Abstract
IMPORTANCE
Use of incretin-based hypoglycemic agents is increasing, but safety data remain limited. We treated a woman with marker-negative autoimmune hepatitis associated with the glucagon-like peptide 1 agonist liraglutide.
OBSERVATIONS
A young woman with type 2 diabetes mellitus and vitiligo presented with a 10-day history of acute hepatitis. Other than starting liraglutide therapy 4 months prior, she reported no changes in medication therapy and no use of supplements. At admission, aspartate aminotransferase level was 991 U/L; alanine aminotransferase level, 1123 U/L; total bilirubin level, 9.5 mg/dL; and international normalized ratio, 1.3. Results of a liver biopsy demonstrated interface hepatitis with prominent eosinophils and rare plasma cells. The patient’s liraglutide therapy was withheld at discharge but her symptoms worsened. A second biopsy specimen revealed massive hepatic necrosis. She started oral prednisone therapy for presumed liraglutide-induced marker-negative autoimmune hepatitis.
CONCLUSIONS AND RELEVANCE
This case represents, to our knowledge, the first report of liraglutide-induced autoimmune hepatitis. Hepatotoxicity may be an incretin analogue class effect with a long latency period. This case raises prescriber awareness about the potential adverse effects of glucagon-like peptide 1 agonists. Postmarketing studies are needed to define the hepatotoxic potential of these agents.
The incidence and prevalence of diabetes mellitus in the United States is increasing rapidly.1 Eleven unique drug classes have been approved by the US Food and Drug Administration for type 2 diabetes mellitus. Incretin-based hypoglycemic agents, including the glucagon-like peptide 1 (GLP-1) agonists and the dipeptidyl peptidase-4 inhibitors, have recently received considerable press. The US Food and Drug Administration released a statement heightening provider awareness of potential adverse events after data linking these drugs to an increased risk for pancreatic cancer surfaced.2 Despite these warnings, the American Association of Clinical Endocrinologists guidelines3 recommend the use of incretin mimetic drugs early in the process of intensifying glycemic control.
Incretin-based hypoglycemic agents have also been implicated in cases of hepatotoxic effects.4,5 Identifying drug-induced liver injury (DILI) is challenging, because cases are rare and can have variable presentations, including hepatic steatosis, acute liver failure, hepatic necrosis, cholestatic hepatitis, and autoimmune hepatitis (AIH).6 A drug’s hepatotoxic potential is often recognized only after approval by the US Food and Drug Administration.6 We present the first case, to our knowledge, of drug-induced marker-negative AIH associated with liraglutide (Victoza; Novo Nordisk), a GLP-1 agonist approved in 2010 for type 2 diabetes mellitus.
Report of a Case
A young Hispanic woman with a medical history of type 2 diabetes mellitus and vitiligo presented with nausea, emesis, and acute hepatitis of 10 days’ duration. Other than switching drug therapy from exenatide, 10 μg twice daily, to liraglutide, 1.2 mg/d, 4 months before presentation, the patient reported no changes in medication use or use of herbal supplements or acetaminophen. For the last 3 years she had taken metformin hydrochloride, 500 mg twice daily, and used topical tacrolimus ointment, 0.1%, for her vitiligo. Her social history was unremarkable other than recent travel to the southern United States. Physical examination findings included mild scleral icterus but no evidence of advanced liver disease, abdominal tenderness, or altered mentation. Admission laboratory data were as follows: aspartate aminotransferase (AST) level, 991 U/L; alanine aminotransferase (ALT) level, 1123 U/L (to convert AST and ALT to microkatals per liter, multiply by 0.0167); total/direct bilirubin levels, 9.5/6.2 mg/dL (to convert to micromoles per liter, multiply by 17.104); alkaline phosphatase level, 90 U/L (to convert to microkatals per liter, multiply by 0.0167); platelet count, 224 × 103/μL (to convert to 109 per liter, multiply by 1.0); and international normalized ratio, 1.3. Doppler ultrasonography of the liver showed increased echogenicity around the portal triads but no biliary ductal dilatation. Results of an extensive virologic workup, including tests for hepatitis A, B, C, D, and E virus; Epstein-Barr virus; cytomegalovirus; and herpes simplex virus, were unremarkable. In initial autoimmune studies, levels of antinuclear antibody, antimitochondrial antibody, anti–smooth muscle antibody, and quantitative immunoglobulins were not significantly elevated. Results of metabolic studies, including levels of thyrotropin, acetaminophen, and salicylate, were also within reference limits.
