Abstract
The current study examined the effects of neonatal amygdala lesions on mother–infant interactions in rhesus monkeys reared in large species-typical social groups. Focal observations of mother–infant interactions were collected in their social group for the first 12 months postpartum on infants that had received amygdala lesions (Neo-A) at 24–25 days of age and control infants. Early amygdala lesions resulted in subtle behavioral alterations. Neo-A females exhibited earlier emergence of independence from the mother than did control females, spending more time away from their mother, whereas Neo-A males did not. Also, a set of behaviors, including coo vocalizations, time in contact, and time away from the mother, accurately discriminated Neo-A females from control females, but not Neo-A and control males. Data suggest that neonatal amygdalectomy either reduced fear, therefore increasing exploration in females, or reduced the positive reward value of maternal contact. Unlike females, neonatal amygdala lesions had little measurable effects on male mother–infant interactions. The source of this sex difference is unknown.
Keywords: development, sex difference, maternal, fear, reward, Macaca mulatta
INTRODUCTION
Early in development mothers or caregivers are the infants’ sole provider of nourishment, warmth, and protection, thus separation from the caregiver or the presence of strangers are potentially dangerous situations that the infant must respond to. Reactivity to maternal separation and the presence of strangers requires that the infant is able to discriminate the mother from other adult females. This filial discrimination develops prior to 3 months of age, and occurs earlier in females than in males (Rosenblum & Andrews, 1994). As filial discrimination develops, infant monkeys also exhibit “stranger anxiety” (Sackett, 1966; Suomi & Harlow, 1976) that further prompts them to maintain a safe distance to the mother, use their mother as a secure base, and establish mother–infant attachment. After 3 months of age, however, infants increasingly explore their environment and develop independence from their mother (Hinde & Spencer-Booth, 1967). Thus, infant independence develops once the infant has mastered the ability to discriminate their mother and detect strangers. Among old world monkeys, the development of independence from the mother differs between sexes, such that males exhibit an earlier independence than do females (Hinde & Spencer-Booth, 1967; Jensen, Bobbitt, & Gordon, 1966, 1968; Rosenblum, 1974). Yet, the neural network that supports these abilities and promote these sex differences in mother–infant interactions are poorly understood, especially in primates.
Extensive evidence has accumulated in the last decades on the contribution of the amygdala to social cognition (Aggleton, 2000; Whalen & Phelps, 2009) and many of its functions are required for the development of normal mother–infant relations (Landers & Sullivan, 2012). The amygdala plays an important role in perceiving and integrating sensory stimuli to guide attention to biologically relevant social information, such as eyes, face, and body movement (Adolphs & Tranel, 2003). Thus, damage to the amygdala, in human and nonhuman primates, has been shown to impart subtle changes in social behavior, such as eye gaze, approaching a stranger, and recognizing emotions in faces (Adolphs, 2010). The amygdala’s role in threat detection and learning the emotional significance of environmental stimuli has also been well studied in rodents and primates. Specifically, the amygdala is recruited during the initial learning period when associations are ambiguous (LaBar et al., 1998) and is essential for the acquisition, storage, and expression of conditioned fear learning (LeDoux, 2007). Finally, the amygdala is essential in adjusting animals’ behavioral and neuroendocrine responses according to the level of threat in their environment (Raper et al., 2013b). Thus, the amygdala is well-suited to modulate the development of social behaviors including those involved in the mother–infant relationship as well as to regulate infants’ emotional reactivity during dramatic changes in mother–infant interactions, yet little is known on this topic in primates (Bachevalier, 2000).
In rhesus monkeys, a number of neonatal amygdala lesion studies have shown that early amygdala damage yields abnormal emotional reactivity to objects and social partners (Bauman et al., 2004a,b; Bliss-Moreau et al., 2010; Prather et al., 2001; Thompson, 1981). These neonatal lesions also alter the magnitude of the expression of emotional and neuroendocrine reactivity to stressors (Raper et al., 2013a,b). Finally, neonatal amygdala lesions also altered the ability to flexibly adjust choices when reward value has changed (Kazama & Bachevalier, 2013). However, the few studies examining the effects of early amygdala damage on mother–infant interactions have produced mixed results. Two studies reported no differences in interactions or attachment to the mother or caregiver (Goursaud & Bachevalier, 2007; Kling & Green, 1967), whereas one study indicated that amygdalectomized infants spent more time in ventral contact with their mothers when in the presence of other animals, even though they did not exhibit the species-typical preference for their mother (Bauman et al., 2004a). One of the major limitations of these earlier studies is the restricted social environment in which the mother–infant interactions were observed. Some studies used nursery surrogate-peer rearing (Goursaud & Bachevalier, 2007; Kling & Green, 1967), one used mother-rearing with individually housed mother–infant pairs (Kling & Green, 1967), still another used mother–infant pairs housed individually but receiving 15 hr/week of social experience with 11 other animals (Bauman et al., 2004a). Since physical and social environment stability is critical for the emergence of infants’ independence from mothers (Rosenblum, 1974; Rosenblum & Andrews, 1994), the socially limited rearing conditions of earlier studies could have obscured the effects of the amygdala lesions. Thus, the goal of the current study was to examine the impact of neonatal amygdala lesions on mother–infant interactions during the first year of life in rhesus infants reared in a complex, species-typical, social environment consisting of a large multigenerational age-graded social group.
METHODS
Thirty-eight infant rhesus monkeys (Macaca mulatta) from middle-ranking multiparous mothers living in long term age-graded groups of monkeys whose social structure duplicated that seen in naturally occurring populations were used in this study (for details see Raper et al., 2013b). Social groups consisted of 19 mother–infant pairs and 70–100 other group members. Animals lived in large outdoor compounds (38 m × 39 m) with attached heated and cooled indoor areas at the Yerkes National Primate Research Center (YNPRC) Field Station (Lawrenceville, GA) of Emory University. At an average of 24.8 ± 1.2 days of age, infants received neonatal neurotoxic lesions of the amygdala (Neo-A; males = 9, females = 7), or sham operations (Neo-C; males = 6, females = 6). An additional 10 animals served as behavioral controls (Neo-BC; males = 6, females = 4) and experienced neonatal maternal separation, anesthesia, and post-operative treatments comparable to the other two groups but no neuroimaging and surgical procedures. All neuroimaging and surgical procedures were performed at the YNPRC Main Station (Atlanta, GA) as described in detail elsewhere (Raper et al., 2013a,b) and are briefly described in Supplemental Materials. Extent of lesions are given in Supplemental Table S1. All procedures and care for the animals followed the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institute for Animal Care and Use Committee of Emory University.
Observational Data Collection
Behavioral data were collected for each subject starting from their return to the social group after surgery (approximately 30 days of age) through 12 months of age. Subjects were identified by a distinctive dye-mark on their body and focally observed twice per week (30-min each) using a detailed ethogram (see Supplemental Table S2) according to published methods (Herman, Measday, & Wallen, 2003). Four trained observers with an inter-rater reliability of Cohen’s κ .82 collected all of the behavioral data in this study.
