Abstract
Objectives
Clinical guidelines for asymptomatic patients with chronic MR utilize ejection fraction (EF) to trigger surgical referral. We hypothesize that 1) EF is not sensitive enough to detect the earliest contractile injury in chronic MR and 2) that the injury associated with chronic MR is not global but heterogeneous, occurring regionally and predictably, prior to the onset of global left ventricular (LV) dysfunction.
Methods
Fifteen patients with chronic MR and normal LV EF by echocardiography underwent cardiac MRI with tissue tagging. Point-specific comparisons (at 15,300 LV grid points) of multiple strain parameters to a normal human strain database allowed normalization of patient-specific regional contractile function. Data were mapped over patient-specific 3D geometry, then averaged across six LV regions.
Results
Global LV longitudinal and circumferential myocardial strains were normal for all 15 MR patients when compared to normal controls (p>0.05). Despite preserved global function, the anteroseptum and posteroseptum demonstrated significantly worse contractile function when compared to other regions of the LV (p=0.003 and p=0.035, respectively). Hyper-contractile regions (lateral walls) appeared to compensate (p=0.002) for the reduced septal contractile function thereby masking injury detection by global indices.
Conclusion
The earliest contractile injury seen in MR patients is heterogeneous, and consistently distributed along the LV septum. Compensatory responses include hypercontractility of other regions. These data suggest that rather than relying upon global LV contractile metrics, which do not detect early injury, patients may be better served by undergoing directed surveillance of “sentinel” LV regions (LV septum) with high-resolution metrics of regional contractile function.
BACKGROUND
Degenerative mitral valve disease is the most common valvular heart disease in America and given its increasing incidence with age, it is estimated that over five million people will have either moderate or severe mitral regurgitation (MR) by the year 2030 1. Several studies have demonstrated that the presence of even moderate MR is associated with increased mortality; and severe MR, even in an asymptomatic patient, is associated with an increase in all-cause mortality, cardiac mortality and cardiac morbidity2. Moreover, symptomatic patients and patients with even mildly depressed ventricular function (ejection fraction <60%) fare much worse with survival as low as 20% at four years 3.
Mitral valve surgery remains the treatment for degenerative mitral valve disease, and advances in surgical technique have allowed experienced centers to achieve high rates of successful valve repair with low observed morbidity and mortality 4-6. The long-term success for patients undergoing mitral valve surgery, however, depends upon the preservation of left ventricular (LV) contractile function. Patients referred for surgery early, before the onset of irreversible contractile injury, have been shown to regain normal life expectancy, and current ACC/AHA guidelines recommend early referral for mitral valve surgery in patients with minimal symptoms or early signs of ventricular embarrassment if mitral valve repair is likely 6-8.
While clinicians agree that surgery for MR should be performed before the onset of significant irreversible myocardial injury, the identification of that specific time point for a given individual patient is problematic. Two fundamental issues contribute to the difficulty of preserving ventricular function in patients with MR: 1) identifying a metric that has the ability to identify the earliest signs of ventricular injury and 2) a lack of knowledge in the manner or pattern by which earliest LV contractile injury presents.
The purpose of this investigation was to test the hypothesis that multiparametric strain analysis by magnetic resonance imaging and tissue tagging could identify early LV dysfunction in minimally or asymptomatic patients, and that the volume loading associated with MR creates a contractile injury that is regionally and heterogeneously distributed in a predictable pattern.
METHODS
Patient Characteristics
Between August 2010 and July 2013, 15 asymptomatic or minimally symptomatic patients with isolated moderate to severe degenerative MR underwent evaluation of their LV function by transthoracic echocardiography and tagged cardiac magnetic resonance imaging. Patient demographics and comorbidities are listed (Table 1). The majority of patients were of NYHA I classification with normal b-type naturetic peptide (BNP) levels. Three (20%) of the 15 patients were receiving afterload reduction medications, 4 (26%) were receiving beta blockers, and 2 (13%) were on diuretic therapy. There were no patients with ischemic mitral regurgitation. All patients with any evidence of coronary artery disease were specifically excluded from the study group.
TABLE 1.
