Abstract
Chronically elevated total peripheral resistance (TPR) is a suspected contributor to the greater rates of hypertension in African Americans. Previous research suggests that over 50% of the variability in measures of vascular resistance may be attributable to genetic effects and genetic effects may play an even greater role in variability of TPR in African Americans. We have previously demonstrated the coherence of a simple equation-based estimate of total peripheral resistance (TPRest) with TPR obtained via a validated method (Hill et al, 2013). We sought further validation by estimating heritability for this measure. Using quantitative genetic analysis, heritabilites were calculated for TPRest during both a resting baseline and orthostasis in a population-based sample of African American mono- and dizygotic twins (mean age = 49.82 ± 14.62). Estimated heritability was greater for males (h2 ≥ .40) both at rest and during orthostasis, compared to all other groups. This value is consistent with previously published point estimates of heritability. Collectively, these findings provide additional support for the validity of TPRest as a practical alternative for deriving additional hemodynamic data from archival sources.
Keywords: Total Peripheral Resistance, Heritability, Genetics
INTRODUCTION
Hypertension, or chronically elevated blood pressure, is a pervasive disease especially among African Americans whom experience the highest prevalence in the world [1]. Persistent or excessive vasoconstriction is a pathognomonic indicator of hypertension reflecting the countervailing force against cardiac blood flow in the microvasculature. Total peripheral resistance (TPR), an index of this force, is an important co-determinant of blood pressure; wherein the mean arterial or average blood pressure (MAP) is determined as the product of TPR and cardiac output (CO), or blood flow from the heart. Elevated blood pressure driven by TPR, compared to CO, has been linked to increased risk of cardiac events and mortality [2].
Chronically elevated TPR is also a major suspected contributor to the greater rates of hypertension in African Americans. In addition, several studies in healthy children, adolescents and young adults have shown African Americans to exhibit higher TPR under both resting conditions and in response to pharmacologic challenge, and physical and mental stress, compared to Whites [3].
Multiple factors influence TPR including blood volume and viscosity as well as circulating levels of hormones and neurotransmitters. Previous research has also investigated the contribution of genes to TPR by estimating heritability (h2), or the relative variability in an observed phenotype or trait (i.e. TPR) that is attributable to genetic factors [4–8].
Studies of related individuals, such as twins, offer an optimal opportunity to investigate genetic effects on a given trait, as mono- and dizygotic twins share 100% and 50% of their genes, respectively. Using samples of twins, as well as non-twin siblings and other family members, previous quantitative genetics studies have shown that as much as 50% of the variability in measures of vascular resistance may be attributable to genetic effects [4–8]. In addition, some evidence suggests that the genetic contribution to TPR may be greater among African Americans [4]; whereas other research indicates similar heritability between African Americans and Whites [8]. Despite these findings, very few studies have examined the heritability of TPR in African Americans. This may, in part, be due to the general lack of twin data and research on this population, but also to methodological limitations in obtaining estimates of TPR.
We have previously described a simple, equation-based estimate of total peripheral resistance (TPRest) and demonstrated its coherence with TPR obtained via the validated, ModelFlow method [10]. In the present research, we aim to provide further validation, by estimating heritability of TPRest. Notably, previous studies have produced point estimates of heritability (h2) ranging between −.05 to .8. Some of the variability in estimate size is likely due to differences between samples (i.e. age, health status) with additional variance attributable to the use of a variety of methodologies including: impedance cardiography/plethysmography and pattern recognition algorithms applied to the oscillometric pressure signal, to obtain TPR. Estimates also tended to differ as a function of posture, or changes in hemodynamic activity in response to experimental stress. Thus, to simulate these differences we will determine heritability estimates based on blood pressure data obtained during both a seated resting baseline period as well as during a mild physical stressor (Orthostasis).
METHODS
Analyses described in this study are based on data from the Carolina African American Twin Study of Aging (CAATSA) [9]. CAATSA was designed to examine health status, cognitive functioning, and physical and psychosocial health in a population-based sample of African American mono- and dizygotic same and opposite sex twins and members of non-intact twin pairs (Age range = 22–92, mean age = 49.82 ± 14.62). Only intact twin pairs were retained for the present analyses.
