Abstract
The accumulation of ubiquitin-positive protein aggregates has been implicated in the pathogenesis of neurodegenerative diseases, heart disease and diabetes. Emerging evidence indicates that the autophagy lysosomal pathway plays a critical role in the clearance of ubiquitin aggregates, a process that is mediated by the ubiquitin binding protein p62. In addition to binding ubiquitin, p62 also interacts with LC3 and transports ubiquitin conjugates to autophagosomes for degradation. The exact regulatory mechanism of this process is still largely unknown. Here we report the identification of Keap1 as a binding partner for p62 and LC3. Keap1 inhibits Nrf2 by sequestering it in the cytosol and preventing its translocation to the nucleus and activation of genes involved in the oxidative stress response. In this study, we found that Keap1 interacts with p62 and LC3 in a stress-inducible manner, and that Keap1 colocalizes with LC3 and p62 in puromycin-induced ubiquitin aggregates. Moreover, p62 serves as a bridge between Keap1 and ubiquitin aggregates and autophagosomes. Finally, genetic ablation of Keap1 leads to the accumulation of ubiquitin aggregates, increased cytotoxicity of misfolded protein aggregates, and defective activation of autophagy. Therefore, this study assigns a novel positive role of Keap1 in upregulating p62-mediated autophagic clearance of ubiquitin aggregates.
Keywords: Keap1, p62, LC3, autophagy, ubiquitin, Nrf2, autophagosome, protein aggregates, oxidative stress
Introduction
Autophagy is essential for survival, differentiation, development and homeostasis of cells through lysosomal degradation. During autophagy, intracellular organelles, protein aggregates, and regions of the cytosol are first sequestered by a double membrane-bound vacuole to form an autophagosome. The autophagosome with its sequestered contents then fuses with a lysosome leading to degradation of its internal components by acid hydrolases.1–4 Without a means of dealing with protein aggregates, autophagy-deficient cells accumulate ubiquitin aggregates.5–7 The failure in the clearance of ubiquitin conjugates by autophagy has been implicated in the pathogenesis of cancer, neurodegeneration, diabetes, aging and heart disease.1–4
The central molecule involved in autophagic clearance of ubiquitin aggregates is p62/SQSM1, a protein possessing dual-binding sites for ubiquitin chains and LC3.8,9 LC3 and its yeast homologue Atg8 are well-characterized autophagosome membrane-linked proteins. LC3/Atg8 conjugates to phosphatidylethanolamine (PE) via a ubiquitin-like reaction and the new LC3-PE conjugate behaves like an integral membrane protein that is highly enriched in the membranes of nascent autophagosomes.10 The enrichment of LC3/Atg8 aids the newly formed autophagosome in cargo docking, which has been proved in yeast in the cytosol-to-vacuole transport (CVT) pathway. In CVT, Atg8 interacts with Atg11, which brings Atg19-associated vacuolar enzyme aminopeptidase I to autophagosomes for packaging.11–14 Recent studies revealed that mitophagy, a specific form of autophagy that mediates the degradation of mitochondria, is also bridged through an interaction between a mitochondrial membrane-associated protein Atg32 and Atg8.15,16
In mammalian cells, p62, as well as NBR1 (next to BRCA1 gene 1 protein) and Alfy (autophagy-linked FYVE protein),17,18 serve as crucial adaptors between LC3-decorated autophagosomes and ubiquitin-conjugated protein aggregates.19,20 p62 binds to LC3 via an LC3-interacting region (LIR) and ubiquitin chains through a UBA (ubiquitin-associated) domain.21 Ubiquitin conjugates accumulate in autophagy knockout mice and lead to cytotoxicity in multiple tissues and cells.5–7 Concomitant deletion of p62 rescued the cytotoxic phenotype of liver cells in autophagy mutant mice.9 Moreover, p62 accumulation is frequently observed in human tumors due to impairment of the autophagy pathway, which leads to the oxidative stress and inflammation responses that correlate with tumorigenesis.22
However, how p62 links autophagy, oxidative stress and ubiquitin aggregates together is still unclear. Here we report that an oxidative stress sensor Kelch-like ECH-associated protein 1 (Keap1) interacts with p62 and LC3. Upon puromycin treatment, the majority of Keap1 localizes to cellular puncta that are also positive for p62, ubiquitin conjugates, and LC3. Furthermore, genetic ablation of Keap1 significantly compromised the clearance of puromycin-induced misfolded protein aggregation. Finally, stress-induced autophagy was defective in Keap1 mutant cells. We therefore propose that Keap1 plays a critical role in protein aggregation clearance through autophagy.