Data Analysis
Behavioral data were divided into two developmental periods for analyses, that is, infancy (2–6 months) and the infancy-juvenile transition (7–12 months) periods, for simplicity during the infancy-juvenile transition animals will be referred to as juveniles. For both periods, preliminary analyses were performed to compare the behavioral control group (Neo-BC) to the sham-operated group (Neo-C). Repeated measures ANOVA (Group × Sex × Age) revealed no significant main effects or interactions for mother-infant interactions (e.g., time spent with or away from the mother, grooming received from the mother). Therefore, data from both groups were combined to create a single control group (Neo-C) for all subsequent analyses. Lastly, one Neo-C male was excluded from the data analysis due to recurring illness unrelated to his treatment. Thus, 37 subjects were included for the final analysis (Neo-C: males = 11, females = 10; Neo-A: males = 9, females = 7).
Given that mother-infant interactions rapidly change during the first 6 months of life as infants transition from relying heavily on the mother to being independent (Hinde, Rowell, & Spencer-Booth, 1964), the data were analyzed for each month such that the Age factor included 5 data points: 2, 3, 4, 5, and 6 months. Therefore, during infancy, behavioral data were examined using repeated measures ANOVAs with Group (Neo-C, Neo-A) and Sex as between subjects factors, and Age (2, 3, 4, 5, and 6 months old) as the within-subjects repeated measure. Interactions were examined with post hoc one-way ANOVAs.
Later in development, as the mother prepares for the arrival of a new infant, juveniles are weaned from their mother. Thus, around 7 months of age, the mothers increasingly reject and punish juveniles when they attempt to contact the mothers’ nipples and in response juveniles emit distress vocalizations and engage in tantrums. These weaning behaviors peak around 9 months of age, and the juveniles’ tantrum responses drop significantly by 12 months of age (Hinde & Spencer-Booth, 1967). Therefore, during the infancy-juvenile transition period (after 6 months of age), two age blocks were created: a 7- to 9-month block (weaning) and a 10- to 12-month block (post-weaning). Average rates of behavior across the individual observations were created within each of these two blocks. Repeated measures ANOVAs were used with Group and Sex as between subjects factors, and Age (7–9 and 10–12 months old) as the within-subjects repeated measure. Interactions were examined with post hoc one-way ANOVAs.
Discriminant function analyses (DFA) were conducted for each developmental period (infancy and juvenile) and for each sex separately and assessed whether behavioral interactions with the mother could be used to accurately classify individual animals according to their Group (Neo-C, Neo-A). Items included in the DFA were coo vocalizations, time in contact with mother, time away from the mother, and amount of grooming received from the mother. In addition, for the infancy-juvenile transition period, a second DFA was conducted including the two combined weaning behaviors (mother’s rejection and punishment, and juvenile’s geckers and tantrums). Behavioral items were selected based on previous studies demonstrating behavioral alterations in similar measures after amygdala damage (Bauman et al., 2004a; Goursaud & Bachevalier, 2007; Kalin, Shelton, & Davidson, 2004; Newman & Bachevalier, 1997). We used Press’s Q statistic to test if the DFA categorized individuals better than would occur by chance (Hair et al., 2009). With the exception of Press’s Q statistic, all statistical comparisons used IBM SPSS statistical package for Windows (IBM, Armonk, NY), p <.05 was considered significant, and data were expressed as means and standards errors.
RESULTS
Infancy Period: 2–6 Months
Distance to Mother
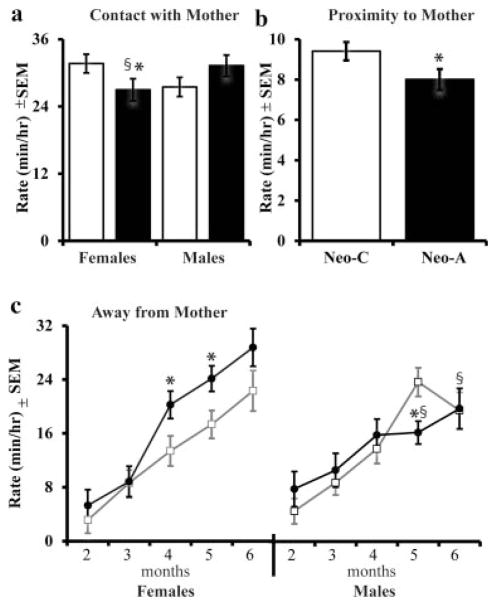
For all infants, the time spent in close contact with their mother decreased from 2 to 6 months (Age: F[4,132] = 37.4, p <.001, η2 =.53; see Tab. 1). However, the significant Group × Sex interaction (F[1,33] = 5.0, p = .032, η2 =.13; Fig. 1a) further indicated that control males spent less time in contact with their mother than did control females, and the reverse was true for the amygdalectomized animals (i.e., Neo-A females spent less time in contact than did Neo-A males). The sex differences in both groups were not significant, but lesion effects were. Neo-A females spent less time in contact with their mother as compared to Neo-C females (F[1,17] = 3.28, p = .05, η2 = .18; Fig. 1a), but there was no group difference for the males (F[1,20] =1.9, p =.17, η2 = .10). When infants are not in close contact with their mother, they typically remain within arm’s reach, but both Neo-A male and female infants spent slightly less time in proximity to their mother than did controls (Group: F [1,33] =4.1, p =.05, η2 =.11; Fig. 1b). Then as infants begin to explore their environment, their time spent within 3 m of the mother increased significantly with age, with the biggest increase between 3 and 4 months (Age: F[4,132] = 5.8, p <.001, η2 =.15; see Tabs. 1 and 2). This was true for control and Neo-A infants alike (Group: F[1,33] = .74, p = .39, η2 = .02).
Table 1.
ANOVA Repeated Contrasts for Age Effects During Infancy (2–6 Months)
| Age
|
|||||
|---|---|---|---|---|---|
| Behavior | df | 2 vs. 3 Months | 3 vs. 4 Months | 4 vs. 5 Months | 5 vs. 6 Months |
| Contact | F[1,33] | 12.6, p = .001, η2 = .3 | 35.5, p <.001, η2 =.5 | 2.6, p = .11, η2 = .07 | .49, p = .5, η2 = .01 |
| 3 m | F[1,33] | 12.0, p = .001, η2 =.27 | 6.9, p =.013, η2 = .17 | .8, p = .39, η2 = .02 | .49, p =.9, η2 =.001 |
| Cradle | F[1,33] | 4.9, p =.033, η2 = .13 | 1.5, p = .22, η2 =.04 | 1.6, p = .21, η2 = .05 | .32, p =.58, η2 = .01 |
| Carry | F[1,33] | 8.11, p = .008, η2 =.19 | .06, p = .8, η2 = .002 | 1.86, p =.18, η2 =.05 | 1.4, p =.25, η2 = .04 |
| Restrain | F[1,33] | 5.4, p =.026, η2 = .14 | 3.7, p = .06, η2 =.10 | 7.2, p = .011, η2 =.18 | 3.4, p =.08, η2 = .09 |
| Mom followf | F[1,33] | 4.67, p = .038, η2 =.12 | .30, p = .56, η2 =.01 | 2.6, p = .12, η2 = .07 | 1.42, p = .24, η2 =.04 |
| Mom followd | F[1,33] | 5.4, p =.027, η2 = .14 | 3.4, p = .08, η2 =.09 | .37, p = .55, η2 = .01 | 1.2, p =.29, η2 = .03 |
| Infant followf | F[1,33] | 6.39, p = .016, η2 = .2 | 5.78, p =.02, η2 = .2 | .05, p = .8, η2 = .001 | 1.73, p = .19, η2 =.05 |
| Infant followd | F[1,33] | 9.51, p = .004, η2 =.22 | 2.75, p = .10, η2 = .08 | 2.20, p =.14, η2 =.06 | 2.31, p = .13, η2 =.07 |
| Retrieve | F[1,33] | 3.32, p = .07, η2 = .09 | 14.7, p = .001, η2 =.3 | 1.93, p = .2, η2 = .06 | 6.05, p = .02, η2 =.16 |
| Touch | F[1,33] | .08, p = .8, η2 = .002 | 18.9, p <.001, η2 = .36 | 5.6, p = .02, η2 = .15 | 1.1, p =.31, η2 = .03 |
| Kidnapf | F[1,33] | .03, p = .9, η2 = .001 | 8.08, p = .008, η2 =.2 | .84, p =.4, η2 = .03 | 2.24, p = .14, η2 =.06 |
Repeated contrasts results for a significant main effect of Age from 2 to 6 months.
