Abstract
Drosophila mojavensis and Drosophila arizonae are cactophilic flies that have been used extensively in speciation studies. Incomplete premating isolation, evidence of reinforcement, and a lack of recent introgression between these species point to a potentially important role for post-zygotic isolating barriers in this system. Other than hybrid male sterility, however, post-zygotic isolation between D. mojavensis and D. arizonae has received little attention. In this study, we examined viability and life-history traits of D. mojavensis/D. arizonae F1 hybrids from sympatric crosses. Specifically, we reared hybrids and purebreds on the natural host cacti of each parental species and compared viability, development time, thorax length, and desiccation resistance between hybrids and purebreds. Interestingly, hybrid females from both crosses performed similarly or even better than purebred females. In contrast, hybrid sons of D. arizonae mothers, in addition to being sterile, had shorter average thorax length than males of both parental species, and hybrid males from both crosses had substantially lower desiccation resistance than D. mojavensis males. The probable cost to hybridization for D. mojavensis females resulting from reduced desiccation resistance of hybrid sons may have been an important selective factor in the history of reinforcement for crosses involving these females.
Keywords: cactus, extrinsic isolation, Haldane’s rule, hybrid fitness, hybrid inviability, speciation
Introduction
Understanding the process of speciation requires the identification of reproductive isolating barriers that prevent gene flow between diverging lineages (Dobzhansky, 1951; Coyne & Orr, 2004). Prezygotic isolating barriers include those that limit the potential for heterospecific matings through ecological, behavioural or mechanical incompatibilities, and those that occur after mating but before zygote formation (post-mating–prezygotic; PMPZ). In contrast, post-zygotic isolating barriers result from the reduced fitness of hybrids relative to pure parental types. Coyne & Orr (2004) further divide post-zygotic isolating barriers into two categories: intrinsic and extrinsic. Intrinsic barriers occur because hybrids have developmental difficulties that render them inviable or sterile, and extrinsic barriers result when hybrids develop normally but have an intermediate phenotype that poses a problem for attracting mates (behavioural sterility) or makes them ecologically unfit (ecological inviability) (Hatfield & Schluter, 1999; Jiggins et al., 2001; Naisbit et al., 2001; Rundle, 2002).
The Drosophila mojavensis/D. arizonae study system has been the focus of speciation studies since 1947 (Baker, 1947). This species pair is relatively young, having diverged less than one million years ago (Reed et al., 2007; Matzkin, 2008). Both species are distributed throughout arid regions of northwestern Mexico and the Southwestern United States, where they breed in decomposing cactus stems (Heed, 1978, 1982). They differ in distribution and pattern of host use across their respective ranges, but are sympatric in much of mainland northwestern Mexico and southern Arizona, USA. Although they favour the use of different host plants in this region, with D. mojavensis specializing on organ pipe cactus (Stenocereus thurberi) and D. arizonae specializing on cina (Stenocereus alamosensis), flies of both species have been collected from the same host plants on occasion (Fellows & Heed, 1972; Ruiz & Heed, 1988).
Previous studies have indicated that prezygotic isolation between D. mojavensis and D. arizonae is strong but incomplete (Wasserman & Koepfer, 1977), and at least some hybrids reach adulthood in laboratory crosses. Nevertheless, despite incomplete prezygotic isolation and some degree of niche overlap in nature, extensive genetic surveys by Counterman & Noor (2006) and Machado et al. (2007) found no evidence for recent introgression, though neither study could completely rule out some low level of gene exchange in the more distant past. This coupled with evidence for a past history of reinforcement in crosses between D. mojavensis females and D. arizonae males (Wasserman & Koepfer, 1977), implies that post-zygotic isolating barriers might play an important role in the divergence of these species. Consistent with this expectation, male sterility is fixed for hybrid crosses involving D. arizonae females. However, hybrid sons of D. mojavensis females are often fertile, and interestingly, the lowest incidence of male sterility results from sympatric crosses where there is an apparent history of reinforcement (Reed & Markow, 2004).
Curiously, a key form of post-zygotic isolation, hybrid inviability, has not been systematically examined in this system. Although clearly at least some hybrids of both sexes are viable, partial hybrid inviability can be common in hybridizations (Lopez-Fernandez & Bolnick, 2007), and may result from either intrinsic or extrinsic factors. In the present study, we examined viability in addition to three life-history traits (development time, thorax length and desiccation resistance) for D. mojavensis/D. arizonae F1 hybrids generated from crosses between sympatric strains of the two parental species. We reared hybrids and purebreds on necrotic tissue of the two parental host plants (organ pipe and cina cactus), which are known to differ in nutritional quality and toxicity (Kircher, 1982). This design allowed us to assess whether reductions in hybrid fitness follow a pattern consistent with extrinsic post-zygotic isolation (i.e. hybrids intermediate between parentals in one or both environments), or intrinsic post-zygotic isolation (i.e. hybrids have lower fitness than parentals in both environments). Moreover, by examining sex-specific effects we were able to assess whether Haldane’s rule, observed for male sterility in this system, also is seen for other post-zygotic phenotypes. Although Haldane’s rule originally described cases of inviability and sterility (Haldane, 1922), it also has been extended to account for sex asymmetries in other traits in some taxa (Davies et al., 1997). Because the earliest manifestations of Haldane’s rule can be environment-dependent (Demuth & Wade, 2007), we expected that the use of naturally stressful rearing environments and stress assays (desiccation) may expose instances of the rule that would not otherwise be apparent.