Results
Identification of Keap1 in the LC3 and p62 protein complex
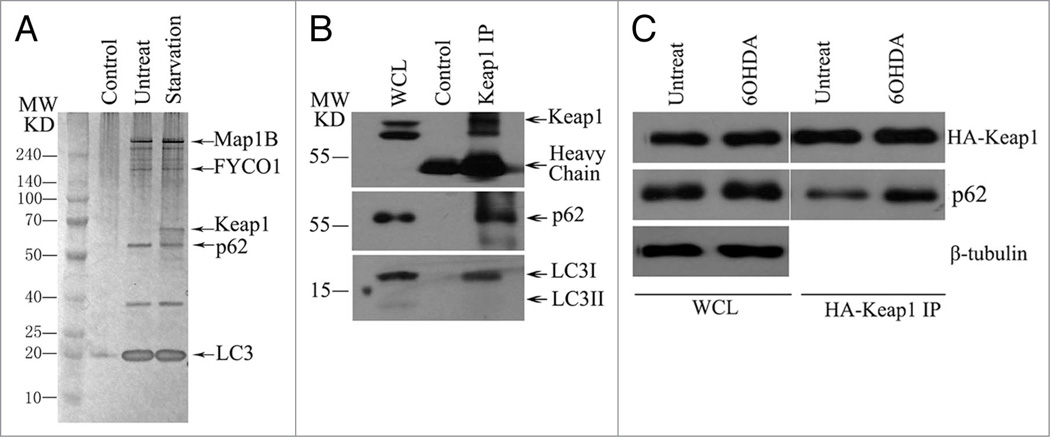
We performed a tandem affinity purification using double-tagged, full-length LC3 (ZZ-FLAG-LC3) as the bait (Fig. 1A) to purify an endogenous complex. We generated an inducible cell line that stably expresses LC3. Expression of LC3 was adjusted by the titration of doxycycline, and a low dose of doxycycline (10 ng/ml) that induces expression of tagged LC3 close to the endogenous level was selected for initiating purification to avoid nonphysiological interactions. We followed a procedure that has been described previously to isolate the LC3 complex from human cells,23,24 and the doxycycline-treated stable cell lines were used to generate cytosolic extracts. IgG-Sepharose affinity chromatography was first applied, and the binding proteins were eluted by TEV protease cleavage, and then TEV-eluted proteins were applied to M2 (anti-FLAG antibody)-agarose beads. The final FLAG peptide eluate was subjected to 4–12% gradient SDS-PAGE, and visualized by silver staining. Specific bands were excised and analyzed by mass spectrometry.
Figure 1.
Keap1 interacts with LC3 and p62 in a stress-inducible manner. (A) Silver staining of LC3 complex after tandem affinity purification, LC3 complex was purified from U2OS cell that stably expresses ZZ-FLAG-LC3 with or without starvation for one hour. Proteins’ ID were identified by mass spectrometry. (B) Keap1 interacts with LC3 and p62 in vivo. HE K293T cells were immunoprecipitated with anti-Keap1 antibody, then immunoblotted with anti-Keap1 or anti-LC3 or anti-p62 antibodies. (C) 6-OHDA induces Keap1-p62 interaction. HA-Keap1 transfected HE K293T cells were treated with 6-OHDA for 6 hours. Cells were then lysed and followed with HA immunoprecipitation. The endogenous p62 pulled down by Keap1 was detected by western blotting. These experiments have been repeated at least three times.