The total sample for the present analysis (N = 207) consisted of 99 monozygotic (37 male-male and 62 female-female) and 108 dizygotic (41 male-male and 67 female-female) same-sex, African American adult twin pairs.
Blood pressure data were obtained during an in-home interview using a validated, oscillometric automated device (A & D model UA-767; Milpitas California). A BP cuff of appropriate size was placed on participants’ bare arms to record BP while the participant was sitting and standing (orthostasis). Three measurements were taken and analyses are based on the average of the three, measures for each position.
TPRest was obtained as the quotient of mean arterial pressure in millimeters of mercury (mmHg) divided by a derived estimate of cardiac output (CO) in liters per minute (L/min| see [10] [11] for further detail).
The contribution of genetic and environmental influences was assessed based on established assumptions about genetic modeling, wherein differences between people on a trait of interest, or phenotype, can be attributed to three sources of variation: (1) additive genetic variance (VA), (2) variance due to common experiences shared by family members living together (VC) and (3) variance due to unique experiences specific to the individual and not shared by the family members (VE)(e.g., work history in adulthood). More explicitly, the phenotypic variance (VP) can be expressed as:
| (eq.#1) |
If each term in the above equation is divided by VP, such that the phenotypic variance now equals unity, the following expression results:
| (eq.#2) |
Where h2 (commonly substituted for a2) is heritability (proportion of variance across individuals attributable to genetic influences), c2 is the proportion of variance attributable to shared environmental influences, and e2 is the proportion of variance attributable to non-shared environmental influences.
Although the components of variance are unobserved (i.e. latent) in quantitative genetic analyses, they nonetheless can be estimated from twin correlations and variances. Intraclass correlations (rICC) were calculated to determine the association of TPRest within twin pairs.
Based on these correlations, quantitative genetic analyses were conducted using structural equation modeling (SEM | Lisrel® software Version 9.1) to obtain coefficient estimates of genetic, shared and non-shared environmental influences on TPRest, at rest and during orthostasis.
RESULTS
Subsample means, standard deviations and n’s are presented in Table 1 by gender and zygosity. Descriptively, means for all hemodynamic parameters were larger in dizygotic twins compared to monozygotes, with the exception of baseline TPRest and SBP and COest during orthostasis in MZ males. In particular, DZ females exhibited significantly higher baseline and standing blood pressure compared to MZ females; whereas, DZ’s in general exhibited significantly higher baseline SBP and higher DBP and MAP at baseline and during standing, all p’s <.05, compared to MZ twins.
Table 1.
Sample n’s, means and standard deviations by gender and zygosity for resting and standing hemodynamic data.
| Monozygotic Twins | Dizygotic Twins | ||||||
|---|---|---|---|---|---|---|---|
| M(SD) | Male | Female | MZ Total | Male | Female | DZ Total | |
| n(pairs) | 37 | 62 | 99 | 41 | 67 | 108 | |
| Age (yrs) | 45.86 (15.73) | 47.50 (14.46) | 46.90 (14.92) | 47.27 (11.76) | 46.54 (13.21) | 46.83 (12.64) | |
| SBP (mmHg) | |||||||
| Baseline | 134.30 (17.47) | 127.18 (19.11) | 129.80(18.80) | 134.63 (18.20) | 131.97 (19.94)a* | 133.00 (19.29)b* | |
