Abstract
Background
Dense deposit disease (DDD) is an ultra-rare renal disease.
Methods
In the study reported here, 98 patients and their families participated in a descriptive patient-centered survey using an online research format. Reports were completed by patients (38%) or their parents (62%). Age at diagnosis ranged from 1.9 to 38.9 years (mean 14 years).
Results
The majority of patients presented with proteinuria and hematuria; 50% had hypertension and edema. Steroids were commonly prescribed, although their use was not evidence-based. One-half of the patients with DDD for 10 years progressed to end-stage renal disease (ESRD), with young females having the greatest risk for renal failure. Of first allografts, 45% failed within 5 years, most frequently due to recurrent disease (70%). Type 1 diabetes (T1D) was present in over 16% of families, which represents a 116-fold increase in incidence compared with the general population (p<0.001).
Conclusions
Based on these findings, we suggest that initiatives are needed to explore the high incidence of T1D in family members of DDD patients and the greater risk for progression to ESRD in young females with DDD. These efforts must be supported by sufficient numbers of patients to establish evidence-based practice guidelines for disease management. An international collaborative research survey should be implemented to encourage broad access and participation.
Keywords: Dense deposit disease, MPGN type II, Patient Information Survey
Introduction
Dense deposit disease (DDD), previously known as membranoproliferative glomerulonephritis type 2 (MPGN type 2), is an ultra-rare renal disease characterized by dense deposits in the lamina densa of the glomerular basement membrane (GBM). The rarity of this disease makes it difficult for clinicians to establish evidence-based clinical practices for its management. In addition, early studies typically grouped DDD with MPGN types 1 and 3, making it difficult to determine an accurate incidence rate for DDD and impossible to establish best practice guidelines [1–4]. Although basic science research over the past decade has increased our understanding of the genetics and pathophysiology of DDD [3, 5], epidemiological information about the disease course, medical history, medications, and clinical presentation has not been collected on large numbers of patients, emphasizing the need for population-based studies.
The purpose of this paper is to report the results of a survey of 98 DDD patients. Specifically, descriptive information is provided on family/patient medical disease history, history of DDD, medications, course of DDD/ progression to end-stage renal disease (ESRD), and history of dialysis and transplantation. This self-reported information provides a broad picture of the disease-related experiences of DDD patients. It is the most comprehensive epidemiological article on DDD, both in terms of the scope of the survey and the number of participants. We believe that by describing the experiences of DDD patients, this paper will provide clinicians, patients, and families with an insight into this ultra-rare disease that can facilitate informed healthcare decision-making.
Background
Dense deposit disease is a glomerular disease characterized by electron-dense deposits (EDD) in the lamina densa of the GBM[1, 4]. These deposits arise secondary to dysregulation of the alternative pathway of the complement cascade. Dysregulation occurs at the level of the C3 convertase and involves a variety of factors, such as C3 nephritic factors (C3Nefs), genetic mutations in complement genes, and autoantibodies to complement proteins, such as complement factor H (fH)[2]. Although previously called MPGN type 2, suggesting a similarity with MPGN types 1 and 3, DDD is not an immune-complex disease (MPGN types 1 and 3 are), and the lesions are not primarily membranoproliferative [3, 6]. These distinctions have led to the adoption of DDD as a more appropriate name for this disease [3, 4]. Recent studies focused only on DDD show that the incidence is between two to three persons per million general population, making DDD rare even amongst the most rarest of diseases [7]. The National Organization for Rare Disorders (NORD; see http://rarediseases.org), for example, defines a rare disease as one that affects fewer than 200,000 people in the USA or 1 in 1,500 people [8].
DDD is most often diagnosed in children between the ages of 5 and 15 years and does not show a sex bias [4]. Although light microscopy and immunofluorescence findings can be suggestive of the diagnosis, electron microscopy (EM) is required and will show ribbon-like dense deposits in the GBM [3, 6]. The presence of C3Nefs in the serum is supportive of the diagnosis, although it is not conclusive [5]. Non-specific symptoms of renal insufficiency, such as hypertension, hematuria, proteinuria, and nephrotic syndrome, are frequently present in DDD patients. Acquired partial lipodystrophy (APL) and drusen (tiny yellow or white accumulations of extracellular material that build up in Bruch’s membrane of the eye) are associated with DDD [9–11]. The treatment of DDD is unsatisfactory and only nonspecific, the goals of which are to control blood pressure, reduce proteinuria, correct electrolyte imbalances, correct anemia, and address growth and other health issues created by declining or failed renal function [4]. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type 1 receptor blockers (ARBs) are commonly prescribed for DDD patients, and although immunosuppression with steroids, specifically prednisone, has been advocated for over 30 years for all types of MPGN and many other renal diseases, there is no evidence for its benefit in DDD [5].
There are no disease-specific treatments for DDD although plasma infusion or exchange to provide intact fH and remove C3Nefs has been used [4, 5]. There are also a few centers around the world using the anti-complement drug eculizumab to treat small groups of DDD patients, but the outcome of this treatment has not yet been reported [12]. Based on the pathophysiology of DDD, successful treatment would be predicted to normalize activity of the alternative complement pathway, suggesting that serum C3 levels may be a biomarker of disease activity [4].