In addition to the known members of a group of LC3 interacting proteins, namely MAP1B,9 FYCO1,25 and p62, we also identified a 60 kD component that associated with LC3 in a starvation-inducible manner (Fig. 1A), and this protein was identified by mass spectrometry as Keap1. The interaction of LC3, p62 and Keap1 was further confirmed by the presence of LC3 and p62 in the eluate of immunoprecipitate pulled down by anti-Keap1 antibody (Fig. 1B). Because Keap1 is a sensor of oxidative stress,26 its activity is tightly regulated by stress,27 and therefore, we hypothesize that the interaction between Keap1 and p62 is also regulated by oxidative stress. In the cells treated with 6-hydroxydopamine (6-OHDA), a neurotoxin-generating active oxygen species, a significantly greater amount of p62 immunoprecipitated with Keap1 compared to that in untreated cells (Fig. 1C), confirming that Keap1 associates with p62 in a stress-inducible manner.
Keap1 colocalizes with p62 and LC3 in ubiquitin-positive protein aggregates
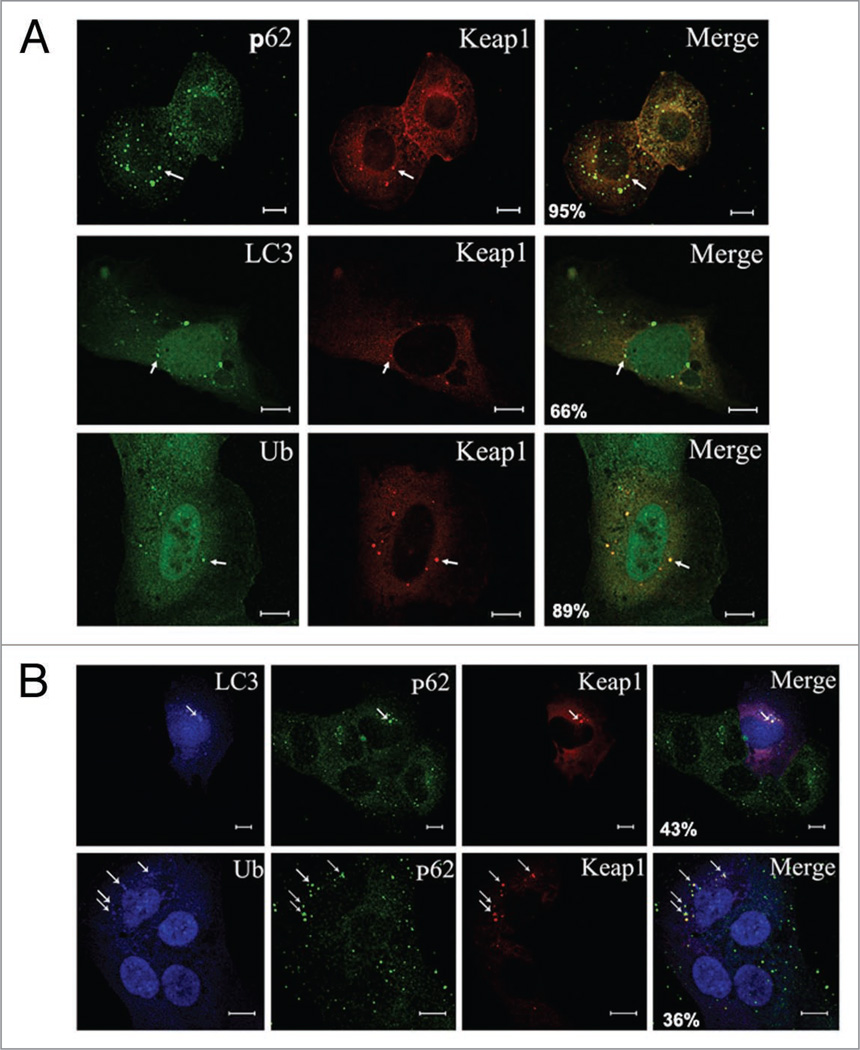
p62 is an adaptor that interacts with LC3 through its LIR and with ubiquitin chains via a UBA domain.21 The interaction is confirmed in vivo by visualizing the colocalization of p62 with LC3 and ubiquitin in the cytosolic puncta.8,9 Given that Keap1 interacts with p62 and LC3, we speculated that Keap1 also colocalizes with LC3 and p62 in the ubiquitin-positive cytosolic puncta. In cells treated with puromycin, an amino acid analog that terminates chain elongation prematurely and therefore accumulates misfolded proteins,7,28–30 Keap1 was shown to be concentrated in the cellular puncta positive for p62, LC3, and ubiquitin conjugates (Fig. 2A), suggesting that Keap1’s possible function may involve the autophagic clearance of ubiquitin protein aggregates.