Indicates the frequency of behavior, whereas
indicates the duration of the same behavior.
FIGURE 1.
Infancy, pre-weaning: Average Rate (min/hr) of contact with the mother (a), proximity to the mother (b), and time away from the mother (c) with control infants (Neo-C, open bars or squares) and neonatal amygdala-lesioned infants (Neo-A, black bars or circles). *Indicates a significant difference from same sex controls (p <.05). §Indicates a significant sex difference.
Table 2.
Behaviors During Infancy (2–6 Months)
| Neo-C
|
Neo-A
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Behavior | Sex | 2 | 3 | 4 | 5 | 6 | 2 | 3 | 4 | 5 | 6 |
| 3 m | F | 4.12 ± 1.4 | 7.37 ± 1.0 | 8.90 ± 1.2 | 8.94 ± 1.5 | 7.61 ± 1.2 | 5.44 ± 1.7 | 7.69 ± 1.2 | 9.65 ± 1.3 | 6.82 ± 1.8 | 6.91 ± 1.5 |
| M | 5.23 ± 1.3 | 7.69 ± 1.0 | 9.25 ± 1.1 | 9.03 ± 1.5 | 10.1 ± 1.2 | 5.21 ± 1.5 | 6.43 ± 1.1 | 8.29 ± 1.2 | 7.70 ± 1.6 | 7.18 ± 1.3 | |
| Carry | F | 5.36 ± 1.1 | 2.69 ± .5 | 1.61 ± .6 | 1.44 ± .4 | .78 ± .4 | 9.36 ± 1.4 | 2.53 ± .6 | 1.53 ± .7 | .99 ± .4 | 1.02 ± .4 |
| M | 5.11 ± 1.1 | 3.41 ± .5 | 2.53 ± .5 | .98 ± .4 | 1.40 ± .4 | 6.95 ± 1.2 | 2.95 ± .5 | 2.24 ± .6 | 2.41 ± .4 | .98 ± .4 | |
| Groom | F | 2.07 ± .6 | 1.98 ± .6 | 1.54 ± .5 | 1.39 ± .5 | 1.44 ± .9 | 2.13 ± .8 | 2.58 ± .7 | 1.37 ± .6 | 1.58 ± .6 | 2.02 ± 1.1 |
| M | 2.70 ± .6 | 1.77 ± .5 | 1.13 ± .5 | 1.03 ± .5 | 1.43 ± .8 | 1.35 ± .7 | 2.10 ± .6 | 1.73 ± .5 | 1.78 ± .5 | 2.26 ± .9 | |
| Restrain | F | 4.99 ± 1.0 | 2.64 ± .8 | 1.75 ± .7 | .86 ± .5 | .83 ± .2 | 4.71 ± 1.2 | 2.74 ± .9 | 3.87 ± .9 | .39 ± .5 | .31 ± .2 |
| M | 3.29 ± 1.0 | 2.57 ± .8 | 1.14 ± .7 | 1.43 ± .5 | .49 ± .2 | 3.91 ± 1.1 | 3.60 ± .8 | 1.61 ± .8 | 1.16 ± .5 | .28 ± .2 | |
| Mom followf | F | .16 ± .1 | .13 ± .1 | .05 ± .02 | .00 ± .0 | .00 ± .0 | .26 ± .2 | .12 ± .1 | .03 ± .03 | .00 ± .0 | .00 ± .0 |
| M | .36 ± .2 | .11 ± .1 | .00 ± .0 | .00 ± .0 | .00 ± .0 | .84 ± .2 | .17 ± .08 | .00 ± .0 | .17 ± .1 | .00 ± .0 | |
| Mom followd | F | .03 ± .01 | .01 ± .001 | .01 ± .001 | .00 ± .0 | .00 ± .0 | .02 ± .02 | .01 ± .01 | .003 ± .001 | .00 ± .0 | .00 ± .0 |
| M | .03 ± .01 | .01 ± .01 | .00 ± .0 | .00 ± .0 | .00 ± .0 | .08 ± .02 | .01 ± .01 | .00 ± .0 | .03 ± .01 | .00 ± .0 | |
| Infant followf | F | 3.00 ± .9 | 5.10 ± .7 | 4.20 ± 1.1 | 6.31 ± 1.2 | 4.97 ± 1.2 | 2.81 ± 1.1 | 4.25 ± .8 | 6.48 ± 1.3 | 6.21 ± 1.4 | 3.88 ± 1.4 |
| M | 3.44 ± .9 | 3.50 ± .7 | 5.80 ± 1.0 | 5.03 ± 1.2 | 6.24 ± 1.1 | 2.77 ± 1.0 | 4.95 ± .7 | 7.17 ± 1.1 | 6.73 ± 1.3 | 5.21 ± 1.3 | |
| Infant followd | F | .34 ± .1 | .65 ± .1 | .51 ± .1 | .89 ± .2 | .59 ± .2 | .32 ± .1 | .66 ± .1 | .70 ± .2 | .77 ± .3 | .53 ± .2 |
| M | .37 ± .1 | .39 ± .1 | .80 ± .1 | .67 ± .2 | .69 ± .2 | .39 ± .1 | .66 ± .1 | .91 ± .1 | 1.23 ± .2 | .91 ± .2 | |
| Retrieve | F | 2.15 ± .6 | 2.16 ± .5 | .58 ± .2 | .58 ± .2 | .14 ± .1 | 1.87 ± .7 | 1.50 ± .6 | .69 ± .3 | .33 ± .3 | .10 ± .1 |
| M | 2.19 ± .5 | .90 ± .5 | .51 ± .2 | .07 ± .2 | .36 ± .1 | 2.52 ± .6 | 1.50 ± .5 | .89 ± .2 | .88 ± .2 | .36 ± .1 | |
| Scream | F | .62 ± .3 | .44 ± .4 | .55 ± .5 | .35 ± .4 | 1.22 ± .7 | .43 ± .4 | 1.88 ± .6 | 1.91 ± .6 | .83 ± .4 | .49 ± .8 |
| M | 1.12 ± .3 | .99 ± .5 | .75 ± .5 | .38 ± .4 | .44 ± .7 | .14 ± .3 | .53 ± .5 | 1.16 ± .5 | 1.01 ± .4 | 1.51 ± .7 | |
| Touch | F | 3.45 ± .9 | 4.73 ± 1.3 | 2.08 ± .6 | .87 ± .3 | .61 ± .2 | 3.41 ± 1.1 | 3.21 ± 1.6 | 1.32 ± .7 | .52 ± .3 | .69 ± .3 |
| M | 3.75 ± .9 | 3.02 ± 1.3 | 1.43 ± .6 | .95 ± .3 | .41 ± .3 | 4.27 ± 1.0 | 3.26 ± 1.4 | .92 ± .7 | .91 ± .3 | .82 ± .3 | |
Mean and SEM rate of behaviors (min/hr) for each month from age 2 through 6 months old. Overall means for control animals (Neo-C) and neonatal amygdala-lesioned animals (Neo-A), separated by sex (females = F and males = M).