Roughly 50% of DDD patients progress to ESRD and require dialysis within 10 years of diagnosis [13, 14]. Various studies have suggested the factors predictive for progression to renal failure include younger age at diagnosis, specific variants of renal pathology, elevated serum creatinine levels and proteinuria at diagnosis, initial presentation with nephrotic and nephritic syndromes, and >20% chronic renal damage on initial biopsy [1, 3, 14–16]. The first factor is noteworthy because Nasr and colleagues [1] have reported that DDD patients diagnosed at>60 years of age have a higher incidence of renal failure, a point neither we nor other investigators have been able to confirm [4, 17]. Progression to ESRD necessitates renal replacement therapy by dialysis or transplantation. Unfortunately, approximately 50% of transplant recipients lose their allografts within 5 years of transplantation, making recurrence of DDD and graft failure a significant issue for DDD patients [16, 18–20].
As a chronic disease, DDD has a significant impact on the entire family, especially if renal failure develops. The aim of the study reported here was to use a patient-driven database to describe the medical experiences of DDD patients to facilitate and optimize their clinical care.
Methods
Participants
Individuals with DDD and their immediate families participated in a secured online research survey (Membranoproliferative Glomerulonephritis–Database Baseline Survey) via the website https://mpgn.nursing.uiowa.edu/. Invitations to participate were extended through contact with health professionals, publications, and websites such as KIDNEEDS (http://www.healthcare.uiowa.edu/kidneeds), a research foundation that provides information on DDD. Information and consent were available on the front page of the Membranoproliferative Glomerulonephritis–Database Baseline Survey. After indicating consent, subjects received passwords to access the research survey. The University of Iowa Human Subject Review Board approved this study.
The Membranoproliferative Glomerulonephritis Database Baseline Survey included DDD patients as well as patients with MPGN types 1 and 3. All DDD patients had a diagnosis verified by biopsy that included EM findings. Patients biopsied prior to 1990 and those who could not provide a pathologic report diagnostic of DDD were not included in the study. Four patients initially diagnosed with DDD based on a primary biopsy were diagnosed with a different disease on a subsequent biopsy and were also excluded. Thirty-eight percent (38%) of surveys were completed by patients and 62% were completed by parents or guardians. There was no age limit for participation.
Instruments
The research team developed the original survey to gather information on patients with DDD and MPGN types 1 and 3. Detailed information on content validity and reliability testing has been published [17], and in this report, we have focused exclusively on DDD patients.
The survey was designed to describe how DDD patients and their families perceive their disease experiences. A revised version of the self-reported survey, with expanded disease categories for health history, lists of medications, and potential treatments, was completed in 2009 with the support of the University of Iowa Information Technology Services. The on-line survey meets the security standards of the University of Iowa and is linked to a highly secured relational database. Data are stored in a Microsoft SQL (Standard query language) server with a functioning relational database schema, which provides efficient data access and storage. Details of the development process will be published separately. Research team members with access to the SQL database can perform simple queries to answer research questions.
The survey consisted of 140 items divided across eight sections: patient information; family/patient health history; history of MPGN; medications; course of MPGN; history of dialysis; history of transplant; conclusion. Throughout the questionnaire, participants respond to closed questions but are also provided with open fields to allow them to contribute additional information.
Procedures
The research team did not send out invitations to participate in the survey. Rather, DDD patients or immediate family members initially contacted researchers by e-mail, phone, or mail to express an interest in the survey. Following completion of the online consent form, if patients had a diagnosis of DDD or MPGN Types 1 and 3, they were e-mailed a computer-generated password to gain access to the survey, which they usually completed online. A paper copy of the questionnaire was available for persons who preferred to complete a paper copy of the survey. If a paper survey was completed, data were entered into the database by a research team member. All responses were confidential, with individual subject codes applied to each survey. Access to the names connected to each coded survey was available only to researcher team members. Access to the online database was secured, password protected, and limited to only a few individuals on the research team.
Data analysis
Data, which were downloaded from the SQL server onto a Microsoft Excel spreadsheet, were analyzed using the Statistical Package for Social Science (SPSS for Windows 17; SPSS, Chicago, IL). The chi-square test was used to compare differences between groups. The two-sample Z test for proportions and one-tailed significant test were used to evaluate the effects of gender and disease duration on progression to renal failure. The Kaplan–Meier survival analysis was used to estimate survival time of native kidneys from date of diagnosis.
Results
Participants
Ninety-eight surveys were completed at the time of data capture (March 2010). Of the patients who responded, 56% (n=55) reported their sex as female and 86% (n=84) reported their ethnicity as ‘White/ Caucasian’. Other reported races included American Indian (3), Hispanic (5), and mixed (4). The mean age at diagnosis was 14 years (male, mean 14.2 years; female, mean 13.9 years). For purposes of evaluating the effect of age-at-diagnosis, we compared the ≤12-year-old group (‘younger population’; n=60; range 1.9–13.0 years) to the ≥13-year-old group (‘older population’; n=38; range 13.1–38.9 years) as this breakpoint approximates puberty. Of the patients, 70% were diagnosed with DDD before age 15 years.
