Abstract
Objective
The aim of this study was to compare the uptake of mail-delivered tests for colorectal cancer screening. We assessed the effect of an advance notification letter and a reminder letter, and analysed the proportion of inappropriately handled tests.
Materials and methods
Fifteen thousand randomly selected residents of Latvia aged 50–74 years were allocated to receive one of three different test systems: either a guaiac faecal occult blood test (gFOBT) or one of two laboratory-based immunochemical tests (FIT) – FOB Gold or OC-Sensor. Half of the target population received an advance notification letter; all nonresponders were sent a reminder letter.
Results
The uptake of screening was 31.2% for the gFOBT, 44.7% for FOB Gold and 47.4% for the OC-Sensor (odds ratio 0.55; 95% confidence interval 0.51–0.60 for gFOBT vs. FOB Gold; odds ratio 0.90; 95% confidence interval 0.83–0.98 for FOB Gold vs. OC-Sensor). The uptake in the gFOBT group was improved by the advance notification letter (7.7%, P<0.0001). 30.9% returned tests were received after the reminder letter. The proportion of tests that could not be analysed because of inadequate handling was 0.9% for gFOBT, 4.4% for FOB Gold and 0.2% for the OC-Sensor (P=0.002 for gFOBT vs. OC-Sensor; P<0.001 for all comparisons vs. FOB Gold).
Conclusion
The use of FIT resulted in higher uptake. Receipt of a reminder letter was critical to participation, but the use of an advance notification letter was important mainly for gFOBT. The proportion of inappropriately handled tests was markedly higher for FOB Gold.
Keywords: advance notification letter, colorectal cancer screening, faecal immunochemical test, faecal occult blood test, uptake
Introduction
Population-based colorectal cancer (CRC) screening is recommended in all countries of the EU 1, and Latvia has declared its intention to implement this type of screening 2. On the basis of the approach used in the Czech Republic 3, nationwide screening in Latvia has been implemented through general practices without a direct invitation to the target population, and uptake has been disappointing – at best 9.6% as of 2013, but 7.6% for the period during which the study was carried out (i.e. 2010) 4 reflecting not only poor organization of the system but also low awareness of screening benefits in the target population as low uptake is characteristic for all cancer screening programmes, and also for those in which invitation letters are being mailed 4. Therefore, changes in the CRC screening system are required. Here, we report the results of a pilot study designed to establish a strategy to optimize population screening in Latvia.
Organized population CRC screening is the approach recommended by the recent European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis 5. However, many European countries are still in the process of implementing organized CRC screening.
In Europe, faecal occult blood tests are recommended for screening and, until recently, guaiac-based tests [guaiac faecal occult blood test (gFOBT)] were recommended 6 because of evidence from randomized-controlled trials of a reduction in mortality 7–9. The recent European Guidelines 5 now encourage the use of quantitative faecal immunochemical tests for haemoglobin (FIT) instead of the traditional gFOBT because of better performance 10–13.
FIT have several other advantages of over gFOBT: restrictions in the use of medications before testing are not necessary, test handling is easier for the participant, the test is specific for human blood, and thus dietary restrictions are not necessary and only one faecal sample is necessary for testing in each episode 14–16. At the same time, there are several disadvantages for FIT use; in particular, increased thickness of the envelope necessary to protect the FIT collection device could increase the risk of damage to the devices when distributed in the mail. Sample stability could be more of an issue with FIT because FIT detect human globin, which is less stable than the haem detected by gFOBT. At room temperature, the manufacturers’ recommended maximum timeframe for analysing FIT may be half that advised for gFOBT. In addition, FIT are more expensive than gFOBT.
Mail delivery of faecal occult blood tests directly to potential screening participants has not been tested in Latvia. The aim of this study was to identify the best approach to maximize uptake in a country where there is a low awareness of the benefits of screening among the target population and low uptake of the current opportunistic screening approach. In addition, we tested the potential gain from advance notification and reminder letters comparing three faecal occult blood tests currently used widely in Europe 5.
Materials and methods
Study population selection
The study population was identified from the Cancer Screening Registry, which is linked to the Latvian Population Registry (including data on all the permanent inhabitants of the country) and the National Cancer Registry. Individuals who, according to data from the National Cancer Registry, had a history of CRC were excluded. In 2011, a total of 15 000 individuals aged 50–74 years were selected at random from the Cancer Screening Registry covering the entire population of Latvia, and then randomized further into three subgroups, of 5000 individuals each, to receive either the gFOBT or one of the FIT kits. In addition, half of the study group (7500 individuals) was assigned randomly to receive an advance notification letter. The letter provided brief information on CRC and the value of screening, and informed the recipient that a screening test would be mailed to the individual unless a refusal to participate was received from him/her. All the nonresponders received a reminder letter, irrespective of whether or not they had received an advance notification letter.
Ethics approval
The study was approved by the Central Medical Ethics Committee of Latvia, protocol NA-10, date 15 December 2010.
Invitation procedure
The cohort that was randomized to receive an advance notification letter was provided an option to refuse participation in the study by telephone. Two weeks following the advance notification letter (June, 2011), a faecal occult blood test, together with instructions, an invitation letter and a postage-paid return envelope, was sent to all individuals who had not refused to participate and whose advance notification letters had not been returned by mail services as being undeliverable. The invitation letter stated that the return of the test kit was considered acceptance of participation in the study.
