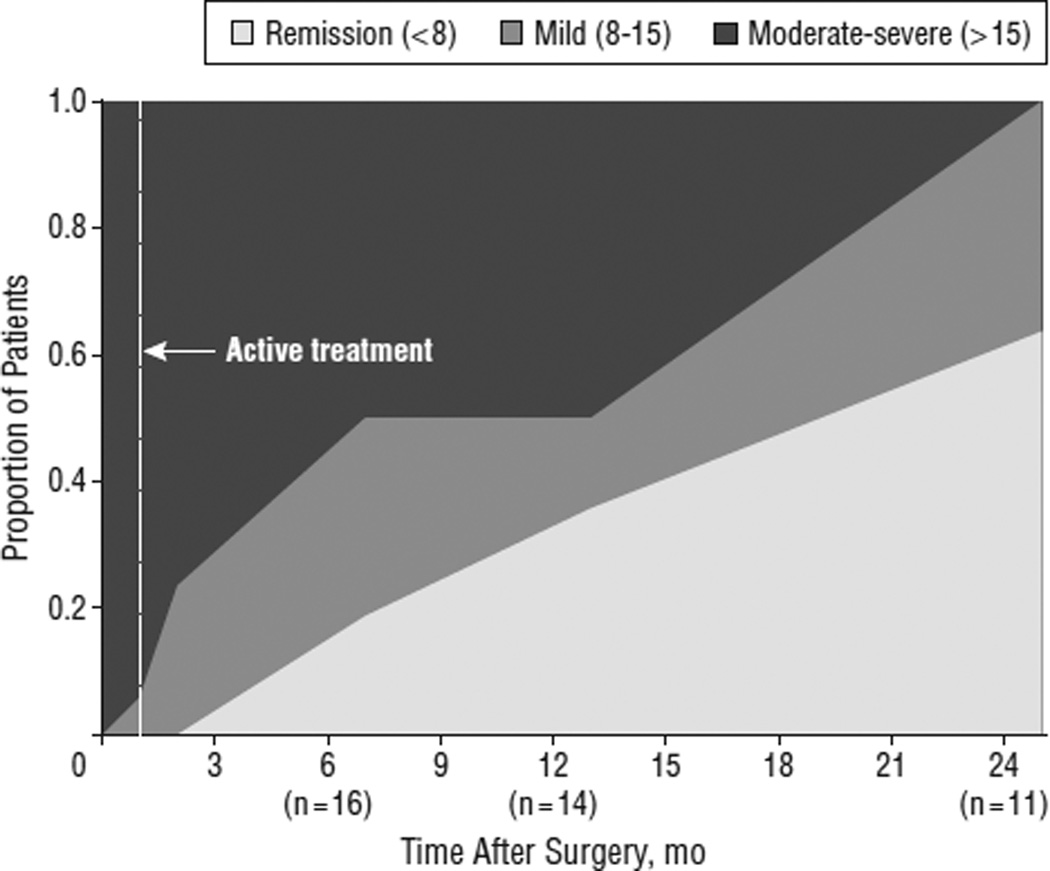
Compared with baseline, the average HDRS score decreased 43.6%, 43.0%, and 70.1% by the 24-week, 1-year, and 2-year time points, respectively. Remission and response were seen in 3 patients (18%) and 7 (41%) after 24 weeks (n=17), 5 (36%) and 5 (36%) after 1 year (n=14), and 7 (58%) and 11 (92%) after 2 years (n=12) of active stimulation. Cutoffs of the HDRS were used to group patients into remission (HDRS, <8), mild depression (HDRS,8–15) or moderate-to-severe depression (HDRS, >15) at each time point (Figure 3). Notably, all patients reaching the 2-year time point (n = 11) were in remission or had only mild depressive symptoms. No patient achieving remission during the study experienced a spontaneous relapse (ie, without cessation of stimulation).
Figure 3.
Depression severity over time using Hamilton Depression Rating Scale (HDRS) cutoffs (moderate-severe [HDRS, >15], mild [HDRS, 8–15], or remission [HDRS, <8]).