Patient/family medical/disease history
Disease histories were obtained on DDD patients and their parents and siblings. Information was also collected on extended family members, including grandparents, aunts, uncles, and cousins. Four of the listed health conditions were reported as co-morbidities by more than five individuals with DDD: fH polymorphism (n=10), depression (n= 9), drusen (n=7), and hypothyroidism (n=5). APL, which has been reported as an autoimmune disease associated with DDD [17], was reported in four DDD patients, two of whom had progressed to ESRD. In three of these patients, the diagnosis of APL anteceded the diagnosis of DDD.
Medical diseases reported by more than ten families are listed in Table 1. Autoimmune diseases were found most commonly. It is noteworthy that 16 families reported family members with type 1 diabetes (T1D) and in seven families two or more blood-related family members carried this diagnosis. Based on data from the Center for Disease Control, the expected prevalence of T1D in the general population is approximately 1.4 per 1,000 U.S. families, suggesting that the diagnosis of DDD is associated with a nearly 116-fold increase in the familial rate of T1D (p<0.001).
Table 1.
Diseases reported in the medical history of more than ten families (total n=98)
| Diseases | n | Percentage |
|---|---|---|
| Autoimmune | ||
| Type 1 diabetes | 16 | 16.3 |
| Autoimmune hypothyroidism | 21 | 21.4 |
| Autoimmune rheumatoid arthritis | 24 | 24.5 |
| Dermatology | ||
| Eczema | 26 | 26.5 |
| Unexplained rashes | 11 | 11.2 |
| Psoriasis | 11 | 11.2 |
| Gastrointestinal | ||
| Irritable bowel syndrome | 12 | 12.2 |
| Genetic | ||
| DDD-associated Factor H polymorphism | 10 | 10.2 |
| Mental health | ||
| Depression | 33 | 33.7 |
| Ophthalmology | ||
| Age-related macular degeneration | 11 | 11.2 |
| Glaucoma | 10 | 10.2 |
DDD, Dense deposit disease
History of DDD
Table 2 shows the symptoms that prompted the healthcare referral ultimately leading to the diagnosis of DDD. The primary symptoms were hematuria (42.9%) and peripheral (37.8%) and facial (31.6%) edema. About one-fifth of DDD patients (21.4%) did not suspect a problem and in this group, signs of kidney disease were detected as part of a routine annual examination. Nausea, vomiting, and fever prompted more visits than did proteinuria and hypertension, although at presentation, 90% of DDD patients had proteinuria, 84% had hematuria, 54% had hypertension, and 40% were edematous. The number of symptoms identified by physicians at diagnosis was greater than the number of symptoms recognized by patients prior to their healthcare referral. In particular, hematuria (X2=35.125, p<0.001), proteinuria (X2=103.753, p<0.001), and hypertension (X2=36.550, p<0.001) were more likely to be identified by physicians than by patients and/or their families (Table 2).
Table 2.
Symptoms present at initial presentation and symptoms identified when diagnosing DDD
| Symptoms present at initial presentation and symptoms identified when diagnosing DDD | Prompted visit | Diagnosed by physiciana | ||
|---|---|---|---|---|
|
|
|
|||
| n | Percentage | n | Percentage | |
| Blood in urine | 42 | 42.9 | 82 | 83.7 |
| Protein in urine | 18 | 18.4 | 89 | 90.8 |
| High fever | 16 | 16.3 | 19 | 19.4 |
| Nausea and vomiting | 19 | 19.4 | 24 | 24.5 |
| Hypertension | 13 | 13.3 | 53 | 54.1 |
| Drusen on retina | 3 | 3.1 | 4 | 4.1 |
| Puffiness around eyes, face | 31 | 31.6 | 38 | 38.8 |
| Puffiness feet, hands, or legs | 37 | 37.8 | 39 | 39.8 |
| No symptom noted by participants; found on routine exam | - | - | 21 | 21.4 |
Patients would have reported macroscopic hematuria and proteinuria. It was not possible to confirm which techniques these doctors used to diagnosed hematuria and proteinuria
Medications
Prescribed medications are listed in Table 3. The most commonly used medications were ACE inhibitors and oral prednisone (ACE: n=76, 77.6%; prednisone use: every other day 55.1%, once daily 35.7%).
Table 3.
Medications taken to treat DDD prior to transplant (n=98)
| Medication | n | Percentage |
|---|---|---|
| Antihypertensives | ||
| ACE Inhibitors | 76 | 77.6 |
| ARB/CCB | 6 | 6.1 |
| Non-ACE Inhibitors (excluding ARB/CCB) | 13 | 13.3 |
| Steroids | ||
| Oral prednisone ( alternate day) | 54 | 55.1 |
| Oral prednisone (every day) | 35 | 35.7 |
| Methylprednisolone | 9 | 9.2 |
| IV steroids | 8 | 8.2 |
| Diuretics | 47 | 48.0 |
| Anticoagulants | ||
| Heparin | 9 | 9.2 |
| Aspirin | 7 | 7.1 |
| Plasma therapy | ||
| Plasmapheresis | 4 | 4.1 |
| Plasma infusion | 3 | 3.1 |
| Immunosuppressives | ||
| Mycophenolate mofetil (MMF) | 4 | 4.1 |
| Cyclosporin | 3 | 3.1 |
| Tacrolimus | 3 | 3.1 |
| Cyclophosphamide | 4 | 4.1 |
| Rituximab | 1 | 1.0 |
| Total | 98 | 100.0 |
ACE, angiotensin-converting enzyme; ARB, angiotension renin blockers; CCB, calcium channel blockers; IV, intravenous
Progression to renal failure
Thirty-five (35.7%) DDD patients had progressed to ESRD: 22 of 55 females (40.0%) and 13 of 43 males (30.2%) (Table 4). There was no statistically significant effect of gender or of age-at-diagnosis, each separately, on the development of renal failure (RF) in the total group. However, in the ‘younger population’, a greater percentage of females than males were on dialysis (females: 50%, n=17/34; males: 27%, n=7/26; Z=1.808, p=0.036) (Table 4). RF was also more common among DDD patients diagnosed for >10 years: 10 of 19 (53%) patients with DDD >10 years verus 25 of 79 (32%) patients with DDD <10 years (two-sample Z test for proportions Z= 1.714, p=0.044) (Table 4).