Figure 2.
Keap1 colocalizes with LC3 and p62 in the ubiquitin aggregates. (A) Keap1 colocalizes with p62, LC3 and ubiquitin in the puromycin-treated cells. U2OS cells were transfected with either DsRED-Keap1 or DsRED-Keap1 together with Myc-LC3. 2 days after transfection, cells were then fixed and stained with antibodies again p62, Myc and FK2 (for poly-ubiquitin chain) respectively. Scale bar 10 µm. Representative puncta are marked by arrows. The percentage of Keap1 puncta that colocalize with p62, LC3 or ubiquitin respectively was shown in the merged images. These data were summarized from at least 200 cells in three different experiments. (B) Coexistence of Keap1, LC3 and p62 in the ubiquitin-positive cellular puncta. U2OS cells were transfected with DsRED-Keap1 and Myc-LC3, followed by treatment with puromycin (10 µg/ml for 4 hours), cells were then fixed and p62 was stained with green fluorescent antibody, while Myc or ubiquitin were stained with blue AMCA conjugated secondary antibody. Scale bar 10 µm. Representative puncta are marked by arrows. The percentage of Keap1 puncta that colocalize with p62 and LC3/ubiquitin was shown in the merged images. These data were summarized from at least 200 cells in three different experiments.
To test if these proteins coexist in the same complex, we labeled Keap1, p62 and LC3 with red, green and blue fluorescence, respectively, in cells treated with puromycin, and we observed that these signals coalesce into the same cellular punctum (Fig. 2B, upper). These cell puncta were also positive for ubiquitin conjugates (Fig. 2B and bottom). These data indicate that the presence of Keap1 overlaps with p62 and LC3 in the ubiquitin-positive protein aggregates.
p62 mediates Keap1 association with ubiquitin aggregates and autophagosomes
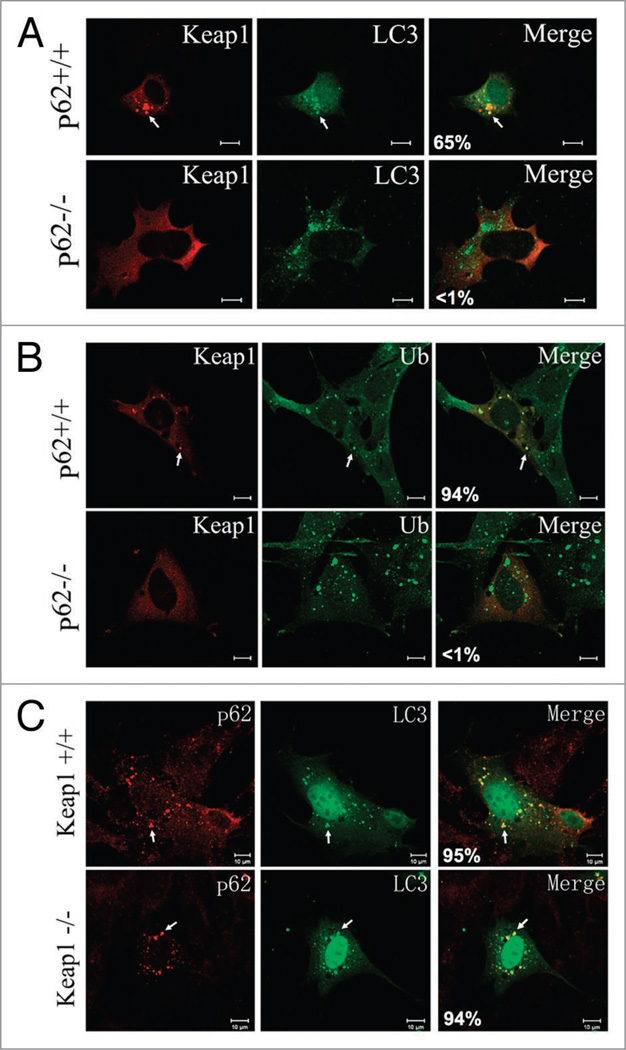
To test whether Keap1 associates with autophagosomes through p62 or independently, we investigated the subcellular localization of Keap1 in p62 knockout mouse embryonic fibroblasts (MEFs). In the absence of p62, Keap1 failed to form cytosolic foci and localize to autophagosomes marked with LC3 (Fig. 3A). In p62 knockout cells, Keap1 also failed to colocalize with ubiquitin aggregates (Fig. 3B). These results reveal that p62 plays an essential role in bridging Keap1’s interaction with ubiquitin aggregates and autophagosomes.