Indicates the frequency of behavior, whereas
indicates the duration of the same behavior.
As infants assert their independence, they venture further away (more than 3 m) from the mother (Hinde et al., 1964; Hinde & Spencer-Booth, 1967; Hansen, 1966). As illustrated in Figure 1c, a significant Group × Sex × Age interaction (F[4,132] = 2.6, p = .04, η2 =.07), indicated that, whereas control animals of both sexes spent the same amount of time away from the mother, Neo-A females spent more time away from their mother than did Neo-A males at 5 and 6 months of age (F[1,15] =9.3, p =.009, η2 =.40; F[1,15] = 3.7, p =.07, η2 = .21, respectively). Neo-A females also spent more time away from their mother than Neo-C females at 4 and 5 months of age (F[1,17] = 4.7, p =.048, η2 = .24; F[1,17] =5.2, p =.037, η2 = .26, respectively) and Neo-A males spent less time away from their mother at 5 months of age compared to Neo-C males (F[1,20] =4.6, p =.045, η2 =.21).
Mother–Infant Interactions
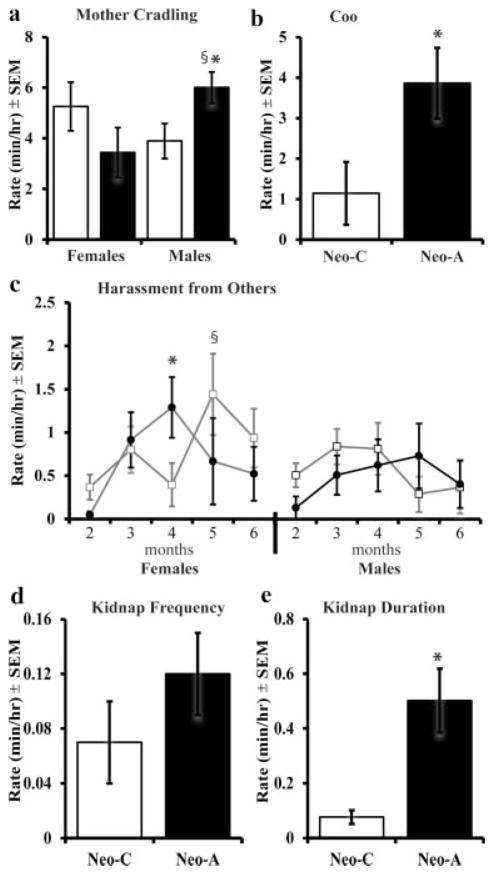
During the first 2 months, the mother spends substantial time cradling and carrying their infants, which decreases as the infants become more independent (Hansen, 1966). Thus, for both groups, the time mothers cradled their infants decreased with age (F[4,132] = 10.9, p <.001, η2 = .25; data not shown). There was a Group × Age interaction for duration of cradles (F[4,132] =2.9, p = .024, η2 =.10) indicating that, at 2 months of age, mothers of Neo-A infants spent less time cradling their infants than did the mothers of Neo-C infants (see Tab. 1). Also, the significant Group × Sex interaction (F[1,33] = 7.9, p =.008, η2 =.20; see Fig. 2a) showed that mothers of Neo-A males cradled them for longer periods than did mothers of Neo-A females or Neo-C males (F [1,16] = 7.7, p = .015, η2 = .36; F[1,20] =5.7, p = .028, η2 =.24, respectively).
FIGURE 2.
Infancy, pre-weaning: Average Rate (min/hr) of cradling from the mother (a), coo vocalizations (b), harassment (c), and frequency (d) and duration (e) of kidnapping from other animals. All other abbreviations are the same as in Figure 1.
Similarly, the amount of time that mothers spent carrying their infants decreased with age (F [4,132] = 57.1, p <.001, η2 =.63), and was also related to both lesion status and age (Group × Age: F [4,132] = 5.03, p = .015, η2 = .13; see Tab. 2). However, the Group × Age interaction indicated that mothers of Neo-A infants carried their infants for longer periods of time than did Neo-C mothers, but only at 2 months of age (see Tab. 1). Neither the sex effect nor its interaction with the other two factors was significant. Finally, there were no Group, Sex, or Age differences in the amount of time that mothers groomed their infants (see Tab. 2).
Rhesus monkey mothers often physically restrain or follow their infants to keep them within maternal protective distances (Hansen, 1966). Mothers restrained and followed their infants more when they were young (Age: F[4,132] = 19.45, p <.001, η2 = .37; F[4,132] = 6.48, p <.001, η2 =.16, respectively) with a steep decline as the infants were becoming independent (see Tabs. 1 and 2). Although mothers of both Neo-C and Neo-A infants exhibited a decline in the amount of time they followed their infants, the frequency of following infants was influenced by age and sex (Age × Sex: F[4,132] = 3.67, p = .007, η2 =.10; see Tab. 2). Mothers initiated follows more frequently when the infant was a 2-month-old male as compared to female infant (see Tab. 1). Infants also contribute to maintaining the maternal protective distances by following their mother, thus both Neo-C and Neo-A infants begin to spend more time following and initiate more frequent following of their mother at an early age (Age: F[4,132] = 6.65, p <.001, η2 = .17; F[4,132] = 6.08, p <.001, η2 = .16, respectively; Tab. 2). The frequency and time spent following the mother increased at 3 months of age and remained high through 6 months of age (see Tab. 1).
Infants use coo or scream vocalizations to communicate location or distress to their mother, often resulting in mothers retrieving their infants (Kalin, Shelton, & Takahashi, 1991; Tomaszycki, Davis, Gouzoules, & Wallen, 2001). One Neo-C male was removed from statistical analyses as an outlier for emitted coo and scream vocalizations two standard deviations above the mean. Neo-A infants emitted more coo vocalizations than did controls (Group: F[1,32] = 5.45, p = .026, η2 = .15; Fig. 2b). There were no Group, Sex, or Age differences in the number of scream vocalizations emitted by infants. Interestingly, the group difference in coo vocalizations was unrelated to the frequency with which mothers retrieved their infants (Group: F[1,33] = .23, p =.64, η2 = .007), but retrievals decreased with age (Age: F[4,132] = 20.62, p <.001, η2 = .39; Tabs. 1 and 2). There were no sex differences and no significant interactions between the three factors.
Infant Interactions With Others
Other females in the social group are attracted to young infants and will touch, harass, and kidnap them (Herman et al., 2003). Both groups received frequent touches, which varied with age (F[4,132] = 18.94, p <.001, η2 = .37), with more touches at 2 and 3 months old, followed by a significant decline at 4 and 5 months old (see Tabs. 1 and 2). Figure 2c illustrates a Group × Sex × Age interaction for the amount of harassment that infants received from other group members (F[4,132] = 2.6, p =.04, η2 = .10). Neo-A females were harassed more than were Neo-C females at 4 months of age (Group: F [1,17] = 3.4, p =.05, η2 = .19), whereas Neo-C females received more harassment than did Neo-C males at 5 months of age (Sex: F[1,21] =5.87, p = .026, η2 =.24). Males did not differ by group in the amount of harassment they received from other animals at any age.