Table 4.
Analysis of factors associated with the development of renal failurea
| General factors | Specific factors | RF | No RF | Total | p |
|---|---|---|---|---|---|
| Age at diagnosis | <13 years | 24 (40.0%) | 36 (60.0%) | 60 (61.2%) | |
| ≥13 years | 11 (28.9%) | 27 (71.1%) | 38 (39.7%) | ||
| Duration of the disease | <10 years | 25 (31.6%) | 54 (68.4%) | 79 (80.6%) | 0.044 |
| ≥10 years | 10 (52.6%) | 9 (47.4%) | 19 (19.4%) | ||
| Gender | Female | 22 (40.0%) | 33 (60.0%) | 55 (56.12%) | |
| Male | 13 (30.2%) | 30 (69.8%) | 43 (43.9%) | ||
| Total | 35 (35.7%) | 63 (64.3%) | |||
| Gender | RF | No RF | Total | p | |
| Age at diagnosis: <13 years | Female | 17 (50.0%) | 17(50.0%) | 34 (56.7%) | 0.036 |
| Male | 7 (26.9%) | 19 (73.1%) | 26 (43.3%) | ||
| Total | 24 (40.0%) | 36 (60.0%) | 60 (100.0%) |
RF, Renal failure
Data are presented as the number of DDD patients, with the percentage of total DDD patients (n=98) given in parenthesis
No other signs and/or symptoms were associated with progression to RF at p<0.05
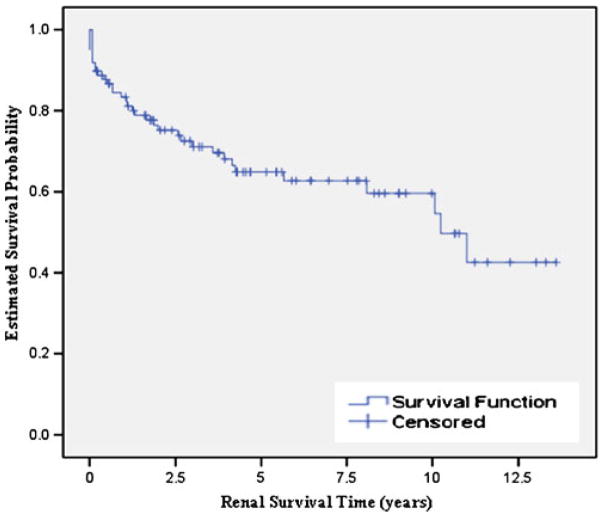
Renal survival time was calculated as the time from diagnosis to ESRD and was defined as a continuous variable. Figure 1 shows the Kaplan–Meier survival estimates for the renal survival time of all participants. Thirty-five DDD patients were in renal failure (35.7%). By using the 63 DDD patients with native kidney function as censored data to estimate the renal survival time of DDD patients, the median survival time to renal failure was calculated to be 10.4 years (standard error 1.401, 95% confidence interval 7.5–13.0 years). Major drops in the survival curve were observed during the first 5 years following diagnosis. For example, 2.5 and 5.0 years after diagnosis, the chance of progression to renal failure approximates 25 and 33%, respectively.
Fig. 1.
Kaplan–Meier survival estimates for renal survival time
Dialysis and transplantation
The characteristics of the 35 DDD patients with RF are presented in Table 5. Thirty-four patients were on dialysis at some time (hemodialysis: n=17, 48.6%; peritoneal dialysis: n=17, 48.6%), and 22 patients (62.8%) had a kidney transplantation either once (n=16, 45.7%) or twice (n=6, 17.1%). The mean time to first transplant from the time of diagnosis was 4.91 years (standard deviation 3). One patient had a preemptive transplantation to avoid dialysis. Of the 28 total kidney transplant events (including 6 who had 2 transplants) among 22 recipients, ten grafts were lost, all which were the first transplant. All losses occurred during the first 5 years following transplantation (X2 =7.368, p=0.007). Of the ten grafts lost, seven (70%) were lost due to recurrent disease. Of the allografts functioning for >5 years (n=9), survival time ranged from 5.1 to 10.2 years.
Table 5.