Materials and methods
Fly collections
We collected D. arizonae in March 2007 from San Carlos, Sonora, Mexico and D. mojavensis in April 2007 from Organ Pipe National Monument, Arizona, USA using traps with fermented bananas. Flies of each species initially were maintained as isofemale lines for three generations before being combined into mass populations (at least 50 lines per species) for our study. We performed two separate experiments. The first, which took place in February/March 2008, included measures of viability, sex ratio, development time and thorax length. In June/July 2008 we conducted the desiccation resistance assay. With the exception of the preparation of cactus food, which was effectively identical for both experiments, we describe protocols for each experiment separately below.
Cactus food preparation
To prepare cactus food we followed a modified protocol established by Matzkin et al. (2006). First, we autoclaved several arms of organ pipe and cina cactus for 15 min at 121 °C. We then added approximately 10 000 cells of one bacterial species (Pectobacterium cacticida) and five species of yeast (Pichia cactophila, P. amethionina, Candida sonorensis, C. ingens and Sporopachydermia cereana) that are common to organ pipe necroses in nature (Starmer, 1982). Although to our knowledge the composition of the microorganismal community of cina has not been reported, the microorganisms we used are common to necroses of all columnar cacti in the Sonoran Desert that have been examined, and are thus also likely to be present in cina rots. We allowed the organ pipe cactus to rot for 21 days (both experiments) and the cina cactus to rot for 10 days in the first experiment and 6 days in the second experiment in an incubator at 30 °C. The cina cactus was kept for a shorter period of time because arms are much thinner and decompose more rapidly than organ pipe arms. We prepared food vials by homogenizing cactus tissue and transferring 16 mL into wide-mouth glass vials. For the second experiment, we combined remaining cactus juice with agar to make 1% agar oviposition plates (see below).
Experiment 1: viability, development time, and thorax length
We set up four crosses, D. mojavensis female-D. mojavensis male, D. arizonae female-D. arizonae male, D. mojavensis female-D. arizonae male (heretofore moj-arz) and D. arizonae female-D. mojavensis male (heretofore arz-moj) by combining 8- to 12-day-old virgin flies in population cages containing Petri plates filled with 1% agar. We cut the outside edge of agar from each plate and spread yeast paste in a small portion in the centre of the plate. To ensure that we had enough hybrids for our experiment, we allowed females to oviposit for approximately 15 h each day before eggs were removed by again cutting away the outside edge of the agar, where nearly all eggs were laid (eggs were discarded from the first day). We then transferred eggs to 3% sterile agar plates where larvae were allowed to hatch. The following day, 30 first instar larvae were transferred to each vial containing cactus food (N = 8 vials for D. arizonae cina; N = 8 for D. arizonae organ pipe; N = 7 for D. mojavensis cina; N = 8 for D. mojavensis organ pipe; N = 7 for arz-moj cina; N = 7 for arz-moj organ pipe; N = 3 for moj-arz cina; N = 3 for moj-arz organ pipe). Developing flies were reared on a 12 : 12 light dark cycle and maintained between 23 °C and 25 °C. Although we acknowledge that this protocol does not allow us to assess egg viability, Kelleher & Markow (2007) demonstrated previously that low hatchability of hybrid eggs results from fertilization failures rather than hybrid inviability.
First larval instar through adult emergence time was assessed by monitoring vials for emerging flies at 8 h intervals (6 AM, 2 PM, 10 PM). Although our development time assay is influenced by the 15 h oviposition period, it is unbiased as all treatments were handled identically. Viability and sex ratio were determined for each vial by counting the number of males and females that emerged. We measured thorax length, from the anterior margin to the tip of the scutellum, to the nearest 0.01 mm using an ocular micrometer in conjunction with a Leica MZ6 dissecting microscope (Leica Microsystems Inc., Bannockburn, IL, USA).
No direct measures of fitness, thorax length and development time are related to fitness in many Drosophila species. In D. mojavensis, male thorax length is positively correlated with mating success (Markow & Ricker, 1992), and female thorax length shows a strong positive correlation with ovariole number (Magnan, 1978). Although the relationship between thorax length or ovariole number and fecundity has not been examined directly in D. mojavensis or D. arizonae, these traits are positively correlated with fecundity in species from the melanogaster group and in D. pseudoobscurra (Robertson, 1957; Tantawy & Vetukhiv, 1960; Tantawy & Rakha, 1964; Cohet & David, 1978; Watada et al., 1986; Lefranc & Bundgaard, 2001). Shorter development time is expected to be advantageous for organisms such as Drosophila that breed in ephemeral resources (Lewontin, 1965; Nylin & Gotthard, 1998). This should be especially true for cactus-breeding Drosophila because shorter development time allows larvae to avoid long periods of competition and exposure to predators (e.g. beetles of the genus Hololepta) that occurs within the diverse arthropod community that inhabits necrotic cactus tissue (Magnan, 1982; Castrezana & Markow, 2001). Although we assume that longer thorax length and faster development time are optimal, we also recognize that these traits may be involved in a trade-off, thus complicating interpretations with respect to fitness. Nevertheless, by collecting data for both traits, we can directly assess this potential trade-off in our interpretation.