The frequency with which infants were kidnapped by other adult and juvenile females depended on the age of the infants (Age: F[4,132] =5.31, p =.007, η2 =.14) but not on their group or sex (Fig. 2d). Therefore, the frequency of kidnaps declined progressively with age in both groups (see Tab. 1). Yet, overall Neo-A infants were kidnapped for longer periods of time as compared to control infants (Group: F [1,33] = 7.47, p = .01, η2 = .19; Fig. 2e).
Discriminant Analysis
A discriminant function analysis (DFA) was performed for each sex to test whether interactions with the mother could accurately classify individual animals into those with an intact amygdala and those with lesions. For the females, a significant overall Wilks’ Lambda (Λ= .398, χ2 [4,N =17] = 11.98, p = .018) indicated that females could be discriminated according to their lesion group based on three behaviors, accounting for 78% of the total variance. The DFA correctly classified 94.1% (16/17) of the females, which differed significantly from chance (Press’s Q = 13.24, df =1, p <.001; see Tab. 3). Specifically, behaviors that best predicted group classification for the females were coo vocalizations (r = .64), time away from mother (r =.46), and time in contact with the mother (r = .38), whereas grooming received from the mother (r =.09) did not. In contrast, the DFA for male infants was not significant (Λ=.67, χ2 [4,N =19] = 6.08, p =.19).
Table 3.
Discriminant Function Analysis: Females During Infancy
| Predicted Classification
|
||
|---|---|---|
| Neo-C | Neo-A | |
| Actual classification | ||
| Neo-C | 10 (10) | 0 (0) |
| Neo-A | 1 (0) | 6 (7) |
Correct classification of 94.1% of original grouped cases based on coo vocalizations, time away from the mother, time in contact with the mother, and time mother spent grooming the infant.
Infancy-Juvenile Transition Period: 7–12 Months
Distance to Mother
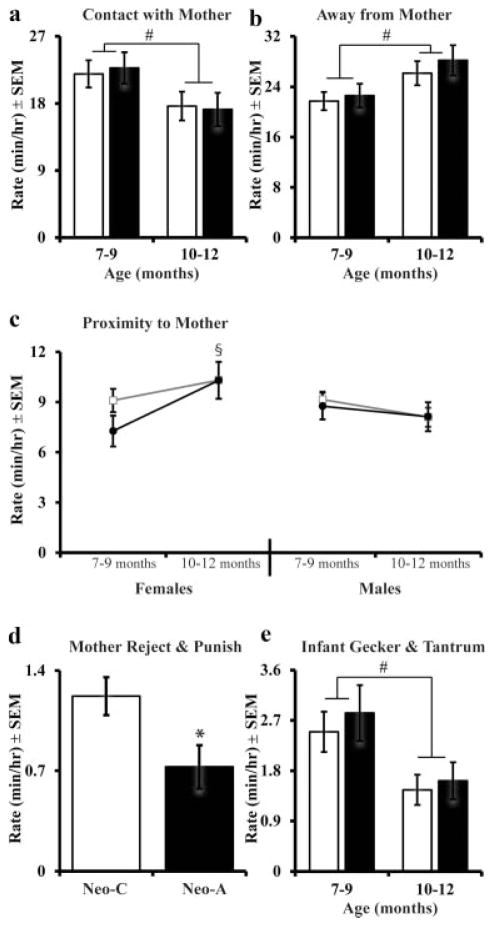
During weaning, mothers begin to deny the young juvenile full body contact and access to nursing, thus the amount of time spent in contact with the mother declined significantly from 7–9 months to 10–12 months (Age: F[1,33] = 7.07, p =.012, η2 =.18; Fig. 3a). The decreased time in contact with mother was paralleled by an increase in the amount of time spent away from the mother (Age: F[1,33] =7.96, p =.008, η2 =.19; Fig. 3b). There were neither Group nor Sex effects nor interactions for time in contact, away, or time within 3 m of the mother (see Tab. 4). Lastly, all females spent more time in proximity to their mother at 10–12 months old as compared to males (Age × Sex: F[1,33] =4.27, p =.047, η2 =.12; Fig. 3c). However, neonatal amygdalectomy did not affect any of these measures.
FIGURE 3.
Infancy-juvenile transition, during weaning: Average Rate (min/hr) of contact with the mother (a), time away from the mother (b), proximity to the mother (c), mothers’ rejecting and punishing (d), and juveniles’ geckering and tantruming (e). #Indicates a significant effect (p <.05) of age. All other abbreviations are the same as in Figure 1.
Table 4.
Behaviors During Infancy-Juvenile Transition (7–12 Months)
| Neo-C
|
Neo-A
|
||||
|---|---|---|---|---|---|
| Behavior | Sex | 7–9 | 10–12 | 7–9 | 10–12 |
| 3 m | F | 6.91 ± .7 | 7.19 ± .8 | 5.66 ± .8 | 5.55 ±1.0 |
| M | 6.83 ± .7 | 6.20 ± .8 | 7.46 ± .8 | 6.81 ±.9 | |
| Groom | F | .68 ± .4 | 1.35 ± .4 | 1.16 ± .5 | 1.41 ±.5 |
| M | 1.00 ± .4 | .85 ± .4 | 1.86 ± .4 | 1.26 ±.4 | |
| Retrieve | F | .02 ± .02 | .00 ± .0 | .12 ± .1 | .00 ±.0 |
| M | .11 ± .07 | .00 ± .0 | .28 ± .08 | .00 ±.0 | |
| Coo | F | 2.23 ± 2.0 | .43 ± .3 | 7.25 ± 4.7 | .53 ±.4 |
| M | 10.9 ± 3.9 | .59 ± .4 | 1.98 ± 1.8 | 1.20 ±.4 | |
| Scream | F | .54 ± .4 | .68 ± .2 | 1.56 ± 1.1 | .46 ±.2 |
| M | 3.21 ± .9 | .38 ± .2 | .91 ± .9 | .61 ±.2 | |
| Infant followf | F | 5.13 ± .7 | 5.18 ± .8 | 5.02 ± .9 | 3.21 ±.9 |
| M | 6.26 ± .7 | 6.04 ± .8 | 4.24 ± .8 | 3.91 ±.8 | |
| Infant followd | F | .59 ± .1 | .71 ± .1 | .51 ± .1 | .48 ±.2 |
| M | .83 ± .1 | .83 ± .1 | .48 ± .1 | .63 ±.1 | |
| Touch | F | .20 ± .07 | .17 ± .1 | .21 ± .08 | .46 ±.1 |
| M | .29 ± .07 | .14 ± .1 | .24 ± .07 | .25 ±.1 | |
| Harass | F | .51 ± .2 | .43 ± .2 | .25 ± .2 | .58 ±.2 |
| M | .36 ± .2 | .20 ± .2 | .27 ± .2 | .35 ±.2 | |
Mean and SEM rate of behaviors (min/hr) for each month from 7 through 12 months of age. All abbreviations are the same as in Table 2.