Information on individuals with R F (n=35) who progressed to dialysis and/or kidney transplantation
| Variable | Variable | n | Percentage |
|---|---|---|---|
| Gender | Female | 22 | 62.9 |
| Male | 13 | 37.1 | |
| Number of transplantations | 0 | 13 | 37.1 |
| 1 | 16 | 45.7 | |
| 2 | 6 | 17.1 | |
| Mean | Standard deviation | Range | |
| Current age (years) (n=35) | 20.8 | 10.5 | 3.7–52.2 |
| Age at diagnosis (years) | 12.9 | 8.7 | 1.9–38.1 |
| Duration of disease (years) | 7.8 | 5.2 | 1.2–18.0 |
| Time to first kidney transplantation (years) (n=22) | 4.9 | 3.3 | 0.8–12.8 |
| Native kidney survival time (years) | 2.4 | 3.1 | 0.0–11.0 |
| Renal survival time (years) | |||
| Age at diagnosis | |||
| <13 years (n=24, 68.57%) | 2.08 | 2.70 | 0.00–10.1 |
| ≥13 years (n=11, 31.43%) | 3.06 | 3.94 | 0.00–11.0 |
Discussion
The goal of this study was to describe the experiences and histories of 98 DDD patients and their families. In so doing, we believe a number of salient findings have emerged that are of importance to healthcare providers. First, and most striking, was the observation that over 16% of families reported at least one family member with T1D. Although Srikanta et al. and Didzar et al. [21, 22] also noted an association between T1D and MPGN, both of these papers describe only case reports linking immune-complex MPGN and T1D. This distinction is important as DDD is NOT an immune-complex MPGN [22]. To the best of our knowledge, therefore, our report is the first to note the association of DDD with T1D. It may be relevant that both T1D and DDD are inflammatory autoimmune diseases as commonalities in genetic predispositions may exist. For example, it has been known for more than three decades that alleles of the major histocompatibility complex contribute to susceptibility to autoimmunity, and with the availability of next-generation sequencing technologies, shared DDD–T1D HLA genotypes should be studied at high resolution to provide insight into this association [23, 24].
Early detection, monitoring, and treatment of the complications of chronic kidney disease (CKD), including hypertension, proteinuria, phosphorus, and calcium dysregulation, and anemia are essential to delay progression to ESRD. Of the 21% of DDD patients in whom renal disease was diagnosed during a routine physical exam, most had proteinuria. Although childhood proteinuria is not rare, if it is persistent or associated with other symptoms of renal disease, such as gross hematuria, hypertension, or persistent hypocomplementemia, a renal biopsy is indicated [25].
ACE inhibitors and prednisone were the most frequently prescribed medications. ACE inhibitors are used to treat hypertension and proteinuria and offer renal-sparing properties in advanced nephropathy beyond benefits that might be expected by a reduction in blood pressure [26, 27]. Prednisone was one of the first drugs used to treat DDD and a number of other inflammatory renal diseases. It is still commonly used to stabilize nephritic/nephrotic range disease [25, 28], although its use in DDD is not evidence-based [5, 7]. If prednisone is used, side effects should be carefully monitored, especially in children [5, 7, 28, 29].
Of the patients diagnosed with DDD for >10 years, 10 of 19 (53%) had progressed to ESRD, a number similar to that reported by several other groups [7, 14, 30]. Of note, we found that DDD in younger patients is more aggressive and that as a group, progression to ESRD is highest in young females. These age-related differences in progression to renal failure may reflect changes in innate immunity that occur with age. For example, innate immune responses may be less robust in older patients, the consequence of which may be a less effective response to a pathogenic insult [31]. Additional research is required to identify why young girls are more likely to develop renal failure [31–33].
Of the 35 DDD patients with RF, 22 underwent transplantation, among whom six had two grafts. All ten graft failures of the first transplants (45%) occurred within 5 years of surgery, a finding consistent with data reported by Braun and colleagues, who reported a 50% loss rate in a group of 75 pediatric patients within 5 years of surgery [20]. Recurrent disease was reported to be the primary cause graft failure in 70% of our patients.
Numerous studies have concluded that preemptive transplant is the renal replacement therapy that offers patients the best survival chances and quality of life [29, 34–37]. Only one person in our survey underwent a preemptive transplant, receiving a living-related donor kidney. Despite information to support preemptive transplantation as the better health option for patients with CKD stage 5, the number of preemptive transplants in the USA is relatively low (15% in the U.S. Renal Data System 2009 report [38]). Reasons for low rates may include: (1) lack of information about preemptive transplants in a timely manner; (2) hesitancy to ask donors due to worries for the donor’s health; (3) financial concerns [35]. In addition to these concerns, DDD patients must also consider the high rate of recurrent disease and graft loss.
Study limitations
The length of our survey may have been a deterrent to its completion. In some cases, participants also encountered problems entering data and did not request help. In addition, even though we offered the option of a paper survey, it is possible that some individuals felt intimidated by the online method and chose not to participate rather than request a paper copy. The majority of participants were English-speaking Caucasians from the USA. Language may therefore have limited the participation of non-English speaking patients. Accurate recollections of the information by the informant can be a limitation. Biopsies were not reviewed by the research team, allowing for possible misdiagnoses. This is particularly true now that C3 glomerulonephritis (C3GN) has recently been recognized as a disease distinct from DDD [5].