The cohort that was randomized to not receive an advance notification letter was sent an identical invitation letter with the faecal occult blood test, instructions and return envelope. As 3-day faecal collection was required for gFOBT, gFOBT kits were mailed 2 days before the FIT; therefore, all the groups were allowed a similar time to respond. The proportion of returned tests was monitored on a daily basis for all groups. When the proportion of returned tests decreased below 0.5%/day and remained at this level for at least 3 consecutive days, a reminder letter was sent to all nonresponders.
Tests returned 3 days or more after the mailing of the reminder letter were considered received ‘after’ the reminder; this lag time was derived by considering the mail system’s operational time and the time needed to complete the test.
Completed tests received up to 60 days after despatch were included in the current analysis.
Test types
We chose two of the quantitative FIT with adjustable cut-off haemoglobin concentrations listed in the European Guidelines 5, that is FOB Gold (Sentinel Diagnostics, Milan, Italy) and OC-Sensor (Eiken Chemical Co., Tokyo, Japan). The gFOBT used most widely in Latvia, HemoCare (Care Diagnostics, Möllersdorf, Austria), was chosen for the third arm. One faecal sample (taken according to the manufacturers’ instructions) was required for the FIT, whereas three consecutive faecal samples (two samples from each) were required for the gFOBT.
The FOB Gold faecal collection device is a round-shaped tube with two screw-threaded caps – one at each end of the collection tube; the green-coloured cap is designed to be removed for sample collection, whereas the other cap is for laboratory testing. The OC-Sensor faecal collection device is a flattened tube with one cap attached to the sampling device. The bottom of the device has a foil seal that is perforated by the analysers in the laboratory to allow access to the sample buffer.
The screening participants were asked to record the sampling date: for the gFOBT and OC-Sensor, the date was recorded on the sample collection device itself, but for FOB Gold, an additional leaflet was provided as space for writing the date on the sample tube was inadequate. Recommendations for dietary restrictions were made according to the manufacturers’ instructions. Only gFOBT carried a requirement to avoid raw or partially raw meat, sausages or horseradish, and to limit the intake of vitamin C or aspirin and to consume more fibre-containing foods.
Evaluation of appropriateness of handling
Each of the tests was evaluated by trained laboratory technicians according to the appropriateness of handling when returned to the laboratory for analysis. The assessment included a review of possible damage to the test in transit, the amount of faecal sample inside the system (visual assessment), whether the system has been opened appropriately and whether the sample had been placed according to the instructions. For FIT, the presence of the buffer in the system was recorded. The presence or absence of the sampling date was also recorded.
Feedback
A telephone helpline was provided for anyone who wanted to refuse participation and for invitees with questions and/or concerns. Every individual who called the helpline and wanted to opt out was asked for the reasons for nonparticipation. Those not willing to participate were classified as ‘rejected’. Other reasons for nonparticipation (e.g. diagnosed with CRC, having recently undergone faecal occult blood testing, etc.) were classified as ‘excluded for other reasons’. Addressees of letters returned undelivered by the mail services were included in the group ‘excluded for other reasons’.
Definition of screening uptake
The screening uptake between the different test groups was defined as the number of returned tests from the number of cases assigned for the particular strategy, that is on an ‘intention-to-test’ basis. The tests that have been handled inappropriately, yet returned according to the instructions, were also included in the group of ‘returned’ tests. Similarly, the gain from the notification letter was calculated on an ‘intention-to-test’ basis. However, no additional randomization was used to evaluate the impact of the reminder letter. For the latter purpose, the proportion of the test kits returned after the reminder letter and following a considerable decrease in return of the test kits initially was considered.
Statistical analysis
Oracle Database PL/SQL Packages and Types Reference 10g Release 2 (version 10.2; Oracle America Inc., Redwood City, California, USA) were used to select the study population from the Cancer Screening Registry database. Further randomization procedures were performed using Microsoft Office Excel 2007 (Microsoft Corporation, Redmond, Washington, USA).
The software and database for data capture and storage were developed using the Sample Information Management System SIMS (a component of the SIMBioMS software suite) 17 by creating an appropriate configuration as well as by adding additional software modules.
Further statistical analysis was carried out using Microsoft Office Excel 2007, statistical package for the social sciences (SPSS version 17.0; SPSS Inc., Chicago, Illinois, USA) and SAS System for Windows, version 9.3 (SAS Institute Inc., Cary, North Carolina, USA).
The homogeneity of the study groups was tested using Pearson’s χ2-test.
Frequency distributions were evaluated for the categorical variables (sex, age group, the place of residence). Age groups were analysed in 5-year age intervals. The place of residence was divided into three groups – group I: Riga, the capital of Latvia; group II: other major cities; and group III: rural areas. Groups I and II were considered urban areas.
Multivariate logistic regression was used to estimate odds ratios and 95% confidence intervals (CIs) controlling for potential confounders (age, sex, place of residence). In addition, the interaction effect of the two randomized factors (test group and advanced notification letter) was explored using a logistic regression model to carry out a sensitivity analysis for main comparisons.
Summary statistics include point estimates and standard deviations or 95% CIs for all variables. All significance levels were set to P less than 0.05, but in the comparison of all three study arms, a Bonferroni multiplicity correction was applied (i.e. significance level of 0.05/3=0.0167).
Results
There was an equal distribution between the three groups (5000 individuals each) randomized to each of the test types in terms of sex (P=0.85), age group (P=0.56) and place of residence (P=0.1). Of the 7500 individuals randomized to receive the advance notification letter, 2506 individuals were in the gFOBT, 2484 individuals were in the FOB Gold and 2510 individuals were in the OC-Sensor arm. Figure 1 presents the number of mailed and received letters/tests for each of the study groups.