Mitral Regurgitation Patient and Healthy Volunteer Characteristics
| Age (years) | 49.3 ± 11.3 | 33.1 ± 10.8 |
| Female Gender | 20% (3/15) | 53% (32/60) |
| Diabetes | 0 | 0 |
| HTN | 13% (2/15) | 0 |
| COPD | 0 | 0 |
| CRI | 0 | 0 |
| Atrial Fibrillation | 0 | 0 |
| NYHA I | 67% (10/15) | 100% (60/60) |
| NYHA II | 33% (5/15) | 0 |
| BNP | 34.0 ± 28.5* | NA |
| Echo Ejection Fraction (percent) | 61.5 ± 5.8 | NA |
| Echo End Systolic Dimension (cm) | 3.4 ± 0.4 | NA |
| Echo Left Atrial Size (cm) | 4.4 ± 0.5 | NA |
| Pulmonary Artery Pressure (mmHg) | 26.1 ± 4.9 | NA |
| Mitral Regurgitation | ||
| Moderate | 13% (2/15) | NA |
| Severe | 87% (13/15) |
HTN: Hypertension, COPD: Chronic Obstructive Pulmonary Disease
CRI: Chronic renal insufficiency, BNP, b-type naturetic peptide
n=9
NA: Not available
Normal Strain Database
Sixty healthy volunteers (Table 1) with no known cardiac disease or hypertension underwent similar myocardial strain analysis and contributed complete LV systolic strain information to a normal human strain database.
The Human Research Protection Office at Washington University (St. Louis, MO) approved this study, and all subjects gave informed written consent. No sex-based or racial/ethnic-based exclusions were present during patient recruitment.
Cardiac Magnetic Resonance Imaging
Electrocardiogram gated short- and long-axis tagged MR images were acquired from end-diastole through systole using a 1.5T scanner (Avanto, Siemens Medical Systems, Malvern, PA). In each imaging plane, a spatial modulation of magnetization (SPAMM) radio-frequency tissue-tagging preparation9 was applied, followed by a 2D balanced steady-state free precession cine image acquisition. Short-axis images covered the entire heart and long-axis images were acquired in four radially oriented planes. Typical imaging parameters were: tag spacing 8mm, slice thickness 8mm, repetition time 32.4ms, echo time 1.52ms, field of view 350 x 350mm and image matrix 192 × 256. In the same breath hold, anatomical and tagged images were acquired at corresponding slice positions.
Strain analysis
Strain measurements were obtained using a previously described and validated method10, 11. Briefly, endocardial and epicardial wall boundaries were manually segmented on the anatomical MR images. These wall boundaries were transferred to the tagged images for the purpose of processing the tagged data. Tag lines were tracked on the images in a semiautomated fashion using an active contour approach. Displacements were calculated from enddiastole to end-systole at each intersection point within the myocardium (Figure 1). For registration, the user identified the posterior and anterior intersection points of the right ventricular free wall with the septum on the most basal short-axis image. Using these two points, and information from the LV geometry, the individual heart was registered to a standard 18 region finite element mesh (Figure 2). Within each element of the finite element mesh, displacements were fit in the least-squares sense to basis functions. Continuity of displacement components was enforced at the element boundaries. The fitting of basis functions to approximate the displacement data and the calculation of longitudinal and circumferential myocardial strain was performed using StressCheck (ESRD, Inc., St. Louis, MO).
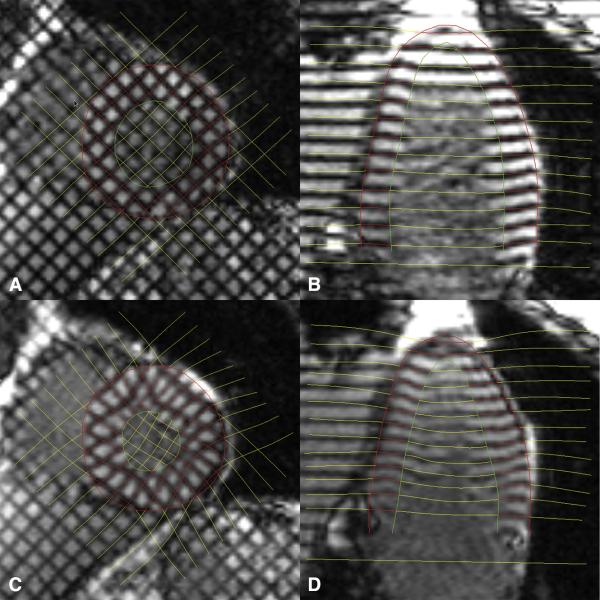
Figure 1.