Implications for practice and research
One-fifth of DDD patients had silent symptoms (microscopic proteinuria, hematuria, and hypertension) that were detected on routine physical examinations, stressing the importance of screening for hematuria, proteinuria, and hypertension even in routine physical checkups for children. The American Academy of Pediatrics recommends yearly blood pressure screening beginning at 3 years of age [39]. Early detection of DDD symptoms and treatment could potentially slow the progression to ESRD[40].
There is a need to increase healthcare provider awareness of DDD to optimize and to provide best practices for the care of DDD patients. Resources include Kidneeds (http://www.healthcare.uiowa.edu/kidneeds), the National Organization for Rare Disorders (https://rarediseases.org), and Gene Reviews (http://www.ncbi.nlm.nih.gov/gooks/NBK1425). An Italian resource related to DDD is PRO-GETTO DDD (http://dddonlus.org). There is also a need to organize all disease-related information and healthcare support in a way that is accessible and understandable by DDD patients and their families. DDD family support groups should be established in different geographical areas to provide both emotional support and the knowledge for coping with different levels of disease progression. There is a private Facebook Group available by invitation only to patients who have completed the survey, and currently, over 55 patients have registered and participate. These patients provide support to each other and exchange information. There are patients from the USA, Italy, UK, and Israel who regularly participate in discussions. Health professionals can provide assistance in identifying resources for these families [41].
There is a need for research to explore the findings of this study, such as the high incidence of T1D in family members and the greater risk for progression to ESRD in young females. Research must be supported by the participation of a sufficient number of DDD patients to establish evidence-based practice guidelines for the management of DDD. Therefore, an international collaborative research survey of DDD patients should be supported. Non-English versions of this survey will encourage broader access and participation. To that end, an Italian translation of this survey has been completed and is available to DDD patients (http://dddonlus.org). Similar expansion of data collection to other countries should be encouraged.
Acknowledgments
The authors would like to acknowledge the generous support from the University of Iowa Information Technology Service, Research Service. Mr. Timothy VanFosson developed the online research survey platform. This work was supported in part by NIH grant DK074409 to RJHS.
Contributor Information
Der-Fa Lu, Email: der-fa-lu@uiowa.edu, University of Iowa College of Nursing, 426 Nursing Building, Iowa City, IA 52242, USA.
Mikyung Moon, University of Iowa College of Nursing, 426 Nursing Building, Iowa City, IA 52242, USA.
Lynne D. Lanning, University of Iowa College of Nursing, 426 Nursing Building, Iowa City, IA 52242, USA. Kidneeds DDD Research Foundation, Greater Cedar Rapids Community Foundation, 324 3rd St SE, Cedar Rapids, IA 52401-1841, USA
Ann Marie McCarthy, University of Iowa College of Nursing, 426 Nursing Building, Iowa City, IA 52242, USA.
Richard J. H. Smith, Departments of Internal Medicine, Molecular Physiology & Biophysics, Pediatrics and Otolaryngology, University of Iowa, 200 Hawkins Drive-21151 A PFP, Iowa City, IA 52242, USA
References
- 1.Nasr S, Valeri A, Appel GB, Sherwinter J, Stokes MB, Said SM, Markowitz GS, D’Agati VD. Dense deposit disease: clinicopathologic study of 32 pediatric and adult patients. Clin J Am Soc Nephrol. 2009;4:22–32. doi: 10.2215/CJN.03480708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strobel S, Zimmering M, Papp K, Prechl J, Jozsi M. Anti-factor B autoantibody in dense deposit disease. Mol Immunol. 2010;47:1476–1483. doi: 10.1016/j.molimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Walker PD, Ferrario F, Joh K, Bonsib SM. Dense deposit disease is not a membranoproliferative glomerulonephritis. Mod Pathol. 2007;20:605–616. doi: 10.1038/modpathol.3800773. [DOI] [PubMed] [Google Scholar]
- 4.Smith RJ, Alexander J, Barlow PN, Botto M, Cassavant TL, Cook HT, de Córdoba SR, Hageman GS, Jokiranta TS, Kimberling WJ, Lambris JD, Lanning LD, Levidiotis V, Licht C, Lutz HU, Meri S, Pickering MC, Quigg RJ, Rops AL, Salant DJ, Sethi S, Thurman JM, Tully HF, Tully SP, van der Vlag J, Walker PD, Würzner R, Zipfel PF Dense Deposit Disease Focus Group. New approaches to the treatment of dense deposit disease. J Am Soc Nephrol. 2007;18:2447–2456. doi: 10.1681/ASN.2007030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith RJ, Harris CL, Pickering MC. Dense deposit disease. Mol Immunol. 2011;48:1604–1610. doi: 10.1016/j.molimm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethi S, Fervenza FC, Zhang Y, Nasr SH, Leung N, Vrana J, Cramer C, Nester CM, Smith RJ. Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin J Am Soc Nephrol. 2011;6:1009–1017. doi: 10.2215/CJN.07110810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appel GB, Cook HT, Hageman G, Jennette JC, Kashgarian M, Kirschfink M, Lambris JD, Lanning L, Lutz HU, Meri S, Rose NR, Salant DJ, Sethi S, Smith RJ, Smoyer W, Tully HF, Tully SP, Walker P, Welsh M, Würzner R, Zipfel PF. Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol. 2005;16:1392–1403. doi: 10.1681/ASN.2005010078. [DOI] [PubMed] [Google Scholar]
- 8.Public law 107–280. Rare Diseases Act of 2002. Public law 107–280. 2002 Available at: http://history.nih.gov/research/downloads/PL107-280.pdf.