Fig. 1.
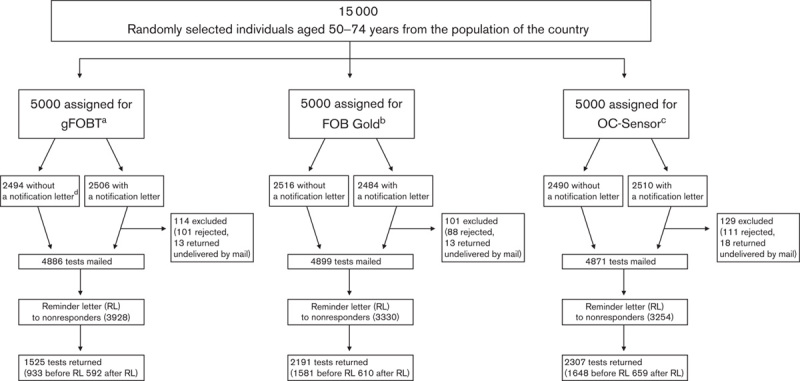
Study design. aGuaiac faecal occult blood test. bFaecal immunochemical test for haemoglobin (Sentinel Diagnostics). cFaecal immunochemical test for haemoglobin (Eiken Chemical Co.). dAdvance notification letter.
In the group that received advance notification letters, 300 (4.0%) of the addressed individuals refused to participate; an additional 44 individuals (0.6%) were excluded because the letters were returned by mail services as being nondeliverable (nonexistent address, addressee not living at the address, addressee deceased).
For all groups, the most common reasons for refusing to participate were a lack of willingness to participate (63.1%), faecal occult blood tests performed during the current year (8.7%), having moved abroad (5.8%), previous colonoscopy (2.3%) or a personal history of CRC (0.9%), the latter indicating discrepancies with the Cancer Registry database as this group should not have been invited otherwise.
An additional 336 refusals (2.3% of those to whom the tests were mailed in each group) were received. For the group that did not receive an advance notification letter, refusal after receipt of the test was 2.1%, whilst for those who did receive an advance notification letter, refusal after receipt of the test was 2.5%.
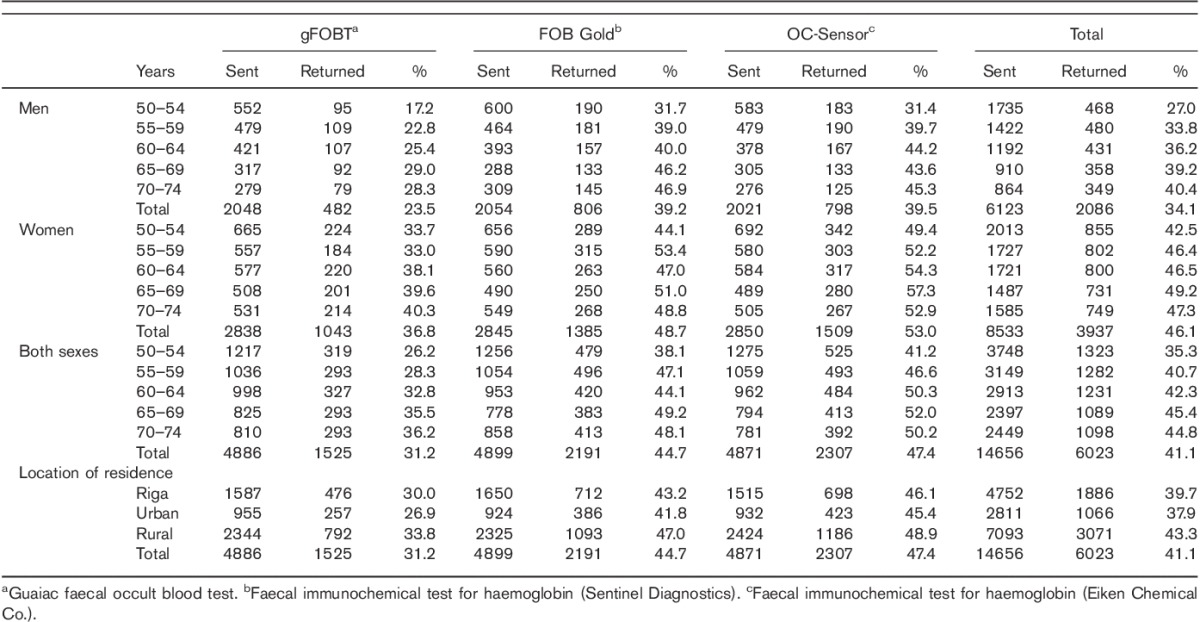
Altogether, 14656 tests were mailed and 6023 tests were returned. Of those returned, two envelopes (0.01%) were damaged (both in the FOB Gold group). Uptake for the entire study population was 41.1% (Table 1). Uptake was 31.2% for gFOBT, 44.7% for FOB Gold and 47.4% for the OC-Sensor [odds ratio (OR) 0.55; 95% CI 0.51–0.60 for gFOBT vs. FOB Gold; OR 0.50; 95% CI 0.46–0.54 for gFOBT vs. the OC-Sensor; OR 0.90; 95% CI 0.83–0.98 for FOB Gold vs. the OC-Sensor]. Uptake for all tests was significantly lower among men (34.1%) than women (46.1%; OR 0.61; 95% CI 0.56–0.65). Uptake of any test was the lowest in the youngest age group (50–54 years) and increased with age until the second highest age group (65–69 years). The differences in uptake between the youngest group and all the other age groups were statistically significant [the largest difference being for 65–69 vs. 50–54 years (OR 1.50; 95% CI 1.35–1.67)].