Images from end-diastole (A and B) and end-systole (C and D) demonstrating deformations of tag lines at during systole.
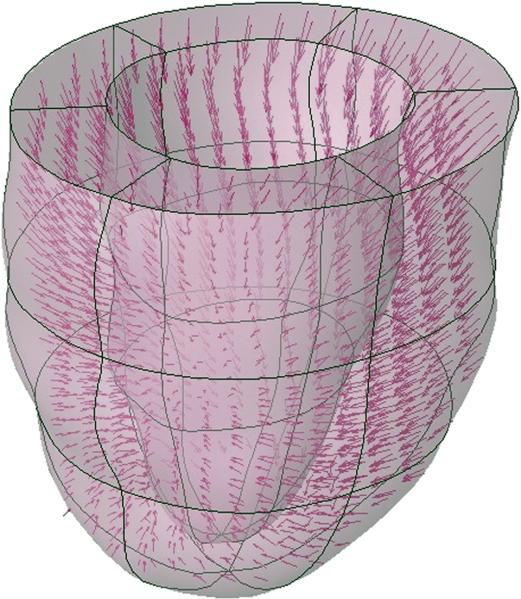
Figure 2.
For each patient, the heart was registered to a standard 18 region finite element mesh and displacements were computed at the intersection points of the tag surfaces. Model predicted displacements and strains were computed from a least squares fitting of the measured displacement data.
MRI-based multiparametric strain z-score analysis
A composite strain index was created utilizing circumferential and longitudinal strains, equally weighted, reflecting contractile function within the circumferential and longitudinal directions. Due to the heterogeneous pattern of normal LV strain, raw values were normalized to supply clinically relevant information. The method to relate individual patient-specific regional contractile function to a normal strain database (multiparametric strain analysis) has previously been described12-16. Briefly, z-scores generated from raw strain values represent the number of standard deviations each raw value is from the mean of the group. The means and standard deviations for both circumferential and longitudinal strain were calculated at each point of an encompassing grid of 15,300 LV points from the 60 normal volunteers. By comparison to this normal strain database, patient-specific z-scores for both strain measures were calculated and averaged to obtain a multiparametric strain z-score representing LV point-specific contractile function relative to normal. For each MR patient, z-scores were averaged over the six regions of the LV model (posteroseptal, anteroseptal, anterior, anterolateral, posterolateral and posterior).
Statistical Analysis
Continuous data are presented as a mean ± standard deviation, while categorical data are presented as a percentage. Global strain comparisons between MR patients and normal controls were performed using an independent samples t-test. Differences in regional normalized contractile function among MR patients were assessed using a repeated-measures analysis of variance (ANOVA). Sphericity of the data was assessed using Mauchly's Test. The Bonferroni method was used for post hoc testing of the regional z-score data. All statistical tests were done using the statistical software package IBM SPSS 21 (IBM SPSS, Inc., Chicago, IL).
RESULTS
Echocardiography Data for MR patients
By clinical echocardiography, left ventricular function, as assessed by LV dimensions and ejection fraction (EF), was within normal limits (Table 1) for this group of patients. Average left atrial size was increased when compared to normal (< 3.8 cm). Mean pulmonary arterial pressures for the entire group were either normal (<25mmHg) or mildly elevated. Mitral regurgitation was severe in 80% of cases (Table 1). The mechanism of MR was posterior leaflet prolapse or flail posterior in 9 (60%) of the studied patients. Five (33%) patients had bileaflet prolapse, and 1 (6%) patient had anterior prolapse.