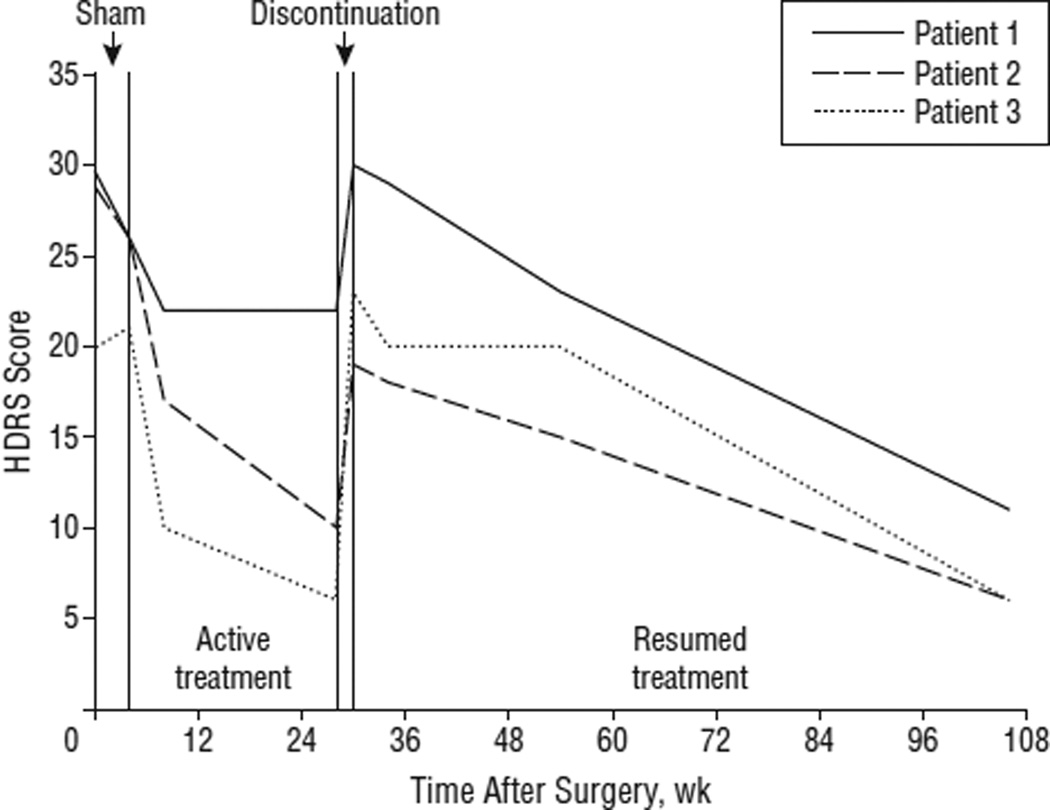
Single-blind discontinuation was associated with recurrence of the full depressive episode in 3 of 3 patients during 2 weeks (Figure 4). These patients were asked to guess whether the stimulation had been discontinued or left on at the beginning of this phase. Two patients guessed that the stimulation had been left on (and that the return of depressive symptoms was spontaneous), and 1 patient guessed that the stimulation had been turned off (based on the return of depressive symptoms after several days). After DBS reinitiation, improvement occurred in all 3 of these individuals, but notably more gradually than with initial stimulation. This was associated with substantial distress and an increase in suicidal ideation in all 3 patients. Because of ethical concerns about patient safety, this study phase was eliminated for subsequent patients.
Figure 4.
The 17-item Hamilton Depression Rating Scale (HDRS) scores in 3 patients who received 4 weeks of sham stimulation followed by 24 weeks of open stimulation and then blinded discontinuation. Stimulation was reinitiated if a patient met criteria for a depressive relapse or if symptom severity returned to baseline level.
Of the 12 patients who had intraoperative testing of individual DBS contacts, 8 spontaneously described acute positive effects, including a sense of increased alertness, less psychic pain, decreased heaviness, and increased interest/motivation. These patients consistently described their mood as less negative but denied feelings of elation, euphoria, or happiness. None of the 12 patients described negative effects of acute stimulation. Patients who experienced intraoperative effects did not differ significantly from patients without these effects on any demographic or baseline clinical variable, nor did they differ significantly in change in HDRS score at any time point (postoperative [presham], after 4weeks of sham, or after active stimulation for 4 weeks, 24 weeks, 1 year, or 2 years). Of note, only 2 of 8 patients experiencing a positive intraoperative effect had similar effects with initiation of stimulation in the open-stimulation phase; each of these patients described a similar experience as during surgery but noted that it seemed less intense the second time.
