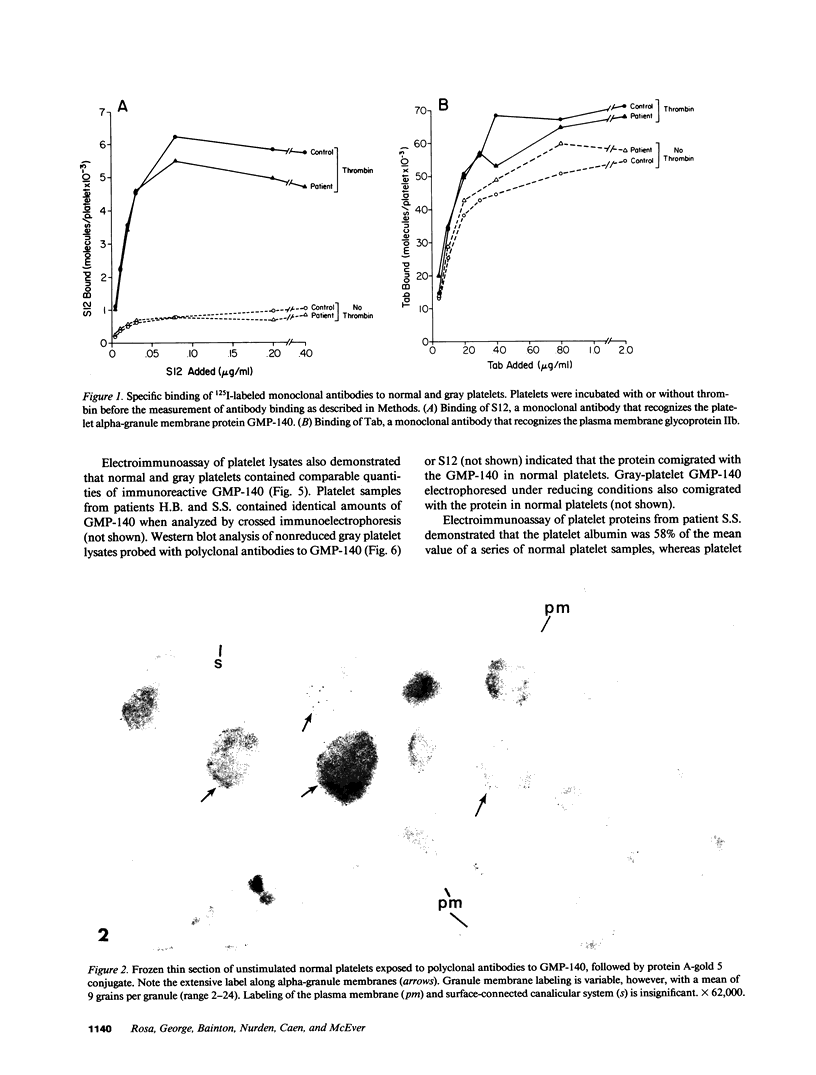
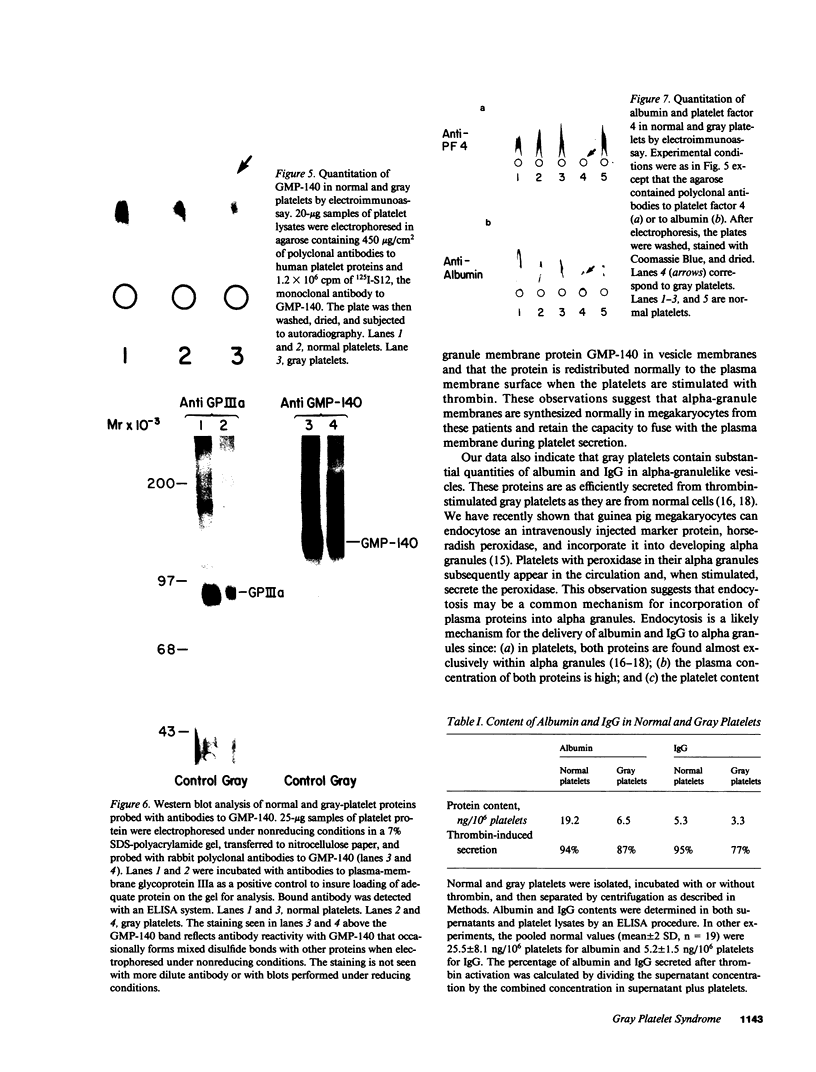
Abstract
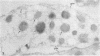
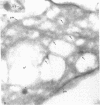
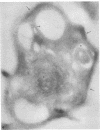
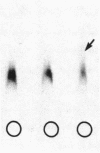
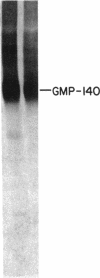
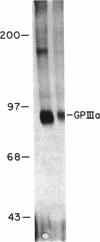
Platelets from patients with the gray platelet syndrome have decreased recognizable alpha granules and are markedly deficient in some alpha-granule secretory proteins. Using immunocytochemical techniques with antibodies to an alpha-granule membrane protein, GMP-140, we identified the membranes of intracellular vesicles in gray platelets as alpha-granule membranes. Gray platelets contained normal amounts of GMP-140 as measured by electroimmunoassay. The activation of gray platelets with thrombin caused GMP-140 to be redistributed to the plasma membrane surface, as in normal platelets. In agreement with previous studies, an endogenously synthesized secretory protein, platelet factor 4, was undetectable in gray platelets. However, the alpha-granule proteins albumin and IgG, which are thought to be derived from endocytosis of plasma proteins into megakaryocytes, were present in substantial quantities and were secreted efficiently from gray platelets. Therefore, the fundamental defect in the gray platelet syndrome may be in the targeting of endogenously synthesized secretory proteins to developing alpha granules in megakaryocytes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckstead J. H., Stenberg P. E., McEver R. P., Shuman M. A., Bainton D. F. Immunohistochemical localization of membrane and alpha-granule proteins in human megakaryocytes: application to plastic-embedded bone marrow biopsy specimens. Blood. 1986 Feb;67(2):285–293. [PubMed] [Google Scholar]
- Belloc F., Hourdille P., Fialon P., Boisseau M. R., Soria J. Fibrinogen synthesis by megakaryocyte rich human marrow cell concentrates. Thromb Res. 1985 May 15;38(4):341–351. doi: 10.1016/0049-3848(85)90133-1. [DOI] [PubMed] [Google Scholar]
- Berman C. L., Yeo E. L., Wencel-Drake J. D., Furie B. C., Ginsberg M. H., Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest. 1986 Jul;78(1):130–137. doi: 10.1172/JCI112542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt M. C., Castaldi P. A., Gordon S., Halley H., McPherson V. J. Morphological and biochemical confirmation of gray platelet syndrome in two siblings. Aust N Z J Med. 1983 Aug;13(4):387–390. doi: 10.1111/j.1445-5994.1983.tb04488.x. [DOI] [PubMed] [Google Scholar]