| Orthostasis | 137.64 (19.39) | 128.90 (18.56) | 132.15 (19.30) | 136.04 (18.53) | 133.54 (19.17)a* | 134.51 (18.93) | |
| DBP (mmHg) | |||||||
| Baseline | 80.75 (11.75) | 78.49 (11.51) | 79.32 (11.62) | 82.37 (11.45) | 82.75 (12.40)a** | 82.60 (12.02)b** | |
| Orthostasis | 85.63 (10.51) | 82.80 (11.44) | 83.85 (11.16) | 87.97 (13.11) | 88.04 (13.88)a** | 88.01 (13.55) b** | |
| MAP (mmHg) | |||||||
| Baseline | 107.52 (13.30) | 102.83 (14.04) | 104.56 (13.92) | 108.50 (13.82) | 107.36 (15.00)a** | 107.80 (14.54)b** | |
| Orthostasis | 102.97 (11.75) | 98.17 (12.69) | 99.95 (12.53) | 103.99 (13.80) | 103.20 (14.41)a** | 103.51 (14.15)b** | |
| COest (L/min) | |||||||
| Baseline | 7.75 (2.39) | 7.26 (2.17) | 7.44 (2.26) | 7.91 (2.72) | 7.50 (2.4) | 7.65 (2.53) | |
| Orthostasis | 8.42 (2.94) | 7.41 (2.33) | 7.79 (2.62) | 8.32 (3.45) | 7.52 (2.41) | 7.84 (2.88) | |
| TPRest (mmHg.s/ml) | |||||||
| Baseline | 0.82 (0.23) | 0.83 (0.22) | 0.83 (0.22) | 0.81 (0.20) | 0.86 (0.23) | 0.84 (0.22) | |
| Orthostasis | 0.81 (0.26) | 0.88 (0.42) | 0.85 (0.37) | 0.84 (0.29) | 0.90 (0.30) | 0.88 (0.30) | |
SBP = Systolic Blood Pressure, DBP = Diastolic Blood Pressure, MAP = Mean Arterial Pressure, mmHg = millimeters of Mercury, COest = estimated Cardiac Output, L/min = liters per minute, TPRest = estimated Total Peripheral Resistance, mmHg.s/ml = millimeters of Mercury per second per milliliter,
= between-zygosity, within-gender comparison,
= between-zygosity comparison,
p ≤.05,
p ≤.01.
Intra-class correlations for TPRest by gender and zygosity are presented in Table 2. Notably, correlations were larger in MZ’s compared to DZ’s both within gender groups and overall. The largest correlations were noted in male MZ twins, rICC = .50 and .54, p < .01, for baseline and orthostasis, respectively. Interestingly, correlations determined by zygosity were similar for baseline in females, MZ and DZ rICC = .32 and .33, p < .01, and overall for orthostasis (MZrICC = .16, p < .05, DZrICC = .13, p > .05).
Table 2.
Intraclass correlations for resting and standing TPRest by gender and zygosity.
| Male | Female | Total | ||||
|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | MZ | DZ | |
| Baseline | 0.50** | 0.09 | 0.32** | 0.33** | 0.39** | 0.25** |
| Orthostasis | 0.54** | −.21 | 0.07 | 0.32** | 0.16* | 0.13 |
p < .05,
p <.01
SEM results are presented in Table 3. With the exception of orthostasis in males, the specified model (i.e. Figure 1) provided acceptable fit (indicated by a non-significant χ2) to the data. Heritability of TPRest was 46% and 40%, respectively for baseline and orthostasis in males. There was no relative genetic contribution to TPRest in females. Overall heritability accounted for 20% of the variability in baseline TPRest and only 6% of the variability in standing TPRest.
Table 3.
Structural equation model (SEM) results of genetic and environmental components of TPRest.
| A | C | E | χ2 | df | P | ||
|---|---|---|---|---|---|---|---|
| Male | Baseline | 0.46 | 0 | 0.54 | 1.012 | 3 | .7984 |
| Orthostasis | 0.40 | 0 | 0.60 | 8.585 | 3 | .0353 | |
| Female | Baseline | 0.00 | 0.32 | 0.68 | .005 | 3 | .999 |
| Orthostasis | 0.00 | 0.20 | 0.80 | 2.355 | 3 | .5021 | |
| Total | Baseline | 0.20 | 0.15 | 0.65 | 0 | 3 | 1.0 |
| Orthostasis | 0.06 | 0.10 | 0.84 | 0 | 3 | 1.0 | |
A = additive genetic influence; C = common or shared environmental influence; E = unique environmental influence, χ2 = chi-square, df = degrees of freedom.