SAFETY
Twenty-two AEs occurred in 11 patients (65%), 12 SAEs occurred in 4 patients (24%), and 13 patients (76%) experienced at least 1 AE or SAE (Table 3). Nine of the 12 SAEs (75%) occurred in 1 patient with BP. No AE or SAE was related to active stimulation. No intraoperative hemorrhages occurred (based on a review of postoperative MRI scans). Eight device- or surgery-related events included 2 SAEs (DBS system infections requiring explantation, both in the same patient) and 6 AEs. No hypomania or mania occurred, and there was no significant change in Young Mania Rating Scale scores in any patient. None of the instances of anxiety was associated with other hypomanic symptoms. Furthermore, episodes of anxiety occurred (and resolved) in the absence of any change to stimulation parameters (≥1 episode was related to benzodiazepine withdrawal in the observational follow-up period). Neuropsychological function either improved or was stable over time (Table 4). Nine patients (53%) required replacement of the implantable pulse generator because of battery depletion after a mean (SD) of 72 (11) weeks of active stimulation. Of note, the majority of these patients reported a mild increase in depressive symptoms prior to the implantable pulse generator replacement.
Table 3.
AEs and SAEs in 17 Patients
| No. | |||
|---|---|---|---|
| Factor | Patients | Events | Device/Surgery Relateda |
| SAEsb | |||
| Infection | 1 | 2 | 2 |
| Anxiety | 2 | 5 | 0 |
| Worsening depression | 1 | 1 | 0 |
| Suicidal ideation | 1 | 2 | 0 |
| Suicide attempt | 2 | 2 | 0 |
| AEs | |||
| System dislodged | 1 | 1 | 1 |
| Extension break | 1 | 1 | 1 |
| Erosion | 1 | 1 | 2 |
| Infection | 1 | 1 | 1 |
| Worsening depression | 1 | 1 | 0 |
| Suicidal ideation | 1 | 1 | 0 |
| Headache | 3 | 3 | 0 |
| Hand numbness/tingling | 2 | 2 | 0 |
| Arm weakness | 1 | 1 | 0 |
| Gait/balance disorder | 1 | 1 | 0 |
| Nausea | 4 | 5 | 1 |
| Infection | 2 | 2 | 0 |
| Chest pain | 1 | 1 | 0 |
| Anemia | 1 | 1 | 0 |
Abbreviations: AE, adverse event; SAE, serious adverse event.
Represents probable or definite relation to surgery or the device.
One patient accounted for 9 of the 12 SAEs.
Table 4.
Neuropsychological Results and Change After 4 Weeks and 24 Weeks of Active Subcallosal Cingulate Deep Brain Stimulation
| Mean (SD) | |||
|---|---|---|---|
| Variable | Baseline (n = 17) |
4 wk (n = 16) |
24 wk (n = 17) |
| Cambridge Gambling Task, normalized score | |||
| Quality of decision makinga | 0.9 (0.1) | 0.9 (0.1) | 1.0 (0.1) |
| Deliberation time, s | 3.0 (2.6) | 2.5 (1.2) | 2.2 (0.7) |
| Risk takingb | 0.5 (0.2) | 0.5 (0.1) | 0.5 (0.1) |
| Risk adjustmentc | 1.6 (0.8) | 1.6 (1.1) | 2.1 (1.2)d |
| Delay aversione | 0.2 (0.2) | 0.2 (0.2) | 0.2 (0.2) |
| Overall proportion betf | 0.5 (0.2) | 0.5 (0.1) | 0.5 (0.1) |
| Graded naming test, % correct | 59.6 (9.5) | 66.9 (9.1)g | 65.7 (10.8)g |
| Intra-/Extra-Dimensional Shift | |||
| Total stages completed | 8.1 (2.0) | 8.8 (0.5) | 8.9 (0.5) |
| Total errors, adjusted | 37.4 (50.9) | 17.7 (15.5) | 16.1 (14.4)d |
| Stockings of Cambridge | |||
| Problems solved in minimal moves, No. | 8.1 (2.2) | 9.4 (2.0)d | 9.2 (2.0)d |
| 5-Move initial thinking, s | 15.8 (13.2) | 17.1 (16.5) | 15.2 (14.5) |
| 5-Move subsequent thinking, s | 2.6 (3.1) | 1.3 (1.3) | 0.8 (1.1)g |
| Verbal Recognition Memory, total correct | |||
| Free recall | 7.6 (2.4) | 7.4 (2.2) | 8.5 (2.2) |
| Recognition | 22.6 (1.2) | 22.9 (1.2) | 23.2 (1.2) |
Higher score indicates better decision making.