- Bray P. F., Rosa J. P., Lingappa V. R., Kan Y. W., McEver R. P., Shuman M. A. Biogenesis of the platelet receptor for fibrinogen: evidence for separate precursors for glycoproteins IIb and IIIa. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1480–1484. doi: 10.1073/pnas.83.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton-Gorius J., Vainchenker W., Nurden A., Levy-Toledano S., Caen J. Defective alpha-granule production in megakaryocytes from gray platelet syndrome: ultrastructural studies of bone marrow cells and megakaryocytes growing in culture from blood precursors. Am J Pathol. 1981 Jan;102(1):10–19. [PMC free article] [PubMed] [Google Scholar]
- Cramer E. M., Vainchenker W., Vinci G., Guichard J., Breton-Gorius J. Gray platelet syndrome: immunoelectron microscopic localization of fibrinogen and von Willebrand factor in platelets and megakaryocytes. Blood. 1985 Dec;66(6):1309–1316. [PubMed] [Google Scholar]
- George J. N., Pickett E. B., Saucerman S., McEver R. P., Kunicki T. J., Kieffer N., Newman P. J. Platelet surface glycoproteins. Studies on resting and activated platelets and platelet membrane microparticles in normal subjects, and observations in patients during adult respiratory distress syndrome and cardiac surgery. J Clin Invest. 1986 Aug;78(2):340–348. doi: 10.1172/JCI112582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. N., Saucerman S., Levine S. P., Knieriem L. K., Bainton D. F. Immunoglobulin G is a platelet alpha granule-secreted protein. J Clin Invest. 1985 Nov;76(5):2020–2025. doi: 10.1172/JCI112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. N., Thoi L. L., McManus L. M., Reimann T. A. Isolation of human platelet membrane microparticles from plasma and serum. Blood. 1982 Oct;60(4):834–840. [PubMed] [Google Scholar]
- Gerrard J. M., Phillips D. R., Rao G. H., Plow E. F., Walz D. A., Ross R., Harker L. A., White J. G. Biochemical studies of two patients with the gray platelet syndrome. Selective deficiency of platelet alpha granules. J Clin Invest. 1980 Jul;66(1):102–109. doi: 10.1172/JCI109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Brands R., Burke B., Louvard D., Warren G. Viral membrane proteins acquire galactose in trans Golgi cisternae during intracellular transport. J Cell Biol. 1982 Dec;95(3):781–792. doi: 10.1083/jcb.