Table 1.
Number of sent and returned tests, proportion of returned tests (uptake) in particular age, sex and location of residence groups
Overall uptake was higher in rural than in urban areas; no difference was found between Riga, the capital of Latvia, and other major cities of the country (Fig. 2).
Fig. 2.
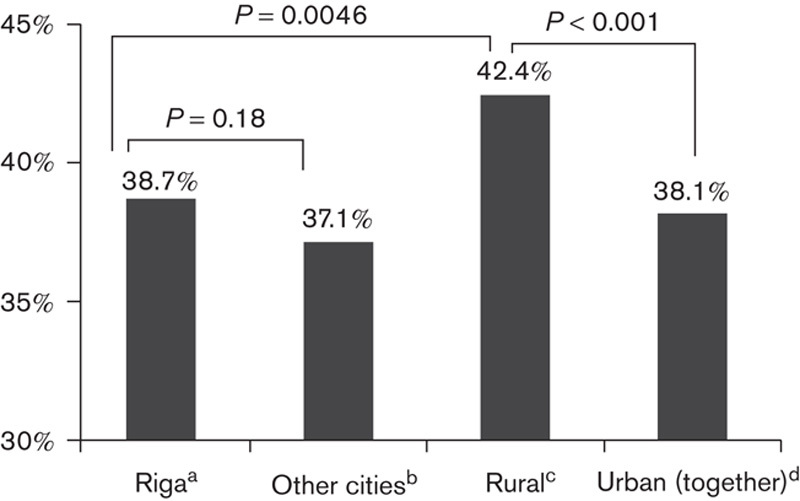
Comparison of uptake between urban and rural inhabitants. aInhabitants of Riga, the capital of the country. bInhabitants of other major cities. cInhabitants of rural territories, including small towns. dInhabitants of Riga and other major cities combined.
In the group that did not receive the advance notification letters, the overall uptake was 38.6% (26.6% for gFOBT, 43.8% for FOB Gold, 45.3% for OC-Sensor tests). Advance notification letters increased uptake by 3.1% (P=0.0001) overall. A marked increase in uptake was observed for the gFOBT group (7.7%, P<0.0001); for the OC-Sensor, the increase in uptake was not statistically significant (1.6%, P=0.5); and for FOB Gold, uptake was unchanged (0.04%, P=0.99).
The reminder letter was mailed on average 20.7 days (median 20, SD 1.8) after the initial test. Altogether, 30.9% of the tests were received following the reminder letter (38.8% for gFOBT, 27.8% for FOB Gold, 28.6% for the OC-Sensor). Figure 3 presents uptake for men and women depending on receipt of advance notification and/or reminder letters (for gFOBT and FIT combined).
Fig. 3.
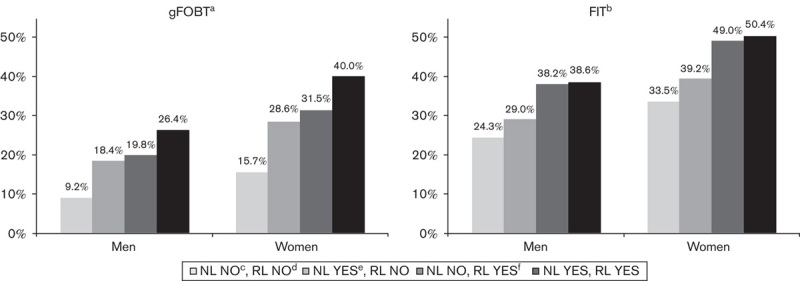
Uptake according to receipt of an advance notification and reminder letters for women and men for gFOBT and for FIT (pooled together). aGuaiac faecal occult blood test. bFaecal immunochemical tests for haemoglobin. cIndividuals having not received an advance notification letter. dIndividuals having not received a reminder letter. eIndividuals having received an advance notification letter. fIndividuals having received a reminder letter.
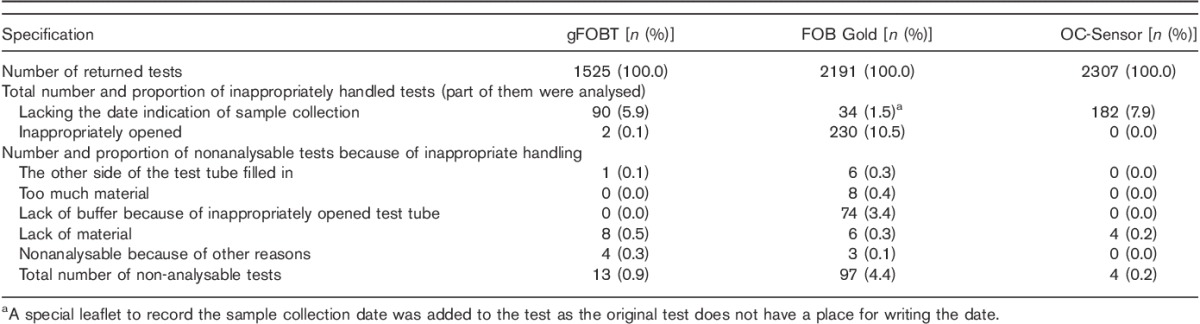
Altogether, 5.1% of the returned analysed tests did not include the sampling date (5.9% for gFOBT, 1.6% for FOB Gold, 7.9% for the OC-Sensor; P=0.03 for gFOBT vs. OC-Sensor, P<0.001 for FOB Gold vs. both the comparisons).