Experiment 2: desiccation resistance assay
Desiccation resistance is expected to be of particular importance to the fitness of desert-adapted flies such as D. mojavensis and D. arizonae. Consistent with this expectation, a recent study of desiccation tolerance that included 23 ecologically diverse fly species revealed that D. mojavensis had the highest resistance to desiccation of any species examined, and D. arizonae was also among the most resistant (Matzkin et al., 2009). We set up the four crosses in the same manner described previously except that, in this experiment, Petri plates contained food made from cactus juice (see above), which encourages oviposition. Plates were checked daily and larvae were transferred as they emerged to vials containing cactus food. Thirty larvae were transferred to most vials though in some cases 30 larvae were not available resulting in some vials that contained less (no fewer than 22).
Flies used for the desiccation assay were collected and sexed within 24 h of emergence and retained for 1 day on cactus food that they emerged from before being transferred to standard cornmeal-molasses food, where they remained until the experiment was performed. To have sufficient numbers for the experiment, we pooled together flies collected over a 3-day period. All flies used in the experiment were 6- to 8-day-old virgins (age was randomized with respect to cross). To test resistance to desiccation, we placed flies in groups of four into empty vials; a foam plug was inserted near the centre of the vial to restrict flies to the bottom half. We added 4 g of Drierite desiccant on top of the plug before sealing the vial with parafilm. Vials were kept on a 12 h light : dark cycle at 22–25 °C. We monitored vials for death every 2 h until all flies had died.
Hybrid verification
To verify the identity of putative hybrids used in the experiments, we designed primers (forward: TTCCCGG TCGRGATTTACT; reverse: GTAGCCTGTGCYTGTGCTCT) to amplify a portion of a nuclear region (locus 5307) that was used in a previous study (Machado et al., 2007). We subjected PCR products to a restriction digestion with the enzyme MWoI (New England Biolabs Inc., Ipswich, MA, USA), which differentially cuts D. mojavensis and D. arizonae fragments, leading to a distinct banding pattern in hybrids. We examined eight to 20 individuals emerging from each population cage. We are confident that this method was robust for detecting contamination; we found two instances where a cage was contaminated, and samples from these cages were not used in the experiments.
Statistical analyses
We used the glimmix procedure (available for download at support.sas.com/rnd/app/da/glimmix.html) in SAS v9.1 (SAS Institute Inc., Cary, NC, USA) to carry out all statistical analyses. This procedure fits generalized mixed models where the response variable can follow any distribution in the exponential family. Unlike other procedures that fit generalized mixed models, the glimmix procedure also allows the inclusion of random effects, which are assumed to be normally distributed. For the survivorship and sex ratio data, our responses were binary (survive = yes or no; sex = male or female), and thus the unit of replication was the individual rather than the vial. The initial model included cross (four levels), cactus (two levels), and the cross by cactus interaction as fixed effects and vial nested within cross and cactus as a random effect. The responses thorax length (log-transformed), development time (log-transformed) and desiccation time (log-transformed time until death) were normally distributed and all models included cross (four levels), cactus (two levels), sex (two levels) and all interactions as fixed effects, and vial nested within cross and cactus as a random effect (thorax length and development time) or vial nested within cross, cactus and sex as a random effect (desiccation time). In all cases, nonsignificant terms (starting with higher order interactions) were eliminated one by one from the model, rerunning the analysis each time until all remaining terms were significant or were involved in a significant interaction. For factors that were significant, we made comparisons among the different levels using the ‘lsmeans’ statement. For significant interactions, we were primarily interested in comparisons among simple effects, which we achieved by using the ‘testslice’ option available in proc glimmix. All post hoc comparisons were corrected using Tukey’s adjustment.
Contribution of different isolating barriers to total isolation
We used our data in conjunction with information from previous studies to calculate the strength of all isolating barriers that have been analyzed to date.
Premating isolation
We estimated the strength of premating isolation by averaging the joint isolation indices for sympatric crosses presented in Table 4 from Wasserman & Koepfer (1977).
Post-mating–prezygotic isolation
For PMPZ isolation we used data presented by Kelleher & Markow (2007) showing that fertility of heterospecifically mated D. mojavensis females was reduced relative to homospecifically mated females. Specifically, we calculated RIPMPZ = 1–fertility of heterospecific crosses/fertility of homospecific crosses. Because that study did not include data on sympatric crosses, we used the highest and lowest reported values to calculate a range for the strength of PMPZ isolation, assuming that sympatric crosses would fall somewhere within this range. Analogous data for the arz-moj cross were not available.
Hybrid sterility
We obtained data on male sterility for crosses involving D. mojavensis females from Organ Pipe National Monument (the same population used in our study) from Reed & Markow (2004). We assumed all females were fertile, and thus calculated RIHS = (1–proportion fertile hybrid males/proportion fertile pure males)/2. For crosses with D. arizonae females sterility is fixed; we therefore used RIHS = 0.5.
Hybrid inviability
As we found no differences between viability of hybrids and purebreds RIHI = 0 for both crosses.
Total reproductive isolation
We calculated the absolute contribution (AC) of each stage (n) of reproductive isolation (RI) to total isolation using equation 4 from Ramsey et al. (2003): . This method of estimation assumes that later acting isolating barriers can only eliminate gene flow that was not already eliminated during previous stages. We calculated total isolation (T) using equation 5 (Ramsey et al., 2003) where for m components of isolation: . Values can range between 0, indicating no isolation, and 1, indicating complete reproductive isolation.
Results
Viability and sex ratio
We found no differences in sex ratio between any of the crosses, irrespective of cactus type (generalized linear mixed model; Table 1). There also were no viability differences between any of the crosses (arz = 84%, N = 480; moj = 81%, N = 450; arz-moj = 84%, N = 390; moj-arz = 89%, N = 180; generalized linear mixed model; Table 1). Overall survivorship, however, was significantly higher onorgan pipe than cina (90% vs. 78%, respectively; generalized linear mixed model; Table 1).