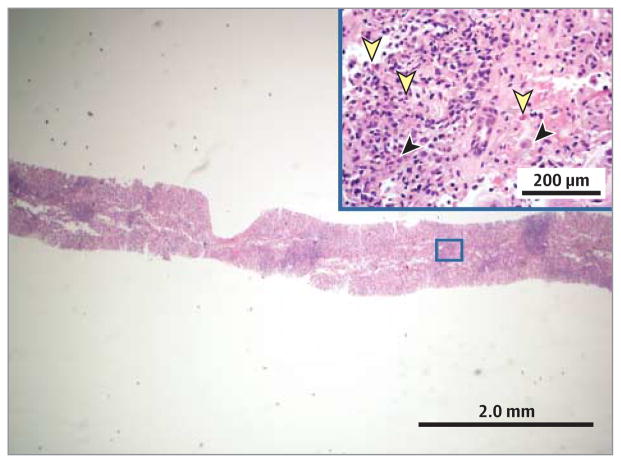
The patient underwent liver biopsy, results of which demonstrated interface hepatitis with a mixed inflammatory infiltrate, prominent intra-acinar eosinophils, canalicular cholestasis, and rare plasma cells (Figure 1). Her symptoms improved with antiemetic therapy and intravenous fluids, and at discharge she was given metformin and insulin. The working differential diagnosis at time of discharge included resolving DILI, marker-negative AIH, or a self-limited viral infection.
Figure 1. Results of the First Liver Biopsy.
Portal and interface inflammation with a mixed inflammatory infiltrate consisting of lymphocytes, occasional plasma cells (black arrowheads), and prominent eosinophils (yellow arrowheads) are seen (hematoxylin-eosin, original magnification ×40). Inset is of the boxed area in the larger slide (original magnification ×200).
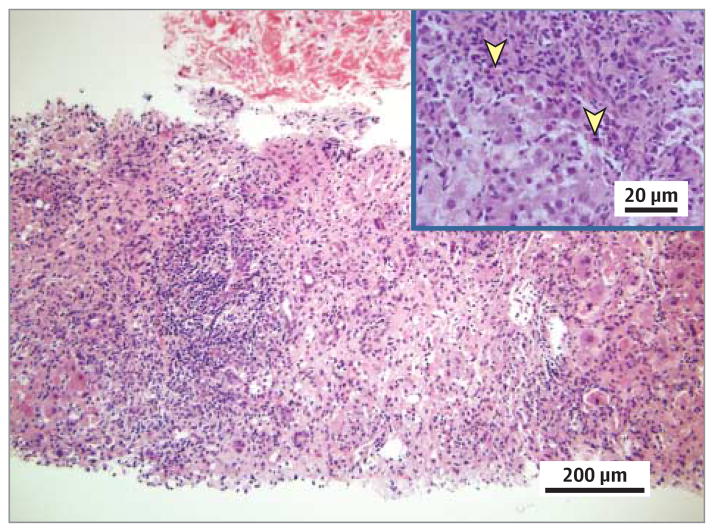
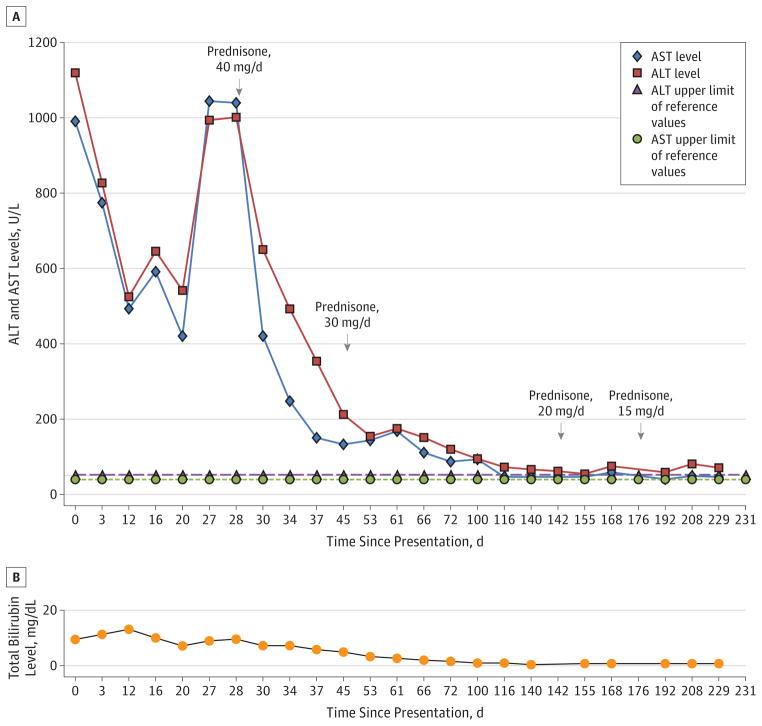
Nine days after discharge, the patient noted worsening jaundice and fatigue. Her AST levels were 492 U/L; ALT levels, 525 U/L; total bilirubin level, 12.8 mg/dL; alkaline phosphatase level, 90 U/L; and international normalized ratio, 1.3. Results of additional studies at that time, including measurement of α1-antitrypsin, ceruloplasmin, and 24-hour urine copper levels and repeated analysis of viral hepatitis serologic markers, were unremarkable. Her symptoms persisted, and 1 month after her initial presentation, she was readmitted. On admission, the AST level was 1045 U/L; ALT level, 994 U/L; total bilirubin level, 9.1 mg/dL; alkaline