Of the returned tests, 0.1% of gFOBT, 10.5% of FOB Gold, but none of the OC-Sensor test kits had been opened inappropriately. For FOB Gold, this meant that the opposite side of the test device was opened, although some of these test samples were still used for the analysis (see Table 2). The proportion of tests that was unreadable because of inadequate sample handling was 0.9% for gFOBT, 4.4% for FOB Gold and 0.2% for the OC-Sensor (P=0.002 for gFOBT vs. the OC-Sensor; P<0.001 for all comparisons vs. FOB Gold). The unreadable tests included those returned without any material in the test tube, a problem that accounted for all unreadable OC-Sensor tests. For the causes of unreadable tests, see Table 2. The majority of tests (89.6%) were analysed within 5 days of sample collection [68.3% for gFOBT (following the first sample placement), 96.5% for FOB Gold, 97.3% for the OC-Sensor]; 97.4% of the tests were analysed within 7 days (92.7% for gFOBT, 98.9% for FOB Gold, 99.1% for the OC-Sensor).
Table 2.
Proportion of inappropriately handled tests
Discussion
Our study showed that CRC screening test mail delivery is a feasible approach in Latvia, and that the uptake can be improved by using FIT instead of gFOBT. Implementation of an organized CRC screening programme is a complex process, and achieving the quality indicators in participation provides only one of the required factors in this chain.
General uptake characteristics
Direct mailing of a faecal occult blood tests, together with instructions, an invitation letter and a postage-paid return envelope with laboratory address, is known to increase uptake significantly 18. The age, sex and residence of the study population were representative of the entire country because of random sampling. The proportion of women in the study population was higher, reflecting the population distribution in this age group.
The current approach to CRC screening in Latvia is opportunistic screening using gFOBT performed from three consecutive faecal samples. As screening was introduced only a few years ago (between 2005 and 2009) and no population-wide campaigns have been conducted during the implementation, awareness of the benefits of screening is low; this is reflected in the low current uptake 4,5. Quantitative FIT have many advantages over gFOBT, including an adjustable cut-off for the haemoglobin concentration that determines a positive result, thereby allowing local decisions on the threshold for referral for colonoscopy according to colonoscopy resource. FIT are now recommended over gFOBT for CRC screening 5.
Our study was designed in three arms – a traditional gFOBT and two quantitative FIT. The overall uptake achieved with targeted mailing (41.1%) was markedly higher than that for the existing opportunistic screening programme in Latvia (7.6%). This study has shown that mail delivery of faecal occult blood tests for screening is feasible in an area with comparatively low awareness of screening benefits. These observations are supported by the results of a randomized study in Belgium: the participation rate was 52.3% with mail-delivered FIT tests compared with 27.7% for tests kits provided by a general practitioner (P<0.001) 19. As has been reported elsewhere 19–23, the participation of women and rural inhabitants was higher when kits were mailed directly to potential participants.
Overall uptake was close to the acceptable minimum requirement (45.0%) set by the European Guidelines 5. The difference in uptake between different tests is an important consideration. Uptake of gFOBT uptake (31.2%) was markedly below 45.0%, whereas there was a slight trend favouring uptake of the OC-Sensor test (47.4%) compared with FOB Gold (44.7%). Therefore, without an extensive public awareness campaign, the recommended minimum acceptable uptake was achievable with FIT. The difference in uptake between gFOBT and FIT reported here is even higher than that reported in other studies in the Netherlands 11,24, Italy 15 or in the USA, Washington State 25. However, a recent study from Israel reported slightly higher uptake for gFOBT (28.8%) than FIT (25.9%; P<0.001); in this study, three faecal samples were analysed for any of the tests 22. It could be speculated that in a low-awareness population, a more easy-to-use test (i.e. FIT test requiring single stool collection and no dietary modifications) could have a higher impact on uptake than in a population with a better understanding of the screening benefits.
The differences in uptake between gFOBT and FIT could be explained by a number of factors. Although modest dietary restrictions for gFOBT may not have critical importance 26, there is at least one study showing the adverse effect of dietary restrictions on uptake 27. The requirement for just one faecal sample for FIT may also improve compliance 14,28. Finally, aversion to sampling faeces is another important barrier 14; this factor could also favour FIT as less contact with faeces is required. Our on-going research will provide more data on patient preferences in our study cohort.
Advance notification letter
The impact of sending an advance notification is two-fold: first, as a tool to increase uptake and second, to avoid the cost of sending the test to individuals who are not motivated to participate. Conversely, there is the possibility that more individuals will decline screening if asked for their preference.
Several studies have suggested that an advance notification letter may increase participation 29. In Australia, an advance notification letter increased uptake from 39.5 to 48.3% (i.e. by 8.8%); this was statistically significant, although the study groups were small 30.
Similarly, in a large screening pilot study in Scotland, an advance notification letter or a letter accompanied by an information booklet increased uptake compared with the invitation letter only (increase by 5.1 and 4.6%, respectively); this difference was apparent for both sexes and also in deprived population groups. In general, uptake was slightly higher (53.9% without an advance notification) than that in our study 31.
Data from the Netherlands have suggested that an advance notification letter increases uptake by 3.3%, and the authors consider that this observation supports recommending that such letters should be incorporated within the standard CRC screening invitation process 32.