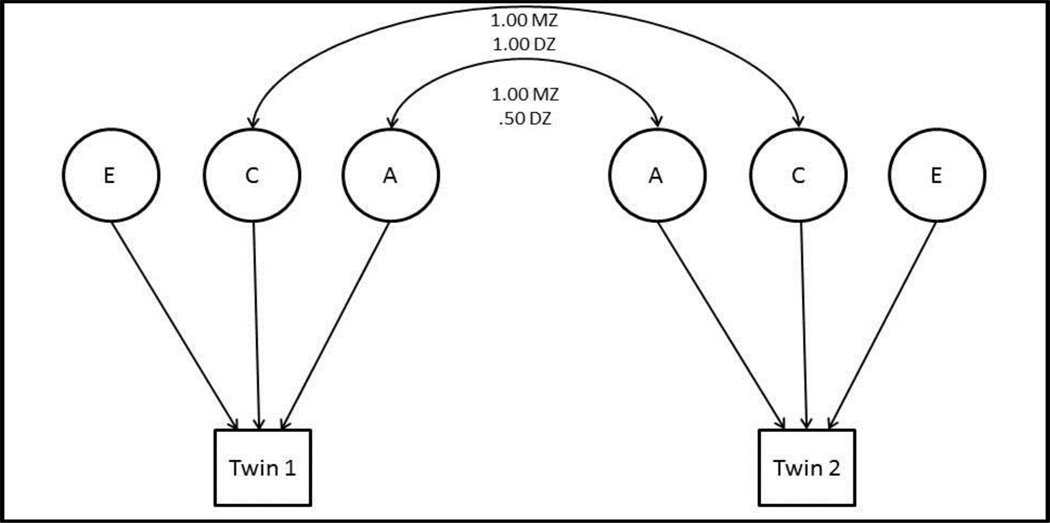
Figure 1. ACE Path Model.
Figure 1 depicts a common ACE path model used in Quantitative Genetic Analysis, where A represents the additive genetic influence, C represents common environmental influences, and E represents unique environmental influences. Expected intraclass correlations for additive genetic influences (lower double-headed arrow) for MZ and DZ twin pairs are 1.00 and .50 respectively; expected intraclass correlations for common environmental influence (upper double-headed arrow) are 1.0 for both MZ and DZ twin pairs.
No shared environmental effects were detected for TPRest in males. In females, 32% and 20% of the variability in baseline and standing TPRest was attributable to shared environmental effects. In analyses collapsed across gender, common environment accounted for 15% and 10% of the variability in resting and standing TPRest.
Non-shared environmental effects accounted for the largest proportion of variance in TPRest, between 54% and 84%, for males and females, as well as overall and for both baseline and orthostasis.
DISCUSSION
African Americans currently experience the highest prevalence of hypertension of any ethnic or cultural group in the world [1]. Total peripheral resistance plays a critical role in the development and maintenance of this disease, and evidence from studies of twins and other related individuals suggest significant genetic influences on TPR [4–8].
Extending our previous research, we sought to evaluate the plausibility of using estimates of total peripheral resistance derived from simple blood pressure measurements to extract additional relevant information from archival data by estimating its heretibility in a sample of middle-aged and older African American twins.
It is notable that with the exception of DZ males, baseline intraclass correlations for all groups were moderate and significant. Moreover, the observed correlation for MZ males at baseline is consistent with similar values reported by others in a younger sample of MZ African American males [4][8]. Correlations for both MZ and DZ males and females during orthostasis were further consonant with twin correlations obtained in MZ and DZ same-sex twins based on a composite of BP measurements taken across exposure to two different stressors [8]. These results are interesting especially given the younger age (mean < 15 years) of the sample in these studies.
Using a relatively simplified model, we obtained estimates of genetic, shared and non-shared environmental influences on TPRest at rest and during a mild physical stressor/posture change. Despite adequate model fit, the overall results for h2 were lower than those reported in other studies [4–8]. Estimates for both shared and non-shared environment were also larger than reported values. It is notable that three of these investigations [4][6][8] contained larger samples (N > 500 twin or sib pairs) and all studies conducted analyses which controlled for gender and employed more validated methods to derive TPR. Despite these differences, we were able to obtain adequate data for the quantitative genetic analysis and derive relatively stable parameter estimates given the overall and respective sub-cell sample sizes.
In addition, an exception to our modest results emerged in the findings for African American males. Particularly, the identification of only genetic (A) and non-shared (E) environmental influences is consistent with previous findings [4][8] in African Americans. Moreover, both the h2 and e2 for baseline are within range of point and interval estimates for resting TPR in these studies [4][8]. Lastly, the variability attributed to additive genetic influences during orthostasis in African American males, h2 = .40, is virtually identical to the value obtained for TPR during stress in another sample [8]. This is promising in consideration of the less than optimal model fit observed for TPRest during orthostasis.