phosphatase level, 118 U/L; and international normalized ratio, 1.1. Studies for the presence of anti–liver-kidney-microsome-1 and F-actin antibodies returned negative results. Findings of a second liver biopsy showed massive hepatic necrosis and an extensive eosinophilic infiltrate (Figure 2). With the biopsy results pending, the patient started oral prednisone therapy, 40 mg/d, for presumed liraglutide-induced marker-negative AIH. She started a prolonged course of the corticosteroid therapy, given initial improvement in her hepatic enzyme levels and symptoms. At present (6 months later), the patient continues to receive prednisone, 15 mg/d, without complete return of enzyme levels to the reference range (Figure 3). She will continue this slow therapy taper and may require a corticosteroid-sparing agent if she has a biochemical relapse.
Figure 2. Results of the Second Liver Biopsy.
Lobular necrosis and portal and interface inflammation with predominant lymphocytes and scattered eosinophils (yellow arrowheads) are seen (hematoxylin-eosin, original magnification ×200). Inset is taken from a portion of the biopsy not captured on the larger slide (original magnification ×400).
Figure 3. Trend in Liver Function Test Results During the Course of the Patient’s Disease.
Results over time for the patient’s alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels (A) and the bilirubin level (B). The upper limit of the reference range for the ALT level is 52 U/L; for AST, 39 U/L. To convert AST and ALT to microkatals per liter, multiply by 0.0167; bilirubin to micromoles per liter, multiply by 17.104.