Figure 3.
p62 is required for Keap1 localization to ubiquitin aggregates and autophagosomes. (A) Wild-type or p62 knockout cells were transfected with DsRED-Keap1 together with Myc-LC3, followed by treatment with rapamycin (2 µM for 4 hours), cells were then fixed and Myc-LC3 was stained with a FITC secondary antibody. Scale bar 10 µm. The images are representative of at least three independent experiments. The percentage of Keap1 puncta that colocalize with LC3 was shown in the merged images. These data were summarized from at least 200 cells in three different experiments. (B) Wild-type or p62 knockout cells were transfected with DsRED-Keap1, followed by treatment with puromycin (10 µg/ml for 4 hours), cells were then fixed and ubiquitin was stained with a FITC conjugated secondary antibody. Scale bar 10 µm. The percentage of Keap1 puncta that colocalize with ubiquitin was shown in the merged images. These data were summarized from at least 200 cells in three different experiments. (C) Wild-type or Keap1 knockout cells were transfected with Myc-LC3, followed by treatment with rapamycin (2 µM for 4 hours). p62 and Myc-LC3 were stained with Rhodamine Red and FITC conjugated secondary antibodies. Scale bar 10 µm. The percentage of p62 puncta that colocalize with LC3 was shown in the merged images. These data were summarized from at least 200 cells in three different experiments.
Alternatively, Keap1 might also regulate the interaction between p62 and ubiquitin aggregates and/or LC3. We examined the colocalization of p62, ubiquitin and LC3 in the Keap1 knockout cells. Ubiquitin conjugates, p62 and LC3, still colocalized in the absence of Keap1 (Fig. 3C), indicating that Keap1 does not affect the interaction among ubiquitin aggregates, p62 adaptor and autophagosomes.
Keap1 is not an autophagic substrate
p62 assists in the assembly of ubiquitin conjugates into large aggregates, but at the same time, is also degraded by autophagy.8,9 Two other proteins involved in the biogenesis of p62 bodies, NBR1 and Alfy, are also degraded via autophagy.17,18 We seek to explore whether Keap1 is also an autophagic substrate.