CONCLUSIONS
Mechanisms underlying the regulation of blood pressure (BP) are of major clinical and research interest, especially in the study of hypertension. In contexts where the resources needed to obtain these parameters are unavailable, it may be both practical and efficient to employ estimation-based methods.
We have previously shown that TPRest provides a conservative, ‘uncalibrated’ approximation to TPR obtained via ModelFlow -- particularly under phasic conditions [10]. The present results extend this work by demonstrating that TPRest may be employed to obtain valid parameter estimates in quantitative genetic analysis and, in the case of African American males, estimates that are comparable to data from more well-powered investigations which used alternative methods to obtain TPR.
Sample size limitations notwithstanding, the present results offer additional support for the utility of TPRest in deriving valid and meaningful information from pre-collected data. This approach may be especially useful in extending the capacity of older, large-scale datasets to explore questions regarding predictors and correlates of basal hemodynamic functioning—particularly in African Americans. Considering the hypertension disparity faced by this population, insights gained from the use of this approach in the re-evaluation of previously collected data may have important implications for shaping future research and treatment.
ACKNOWLEDGMENTS
We would like to acknowledge the CAATSA study participants. CAATSA was funded by a grant from the National Institute on Aging (1R01-AG13662-01A2) to K.E.W. Research conducted by L.K.H was supported by National Institute of Aging grant (5T32AG000029-37).
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Julius S. Transition from high cardiac output to elevated vascular resistance in hypertension. American Heart Journal. 1988;116(2):600–606. doi: 10.1016/0002-8703(88)90557-1. [DOI] [PubMed] [Google Scholar]
- 3.Taherzadeh Z, Brewster LM, Van Montfrans GA, VanBavel E. Function and structure of resistance vessels in black and white people. J. Clinical Hypertension. 2010;12(6):431–438. doi: 10.1111/j.1751-7176.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African-and European-American youth. Hypertension. 2003;41(6):1196–1201. doi: 10.1161/01.HYP.0000072269.19820.0D. [DOI] [PubMed] [Google Scholar]
- 5.Seasholtz TM, Wessel J, Rao F, Rana BK, Khandrika S, Kennedy BP, O’Connor DT. Rho Kinase Polymorphism Influences Blood Pressure and Systemic Vascular Resistance in Human Twins Role of Heredity. Hypertension. 2006;47(5):937–947. doi: 10.1161/01.HYP.0000217364.45622.f0. [DOI] [PubMed] [Google Scholar]
- 6.Davis JT, Rao F, Naqshbandi D, Fung MM, Zhang K, Schork AJ, O'Connor DT. Autonomic and Hemodynamic Origins of Pre-Hypertension:Central Role of Heredity. J. American College of Cardiology. 2012;59(24):2206–2216. doi: 10.1016/j.jacc.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotchen TA, Kotchen JM, Grim CE, George V, Kaldunski ML, Cowley AW, Chelius TH. Genetic Determinants of Hypertension Identification of Candidate Phenotypes. Hypertension. 2000;36(1):7–13. doi: 10.1161/01.hyp.36.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Wu T, Treiber FA, Snieder H. Genetic Influence on Blood Pressure and Underlying Hemodynamics Measured at Rest and During Stress. Psychosomatic Medicine. 2013;75(4):404–412. doi: 10.1097/PSY.0b013e31828d3cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitfield KE, Brandon DT, Wiggins S, Vogler G, McClearn G. Does intact pair status matter in the study of African American twins? The Carolina African American Twin Study of Aging. Experimental aging research. 2003;29(4):407–423. doi: 10.1080/03610730303699. [DOI] [PubMed] [Google Scholar]
- 10.Hill LK, Sollers JJ, III, Thayer JF. Resistance Reconstructed: Estimation of Total Peripheral Resistance from Computationally-derived Cardiac Output. Biomed Sci Instrum. 2013;49:216–223. [PMC free article] [PubMed] [Google Scholar]
- 11.Hill LK, Sollers JJ, III, Thayer JF. Evaluation of a Simple Estimation Method for the Derivation of Cardiac Output from Arterial Blood Pressure and Heart Rate. Biomed Sci Instrum. 2012;48:165–170. [PubMed] [Google Scholar]