Global Strain for MR patients vs. Normal Controls
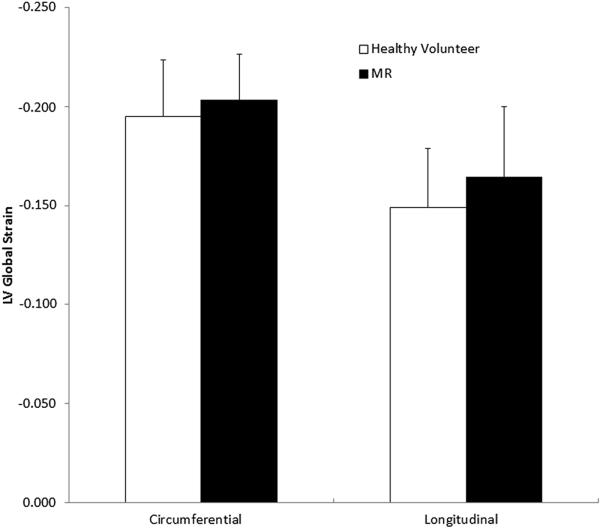
Left ventricular function as assessed by global myocardial strain and compared to normal controls is shown (Figure 3). Both circumferential strain (−0.20 ± 0.02 vs. −0.19 ± 0.03) and longitudinal strain (−0.16 ± 0.03 vs. −0.15 ± 0.03) were greater in absolute value compared to normal controls, but neither difference was found to be significant (p = 0.3 for circumferential and p = 0.08 for longitudinal).
Figure 3.
Global left ventricular function as assessed by global myocardial circumferential and longitudinal strains when compared to normal controls.
Regional Multiparametric Strain
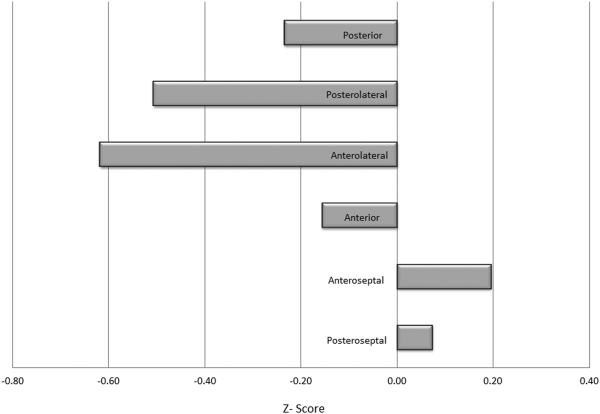
Regional multiparametric z-scores were positive (least contractile) in the septal regions of the LV and were maximally negative (most contractile) in the lateral walls (Figure 4). Standard deviations ranged from a low of 0.39 in the anteroseptal wall to a high of 0.71 in the anterior region. A one-way repeated-measures ANOVA was applied to test for differences in multiparametric z-scores among the six ventricular regions. Mauchly's test indicated the assumption of sphericity was violated, χ2 (14) = 33.51, p = 0.003. As a result, the degrees of freedom were adjusted using the Greenhouse-Geisser estimate of sphericity (ε = 0.562). Overall, multiparametric z-scores were found to vary significantly by region, F (2.81, 39.37) = 7.21, p = 0.001. The anteroseptum was the least contractile region of the LV and significantly less contractile when compared to the anterolateral (p=0.003), posterior (p=0.049) and posterolateral walls (p=0.009). The posteroseptum was the next least contractile segment and specifically less contractile compared to the anterolateral wall (p=0.035). The anterolateral segment was the most contractile of all segments and significantly greater than its anterior counterpart (p=0.002). While the posterolateral walls also demonstrated greater contractility when compared to the posterior segment this comparison failed to reach statistical significance (p=ns).
Figure 4.
Regional ventricular function among mitral regurgitation patients as assessed by multiparametric Z score analysis.
DISCUSSION
Multiple studies have now addressed the limitations of traditional echocardiographic metrics in evaluating subclinical LV dysfunction among asymptomatic patients with MR. The inference, however, that the incorporation of global strain indices or neurohumoral markers in the evaluation of MR patients in order to minimize the risk of developing permanent LV contractile injury may be intrinsically flawed 15, 17-19. A principal finding of this investigation is that in patients with severe degenerative MR, global contractile function, even as assessed by multiparametric strain analysis and high-resolution cardiac magnetic resonance tissue tagging, is preserved in early stages of the disease. This finding is not contradictory to literature that demonstrates decreased global strains prior to detectable changes in EF or chamber dilatation, but more so in agreement with studies that demonstrate in minimally symptomatic patients, with preserved EF, global strains may not only be easily preserved but even hypercontractile when compared to healthy volunteers 20, 21. Similarly, while elevated BNP levels among MR patients portend a worse prognosis, BNP at its core, remains a neurohumoral marker of LV remodeling, suggesting that its elevation is unfortunately indicative of a maladaptive response that has already occurred within the LV 17, 19, 22. The normal BNP levels and preserved global contractile function seen in this investigation suggest that the MR patients within this study were evaluated relatively early in the course of their disease and that the utility of global metrics in this setting may be limited.