Mother–Juvenile Interactions
Mothers of Neo-A juveniles exhibited significantly less rejection and punishment as compared to mothers of control animals (Group: F[1,33] = 6.02, p =.02, η2 = .15; see Fig. 3d). When rejected by their mothers, juveniles of both groups expressed similar amounts of geckers and tantrums and did not differ by group or sex (Group: F [1,33] =.24, p =.63, η2 =.007; Sex: F[1,33] =.001, p =.97, η2 =.001). Therefore, all juveniles exhibited more geckers and tantrums at 7–9 months during the weaning period, than at 10–12 months during the post-weaning period (Age: F[1,33] =9.61, p =.004, η2 = .23; Fig. 3e). Table 4 shows that juveniles emitted more coo and scream vocalizations at the beginning of weaning (Age: F[1,32] =5.35, p =.027, η2 =.14; F [1,32] =4.17, p =.05, η2 =.12, respectively), and again the group and sex factors and interactions were not significant. In addition, Neo-C juveniles spent more time following their mother and initiated following more frequently as compared to Neo-A juveniles (Group: F[1,33] = 5.46, p = .026, η2 = .14; F[1,33] = 5.61, p = .024, η2 =.15, respectively; see Tab. 4), but there was no effect of Age or Sex.
During this period, there was a significant decrease in the frequency that mothers retrieved their juveniles (Age: F[1,33] = 9.14, p =.005, η2 = .22; see Tab. 4) with no Group or Sex differences. Additionally, mothers groomed and carried their juveniles at comparable levels across the weaning stages with no differences between Groups, Sex, or Age and no interactions between factors.
Juvenile Interactions With Others
As juveniles became more independent, they are less attractive to other adult and juvenile females, such that the levels of touching, harassment, and kidnapping they received were consistently low with no differences between Groups, Sex, Age, or interactions (see Tab. 4).
Discriminant Function Analyses
Unlike during infancy, in the infancy-juvenile transition period, the overall Wilks’ Lambda for the females was not significant (Λ = .75, χ2 [4,N = 17] =3.73, p = .44), indicating that females could not be discriminated by lesion status. The addition of weaning behaviors to the DFA did not better classify the females (Λ= .68, χ2 [6,N =17] = 4.72, p = .58). Similar to infancy, males still could not be classified based on these behaviors (Λ= .87, χ2 [4,N = 19] =2.09, p = .72), even with the addition of the weaning behaviors to the analyses (Λ= .66, χ2 [6,N = 19] =5.89, p = .44).
DISCUSSION
The present study demonstrates that early insult to the amygdala subtly, but significantly, alters mother–infant interactions of rhesus macaques living in large species-typical social groups. The loss of the amygdala early in infancy altered mother–infant interactions in a sex dependent manner, such that it sex-reversed the typical sexually differentiated pattern of the development of infant independence. These changes in infant independence also likely affected behavior with other animals. For example Neo-A infants were kidnapped for longer periods of time, which could be explained by either reduced fear or lack of motivation to return to the mother after early amygdala damage. Nevertheless, only females, not males, could be accurately classified by lesion status based on their behavior with their mother during early infancy. These subtle changes were transient and no longer evident during the infancy-juvenile transition period.
At approximately 5–6 months, rhesus infants become progressively more independent from their mother. Studies have shown that the timing of infant independence differs between sexes with males exhibiting earlier independence than females (Hinde & Spencer-Booth, 1967; Jensen et al., 1966, 1968; Rosenblum, 1974). Although this sex difference was not replicated in the control animals of the current study, neonatal amygdalectomy impacted the emergence of infant independence from the mother in a sex-dependent manner. Thus, Neo-A females exhibited an earlier onset of independence starting at 4 months of age as compared to control animals and Neo-A males. The precocious independence coincides with increased harassment they received from other individuals of the group at the same age. The increased harassment likely reflects maternal independence as control females received more harassment when they started spending more time off their mother at 5 months of age. Conversely, Neo-A males were harassed less and exhibited more dependence on their mother (i.e., received more cradling from the mother and spent less time away from the mother) when 5 months old as compared to control males. Although the protracted emergence of independence in the Neo-A males could have resulted from the increased cradling received from their mothers, it is as likely that the tendency of Neo-A males to not assert independence from their mother offered the mother’s more opportunity to cradle them. It appears that the Neo-A males were willing to spend more time with their mothers as there were no group differences in how often the mothers restrained or retrieved their infants. Thus, the precocious independence in Neo-A females and delayed independence in Neo-A males seems driven primarily by sex specific alterations in infant behavioral responses rather than by changes in the mother’s behavior. The increased harassment that both control and Neo-A females received in comparison to the males parallels previous findings in normally developing monkeys (Frodi & Lamb, 1978; Herman et al., 2003; Lovejoy & Wallen, 1988).
The sex specific difference in the emergence of independence in Neo-A infants differs from results in earlier studies where both males and females with neonatal amygdalectomy spent more time on the mothers ventrum when in the presence of other animals (Bauman et al., 2004a). However, in this previous study the Neo-A lesioned infants were just exposed to other animals for 15 hr/week as compared to 168 hr/week in the present study. Therefore the different outcomes between the two studies may relate to the differences in the complexity of the social environment in which the infants were reared. Other studies have shown that sex differences in the emergence of infant independence are more or less pronounced, or not expressed at all, as a function of the specific social environment (Rosenblum & Andrews, 1994; Wallen, 1996).
Neonatal amygdala lesions also altered distress vocalizations, such that both Neo-A females and males emitted more coo vocalizations in infancy as compared to controls. This increased cooing in an unconstrained social context is consistent with similar increased cooing seen in the same animals (Raper et al., 2013b), as well as adult animals with amygdala lesions (Kalin et al., 2004) when tested in the more constrained Human Intruder paradigm. However, this increase in vocalizations did not seem associated with separation from the mother, given that both Neo-A males and females emit increased coos, even though Neo-A males spent more time near their mothers. The alterations in emotional vocalizations may reflect the influence of the amygdala on the periaqueductal gray matter (Jürgens & Pratt, 1979; Jürgens, 1994), which represents a crucial relay station of all descending vocalization-controlling limbic forebrain structures (amygdala, anterior cingu-late cortex, nucleus accumbens, and preoptic area).
Overall, these data indicate that in the first 6 months of life neonatal amygdala lesions affect mother–infant relations in the female infants more so than male infants; a finding that was confirmed by the discriminant function analyses. These analyses showed that an animal’s expression of coo vocalizations, time in contact, and time away from the mother in early infancy could accurately classify 94.1% of female infants, but not the males. Yet, caution is warranted due to our relatively small sample size (n =17), although this analysis complements the results reported above and provides a valuable tool for identifying the role of the amygdala in behavioral development. Indeed, just as Neo-A females could statistically be discriminated from females with an intact amygdala, it seems likely that other members of their social group could detect behavioral changes in animals with amygdala lesions using a combination of behaviors, rather than changes in any single behavior.
The current study replicated previous reports that during the infancy-juvenile transition period, juvenile animals exhibited less contact with the mother, spent more time away from the mother, and emitted more vocalizations (Hinde & Spencer-Booth, 1967). These juvenile behavioral changes were not altered by amygdalectomy and this lack of noticeable effects of amygdala lesions was confirmed by the nonsignificant discriminant function analyses.