Table 1.
Final terms and their associated P-values for generalized linear mixed models. Nonsignificant terms were eliminated from the model beginning with higher order interactions.
| Response | Final model | Num d.f., Den d.f. | F | P |
|---|---|---|---|---|
| Sex ratio | Cross | 3, 45 | 1.35 | 0.270 |
| Cactus | 1, 45 | 0.09 | 0.769 | |
| Viability | Cross | 3, 45 | 0.89 | 0.452 |
| Cactus | 1, 45 | 14.94 | < 0.0001 | |
| Development time | Cross | 3, 1230 | 81.93 | < 0.0001 |
| Cactus | 1, 1230 | 9.32 | 0.002 | |
| Sex | 1, 1230 | 5.13 | 0.024 | |
| Cross × Cactus | 3, 1230 | 10.67 | < 0.0001 | |
| Cross × Sex | 3, 1230 | 18.65 | < 0.0001 | |
| Cactus × Sex | 1, 1230 | 6.34 | 0.012 | |
| Thorax length | Cross | 3, 46 | 4.39 | 0.009 |
| Sex | 1, 1145 | 2674.93 | < 0.0001 | |
| Cross × sex | 3, 1145 | 27.45 | < 0.0001 | |
| Desiccation resistance | Cross | 3, 160 | 56.27 | < 0.0001 |
| Cactus | 1, 160 | 22.13 | < 0.0001 | |
| Sex | 1, 160 | 59.52 | < 0.0001 | |
| Cross × Sex | 3, 160 | 14.81 | < 0.0001 | |
| Cactus × Sex | 1, 160 | 22.51 | < 0.0001 |
Development time
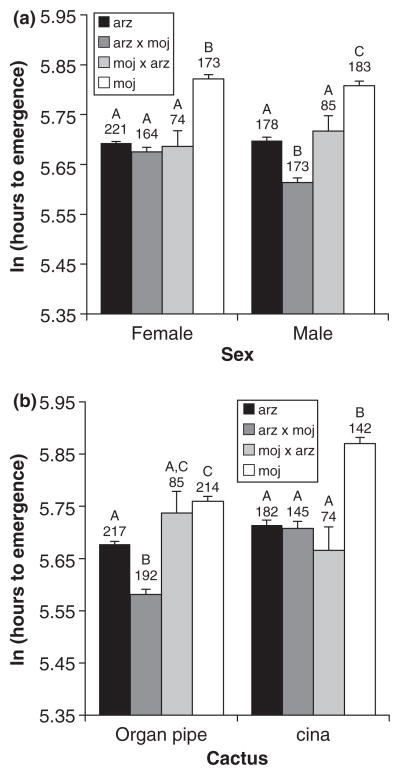
For development time, all two-way interactions and all main effects were significant after eliminating the nonsignificant three way interaction (generalized linear model; Table 1). Because all main effects also were involved in at least one significant interaction, we further examined the nature of these interactions, with particular interest in the two interactions involving the ‘cross’ model term (cross × sex and cross × cactus). We first compared cross development time separately for each sex, which revealed that females of D. arizonae and both hybrids developed faster than D. mojavensis females, but were not different from each other (lsmeans comparions with Tukey’s adjustment; Fig. 1a). The difference between the fastest developing female (arz-moj) and the D. mojavensis females was approximately 44 h. Drosophila mojavensis males again developed more slowly than all other males, but, in this case, arz-moj males developed faster by about 24 h than both D. arizonae and the moj-arz hybrid (lsmeans comparisons with Tukey’s adjustment; Fig. 1a). Next, we compared crosses separately for each cactus, finding that, whereas on cina D. arizonae and the two hybrids developed faster than D. mojavensis, on organ pipe the arz-moj hybrid developed faster than all others and D. arizonae developed faster than D. mojavensis (lsmeans comparisons with Tukey’s adjustment; Fig. 1b). There was also a significant interaction between cactus and sex, which reflected slightly longer development time of females relative to males on cina but not organ pipe cactus (lsmeans comparisons with Tukey’s adjustment; data not shown).
Fig. 1.
Mean development time (log-transformed) for Drosophila mojavensis, Drosophila arizonae, and the reciprocal hybrids by (a) sex and (b) cactus. Groupings under a different letter indicate those that were significantly different. Sample sizes are given under the grouping labels and error bars represent standard errors.
Thorax length
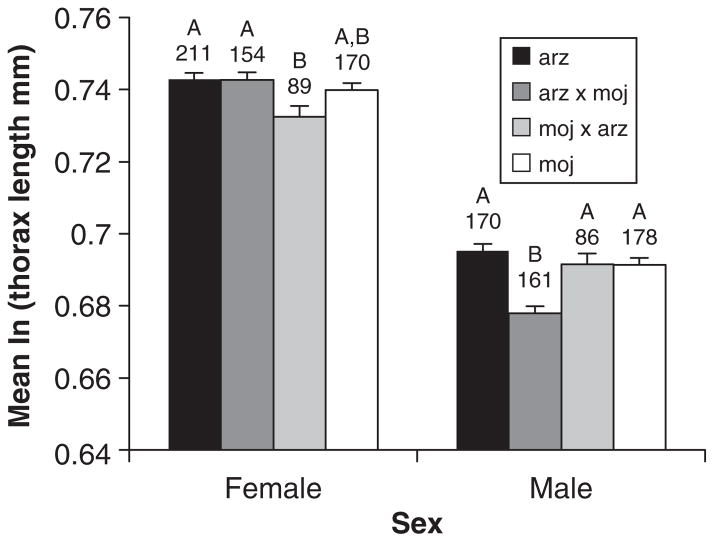
Analysis of thorax length data revealed a significant cross by sex interaction in addition to significant main effects of these two factors (generalized linear mixed model, Table 1). We further explored the nature of the interaction by comparing crosses separately for each sex (we were less interested in differences between the sexes). Female offspring of the moj-arz cross were significantly smaller than both D. arizonae purebreds and arz-moj hybrids (lsmeans with Tukey’s adjustment, P < 0.05; Fig. 2), whereas no other comparisons differed. In contrast, arz-moj males were smaller than all other males (lsmeans with Tukey’s adjustment, P < 0.05; Fig. 2), whereas there were no significant differences among any other comparisons.