The higher impact of a notification letter on gFOBT users than for FIT could be because gFOBT is more difficult to use, and that advanced information to that group might have a greater influence than on FIT users. Therefore, although it might be rational to use advance notification letters in a gFOBT-based programme, the evidence is less convincing for FIT.
Reminder letters
The meta-analysis carried out by Stone et al. 33 showed the role of patient reminders (adjusted OR 2.75; 95% CI: 1.90–3.97) and provider reminders (adjusted OR 1.46; 95% CI: 1.15–1.85) in increasing the target population compliance.
Marked differences in the gain from reminder letters have been reported in different studies. In US-based studies, reminder letters yielded additional uptake ranging from 5.9% 34 to 16.2% 35, but the authors of a study from the Netherlands with a design similar to ours 32 reported that 12.9% of all returned tests were received following the reminder letter. In our study, a higher proportion of tests (30.9%) was received following the reminder letter; therefore, there could be marked differences in the gain from the reminder letters in different European populations, and local pilot studies should be carried out to evaluate the local results.
Variable timing for reminder letters has been trialled in different studies, starting from 10 days after receipt of the faecal occult blood tests kits 35 up to 60 days in the study from the Netherlands 32 and even 6 months in another US study 36. Compared with some studies, our reminder letter was sent comparatively early (21 days after mailing the tests) as we considered it important to send the reminder letters before the test kits were discharged as the reminder letters did not contain an extra test.
The sending of reminder letters was of critical importance in our population as a large proportion of tests was returned following the reminder. In this population, a screening approach that did not include a reminder letter would not achieve the minimum uptake requirements set in the European Guidelines.
In general, our data suggest that the yield of a reminder letter could be higher than that of an advance notification letter; therefore, under the circumstances of limited healthcare budgets, we would suggest the use of a reminder letter as the initial step to increase uptake.
Appropriateness of test handling faecal immunochemical tests
Individuals returning FOB Gold tests were better at recording the date of sampling than those returning either gFOBT or the OC-Sensor. For FOB Gold tests, there is no manufacturer-assigned space to record the date or any other information on the sampling tube, and so a special research-designed leaflet was added to the letter. Sampling dates for tests sent with a separate leaflet were better documented than those with a designated space on the test kit. It is likely that the limited space available for recording the date on gFOBT and OC-Sensor tests could cause problems for elderly individuals. The possibility of sending tests with a separate leaflet for recording sampling dates should be considered.
A laboratory-based comparison of the two quantitative FIT listed in the European Guidelines has been performed by Lamph et al. 37, although, to our knowledge, our data provide the first clinical comparison between FOB Gold and the OC-Sensor in a randomized population setting. The third laboratory-based test system (HemSp/Magstream HT; Fujirebio Inc., Tokyo, Japan) has a nonadjustable cut-off haemoglobin concentration and is therefore not the optimal screening tool as adjustment of the cut-off haemoglobin concentrations should be possible to optimize the referral rate 37.
The laboratory examinations have indicated the potential risk of leakage from FOB Gold tubes as the buffer compartment of this test tube has a cap that could be removed in error by the participant. OC-Sensor sample tubes have a robust construction and are unlikely to leak in transit 37. Our clinical data support this concern – more than 10.0% of the FOB Gold sample tubes had been opened inappropriately, whereas none of the OC-Sensor sample tubes was mishandled. In 0.3% of the FOB Gold tests, the wrong end of the tube had been filled with the faecal material (Table 2), rendering the test unreadable.
Although in laboratory settings FOB Gold and OC-Sensor collecting systems are designed to sample an identical amount of faecal material (10 mg) 37, in our cohort, an excessive amount of faecal material had been placed in 0.4% of FOB Gold sample tubes. None of the OC-Sensor sample tubes contained excess samples.
98.9% of FOB Gold and 99.1% of OC-Sensor tests were analysed within 7 days of the sampling date, and 96.5 and 97.3% within 5 days, respectively. This is in line with the manufacturers’ recommendations if the samples are stored in a refrigerator and allowing for 2–3 days of this period in the mail system at higher temperatures. The laboratory tests have, however, shown that the performance of FOB Gold deteriorates during the 7-day period at 4–8°C temperature and 3 days at 23–26°C temperature 37.
For the purposes of this study, we packed the sampling tubes in standard envelopes to avoid differences between the study groups. The proportion of damaged envelopes in any group was very low and did not create problems for the study. The thickness (maximum outer diameter) of the FOB Gold sampling tube reaches 15 mm, and according to the postal regulations in the EU, this size may be required to be sent in a postal parcel, not a standard envelope; this would increase the mailing costs considerably. At the same time, our results indicate that a normal envelope is sufficient for any of the evaluated tests.
Our findings are limited to the current test design used in the study. Manufacturers are continuously updating the design of their products and further studies may be required to ensure that these observations remain valid.
Conclusion
In conclusion, mail-delivered faecal occult blood tests were proven to improve uptake of CRC screening in Latvia. The uptake of FIT was better than for gFOBT. There was a small difference in uptake between the FOB Gold and OC-Sensor tests and the proportion of inadequately handled tests was markedly higher for FOB Gold. Using a separate leaflet to record the sampling date increased the proportion of tests with the sampling date. The use of an advance notification letter was found to be more effective for gFOBT than FIT, whereas a reminder letter was of critical importance to ensure an acceptable uptake.
We consider that the results obtained are important not only to guide the implementation of an organized screening programme in Latvia but also for other countries with similar socioeconomic conditions and awareness of the CRC screening target population.