Although the impact of neonatal amygdala lesions was not apparent in the juveniles, the mothers’ expression of weaning behaviors differed with lesion status. Thus, mothers of Neo-A infants displayed less weaning behavior as compared to mothers of control infants. This decrease in weaning behaviors may have been influenced, at least among Neo-A females, by the earlier emergence of infant independence, which could have in turn reduced maternal opportunity or need to wean their infants. Additionally, despite receiving less weaning behavior from their mothers, Neo-A juveniles of both sexes produced similar amounts of geckers and tantrums as did control juveniles, with a decline at 10–12 months old. This dichotomy between the weaning behavior received from the mother and the juveniles’ production of geckers and tantrums suggests that the increased vocalizations by Neo-A animals are not necessarily triggered by the mother’s weaning behavior. Although it is difficult to identify the cause of the increased vocalizations, one possibility may be that neonatal amygdalectomy increased the emotional reactivity of the infants, especially when exposed to the social demands of constant daily contact with a large number of other monkeys after weaning. There is indeed some support for this proposal since earlier reports have demonstrated an increase in anxious behaviors after neonatal amygdala lesions, specifically when the animals interacted with the other peers or members of a small social group (Bauman et al., 2004b; Prather et al., 2001). However, this explanation seems contradicted by evidence that at approximately the same age these same Neo-A animals exhibited less anxious behaviors compared to controls when faced with a threatening human intruder (Raper et al., 2013b) and showed less emotional reactivity than controls during a mother-preference task (Goursaud, Wallen, & Bachevalier, this issue). Although a study in adult monkeys with amygdala lesions also exhibited a similar dichotomy of increased anxiety with peers and reduced fearfulness when tested alone (Rosvold, Mirsky, & Pribram, 1954). Thus, it is possible that the species-typical social environment in which these animals live is so socially intense that it revealed a tendency toward hyper-emotionality that was not evident in more socially constrained environments. If true this would suggest that neonatal amygdalectomy results in an emotional vulnerability that is only evident when social conditions require full use of social coping mechanisms.
Considering the amygdala’s diverse contributions to social cognition, it is presently difficult to propose any single cognitive process that has been altered by the neonatal amygdala lesions. Nevertheless, there are several amygdala functions that likely interact for the maintenance of normal mother–infant interactions and the development of emotional regulation in the infants and that could have been affected by the early lesions. First, given the critical role of the amygdala in the regulation of fear and anxious behaviors, one possibility is that neonatal lesions alter the detection of potential danger when animals navigate in a complex social group. Another possibility is that neonatal amygdala lesions disrupted social motivation. The amygdala guides gaze and attention toward socially salient information (Adolphs, 2010), thus neonatal amygdala damage could have altered the early developing ability to detect social signals provided by the mother and resulted in impaired filial discrimination and attachment. If correct this proposal suggests that our findings imply that amygdala-lesioned females were less attached to their mother, whereas the amygdala-lesioned males exhibited an increased maternal attachment. Yet, controls and Neo-A animals of both sexes exhibited a significant preference for their mother over a familiar female (Goursaud et al., this issue), although Neo-A infants spent less time in physical contact with their mother and reached for their mother less often than did controls. Therefore Neo-A infants can discriminate their mother from others and even form an attachment. However, the decreased physical contact and attempts to reach for the mother by Neo-A animals indicates that they did not use their mothers as a social buffer during this potentially stressful situation (Wiener, Johnson, & Levine, 1987; Winslow et al., 2003). Possibly the Neo-A infants did not find the maternal preference situation as stressful as did the controls. Alternatively, Neo-A infants may not have found physical contact with the mother as rewarding as did controls. Support for this latter idea comes from the amygdala’s role in reward processing such that damage reduced the rewarding properties of stimuli (Kazama & Bachevalier, 2013; Murray, 2007).
Lastly, the transient aspect of the changes in mother–infant interactions is intriguing and may be explained in several ways. By the time our subjects reached the infancy-juvenile transition period, the control animals exhibited independence from mother similar to that of the Neo-A juveniles, whereas Neo-A juveniles could not become any more independent thus eliminating the group difference. Alternatively, the rich and complex rearing environment allowed the Neo-A juveniles to adapt and compensate for the lack of a functioning amygdala, thereby presenting fewer differences from control animals. It is also possible that group or family members could alter their behavior towards the Neo-A animals and thus socially compensate for the missing amygdala functionality. Indeed, the subtle behavioral changes described here differ greatly from the reported impact of neonatal amygdalectomy in previous studies carried out in a more restricted social environments, reducing the possibilities that others in the social environment could compensate for social deficits in the lesioned animals (Bachevalier, 1994; Bauman et al., 2004b; Kling & Green, 1967; Prather et al., 2001; Thompson, 1981). Evidence from the rodent literature has already demonstrated the significant and positive impact of enriched environments have on cognitive functioning in normal, neonatal and adult ischemic, and prenatally stressed animals (Pereira et al., 2007; Sozda et al., 2010; Qian et al., 2008). Therefore, it is possible that the species-typical environment of a large multigenerational age-graded social group provides enhanced stimulation that compensates for the effects of early amygdala damage on social cognition. Yet, it remains a possibility that as the animals reach puberty and adulthood, a period during which they must discriminate and interpret more challenging and ambiguous social signals from other group members, without maternal support, the impact of early amygdala damage may become more profound and increasingly apparent and more consistent with previously reported results (Bauman et al., 2004b, 2006, 2008; Thompson, 1981).
Supplementary Material
Acknowledgments
Authors are grateful to Amy Henry and Trina Villarreal for their invaluable assistance with mother–infant reunions, reintroductions to the social groups, and data collection. Special thanks to all members of the Bachevalier Laboratory who helped with the neuroimaging and surgical procedures on the infant monkeys. We also thank Rebecca Herman, Ph.D., for her assistance in programming and data extraction. This research was supported by the National Institute for Mental Health (MH050268). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIMH, or the National Institutes of Health. The studies were also supported in part by the Center for Behavioral Neuroscience (NSF IBN 9876754), and Integrated Training in Psychobiology and Psychopathology Fellowship (NIMH T32 MH732525), as well as by the National Center for Research Resources to the Yerkes National Research Center (P51 RR00165; YNRC Base grant), which is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132. The YNPRC is fully accredited by the American for the Assessment and Accreditation of Laboratory Care, International.