Fig. 2.
Mean thorax length (log-transformed) for Drosophila mojavensis, Drosophila arizonae, and the reciprocal hybrids by sex. Groupings under a different letter indicate those that were significantly different. Sample sizes are given under the grouping labels and error bars represent standard errors.
Desiccation resistance
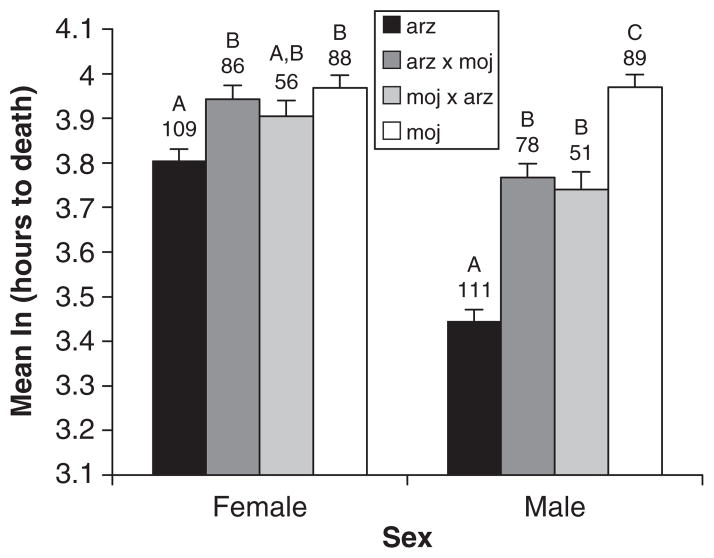
We observed significant interactions between cross and sex and cactus and sex, in addition to several significant main effects (generalized linear mixed model; Table 1). We thus compared crosses separately for each sex (again, we were less interested in comparisons between sexes). This analysis showed that hybrid males were intermediate between D. arizonae and D. mojavensis, with all comparisons being significant except for that between the two hybrids (lsmeans with Tukey’s adjustment; Fig. 3). The difference between D. mojavensis and D. arizonae males in mean time to death was approximately 22 h. For females, D. arizonae purebreds died earlier than both arz-moj hybrids and D. mojavensis (lsmeans with Tukey’s adjustment, P < 0.05), but differences between other crosses were not significant. The difference between D. arizonae and D. mojavensis females was much smaller than that observed between males (approximately 8 h). The significant cactus by sex interaction reflected the fact that females died earlier when reared on cina cactus compared with organ pipe cactus, whereas there was no difference between males reared on the two cacti (lsmeans comparisons with Tukey’s adjustment; data not shown).
Fig. 3.
Mean time to death by desiccation (log-transformed) for Drosophila mojavensis, Drosophila arizonae, and the reciprocal hybrids by sex. Groupings under a different letter indicate those that were significantly different. Sample sizes are given under the grouping labels and errors bars represent standard errors.
Summary of performance data
Performance data are summarized in Table 2, where, for each interspecific cross, we compare the performance of hybrid offspring relative to that of offspring that would result from an intraspecific cross involving the same mother. This depiction assumes that any costs to hybridizing would be experienced disproportionately by females. Thus, it is particularly useful for identifying selective factors that may have contributed to reinforcement because the increased premating isolation observed for the moj-arz cross in sympatry is apparently the result of selection on D. mojavensis female mate preference rather than D. arizonae male mate preference (Wasserman & Koepfer, 1977; Markow, 1981).
Table 2.
Comparison of trait values for hybrid offspring relative to purebred offspring that would result from a homospecific cross with the same mother (e.g. the arz-moj column compares the performance of hybrid offspring with that of Drosophila arizonae purebred offspring).
| arz-moj
|
moj-arz
|
|||
|---|---|---|---|---|
| Organ pipe | Cina | Organ pipe | Cina | |
| Survival | Same | Same | Same | Same |
| Development time | ||||
| Female | Faster | Same | Same | Faster |
| Male | Faster | Same | Same | Faster |
| Thorax length | ||||
| Female | Same | Same | Same | Same |
| Male | Smaller | Smaller | Same | Same |
| Desiccation resistance | ||||
| Female | Higher | Higher | Same | Same |
| Male | Higher | Higher | Lower | Lower |
Contribution of different isolating barriers to total isolation
Total reproductive isolation between D. mojavensis and D. arizonae in sympatry is high (Table 3). Reproductive isolation for the moj-arz cross is nearly complete (T = 0.994–0.999), whereas total isolation for the arzmoj cross is somewhat lower (T = 0.950). Total isolation would increase, however, if there is additional isolation resulting from PMPZ mechanisms.