Acknowledgements
The authors acknowledge the Latvian Colorectal Cancer Screening Study Group: Valda Ansevica, Sintija Balode, Tatjana Baranovska, Rita Baurovska, Iveta Bebrisa, Normunds Belskis, Eriks Cems, Martins Dinka, Evija Dompalma, Janis Eglitis, Ludmila Engele, Liga Gaigala, Marina Grisanovica, Reinis Joksts, Aija Karklina, Edgars Kasalis, Ilze Kikuste, Una Kojalo, Mairita Kruka-Zalcmane, Anita Lapina, Inta Liepniece-Karele, Atis Martinsons, Signe Mezinska, Janis Misins, Indrikis Muiznieks, Solvita Olsena, Dace Osite, Aija Ozola-Priedite, Liga Panina, Anzela Pavlovica, Karlis Purmalis, Viesturs Putnins, Evija Rikveile, Dace Rudzite, Ingrida Rumba-Rozenfelde, Linda Sosare, Dans Stirna, Armands Strauss, Andris Tjunitis, Ivars Tolmanis, Liga Ungure, Aigars Vanags, Uldis Vikmanis, Ilze Viberga, Juris Viksna. Our special thanks to Helen Seaman for the advice in improving the manuscript and the language. The authors also acknowledge Ernst Kuipers for the advice with the study design, Stephen Halloran for the methodological advice; Ministry of Health of Latvia and National Health Services of Latvia for the support and availability of the screening/manipulation database; ‘Latvijas Pasts’ (Latvia Postage Services) for the involvement and collaboration.
M. Leja conceived the idea for the study; M. Leja, D. Santare, T. Huttunen, V. Boka designed the study protocol; D. Santare, M. Leja designed the letters to address to the target individuals; I. Kojalo performed the randomization; S. Rikacovs, P. Rucevskis, I. Kojalo were responsible for the study database; D. Santare was responsible for the execution and co-ordination of the study, M. Leja for the supervision of the study; T. Huttunen, I. Kojalo, D. Santare carried out the statistical analysis.
The study was funded in part by the project of the European Social Fund No. 009/0220/1DP/1.1.1.2.0/09/APIA/VIAA/016 ‘Multidisciplinary research group for early cancer detection and cancer prevention’. Immunochemical tests and reagents were partly supplied by Eiken Chemical Co. (Tokyo, Japan) and Sentinel Diagnostics (Milan, Italy).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lynge E. Recommendations on cancer screening in the European union. Advisory Committee on Cancer Prevention. Eur J Cancer 2000; 36:1473–1478. [DOI] [PubMed] [Google Scholar]
- 2.Valsts organizētais vēža skrīnings. 37.pielikums MK 2006.gada 19.decembra noteikumiem Nr.1046. 2010. (State Organized Cancer Screening. The Cabinet of Ministers of the Republic of Latvia. The Supplement No. 37 to the Regulations 1046.).
- 3.Seifert B, Zavoral M, Fric P, Bencko V. The role of primary care in colorectal cancer screening: experience from Czech Republic. Neoplasma 2008; 55:74–80. [PubMed] [Google Scholar]
- 4.National Health Service, Latvia: Veža savlaicigas atklašanas programmas rezultati. (Results of the National screening program) Available at: http://www.vmnvd.gov.lv/lv/469-veselibas-aprupes-pakalpojumi/veza-savlaicigas-atklasanas-programma/626-veza-savlaicigas-atklasanas-programmas-rezultati [Accessed 2 December 2013]. [Google Scholar]
- 5.Segnan N, Patnick J, von Karsa L. European guidelines for quality assurance in colorectal cancer screening and diagnosis. Luxembourg: Office for Official Publications of the European Communities; 2010. Available at: http://bookshop.europa.eu/is-bin/INTERSHOP.enfinity/WFS/EU-Bookshop-Site/en_GB/-/EUR/ViewPublication-Start?PublicationKey=ND3210390. [Accessed 17 February 2012]. [Google Scholar]
- 6.Council of the European Union. Council Recommendation of 2 December 2003 on cancer screening. Official Journal L 327 of 16.12.2003.