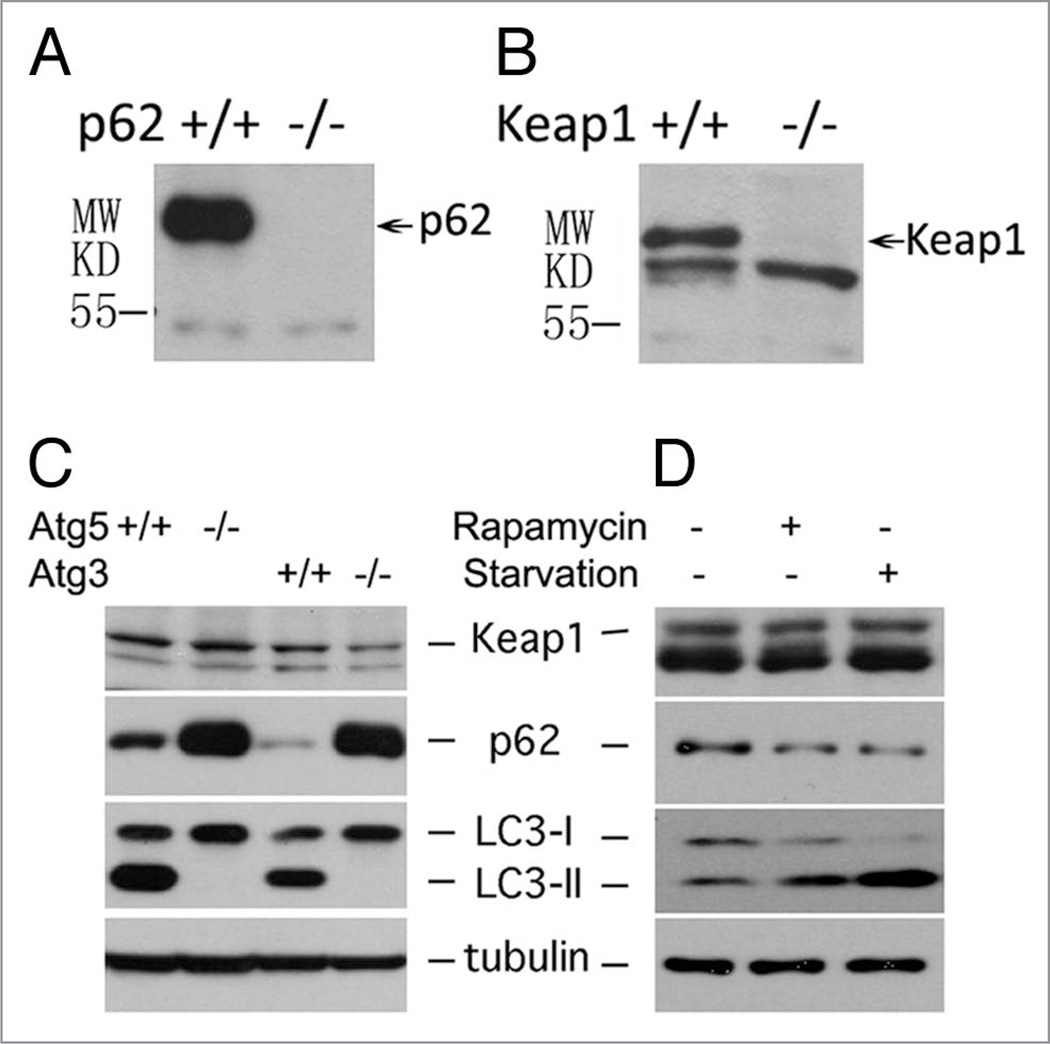
To study the protein turnover of Keap1 and p62, we first examined the specificity of anti-p62 and anti-Keap1 antibodies. The expression of endogenous p62 or Keap1 in p62 or Keap1 wild-type and knockout cells was checked. As expected, p62 was absent from p62 knockout cells (Fig. 4A) and Keap1 was missing from Keap1 knockout cells (Fig. 4B), proving that these antibodies were indeed specific for their respective proteins. We further tested the protein levels of Keap1 and p62 in autophagy-deficient cells. In both Atg3 and Atg5 knockout MEFs, autophagosome formation is completely abolished.31,32 As a positive control, p62 proteins were accumulated in both Atg5 and Atg3 knockout MEFs (Fig. 4C). However, no obvious accumulation of Keap1 was observed in these cells (Fig. 4C), indicating that Keap1 is unlikely to be an autophagic substrate. This notion was further supported by an observation in which Keap1 protein levels remained unchanged in HEK293T cells upon treatments with starvation or rapamycin (Fig. 4D). As controls for autophagy activation in the same HEK293T cells, p62 levels were decreased while LC3 II levels were increased (Fig. 4D).
Figure 4.
Keap1 is not an autophagic substrate. (A) p62 expression was examined in wild-type or p62 knock out cells. (B) Keap1 expression was examined in wild-type or Keap1 knockout cells. (C) The protein level of Keap1, p62, tubulin and LC3 in Atg5+/+ and Atg5−/−, or Atg3+/+ and Atg3−/− mouse embryonic fibroblasts were detected by western blotting. (D) HE K293T cells were treated with either 2 µM rapamycin for 4 hours or starved by EBSS for 2 hours. After treatment, cells were lysed in 1× SDS, and Keap1, p62, tubulin and LC3 levels were detected by western blotting. All data shown in (C) and (D) are representative of at least three independent experiments.