- 9.Misra A, Peethambaram A, Garg A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine (Baltimore) 2004;83:18–34. doi: 10.1097/01.md.0000111061.69212.59. [DOI] [PubMed] [Google Scholar]
- 10.D’souza YB, Jones CJ, Short CD, Roberts IS, Bonshek RE. Oligosaccharide composition is similar in drusen and dense deposits in membranoproliferative glomerulonephritis type II. Kidney Int. 2009;75:824–827. doi: 10.1038/ki.2008.658. [DOI] [PubMed] [Google Scholar]
- 11.Smith RJH, Sethi S, Zipfel PF. [Accessed 2 July 2011];Dense deposit disease/membranoproliferative glomerulonephritis type II. 2011 Available at: http://history.nih.gov/research/downloads/PL107-280.pdf. Updated 2011.
- 12.Pickering MC, Warren J, Rose KL, Carlucci F, Wang Y, Walport MJ, Cook HT, Botto M. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci USA. 2006;103:9649–9654. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponticelli C, Glassock RJ. Treatment of Primary Glomerulonephritis. 2. Oxford University Press; Oxford: 1997. [Google Scholar]
- 14.Cameron JS, Turner DR, Heaton J, Williams DG, Ogg CS, Chantler C, Haycock GB, Hicks J. Idiopathic mesangiocapillary glomerulonephritis. Comparison of types I and II in children and adults and long-term prognosis. Am J Med. 1983;74:175–192. doi: 10.1016/0002-9343(83)90606-x. [DOI] [PubMed] [Google Scholar]
- 15.Liu JC, Yang JY, Xiao HJ, Huang JP, Yao Y, Li X, Wang SX. Clinical and pathological characteristics of children with dense deposit disease. Zhonghua Er Ke Za Zhi. 2009;47:593–597. [PubMed] [Google Scholar]
- 16.Cansick JC, Lennon R, Cummins CL, Howie AJ, McGraw ME, Saleem MA, Tizard EJ, Hulton SA, Milford DV, Taylor CM. Prognosis, treatment and outcome of childhood mesangiocapillary (membranoproliferative) glomerulonephritis. Nephrol Dial Transplant. 2004;19:2769–2777. doi: 10.1093/ndt/gfh484. [DOI] [PubMed] [Google Scholar]
- 17.Lu DF, McCarthy AM, Lanning LD, Delaney C, Porter C. A descriptive study of individuals with membranoproliferative glomerulonephritis. Nephrol Nurs J. 2007;34:295–302. quiz 303. [PubMed] [Google Scholar]
- 18.Little MA, Dupont P, Campbell E, Dorman A, Walshe JJ. Severity of primary MPGN, rather than MPGN type, determines renal survival and post-transplantation recurrence risk. Kidney Int. 2006;69:504–511. doi: 10.1038/sj.ki.5000084. [DOI] [PubMed] [Google Scholar]
- 19.Schwertz R, de Jong R, Gretz N, Kirschfink M, Anders D, Scharer K. Outcome of idiopathic membranoproliferative glomerulonephritis in children. Arbeitsgemeinschaft Pädiatrische Nephrologie Acta Paediatr. 1996;85:308–312. doi: 10.1111/j.1651-2227.1996.tb14022.x. [DOI] [PubMed] [Google Scholar]
- 20.Braun MC, Stablein DM, Hamiwka LA, Bell L, Bartosh SM, Strife CF. Recurrence of membranoproliferative glomerulonephritis type II in renal allografts: The North American Pediatric Renal Transplant Cooperative Study experience. J Am Soc Nephrol. 2005;16:2225–2233. doi: 10.1681/ASN.2005020175. [DOI] [PubMed] [Google Scholar]
- 21.Dizdar O, Kahraman S, Gençtoy G, Ertoy D, Arici M, Altun B, Yasavul U, Turgan C. Membranoproliferative glomerulonephritis associated with type 1 diabetes mellitus and Hashimoto’s thyroiditis. Nephrol Dial Transplant. 2004;19:988–989. doi: 10.1093/ndt/gfh059. [DOI] [PubMed] [Google Scholar]
- 22.Srikanta S, Malaviya AN, Rajagopalan P, Bhuyan UN, Ahuja MM. Association of type I (insulin-dependent) diabetes mellitus, autoimmunity, antinuclear antibody, and membranoproliferative glomerulonephritis. Diabetes Care. 1983;6:71–74. doi: 10.2337/diacare.6.1.71. [DOI] [PubMed] [Google Scholar]