- 7.Hewitson P, Glasziou P, Irwig L, Towler B, Watson E. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev 2007; CD001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerr J, Day P, Broadstock M, Weir R, Bidwell S. Systematic review of the effectiveness of population screening for colorectal cancer. N Z Med J 2007; 120:U2629. [PubMed] [Google Scholar]
- 9.Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg 2008; 95:1029–1036. [DOI] [PubMed] [Google Scholar]
- 10.Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst 2007; 99:1462–1470. [DOI] [PubMed] [Google Scholar]
- 11.Van Rossum LG, van Rijn AF, Laheij RJ, van Oijen MG, Fockens P, van Krieken HH, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008; 135:82–90. [DOI] [PubMed] [Google Scholar]
- 12.Wong BC, Wong WM, Cheung KL, Tong TS, Rozen P, Young GP, et al. A sensitive guaiac faecal occult blood test is less useful than an immunochemical test for colorectal cancer screening in a Chinese population. Aliment Pharmacol Ther 2003; 18:941–946. [DOI] [PubMed] [Google Scholar]
- 13.Dancourt V, Lejeune C, Lepage C, Gailliard MC, Meny B, Faivre J. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. Eur J Cancer 2008; 44:2254–2258. [DOI] [PubMed] [Google Scholar]
- 14.Cole SR, Young GP, Esterman A, Cadd B, Morcom J. A randomised trial of the impact of new faecal haemoglobin test technologies on population participation in screening for colorectal cancer. J Med Screen 2003; 10:117–122. [DOI] [PubMed] [Google Scholar]
- 15.Federici A, Giorgi Rossi P, Borgia P, Bartolozzi F, Farchi S, Gausticchi G. The immunochemical faecal occult blood test leads to higher compliance than the guaiac for colorectal cancer screening programmes: a cluster randomized controlled trial. J Med Screen 2005; 12:83–88. [DOI] [PubMed] [Google Scholar]
- 16.Grazzini G, Visioli CB, Zorzi M, Ciatto S, Banovich F, Bonanomi AG, et al. Immunochemical faecal occult blood test: number of samples and positivity cutoff. What is the best strategy for colorectal cancer screening? Br J Cancer 2009; 100:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krestyaninova M, Zarins A, Viksna J, Kurbatova N, Rucevskis P, Neogi SG, et al. A System for Information Management in BioMedical Studies – SIMBioMS. Bioinformatics 2009; 25:2768–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Church TR, Yeazel MW, Jones RM, Kochevar LK, Watt GD, Mongin SJ, et al. A randomized trial of direct mailing of fecal occult blood tests to increase colorectal cancer screening. J Natl Cancer Inst 2004; 96:770–780. [DOI] [PubMed] [Google Scholar]
- 19.Van Roosbroeck S, Hoeck S, Van Hal G. Population-based screening for colorectal cancer using an immunochemical faecal occult blood test: A comparison of two invitation strategies. Cancer Epidemiol 2012; 36:e317–e324. [DOI] [PubMed] [Google Scholar]
- 20.Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C, et al. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012; 61:1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martini A, Javanparast S, Ward PR, Baratiny G, Gill T, Cole S, et al. Colorectal cancer screening in rural and remote areas: analysis of the National Bowel Cancer Screening Program data for South Australia. Rural Remote Health 2011; 11:1648. [PubMed] [Google Scholar]
- 22.Levi Z, Birkenfeld S, Vilkin A, Bar-Chana M, Lifshitz I, Chared M, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer 2011; 128:2415–2424. [DOI] [PubMed] [Google Scholar]
- 23.Digby J, McDonald PJ, Strachan JA, Libby G, Steele RJ, Fraser CG. Use of a faecal immunochemical test narrows current gaps in uptake for sex, age and deprivation in a bowel cancer screening programme. J Med Screen 2013; 20:80–85. [DOI] [PubMed] [Google Scholar]
- 24.Hol L, van Leerdam ME, van Ballegooijen M, van Vuuren AJ, van Dekken H, Reijerink JC, et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut 2010; 59:62–68. [DOI] [PubMed] [Google Scholar]
- 25.Chubak J, Bogart A, Fuller S, Laing SS, Green BB. Uptake and positive predictive value of fecal occult blood tests: a randomized controlled trial. Prev Med 2013; 57:671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pignone M, Campbell MK, Carr C, Phillips C. Meta-analysis of dietary restriction during fecal occult blood testing. Eff Clin Pract 2001; 4:150–156. [PubMed] [Google Scholar]
- 27.Cole SR, Young GP. Effect of dietary restriction on participation in faecal occult blood test screening for colorectal cancer. Med J Aust 2001; 175:195–198. [DOI] [PubMed] [Google Scholar]
- 28.Federici A, Giorgi Rossi P, Bartolozzi F, Farchi S, Borgia P, Guasticchi G. Survey on colorectal cancer screening knowledge, attitudes, and practices of general practice physicians in Lazio, Italy. Prev Med 2005; 41:30–35. [DOI] [PubMed] [Google Scholar]
- 29.Camilloni L, Ferroni E, Cendales BJ, Pezzarossi A, Furnari G, Borgia P, et al. Methods to increase participation in organised screening programs: a systematic review. BMC Public Health 2013; 13:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole SR, Smith A, Wilson C, Turnbull D, Esterman A, Young GP. An advance notification letter increases participation in colorectal cancer screening. J Med Screen 2007; 14:73–75. [DOI] [PubMed] [Google Scholar]
- 31.Libby G, Bray J, Champion J, Brownlee LA, Birrell J, Gorman DR, et al. Pre-notification increases uptake of colorectal cancer screening in all demographic groups: a randomized controlled trial. J Med Screen 2011; 18:24–29. [DOI] [PubMed] [Google Scholar]
- 32.van Roon AH, Hol L, Wilschut JA, Reijerink JC, van Vuuren AJ, van Ballegooijen M, et al. Advance notification letters increase adherence in colorectal cancer screening: a population-based randomized trial. Prev Med 2011; 52:448–451. [DOI] [PubMed] [Google Scholar]
- 33.Stone EG, Morton SC, Hulscher ME, Maglione MA, Roth EA, Grimshaw JM, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med 2002; 136:641–651. [DOI] [PubMed] [Google Scholar]
- 34.Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med 2009; 169:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JK, Groessl EJ, Ganiats TG, Ho SB. Cost-effectiveness of a mailed educational reminder to increase colorectal cancer screening. BMC Gastroenterol 2011; 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sequist TD, Franz C, Ayanian JZ. Cost-effectiveness of patient mailings to promote colorectal cancer screening. Med Care 2010; 48:553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamph SA, Bennitt WE, Brannon CR, Halloran SP. Evaluation report: Immunochemical faecal occult blood tests. Reading, UK: NHS Purchasing and Supply Agency; 2009. Available at: http://www.cancerscreening.nhs.uk/bowel/ifobt.pdf [Accessed 20 February 2012]. [Google Scholar]