Discussion
The constellation of findings from this patient’s history and the evolution of laboratory test and liver biopsy results suggest that marker-negative AIH secondary to the GLP-1 agonist liraglutide was the most probable diagnosis. Drug-induced immune-mediated liver injury occurs when the immune system mounts a response against hepatic proteins, causing a clinical syndrome of AIH. Approximately 9% of AIH cases are drug induced.7 The exact trigger for drug-induced AIH is unknown, but several potential mechanisms exist. Drugs or their metabolites may bind to liver proteins, forming drug-protein products that trigger an immunologic response.6 Drug-induced AIH is most common with drugs metabolized by the cytochrome P450 enzymes, although it can occur whenever drug-protein complexes are formed.6,8
The clinical presentation of drug-induced AIH is similar to that of classic AIH. More than half of the patients are female and 80% are younger than 65 years.9 Approximately 20% to 40% of drug-induced AIH cases lack typical serologic markers, and high-acuity cases may have serum γ-globulin levels within reference ranges.10 Liver tissue examination is essential for the diagnosis of AIH because results demonstrate the histologic hallmark of a lymphoplasmacytic peri-portal infiltrate invading the limiting plate, referred to as interface hepatitis.10 A drug-related cause should be suspected when eosinophils are a prominent feature of the inflammatory infiltrate, although previous studies11,12 suggest that the presence of eosinophils may not reliably distinguish DILI from AIH. Treatment of drug-induced AIH depends on the disease severity.8 Patients with asymptomatic or mild cases can discontinue therapy with the offending agent and undergo close monitoring, whereas those with more severe disease require corticosteroid therapy.7,8 Histologic improvement lags behind clinical and laboratory resolution by 3 to 8 months; thus, therapy should be continued for at least 6 months beyond the time clinical and laboratory findings normalize.8 If corticosteroid therapy cannot be tapered, or if patients relapse after corticosteroid therapy withdrawal, then they are treated as having classic AIH and receive corticosteroids in combination with a corticosteroid-sparing agent such as azathioprine.8
To our knowledge, this patient constitutes the first reported case of hepatotoxic effects associated with the GLP-1 agonist liraglutide. Approximately 363 000 patients have started liraglutide therapy since its approval in 2010 (Tor Constantino, MBA, written communication, February 25, 2014). Other incretin-based hypoglycemic agents, by contrast, have been implicated in cases of hepatotoxic effects. Trials of vildagliptin, a dipeptidyl peptidase-4 inhibitor currently approved by the European Medicines Agency, have reported hepatic dysfunction and hepatitis.4 The dipeptidyl peptidase-4 inhibitor sitagliptin phosphate has also been linked to a case of DILI.5 We speculate that liraglutide triggers immune-mediated liver injury owing to the formation of antiliraglutide antibodies. Liraglutide is a recombinant peptide that is 97% homologous with human GLP-1.13 Approximately 8% of patients will form antiliraglutide antibodies, and more than half of these antibodies cross-react with native GLP-1, which has receptors on human hepatocytes.13 The interaction between the antiliraglutide antibody and native GLP-1 at the site of its hepatocyte receptor may form a neoantigen against which the immune system reacts, causing an AIH syndrome. This mechanism is speculative, and antiliraglutide antibody levels were not measured in our patient. Further studies are needed to explore this potential pathway of injury.
Our case report strongly implicates liraglutide in the etiology of the patient’s marker-negative AIH, although a causal relationship cannot be firmly established. A number of scoring systems exist to help clinicians categorize acute liver injury. The Roussel Uclaf Causality Assessment Method is used to define DILI,14 and the International Autoimmune Hepatitis Group scoring system is used to identify AIH.15 Based on her presentation, our patient would have a “probable” autoimmune cause15 and a “possible” drug contribution to her presentation.14 The patient did not undergo drug rechallenge, results of which would strengthen the association between liraglutide and the patient’s liver injury.
Conclusions
This case study reports the first known instance of liraglutide-induced hepatotoxicity and suggests that liver injury may occur with a long latency period. The patient’s presentation also illustrates the complexity of distinguishing drug-induced AIH from other entities such as acute classic AIH, marker-negative AIH, and other forms of DILI. Larger postmarketing studies are needed to better define the hepatotoxic potential of GLP-1 agonists.
Acknowledgments
Funding/Support: This study was supported by grant 1 F32 HL116151-01 from the National Institutes of Health and by a postdoctoral research fellowship award from the American Liver Foundation (Dr VanWagner).
Footnotes
Conflict of Interest Disclosures: None reported.