The most important finding of this investigation is that the earliest contractile injury seen in degenerative MR patients with normal global contractile function is regionally and predictably distributed in the LV septum. These data further suggest that a compensatory increase in lateral wall contractility may exist, providing an explanation for the preserved global strain values demonstrated in the series. To our knowledge this is the first reported finding of such a regional contractile pattern demonstrated in MR patients, particularly when overall contractile metrics, are normal. These results build upon data demonstrating regional contractile dysfunction that has been observed in mitral valve prolapse or within the setting of congestive heart failure and dilated cardiomyopathy 15, 23. This heterogeneous injury pattern is furthermore consistent with experimental animal data that suggests that the earliest contractile injury in MR is not only likely to be regional but may also vary across LV wall thickness 24.
While the ideal strain marker to detect subclinical LV dysfunction remains to be determined, the use of circumferential and longitudinal strains may be particularly appealing due to their relative simplicity. These strain components are conceptually straightforward and when focused upon a specific anatomic region of interest (i.e. LV septum) that is easily identifiable by both cardiac MRI and echocardiography, these metrics have particular appeal for both repeatability and subsequent adoption by health care providers. The finding of diminished contractile function along the LV septum while not explained by the results of this investigation is certainly hypothesis generating and may reflect regional heterogeneity in injury susceptibility or stress distribution.
The findings of this study suggest that EF may not be the ideal metric to determine surgical referral in asymptomatic patients with chronic mitral regurgitation. Patient long-term outcomes remain variable when surgical referral of asymptomatic patients is based upon traditional echocardiography triggers as EF or chamber dilatation 18, 20, 22, 25. Most critically, it has been shown that preserved preoperative EF, does not assure postoperative preservation of contractile function 26. Moreover, the regional heterogeneity of contractile injury confirmed in this study highlights the presence of yet unidentified factors that induce non-uniform myocardial injury despite the global pathologic volume loading of MR upon the LV. The importance of using regional metrics of contractile function in assessing MR patients is hereby suggested, as the septum appears reproducibly vulnerable while the lateral wall may well have an early compensatory role in maintaining global contractile function.
In an effort to preserve LV contractile function and optimize MV repair results, this study would suggest that it may be possible to track the earliest of contractile injury among MR patients with serial imaging and that subsequent investigational efforts should be geared towards identifying when regional septal dysfunction either becomes more widespread across the LV or irreversible 26. Certainly waiting for global decreases in myocardial strain is unlikely to be the ideal solution as many patients with reduced global strains preoperatively, fail to normalize LV function postoperatively21, 22, 27. However, with an improved understanding of the injury pattern and progression associated with MR, coupled with the use of more sophisticated metrics of regional contractile function, the preservation of LV function and subsequent improved patient survival after MV surgery may become more standardized.
LIMITATIONS
This study is limited by the MRI data acquisition process and the complexity of the data analysis that have allowed only 15 MR patients to contribute to the outcome analysis (60 normal volunteers contributed to the normal human strain database). Continued efforts to fully automate the data analysis may extend this investigative tool to larger patient populations. As an initial pilot study this investigation lacks a true longitudinal clinical comparison of strain values patients over time. We are currently targeting patients both earlier in disease presentation and later in an attempt to more accurately define the contractile injury pattern by MR type (prolapse vs flail) and as MR evolves and progresses. The terms asymptomatic and minimally symptomatic while helpful are clearly subjective and difficult to standardize. That the patients chosen for study demonstrated normal global function by echo and multiparametric strain analysis and had a normal BNP level is reassuring that a relatively homogeneous group of patients with early MR were chosen for study.
CONCLUSION
Multiparametric strain analysis based upon high-resolution cardiac magnetic resonance imaging and tissue tagging demonstrated a regional pattern of contractile injury in minimally symptomatic and asymptomatic chronic MR patients that was not identifiable by any global contractile or biochemical marker. The LV septum appears to demonstrate contractile injury earliest in response to chronic MR and compensatory mechanisms to maintain overall systolic function may include hypercontractile compensation by other regions. An alternative paradigm for triggering earlier surgical referral to preserve LV contractile function in asymptomatic chronic MR patients should probably include the directed surveillance of early injury (or “sentinel”) LV regions (LV septum) with high-resolution metrics of regional contractile function.