Table 3.
The strength and absolute contribution of different types of reproductive isolating barriers to total isolation between Drosophila mojavensis and Drosophila arizonae. The absolute contribution of each barrier and total reproductive isolation were computed using equations 4 and 5 from Ramsey et al., 2003.
Discussion
As the first comprehensive assessment of D. mojavensis/D. arizonae hybrid performance, our study provides new insight into the role of post-zygotic isolating barriers in the evolution of reproductive isolation between these species.
Post-zygotic isolation and reinforcement
Despite evidence that other types of isolating barriers may drive the process of reinforcement, empirical studies in Drosophila have focused almost exclusively on hybrid sterility and inviability (Servedio & Noor, 2003). Here, we show that hybrid inviability per se was not a factor in the apparent history of reinforcement for the moj-arz cross. However, an analysis of hybrid life-history traits revealed a potential cost to hybridization for D. mojavensis females, as their hybrid male offspring had lower resistance to desiccation than purebred males (Table 2). The reduction in desiccation resistance of hybrid males was substantial (approximately 11 h) and is therefore likely to have a negative impact on fitness given the arid natural environment of these flies. Although this cost may be counterbalanced by the advantages of faster development time, this was only the case in cina cactus, which is not D. mojavensis’s preferred host. Our findings, along with those from previous studies showing strong PMPZ isolation (Kelleher & Markow, 2007) and some male sterility (Reed & Markow, 2004), indicate that multiple factors may have played a significant role in reinforcement.
Effects of ecological factors on reproductive isolation
Drosophila have served as an important model system for studies of speciation, but few studies have examined the possible role of ecology in post-zygotic isolation (but see Soto et al., 2007a,b). Here, we demonstrate that post-zygotic isolation between D. mojavensis and D. arizonae was not ecologically-dependent, as nearly all cross by cactus interactions in our fitness assays were not significant. The only exception was for development time, but even for this trait hybrids performed at least as well as purebreds on both hosts. The lack of extrinsic isolation reflects the fact that despite differences in host plant chemistry (Kircher, 1982) and divergent host plant preferences in nature, both D. mojavensis and D. arizonae performed similarly on the two hosts, with both showing higher viability on organ pipe. One caveat to this result is that although the microorganisms we used to create cactus rots are common in necroses of many columnar cacti, we cannot rule out the possibility that there are other important quantitative and/or qualitative differences in the microorganismal communities of cina and organ pipe that might affect larval performance. Moreover, in nature larvae would be in competition with each other and with other arthropods in the necrotic cactus community (Castrezana & Markow, 2001), and thus future studies that include competition may well reveal additional fitness differences among hybrids and purebreds that might be ecologically-dependent.
Sex-specific effects
We found no differences in viability or sex ratio between hybrids and purebreds, indicating that hybrid inviability of either sex has not evolved. These results are consistent with the general pattern observed in Drosophila and other animal groups that hybrid male sterility evolves more rapidly than inviability (Coyne & Orr, 1997; Sasa et al., 1998; Presgraves, 2002; Price & Bouvier, 2002; Russell, 2003). We also examined sex-specific differences for three life-history traits (development time, thorax length and desiccation resistance), to determine whether hybrid males showed reduced performance compared with both parental species (a pattern consistent with Haldane effects). In fact, arz-moj males were smaller than both D. mojavensis and D. arizonae males, a result that was not environment dependent (Fig. 2). Interestingly, these males are also sterile, and thus appear to be compromised in multiple ways. Nevertheless, with regard to Haldane’s rule, we also note that females (but not males) from the reciprocal cross also had a shorter average thorax length than both purebreds, though only one of the comparisons was statistically significant (Fig. 2). Considering this, overall support for Haldane’s rule is equivocal at best for this trait.
Synthesis with existing data
Comprehensive data on the strength of different forms of reproductive isolation, including intrinsic and extrinsic post-zgyotic barriers, are not available for most study systems, despite the fact that such data are critical for understanding the processes involved in a particular speciation event. Combining our results with those from previous studies on other forms of reproductive isolation in this system reveals that total isolation between sympatric strains of D. mojavensis and D. arizonae is high (Table 3). These levels of isolation are apparently sufficient for maintaining species boundaries, as there is no evidence for recent introgression between the species (Counterman & Noor, 2006; Machado et al., 2007). Clearly, prezygotic barriers, including premating and PMPZ mechanisms, represent the most formidable barriers to current gene exchange. Although prezygotic barriers are expected to be relatively more important in restricting current gene flow simply because they occur earlier, in this case they are also stronger than post-zygotic barriers in the absolute sense. This is consistent with the general pattern in Drosophila that prezygotic isolation evolves more rapidly than intrinsic post-zygotic isolation when taxa are sympatric, presumably due to reinforcement (Coyne & Orr, 1989, 1997). Our results are among the first to support this pattern even when extrinsic post-zygotic isolation is considered, although we do acknowledge that additional studies on hybrid mating success and fitness of F2 backcross hybrids are necessary to be fully confident in this assertion. Although prezygotic isolating mechanisms appear to play a stronger role in present day isolation between D. mojavensis and D. arizonae, this does not preclude an important role for post-zygotic isolation during the initial stages of the speciation process, especially considering the fact some hybrids have reduced fitness and that there is evidence for reinforcement.