Higher score indicates greater risk taking.
Higher score indicates greater adjustment of bet based on risk.
P < .05 vs baseline.
Higher score indicates greater aversion to delay.
Higher score indicates larger average bet.
P < .01 vs baseline.
There were 2 suicide attempts; each was temporally associated with a significant psychosocial stressor. One suicide attempt in a patient with MDD occurred after 1 week of active stimulation, but the suicidal ideation resolved without stimulation parameter or medication change; this patient was a responder at the 24-week time point and a remitter at the 1- and 2-year time points. The other suicide attempt in a patient with BP occurred 54 weeks into the observational follow-up phase and was not associated with any treatment change; this patient was a responder at the 2-year time point.
PARAMETER ADJUSTMENTS AND MEDICATION CHANGES
During the 24-week active stimulation phase, stimulation parameter adjustments were made in 12 of 17 patients according to the planned algorithm, including contact changes in 5 patients. After 24 weeks of active stimulation, 6 patients were receiving 6 mA and 10 patients were receiving 8 mA (1 patient exited this phase early because of infection; this patient had been receiving 8 mA prior to phase exit). By 1 year, additional adjustments were made in 8 of 14 patients in an attempt to maximize efficacy, including contact changes in 7 patients; at this time point, 6 patients were receiving 6 mA, 1 patient was receiving 7 mA, 6 patients were receiving 8 mA, and 1 patient was receiving 10 mA. By 2 years, further adjustments were made in 5 of 12 patients, including contact changes in 2 patients; 1 patient was receiving 5 mA, 9 patients were receiving 6 mA, and 1 patient was receiving 8 mA current (1 patient exited the study between the 1- and 2-year time points). Patients were receiving 130-Hz, 91-µs stimulation at all primary time points.
No psychotropic medications were added and no dosages were increased for any patient during the 4 weeks prior to surgery until entry into the observational phase after 24 weeks of active stimulation. One patient discontinued escitalopram during the open-label stimulation phase because of increased emotional blunting, which improved with medication discontinuation. From the end of the 24-week active stimulation phase to the 1-year time point, medication changes were made in 9 of 14 patients, and 1 patient began psychotherapy. Between the 1- and 2-year time points, medication changes were made in 8 of 12 patients. One patient with MDD remained medication free and was not in psychotherapy throughout the study: this patient was a responder after 24 weeks of active stimulation and a remitter at the 1- and 2-year time points. A second patient with MDD was medication free until after 24 weeks of active DBS but started medication before reaching the 1-year time point. This patient was not a responder at 1 year but was a responder (but not remitter) at 2 years.
COMMENT
These findings support the long-term safety and efficacy of SCC DBS for TRD and suggest similar effectiveness for TRD in patients with BP. After 2 years of chronic stimulation, response and remission rates were high (92% and 58%, respectively), and no patient was in a moderate or severe depressive episode after 2 years of chronic stimulation. One important observation is that no patient achieving remission experienced a spontaneous depressive relapse.
Use of SCC DBS was safe and well tolerated. Importantly, no patient experienced a hypomanic or manic episode during the study, and there was no significant change in the Young Mania Rating Scale score. No AE or SAE was directly related to acute or chronic stimulation. There were 2 suicide attempts unrelated to the device or stimulation. In one case, the attempt occurred in close proximity to the onset of SCC DBS, but suicidal ideation resolved spontaneously without cessation of stimulation, a change in parameters, or the addition of medications. The other attempt occurred more than 18 months into the study and was not associated with any change in treatment. There were 2 infections (occurring in the same patient) requiring partial or complete explantation of the system.