Acknowledgments
Funding Sources: This work was supported by grant funding from the American Heart Association (11CRP7380050) and partially by funding from the National Institutes of Health (HL064869 and HL069967)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Michael K. Pasque, MD, Brian P. Cupps, PhD, and Washington University may receive income based on a license of related technology by the University to CardioWise, LLC. CardioWise, LLC did not support this work.
REFERENCES
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. The New England journal of medicine. 2005;352:875–83. doi: 10.1056/NEJMoa041451. [DOI] [PubMed] [Google Scholar]
- 3.Ling LH, Enriquez-Sarano M, Seward JB, Tajik AJ, Schaff HV, Bailey KR, et al. Clinical outcome of mitral regurgitation due to flail leaflet. The New England journal of medicine. 1996;335:1417–23. doi: 10.1056/NEJM199611073351902. [DOI] [PubMed] [Google Scholar]
- 4.David TE, Ivanov J, Armstrong S, Christie D, Rakowski H. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. The Journal of thoracic and cardiovascular surgery. 2005;130:1242–9. doi: 10.1016/j.jtcvs.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 5.Gillinov AM, Blackstone EH, Nowicki ER, Slisatkorn W, Al-Dossari G, Johnston DR, et al. Valve repair versus valve replacement for degenerative mitral valve disease. The Journal of thoracic and cardiovascular surgery. 2008;135:885–93, 93 e1-2. doi: 10.1016/j.jtcvs.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Gillinov AM, Mihaljevic T, Blackstone EH, George K, Svensson LG, Nowicki ER, et al. Should patients with severe degenerative mitral regurgitation delay surgery until symptoms develop? The Annals of thoracic surgery. 2010;90:481–8. doi: 10.1016/j.athoracsur.2010.03.101. [DOI] [PubMed] [Google Scholar]
- 7.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr., Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2006;48:e1–148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Topilsky Y, Suri R, Schaff HV, Enriquez-Sarano M. When to intervene for asymptomatic mitral valve regurgitation. Semin Thorac Cardiovasc Surg. 2010;22:216–24. doi: 10.1053/j.semtcvs.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Axel L, Dougherty L. Heart wall motion: improved method of spatial modulation of magnetization for MR imaging. Radiology. 1989;172:349–50. doi: 10.1148/radiology.172.2.2748813. [DOI] [PubMed] [Google Scholar]
- 10.Moulton MJ, Creswell LL, Downing SW, Actis RL, Szabo BA, Vannier MW, et al. Spline surface interpolation for calculating 3-D ventricular strains from MRI tissue tagging. Am J Physiol. 1996;270:H281–97. doi: 10.1152/ajpheart.1996.270.1.H281. [DOI] [PubMed] [Google Scholar]
- 11.Moustakidis P, Cupps BP, Pomerantz BJ, Scheri RP, Maniar HS, Kates AM, et al. Noninvasive, quantitative assessment of left ventricular function in ischemic cardiomyopathy. The Journal of surgical research. 2004;116:187–96. doi: 10.1016/j.jss.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Brady BD, Knutsen AK, Ma N, Gardner R, Taggar AK, Cupps BP, et al. MRI-based multiparametric strain analysis predicts contractile recovery after aortic valve replacement for aortic insufficiency. J Card Surg. 2012;27:415–22. doi: 10.1111/j.1540-8191.2012.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cupps BP, Bree DR, Wollmuth JR, Howells AC, Voeller RK, Rogers JG, et al. Myocardial viability mapping by magnetic resonance-based multiparametric systolic strain analysis. The Annals of thoracic surgery. 2008;86:1546–53. doi: 10.1016/j.athoracsur.2008.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cupps BP, Taggar AK, Reynolds LM, Lawton JS, Pasque MK. Regional myocardial contractile function: multiparametric strain mapping. Interact Cardiovasc Thorac Surg. 2010;10:953–7. doi: 10.1510/icvts.2009.220384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph S, Moazami N, Cupps BP, Howells A, Craddock H, Ewald G, et al. Magnetic resonance imaging-based multiparametric systolic strain analysis and regional contractile heterogeneity in patients with dilated cardiomyopathy. J Heart Lung Transplant. 