Acknowledgments
We would like to thank members of the Markow lab for useful discussions on the experiments and the manuscript. We also thank Tom Starmer for graciously donating the yeasts and bacteria used to make cactus rots, and Erin Kelleher and Laura Reed for sharing data used in our calculations of total reproductive isolation. J.M.B was supported by a NIH funded Postdoctoral Excellence in Research and Teaching Fellowship through the University of Arizona.
References
- Baker WK. A study of isolating mechanisms found in Drosophila arizonensis and D. mojavensis. Univeristy of Texas Publications. 1947;4720:126–136. [Google Scholar]
- Castrezana S, Markow TA. Arthropod diversity in necrotic columnar cacti of the Sonoran Desert. Can Entomol. 2001;133:301–309. [Google Scholar]
- Cohet Y, David S. Control of the adult reproductive potential by preimaginal thermal conditions. Oecologia. 1978;12:295–306. doi: 10.1007/BF00348055. [DOI] [PubMed] [Google Scholar]
- Counterman BA, Noor MAF. Multilocus test for introgression between the cactophilic species Drosophila mojavensis and Drosophila arizonae. Am Nat. 2006;168:682–696. doi: 10.1086/508632. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Patterns of speciation in Drosophila. Evolution. 1989;43:362–381. doi: 10.1111/j.1558-5646.1989.tb04233.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. “Patterns of speciation in Drosophila” revisited. Evolution. 1997;51:295–303. doi: 10.1111/j.1558-5646.1997.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sinauer Associates Inc; Sunderland: 2004. [Google Scholar]
- Davies N, Aiello A, Mallet J, Pomiankowski A, Silberglied RE. Speciation in two neotropical butterflies: extending Haldane’s rule. Proc R Soc Lond B Biol Sci. 1997;264:845–851. [Google Scholar]
- Demuth JP, Wade MJ. Population differentiation in the beetle Tribolium castaneum. II Haldane’s rule and incipient speciation. Evolution. 2007;61:694–699. doi: 10.1111/j.1558-5646.2007.00049.x. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. Genetics and the Origin of Species. 3. Columbia University Press; New York: 1951. [Google Scholar]
- Fellows DP, Heed WB. Factors affecting host plant selection in desert-adapted cactophilic Drosophila. Ecology. 1972;53:850–858. [Google Scholar]
- Haldane JBS. Sex ratio and the unisexual sterility in animal hybrids. J Genet. 1922;12:101–109. [Google Scholar]
- Hatfield T, Schluter D. Ecological speciation in sticklebacks: environment-dependent hybrid fitness. Evolution. 1999;53:866–873. doi: 10.1111/j.1558-5646.1999.tb05380.x. [DOI] [PubMed] [Google Scholar]
- Heed WB. Ecology and genetics of sonoran desert Drosophila. In: Brussard PF, editor. Ecological Genetics: The Interface. Springer-Verlag; New York: 1978. pp. 109–126. [Google Scholar]
- Heed WB. The origin of Drosophila in the Sonoran Desert. In: Barker JSF, Starmer WT, editors. Ecological Genetics and Evolution: The Cactus-Yeast-Drosophila Model System. Academic Press; Sydney: 1982. pp. 65–80. [Google Scholar]
- Jiggins CD, Naisbit RE, Coe RL, Mallet J. Reproductive isolation caused by colour pattern mimicry. Nature. 2001;411:302–305. doi: 10.1038/35077075. [DOI] [PubMed] [Google Scholar]
- Kelleher ES, Markow TA. Reproductive tract interactions contribute to isolation in Drosophila. Fly. 2007;1:33–37. doi: 10.4161/fly.3840. [DOI] [PubMed] [Google Scholar]
- Kircher HW. Chemical composition of cacti and its relationship to Sonoran Desert Drosophila. In: Barker JSF, Starmer WT, editors. Ecological Genetics and Evolution: The Cactus-Yeast-Drosophila Model System. Academic Press; New York: 1982. pp. 143–158. [Google Scholar]
- Lefranc A, Bundgaard J. The influence of male and female body size on copulation duration and fecundity in Drosophila melanogaster. Hereditas. 2001;132:243–247. doi: 10.1111/j.1601-5223.2000.00243.x. [DOI] [PubMed] [Google Scholar]
- Lewontin RC. Selection for colonizing ability. In: Baker HG, Stebbings G, editors. The Genetics of Colonizing Species. Academic Press; New York: 1965. pp. 77–94. [Google Scholar]
- Lopez-Fernandez H, Bolnick DI. What causes partial F1 hybrid viability? Incomplete penetrance versus genetic variation. PLoS ONE. 2007;2:e1294. doi: 10.1371/journal.pone.0001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CA, Matzkin LM, Reed LK, Markow TA. Multilocus nuclear sequences reveal intra- and interspecific relationships among chromosomally polymorphic species of cactophilic Drosophila. Mol Ecol. 2007;16:3009–3024. doi: 10.1111/j.1365-294X.2007.03325.x. [DOI] [PubMed] [Google Scholar]
- Magnan RL. PhD thesis. University of Arizona; Tucson: 1978. Competitive Interactions among Host Plant Specific Drosophila Species. [Google Scholar]
- Magnan RL. Adaptations to competition in cactus breeding Drosophila. In: Barker JSF, Starmer WT, editors. Ecological Genetics and Evolution: The Cactus-Yeast-Drosophila Model System. Academic Press; New York: 1982. pp. 257–272. [Google Scholar]
- Markow TA. Courtship behavior and control of reproductive isolation between Drosophila mojavensis and Drosophila arizonensis. Evolution. 1981;35:1022–1026. doi: 10.1111/j.1558-5646.1981.tb04968.x. [DOI] [PubMed] [Google Scholar]