The clinical improvements in this study could be explained by a sham stimulation effect that persisted beyond the 4-week sham lead-in; a longer, randomized, sham-controlled trial would be needed to adequately test this. However, these findings overall argue against a clinically significant sham DBS effect. Although depression severity was statistically significantly lower after 4 weeks of sham stimulation compared with baseline, the mean decrease in HDRS score (3.3 points [14%]) was small and not clinically significant. Furthermore, in 11 patients with available data, a decrease in depression severity occurred after surgery but before the sham stimulation phase, and depression severity was not significantly lower following sham stimulation compared with the presham ratings. This suggests a modest antidepressant effect from the surgery. Beneficial effects have been seen following DBS surgery (but without ongoing stimulation) for Parkinson disease,20,21 epilepsy,22 and TRD (at another target).23 It is unclear whether these insertional effects are the result of a “microlesion” occurring during implantation, postoperative edema, carryover of an intraoperative stimulation effect, eligibility creep,24 or (in this study) a decrease in anticipatory anxiety associated with surviving an invasive and relatively high-risk procedure. The consistent subjective increase in depressive symptoms with battery depletion further supports an antidepressant effect of chronic, active SCC DBS. Blinded discontinuation of chronic, active DBS resulted in relapse of depression in 3 of 3 patients, and stimulation reinitiation was associated with return of efficacy in all. Although the increase in depression may have been due to withdrawal effects associated with stimulation cessation, the relatively slow return of symptoms after discontinuation and the decrease in symptoms with reinitiation argue against this. All patients experienced a return of only depressive symptoms, and no patient experienced symptoms akin to antidepressant withdrawal (eg, a serotonin withdrawal syndrome). The delayed return of antidepressant efficacy may have been partially related to psychological distress experienced by these patients when efficacy did not return within hours to days (as is seen with DBS for other indications, eg, Parkinson disease).
Nearly all patients in this study showed some degree of improvement in depression severity, with the majority achieving remission after 2 years of chronic stimulation. However, improvement occurred over a longer time course in some patients. The reasons for this are not clear. Several patients had medication and psychotherapy changes after 24 weeks of chronic DBS, limiting our ability to attribute long-term improvement to SCC DBS alone. However, it is improbable that patients with this degree of chronicity and treatment resistance would have otherwise achieved and maintained such significant improvement in depression.25,26 It is possible that chronic DBS enhances the antidepressant effects of concomitant treatments. It is also likely that premorbid functioning, psychosocial support, and personality/temperament contribute to the rate of recovery. To this last point, it is possible that adjunctive psychotherapeutic rehabilitation might optimize and hasten recovery in patients with chronic DBS (akin to physical and occupational therapy following a hip replacement).
Primary limitations of this study include small sample size and the limited duration and single-blind design of the sham control periods. Furthermore, if the blinded discontinuation phase had occurred in all patients, a stronger statement could be made about the efficacy of active vs sham stimulation. Finally, this study was designed to assess the preliminary safety of SCC DBS in patients with BP, given reports of manic symptoms with DBS of other targets.5,6 Therefore, this study was powered to find only large differences in efficacy between the MDD and BP group; a larger trial would be needed to identify small-to-moderate differences in effectiveness.
Taken together, these results support the long-term safety and antidepressant efficacy of SCC DBS for TRD, building on previous reports of long-term efficacy in MDD.7 Unique to this study was the demonstration of comparable antidepressant efficacy in patients with BP, with no manic or hypomanic episodes associated with stimulation or parameter adjustments. Next steps in developing this intervention include double-blind trials with a longer sham stimulation period, careful attention to potential demographic and clinical predictors of response and remission, and efforts aimed at decreasing time to remission, such as adjunctive psychotherapeutic rehabilitation.
Supplementary Material
Acknowledgments
Dr Holtzheimer has received grant funding from the Greenwall Foundation, NARSAD, National Institutes of Health Loan Repayment Program, and National Institute of Mental Health; he has received consulting fees from St. Jude Medical Neuromodulation. Dr Gross has received consulting fees from St Jude Medical Neuromodulation, Boston Scientific, and Bayer Healthcare, and he has equity in Neurovista. Dr Mayberg has a consulting agreement with St Jude Medical Neuromodulation, which has licensed her intellectual property to develop SCC DBS for the treatment of severe depression (US 2005/0033379A1). Dr Mayberg has current grant funding from the Dana Foundation, NARSAD, National Institute of Mental Health, Stanley Medical Research Institute, and Woodruff Foundation.
Funding/Support: This study was funded by grants from the Dana Foundation (Dr Mayberg), Stanley Medical Research Institute (Dr Mayberg), Woodruff Foundation (Dr Mayberg), Emory Healthcare (Dr Mayberg), and K23 MH077869 (Dr Holtzheimer). Devices were donated by Advanced Neuromodulation Systems/St Jude Medical Neuromodulation.