2009;28:388–94. doi: 10.1016/j.healun.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutsen AK, Ma N, Taggar AK, Brady BD, Cupps BP, Pasque MK. Heterogeneous distribution of left ventricular contractile injury in chronic aortic insufficiency. The Annals of thoracic surgery. 2012;93:1121–7. doi: 10.1016/j.athoracsur.2011.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Detaint D, Messika-Zeitoun D, Avierinos JF, Scott C, Chen H, Burnett JC, Jr., et al. B-type natriuretic peptide in organic mitral regurgitation: determinants and impact on outcome. Circulation. 2005;111:2391–7. doi: 10.1161/01.CIR.0000164269.80908.9D. [DOI] [PubMed] [Google Scholar]
- 18.Mankad R, McCreery C, Rogers WJ, RJ W, Savage E, Reichek N, et al. Regional Myocardial Strain Before and After Mitral Valve Repair for Severe Mitral Regurgitation. Journal of Cardiovascular Magnetic Resonance. 2001;3:257–66. doi: 10.1081/jcmr-100107474. [DOI] [PubMed] [Google Scholar]
- 19.Pizarro R, Bazzino OO, Oberti PF, Falconi M, Achilli F, Arias A, et al. Prospective validation of the prognostic usefulness of brain natriuretic peptide in asymptomatic patients with chronic severe mitral regurgitation. Journal of the American College of Cardiology. 2009;54:1099–106. doi: 10.1016/j.jacc.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Witkowski TG, Thomas JD, Delgado V, van Rijnsoever E, Ng AC, Hoke U, et al. Changes in left ventricular function after mitral valve repair for severe organic mitral regurgitation. The Annals of thoracic surgery. 2012;93:754–60. doi: 10.1016/j.athoracsur.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Zito C, Carerj S, Todaro MC, Cusma-Piccione M, Caprino A, Di Bella G, et al. Myocardial deformation and rotational profiles in mitral valve prolapse. The American journal of cardiology. 2013;112:984–90. doi: 10.1016/j.amjcard.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Hwang IC, Kim YJ, Kim KH, Lee SP, Kim HK, Sohn DW, et al. Prognostic value of B-type natriuretic peptide in patients with chronic mitral regurgitation undergoing surgery: mid-term follow-up results. Eur J Cardiothorac Surg. 2013;43:e1–6. doi: 10.1093/ejcts/ezs513. [DOI] [PubMed] [Google Scholar]
- 23.Malev E, Zemtsovsky E, Pshepiy A, Timofeev E, Reeva S, Prokudina M. Evaluation of left ventricular systolic function in young adults with mitral valve prolapse. Exp Clin Cardiol. 2012;17:165–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Carlhall CJ, Nguyen TC, Itoh A, Ennis DB, Bothe W, Liang D, et al. Alterations in transmural myocardial strain: an early marker of left ventricular dysfunction in mitral regurgitation? Circulation. 2008;118:S256–62. doi: 10.1161/CIRCULATIONAHA.107.753525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enriquez-Sarano M, Sundt TM., 3rd. Early surgery is recommended for mitral regurgitation. Circulation. 2010;121:804–11. doi: 10.1161/CIRCULATIONAHA.109.868083. discussion 12. [DOI] [PubMed] [Google Scholar]
- 26.Marciniak A, Sutherland GR, Marciniak M, Kourliouros A, Bijnens B, Jahangiri M. Prediction of postoperative left ventricular systolic function in patients with chronic mitral regurgitation undergoing valve surgery--the role of deformation imaging. Eur J Cardiothorac Surg. 2011;40:1131–7. doi: 10.1016/j.ejcts.2011.02.049. [DOI] [PubMed] [Google Scholar]
- 27.Pomerantz BJ, Wollmuth JR, Krock MD, Cupps BP, Moustakidis P, Kouchoukos NT, et al. Myocardial systolic strain is decreased after aortic valve replacement in patients with aortic insufficiency. The Annals of thoracic surgery. 2005;80:2186–92. doi: 10.1016/j.athoracsur.2005.05.095. [DOI] [PubMed] [Google Scholar]