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handagama P. J., George J. N., Shuman M. A., McEver R. P., Bainton D. F. Incorporation of a circulating protein into megakaryocyte and platelet granules. Proc Natl Acad Sci U S A. 1987 Feb;84(3):861–865. doi: 10.1073/pnas.84.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu-Lin S., Berman C. L., Furie B. C., August D., Furie B. A platelet membrane protein expressed during platelet activation and secretion. Studies using a monoclonal antibody specific for thrombin-activated platelets. J Biol Chem. 1984 Jul 25;259(14):9121–9126. [PubMed] [Google Scholar]
- Isenberg W. M., McEver R. P., Shuman M. A., Bainton D. F. Topographic distribution of a granule membrane protein (GMP-140) that is expressed on the platelet surface after activation: an immunogold-surface replica study. Blood Cells. 1986;12(1):191–204. [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes in normal and disease states. J Clin Invest. 1986 Jan;77(1):1–6. doi: 10.1172/JCI112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M., Hellstern P., Morgenstern E., Mueller-Eckhardt C., Berberich R., Meiser R. J., Scheffler P., Wenzel E. Gray platelet syndrome: selective alpha-granule deficiency and thrombocytopenia due to increased platelet turnover. Blut. 1985 Jun;50(6):331–340. doi: 10.1007/BF00320926. [DOI] [PubMed] [Google Scholar]
- Leven R. M., Schick P. K., Budzynski A. Z. Fibrinogen biosynthesis in isolated guinea pig megakaryocytes. Blood. 1985 Feb;65(2):501–504. [PubMed] [Google Scholar]
- Levine S. P., Krentz L. S. Development of a radioimmunoassay for human platelet factor 4. Thromb Res. 1977 Nov;11(5):673–686. doi: 10.1016/0049-3848(77)90025-1. [DOI] [PubMed] [Google Scholar]
- Levy-Toledano S., Caen J. P., Breton-Gorius J., Rendu F., Cywiner-Golenzer C., Dupuy E., Legrand Y., Maclouf J. Gray platelet syndrome: alpha-granule deficiency. Its influence on platelet function. J Lab Clin Med. 1981 Dec;98(6):831–848. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Baenziger N. L., Majerus P. W. Isolation and quantitation of the platelet membrane glycoprotein deficient in thrombasthenia using a monoclonal hybridoma antibody. J Clin Invest. 1980 Dec;66(6):1311–1318. doi: 10.1172/JCI109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- McEver R. P., Martin M. N. A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem. 1984 Aug 10;259(15):9799–9804. [PubMed] [Google Scholar]