Additional Contributions: Eundria Hill, MSW, Rebecca de Mayo, MA, Mustafa Mufti, MD, and Sinéad Quinn, BA, provided research coordination; Sabrina Starr assisted with data entry; and Kate Rahimzadeh, RN, acted as the research nurse. Mahlon DeLong, MD, gave invaluable support and assistance.
Footnotes
Financial Disclosure: The terms of these arrangements have been reviewed and approved by Emory University in accordance with their conflict-of-interest policies (including an independent review of the manuscript by Kirk Easley, MS, Department of Biostatistics and Bioinformatics, Emory University School of Public Health).
Online-Only Material: The eFigure is available at http: //www.archgenpsychiatry.com.
REFERENCES
- 1.Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34(1):1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crown WH, Finkelstein S, Berndt ER, Ling D, Poret AW, Rush AJ, Russell JM. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63(11):963–971. doi: 10.4088/jcp.v63n1102. [DOI] [PubMed] [Google Scholar]
- 3.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64(6):461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Malone DA, Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX, Brockmann H, Lenartz D, Sturm V, Schlaepfer TE. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, Lozano AM. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502–510. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 8.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Disorders—Research Version (SCID-I, Version 2.0) New York: Biometrics Research Dept, New York State Psychiatric Institute; 1996. [Google Scholar]
- 9.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10–17. [PubMed] [Google Scholar]
- 10.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diagnostic and Statistical Manual of Mental Disorders—Text Revision (DSM-IV-TR) 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 12.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Disorders—Research Version (SCID-II, Version 2.0) New York: Biometrics Research Dept, New York State Psychiatric Institute; 1994. [Google Scholar]
- 13.Hamani C, Mayberg H, Snyder B, Giacobbe P, Kennedy S, Lozano AM. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111(6):1209–1215. doi: 10.3171/2008.10.JNS08763. [DOI] [PubMed] [Google Scholar]
- 14.Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18(6):1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS. A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry. 2009;65(4):276–282. doi: 10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory II (BDI-II) San Antonio, TX: Psychological Corp; 1996. [Google Scholar]
- 17.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 18.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3(2):129–136. [Google Scholar]
- 19.Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, Rabbitt PM. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers. J Int Neuropsychol Soc. 1998;4(5):474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- 20.Mann JM, Foote KD, Garvan CW, Fernandez HH, Jacobson CE, IV, Rodriguez RL, Haq IU, Siddiqui MS, Malaty IA, Morishita T, Hass CJ, Okun MS. Brain penetration effects of microelectrodes and DBS leads in STN or GPi. J Neurol Neurosurg Psychiatry. 2009;80(7):794–797. doi: 10.1136/jnnp.2008.159558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morishita T, Foote KD, Wu SS, Jacobson CE, IV, Rodriguez RL, Haq IU, Siddiqui MS, Malaty IA, Hass CJ, Okun MS. Brain penetration effects of microelectrodes and deep brain stimulation leads in ventral intermediate nucleus stimulation for essential tremor. J Neurosurg. 2010;112(3):491–496. doi: 10.3171/2009.7.JNS09150. [DOI] [PubMed] [Google Scholar]
- 22.Fisher R, Salanova V,Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, De-Salles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N SANTE Study Group. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 23.Jiménez F, Velasco F, Salin-Pascual R, Hernández JA, Velasco M, Criales JL, Nicolini H. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585–593. doi: 10.1227/01.neu.0000170434.44335.19. [DOI] [PubMed] [Google Scholar]
- 24.Hick J, Feldman SR. Eligibility creep: a cause for placebo group improvement in controlled trials of psoriasis treatments. J Am Acad Dermatol. 2007;57(6):972–976. doi: 10.1016/j.jaad.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 25.George MS, Rush AJ, Marangell LB, Sackeim HA, Brannan SK, Davis SM, Howland R, Kling MA, Moreno F, Rittberg B, Dunner D, Schwartz T, Carpenter L, Burke M, Ninan P, Goodnick P. A one-year comparison of vagus nerve stimulationwith treatment as usual for treatment-resistant depression. Biol Psychiatry. 2005;58(5):364–373. doi: 10.1016/j.biopsych.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Dunner DL, Rush AJ, Russell JM, Burke M, Woodard S, Wingard P, Allen J. Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J Clin Psychiatry. 2006;67(5):688–695. doi: 10.4088/jcp.v67n0501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.