Previous Presentation: This paper was presented in poster format at the 78th Annual Meeting of the College of Gastroenterology; October 14, 2013; San Diego, California.
Author Contributions: Dr Rinella had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kern, VanWagner, Rinella.
Acquisition, analysis, or interpretation of data: Kern, VanWagner, Yang.
Drafting of the manuscript: Kern, VanWagner.
Critical revision of the manuscript for important intellectual content: All authors.
Obtained funding: VanWagner.
Administrative, technical, or material support: VanWagner, Yang, Rinella.
Study supervision: VanWagner, Rinella.
Role of the Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Contributor Information
Emily Kern, Department of Internal Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Lisa B. VanWagner, Division of Gastroenterology and Hepatology, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Guang-Yu Yang, Department of Pathology, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Mary E. Rinella, Division of Gastroenterology and Hepatology, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
References
- 1.Qaseem A, Humphrey LL, Sweet DE, Starkey M, Shekelle P Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156(3):218–231. doi: 10.7326/0003-4819-156-3-201202070-00011. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. FDA drug safety communication. [Accessed October 21, 2013];FDA investigating reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin mimetic drugs for type 2 diabetes. 2013 Mar 14; http://www.fda.gov/drugs/drugsafety/ucm343187.htm.
- 3.Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr Prac. 2013;19(2):327–336. doi: 10.4158/endp.19.2.a38267720403k242. [DOI] [PubMed] [Google Scholar]
- 4.Novartis Euroharm Ltd. [Accessed July 20, 2013];Galvus: summary of product characteristics (SmPC) for the European Union. 2013 http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_information/human/000771/WC500020327.pdf.
- 5.Toyoda-Akui M, Yokomori H, Kaneko F, et al. A case of drug-induced hepatic injury associated with sitagliptin. Intern Med. 2011;50(9):1015–1020. doi: 10.2169/internalmedicine.50.5057. [DOI] [PubMed] [Google Scholar]
- 6.Tujios S, Fontana RJ. Mechanisms of drug-induced liver injury: from bedside to bench. Nat Rev Gastroenterol Hepatol. 2011;8(4):202–211. doi: 10.1038/nrgastro.2011.22. [DOI] [PubMed] [Google Scholar]
- 7.Björnsson E, Talwalkar J, Treeprasertsuk S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51(6):2040–2048. doi: 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
- 8.Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci. 2011;56(4):958–976. doi: 10.1007/s10620-011-1611-4. [DOI] [PubMed] [Google Scholar]
- 9.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Drug Induced Liver Injury Network (DILIN) Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924–1934. e1–e4. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara K, Fukuda Y, Yokosuka O. Precise histological evaluation of liver biopsy specimen is indispensable for diagnosis and treatment of acute-onset autoimmune hepatitis. J Gastroenterol. 2008;43(12):951–958. doi: 10.1007/s00535-008-2254-x. [DOI] [PubMed] [Google Scholar]
- 11.Pham BN, Bemuau J, Durand F, et al. Eotaxin expression and eosinophil infiltrate in the liver of patients with drug-induced liver disease. J Hepatol. 2001;34(4):537–547. doi: 10.1016/s0168-8278(00)00057-x. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki A, Brunt EM, Kleiner DE, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54(3):931–939. doi: 10.1002/hep.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buse JB, Garber A, Rosenstock J, et al. Liraglutide treatment is associated with a low frequency and magnitude of antibody formation with no apparent impact on glycemic response or increased frequency of adverse events: results from the Liraglutide Effect and Action in Diabetes (LEAD) trials. J Clin Endocrinol Metab. 2011;96(6):1695–1702. doi: 10.1210/jc.2010-2822. [DOI] [PubMed] [Google Scholar]
- 14.Danan G, Benichou C. Causality assessment of adverse reactions to drugs, I: a novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46(11):1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 15.Czaja AJ. Autoantibody-negative autoimmune hepatitis. Dig Dis Sci. 2012;57(3):610–624. doi: 10.1007/s10620-011-2017-z. [DOI] [PubMed] [Google Scholar]