- Nachman R., Levine R., Jaffe E. A. Synthesis of factor VIII antigen by cultured guinea pig megakaryocytes. J Clin Invest. 1977 Oct;60(4):914–921. doi: 10.1172/JCI108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden A. T., Kunicki T. J., Dupuis D., Soria C., Caen J. P. Specific protein and glycoprotein deficiencies in platelets isolated from two patients with the gray platelet syndrome. Blood. 1982 Apr;59(4):709–718. [PubMed] [Google Scholar]
- Paul J. I., Schwarzbauer J. E., Tamkun J. W., Hynes R. O. Cell-type-specific fibronectin subunits generated by alternative splicing. J Biol Chem. 1986 Sep 15;261(26):12258–12265. [PubMed] [Google Scholar]
- Raccuglia G. Gray platelet syndrome. A variety of qualitative platelet disorder. Am J Med. 1971 Dec;51(6):818–828. doi: 10.1016/0002-9343(71)90311-1. [DOI] [PubMed] [Google Scholar]
- Ryo R., Nakeff A., Huang S. S., Ginsberg M., Deuel T. F. New synthesis of a platelet-specific protein: platelet factor 4 synthesis in a megakaryocyte-enriched rabbit bone marrow culture system. J Cell Biol. 1983 Feb;96(2):515–520. doi: 10.1083/jcb.96.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixma J. J., van den Berg A., Schiphorst M., Geuze H. J., McDonagh J. Immunocytochemical localization of albumin and factor XIII in thin cryo sections of human blood platelets. Thromb Haemost. 1984 Jul 29;51(3):388–391. [PubMed] [Google Scholar]
- Stenberg P. E., Beckstead J. H., McEver R. P., Levin J. Immunohistochemical localization of membrane and alpha-granule proteins in plastic-embedded mouse bone marrow megakaryocytes and murine megakaryocyte colonies. Blood. 1986 Sep;68(3):696–702. [PubMed] [Google Scholar]
- Stenberg P. E., McEver R. P., Shuman M. A., Jacques Y. V., Bainton D. F. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985 Sep;101(3):880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg P. E., Shuman M. A., Levine S. P., Bainton D. F. Redistribution of alpha-granules and their contents in thrombin-stimulated platelets. J Cell Biol. 1984 Feb;98(2):748–760. doi: 10.1083/jcb.98.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- White J. G. Ultrastructural studies of the gray platelet syndrome. Am J Pathol. 1979 May;95(2):445–462. [PMC free article] [PubMed] [Google Scholar]
- Woods V. L., Jr, Wolff L. E., Keller D. M. Resting platelets contain a substantial centrally located pool of glycoprotein IIb-IIIa complex which may be accessible to some but not other extracellular proteins. J Biol Chem. 1986 Nov 15;261(32):15242–15251. [PubMed] [Google Scholar]