- 23.Heinig M, Petretto E, Wallace C, Bottolo L, Rotival M, Lu H, Li Y, Sarwar R, Langley SR, Bauerfeind A, Hummel O, Lee YA, Paskas S, Rintisch C, Saar K, Cooper J, Buchan R, Gray EE, Cyster JG, Erdmann J, Hengstenberg C, Maouche S, Ouwehand WH, Rice CM, Samani NJ, Schunkert H, Goodall AH, Schulz H, Roider HG, Vingron M, Blankenberg S, Münzel T, Zeller T, Szymczak S, Ziegler A, Tiret L, Smyth DJ, Pravenec M, Aitman TJ, Cambien F, Clayton D, Todd JA, Hubner N, Cook SA Cardiogenics Consortium. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature. 2010;467:460–464. doi: 10.1038/nature09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holcomb CL, Hoglund B, Anderson MW, Blake LA, Böhme I, Egholm M, Ferriola D, Gabriel C, Gelber SE, Goodridge D, Hawbecker S, Klein R, Ladner M, Lind C, Monos D, Pando MJ, Pröll J, Sayer DC, Schmitz-Agheguian G, Simen BB, Thiele B, Trachtenberg EA, Tyan DB, Wassmuth R, White S, Erlich HA. A multi-site study using high-resolution HLA genotyping by next generation sequencing. Tissue Antigens. 2011;77:206–217. doi: 10.1111/j.1399-0039.2010.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung AK, Wong AH. Proteinuria in children. Am Fam Physician. 2010;82:645–651. [PubMed] [Google Scholar]
- 26.Kalaitzidis R, Bakris GL. Effects of angiotensin II receptor blockers on diabetic nephropathy. J Hypertens Suppl. 2009;27:S15–S21. doi: 10.1097/01.hjh.0000357904.71080.7d. [DOI] [PubMed] [Google Scholar]
- 27.Galle J. Reduction of proteinuria with angiotensin receptor blockers. Nat Clin Pract Cardiovasc Med. 2008;5(Suppl 1):S36–S43. doi: 10.1038/ncpcardio0806. [DOI] [PubMed] [Google Scholar]
- 28.Alchi B, Jayne D. Membranoproliferative glomerulonephritis. Pediatr Nephrol. 2010;25:1409–1418. doi: 10.1007/s00467-009-1322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donadio JV, Jr, Offord KP. Reassessment of treatment results in membranoproliferative glomerulonephritis, with emphasis on life-table analysis. Am J Kidney Dis. 1989;14:445–451. doi: 10.1016/s0272-6386(89)80143-x. [DOI] [PubMed] [Google Scholar]
- 30.West CD. Idiopathic membranoproliferative glomerulonephritis in childhood. Pediatr Nephrol. 1992;6:96–103. doi: 10.1007/BF00856851. [DOI] [PubMed] [Google Scholar]
- 31.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gameiro C, Romao F. Changes in the immune system during menopause and aging. Front Biosci (Elite Ed) 2010;2:1299–1303. doi: 10.2741/e190. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez DA, Diaz BB, del Rodriguez Perez MC, Hernandez AG, Chico BN, de Leon AC. Sex hormones and autoimmunity. Immunol Lett. 2010;133:6–13. doi: 10.1016/j.imlet.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Riaño-Galán I, Málaga S, Rajmil L, Ariceta G, Navarro M, Loris C, Vallo A. Quality of life of adolescents with end-stage renal disease and kidney transplant. Pediatr Nephrol. 2009;24:1561–1568. doi: 10.1007/s00467-009-1175-0. [DOI] [PubMed] [Google Scholar]
- 35.Pradel FG, Jain R, Mullins CD, Vassalotti JA, Bartlett ST. A survey of nephrologists’ views on preemptive transplantation. Clin J Am Soc Nephrol. 2008;3:1837–1845. doi: 10.2215/CJN.00150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boehm M, Winkelmayer WC, Arbeiter K, Mueller T, Aufricht C. Late referral to paediatric renal failure service impairs access to pre-emptive kidney transplantation in children. Arch Dis Child. 2010;95:634–638. doi: 10.1136/adc.2009.174581. [DOI] [PubMed] [Google Scholar]
- 37.Davis CL. Preemptive transplantation and the transplant first initiative. Curr Opin Nephrol Hypertens. 2010;19:592–597. doi: 10.1097/MNH.0b013e32833e04f5. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Renal Data System. USRDS 2009 annual data report: atlas of end-stage renal disease in the United States. National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; Bethesda: 2009. [Google Scholar]
- 39.American Academy of Pediatrics. [Accessed 12 Aug 2011];Recommendations for preventive pediatric health care. Available at: http://brightfutures.aap.org/pdfs/AAP%20Bright%20Futures%20Periodicity%20Sched%20101107.pdf. Updated 2008.
- 40.Iitaka K, Igarashi S, Sakai T. Hypocomplementaemia and membranoproliferative glomerulonephritis in school urinary screening in Japan. Pediatr Nephrol. 1994;8:420–422. doi: 10.1007/BF00856519. [DOI] [PubMed] [Google Scholar]
- 41.Putkowski S. The National Organization for Rare Disorders (NORD): providing advocacy for people with rare disorders. NASN School Nurse. 2010;25:38–41. doi: 10.1177/1942602X09352796. Available at: http://search.ebscohost.com/login.aspx?direct=true&db=jlh&AN=2010524084&site=ehost-live. [DOI] [PubMed] [Google Scholar]