- Markow TA, Ricker JP. Male size, developmental stability, and mating success in natural populations of three Drosophila species. Heredity. 1992;69:122–127. doi: 10.1038/hdy.1992.104. [DOI] [PubMed] [Google Scholar]
- Matzkin LM. The molecular basis of host adaptation in cactophilic Drosophila: molecular evolution of a glutathione S-transferase gene (GstD1) in Drosophila mojavensis. Genetics. 2008;178:1073–1083. doi: 10.1534/genetics.107.083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzkin LM, Watts TD, Bitler BG, Machado CA, Markow TA. Functional genomics of cactus host shifts in Drosophila mojavensis. Mol Ecol. 2006;15:4635–4643. doi: 10.1111/j.1365-294X.2006.03102.x. [DOI] [PubMed] [Google Scholar]
- Matzkin LM, Watts TD, Markow TA. Evolution of stress resistance in Drosophila: interspecific variation in tolerance to desiccation and starvation. Funct Ecol. 2009 doi: 10.1111/j.1365-2435.2008.01533. [DOI] [Google Scholar]
- Naisbit RE, Jiggins CD, Mallet JLB. Disruptive sexual selection against hybrids contributes to speciation between Heliconius cydno and H. melpomene. Proc R Soc Lond B, Biol Sci. 2001;268:1–6. doi: 10.1098/rspb.2001.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylin S, Gotthard K. Plasticity in life-history traits. Annu Rev Entomol. 1998;43:63–83. doi: 10.1146/annurev.ento.43.1.63. [DOI] [PubMed] [Google Scholar]
- Presgraves DC. Patterns of postzygotic isolation in Lepidoptera. Evolution. 2002;56:1168–1183. doi: 10.1111/j.0014-3820.2002.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Price TD, Bouvier MM. The evolution of F-1 postzygotic incompatibilities in birds. Evolution. 2002;56:2083–2089. [PubMed] [Google Scholar]
- Ramsey J, Bradshaw HD, Schemske W. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae) Evolution. 2003;57:1520– 1534. doi: 10.1111/j.0014-3820.2003.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Reed LK, Markow TA. Early events in speciation: Polymorphism for hybrid male sterility in Drosophila. Proc Natl Acad Sci USA. 2004;101:9009–9012. doi: 10.1073/pnas.0403106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LK, Nyboer M, Markow TA. Evolutionary relationships of Drosophila mojavensis geographic host races and their sister species Drosophila arizonae. Mol Ecol. 2007;16:1007– 1022. doi: 10.1111/j.1365-294X.2006.02941.x. [DOI] [PubMed] [Google Scholar]
- Robertson FW. Studies in quantitative inheritance. XI Genetic and environmental correlation between body size and egg production in Drosophila melanogaster. J Genet. 1957;55:428– 443. doi: 10.1007/BF02715825. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Heed WB. Host-plant specificity in the cactophilic Drosophila mulleri species complex. J Anim Ecol. 1988;57:237–249. [Google Scholar]
- Rundle HD. A test of ecologically dependent postmating isolation between sympatric sticklebacks. Evolution. 2002;56:322– 329. doi: 10.1111/j.0014-3820.2002.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Russell ST. Evolution of intrinsic post-zygotic reproductive isolation in fish. Ann Zool Fenn. 2003;40:321–329. [Google Scholar]
- Sasa M, Chippendale PT, Johnson NA. Patterns of postzyotic isolation in frogs. Evolution. 1998;52:1811–1820. doi: 10.1111/j.1558-5646.1998.tb02258.x. [DOI] [PubMed] [Google Scholar]
- Servedio MR, Noor MAF. The role of reinforcement in speciation: theory and data. Annu Rev Ecol Syst. 2003;34:339– 364. [Google Scholar]
- Soto EM, Soto IM, Carreira VP, Fanara JJ, Hasson E. Host-related life history traits in interspecific hybrids of cactophilic Drosophila. Entomol Exp Appl. 2007a;126:18–27. [Google Scholar]
- Soto IM, Manfrin MH, Sene FM, Hasson E. Viability and development time in cactophilic Drosophila gouveai and Drosophila antonietae (Diptera: Drosophilidae) are dependent on the cactus host. Ann Entomol Soc Am. 2007b;100:490– 496. [Google Scholar]
- Starmer WT. Associations and interactions among yeasts, Drosophila and their habiats. In: Barker JSF, Starmer WT, editors. Ecological Genetics and Evolution: The Cactus-Yeast-Drosophila Model System. Academic Press; New York: 1982. pp. 159–174. [Google Scholar]
- Tantawy AO, Rakha FA. Studies on natural populations of DrosophilaIV Genetic variances of correlations between four characters in D melanogaster andD. simulans. Genetics. 1964;30:1349–1355. doi: 10.1093/genetics/50.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantawy AO, Vetukhiv MO. Effects of size on fecundity, longevity and viability in populations of Drosophila pseudoobscura. Am Nat. 1960;94:395–403. [Google Scholar]
- Wasserman M, Koepfer HR. Character displacement for sexual isolation between Drosophila mojavensis and Drosophila arizonensis. Evolution. 1977;31:812–823. doi: 10.1111/j.1558-5646.1977.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Watada M, Ohba S, Tobari YN. Genetic differentiation in Japanese populations of Drosophila simulans and D. melanogaster. Jap J Genet. 1986;61:469–480. [Google Scholar]