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D. Amygdala damage impairs emotion recognition from scenes only when they contain facial expressions. Neuropsychologia. 2003;41:1281–1289. doi: 10.1016/s0028-3932(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, editor. The amygdala. A functional analysis. 2. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Bachevalier J. Medial temporal lobe structures and autism: A review of clinical and experimental findings. Neuropsychologia. 1994;32:627–648. doi: 10.1016/0028-3932(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Bachevalier J. The amygdala, social cognition, and autism. In: Aggleton JP, editor. The amygdala: A functional analysis. New York, NY: Oxford University Press; 2000. pp. 509–544. [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother–infant interactions after neonatal amygdala lesions in rhesus monkeys. Journal of Neuroscience. 2004a;24(3):711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. Journal of Cognitive Neuroscience. 2004b;16(8):1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Babineau BA, Mason WA, Amaral DG. Emergence of stereotypies in juvenile monkeys (Macaca mulatta) with neonatal amygdala or hippocampus lesions. Behavioral Neuroscience. 2008;122:1005–1015. doi: 10.1037/a0012600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Mason WA, Lavenex P, Amaral DG. The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2006;120:749–760. doi: 10.1037/0735-7044.120.4.749. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Developmental Psychobiology. 2010;52:487–503. doi: 10.1002/dev.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodi AM, Lamb ME. Sex-differences in responsiveness to infants—Developmental study of psychophysiological and behavioral responses. Child Development. 1978;49:1182–1188. [PubMed] [Google Scholar]
- Goursaud APS, Bachevalier J. Social attachment in juvenile monkeys with neonatal lesion of the hippocampus, amygdala, and orbital frontal cortex. Behavior Brain Research. 2007;176:75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Goursaud A-PS, Wallen K, Bachevalier J. Neonatal amygdala lesion and the development of filia attachment in rhesus macaques (Macaca mulatta) raised in a species-typical environment. Developmental Psychobiology. 2014 doi: 10.1002/dev.21233. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair JF, Black WC, Babin BJ, Anderson RE. Multivariate data analysis. 7. New Jersey: Prentice Hall; 2009. p. 263. [Google Scholar]
- Hansen EW. The development of maternal and infant behavior in the rhesus monkey. Behaviour. 1966;27:107–149. doi: 10.1163/156853966x00128. [DOI] [PubMed] [Google Scholar]
- Herman RA, Measday MA, Wallen K. Sex differences in interest in infants in juvenile rhesus monkeys: Relationship to prenatal androgen. Hormones and Behavior. 2003;43:573–583. doi: 10.1016/s0018-506x(03)00067-9. [DOI] [PubMed] [Google Scholar]
- Hinde RA, Rowell TE, Spencer-Booth Y. Behavior of socially living rhesus monkey in their first six months. Proceedings of the Zoological Society London. 1964;143:609–649. [Google Scholar]
- Hinde RA, Spencer-Booth Y. The behaviour of socially living rhesus monkeys in their first two and a half years. Animal Behaviour. 1967;15:169–196. doi: 10.1016/s0003-3472(67)80029-0. [DOI] [PubMed] [Google Scholar]
- Jensen GD, Bobbitt RA, Gordon BN. Sex differences in social interaction between infant monkeys and their mothers. Recent Advances in Biological Psychiatry. 1966;9:283–293. doi: 10.1007/978-1-4684-8228-7_21. [DOI] [PubMed] [Google Scholar]
- Jensen GD, Bobbitt RA, Gordon BN. Sex difference in the development of independence of infant monkeys. Behaviour. 1968;30:1–14. doi: 10.1163/156853968x00144. [DOI] [PubMed] [Google Scholar]
- Jürgens U. The role of the periaqueductal grey in vocal behavior. Experimental Brain Research. 1994;62:107–117. doi: 10.1016/0166-4328(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Jürgens U, Pratt R. Role of the periaqueductal grey in vocal expression of emotion. Brain Research. 1979;167:367–378. doi: 10.1016/0006-8993(79)90830-8. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: Ontogeny and context-dependent selective expression. Child Development. 1991;62:1175–1183. [PubMed] [Google Scholar]
- Kazama AM, Bachevalier J. Effects of selective neonatal amygdala damage on concurrent discrimination learning and reinforcer devaluation in monkeys. Journal of Psychology and Psychotherapy. 2013;S7:1–9. doi: 10.4172/2161-0487.S7-005. doi:org/10.4172/2161-048.S7-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling A, Green PC. Effects of neonatal amygdalaectomy in maternally reared and maternally deprived macaque. Nature. 1967;213:742–743. doi: 10.1038/213742b0. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Developmental Neuroscience. 2012;34:101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. The amygdala. Current Biology. 2007;17(20):R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lovejoy J, Wallen K. Sexually dimorphic behavior in grouphoused rhesus monkeys (Macaca mulatta) at 1 year of age. Psychobiology. 1988;16:348–356. [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Newman JD, Bachevalier J. Neonatal ablations of the amygdala and inferior temporal cortex alter the vocal response to social separation in rhesus macaques. Brain Research. 1997;758:180–186. doi: 10.1016/s0006-8993(97)00212-6. [DOI] [PubMed] [Google Scholar]
- Pereira LO, Arteni NS, Petersen RC, da Rocha AP, Achaval M, Netto CA. Effects of daily environmental enrichment on memory deficits and brain injury following neonatal hypoxia-ischemia in the rat. Neurobiology of Learning and Memory. 2007;87:101–108. doi: 10.1016/j.nlm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Qian J, Zhou D, Pan F, Liu CX, Wang YW. Effect of environmental enrichment on fearful behavior and gastrin-releasing peptide receptor expression in the amygdala of prenatal stressed rats. Journal of Neuroscience Research. 2008;86:3011–3017. doi: 10.1002/jnr.21736. [DOI] [PubMed] [Google Scholar]
- Raper J, Bachevalier J, Wallen K, Sanchez M. Neonatal amygdala lesions alter basal cortisol levels in infant rhesus monkeys. Psychoneuroendocrinology. 2013a;38:818–829. doi: 10.1016/j.psyneuen.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wallen K, Sanchez MM, Stephens SB, Henry A, Villareal T, Bachevalier J. Sex-dependent role of the amygdala in the development of emotional and neuroendocrine reactivity to threatening stimuli in infant and juvenile rhesus monkeys. Hormones and Behavior. 2013b;63:646–658. doi: 10.1016/j.yhbeh.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum LA. Sex differences, environmental complexity, and mother–infant relations. Archives of Sexual Behavior. 1974;3:117–128. doi: 10.1007/BF01540995. [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Andrews MW. Influences of environmental demand on maternal behavior and infant development. Acta Paediatrica Supplement. 1994;397:57–63. doi: 10.1111/j.1651-2227.1994.tb13266.x. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. Journal of Comparative and Physiological Psychology. 1954;47:173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- Sackett GP. Monkeys reared in isolation with pictures as visual input: Evidence for an innate releasing mechanism. Science. 1966;154:1468–1472. doi: 10.1126/science.154.3755.1468. [DOI] [PubMed] [Google Scholar]
- Sozda CN, Hoffman AN, Olsen AS, Cheng JP, Zafonte RD, Kline AE. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. Journal of Neurotrauma. 2010;27:1047–1057. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ, Harlow HF. The facts and functions of fear. In: Zuckerman M, Speilberger CD, editors. Emotions and anxiety: New concepts, methods, and applications. Hillsdale, NJ: Erlbaum; 1976. pp. 3–34. [Google Scholar]
- Thompson CI. Learning in rhesus monkeys after amygdalectomy in infancy or adulthood. Behavioral Brain Research. 1981;2:81–101. doi: 10.1016/0166-4328(81)90039-5. [DOI] [PubMed] [Google Scholar]
- Tomaszycki ML, Davis JE, Gouzoules H, Wallen K. Sex differences in infant rhesus macaque separation-rejection vocalizations and effects of prenatal androgens. Hormones and Behavior. 2001;39:267–276. doi: 10.1006/hbeh.2001.1659. [DOI] [PubMed] [Google Scholar]
- Wallen K. Nature needs nurture: The interaction of hormonal and social influences on the development of behavioral sex differences in rhesus monkeys. Hormones and Behavior. 1996;30:364–378. doi: 10.1006/hbeh.1996.0042. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Phelps EA. The human amygdala. New York, NY: The Guilford Press; 2009. [Google Scholar]
- Wiener SG, Johnson DF, Levine S. Influence of postnatal rearing conditions on the response of squirrel monkey infants to brief perturbations in mother–infant relationships. Physiology and Behavior. 1987;39:21–26. doi: 10.1016/0031-9384(87)90339-8. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.