Keap1 deletion leads to ubiquitin aggregate accumulation and cytotoxicity
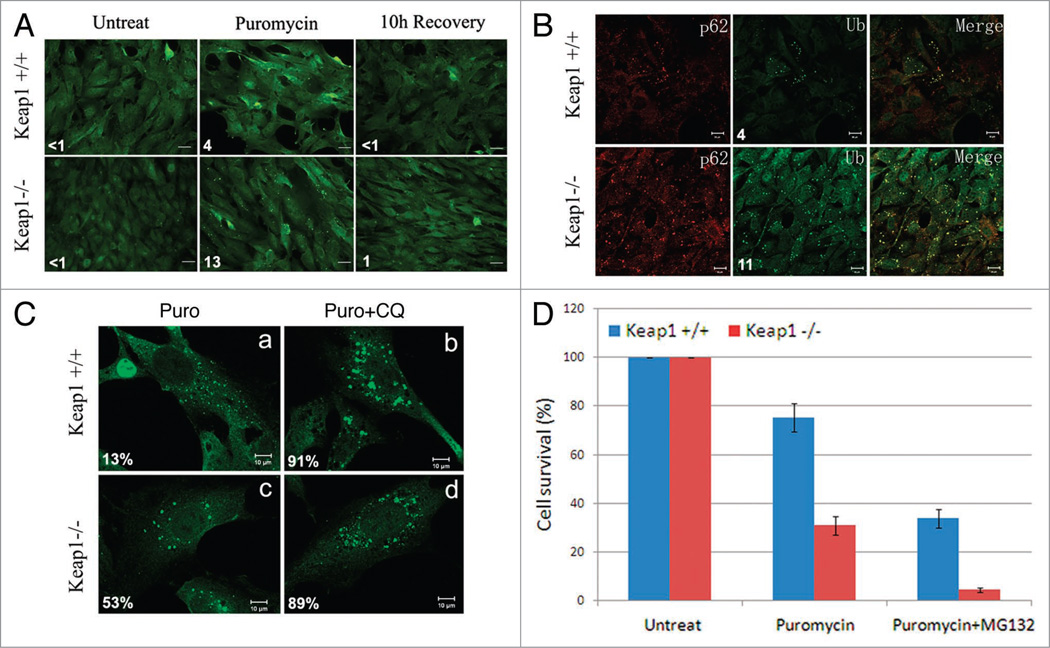
Keap1 encodes an essential protein that is required for Nrf2 suppression in oxidative and xenobiotic stress.33 Based on its interaction and colocalization with LC3 and p62, we speculated that Keap1 also plays a role in ubiquitin aggregate clearance. We tested this hypothesis in a Keap1 knockout MEF and a wild-type MEF. Upon puromycin treatment, significantly more ubiquitin-positive aggregates accumulated in the Keap1 knockout MEFs than in wild-type MEFs (Fig. 5A). These ubiquitin-positive aggregates were also positive for p62 (Fig. 5B). These results suggest that Keap1 is involved in the efficient degradation of ubiquitin aggregates.
Figure 5.
Keap1 ablation leads to ubiquitin aggregates accumulation and cytotoxicity. (A) Ubiquitin aggregates in Keap1 knockout cells. Keap1+/+ and Keap1−/− MEF cells were treated with 10 µg/ml puromycin for 4 hours, and recovered in normal DMEM for another 10 hours. Cell were then fixed and stained with FK2 antibody. These images are representative of at least three independent experiments. The numbers of ubiquitin puncta per cell were summarized from at least 200 cells in three different experiments. (B) Keap1 wild-type and knockout MEFs were treated with puromycin for 4 hours. p62 and ubiquitin (Ub) were stained by anti-p62 or FK2 antibodies respectively. The numbers of ubiquitin puncta per cell were summarized from at least 200 cells in three different experiments. (C) Keap1+/+ and Keap1−/− cells were either treated with 10 µg/ml puromycin for 4 hours, or with 10 µg/ml puromycin and 200 µM chloroquine for 4 hours. Cells were then fixed and stained with FK2 antibody. These images are representative of at least three independent experiments. The percentage of large aggregates (twice the size of smaller ones) in total aggregates was summarized from at least 200 cells. (D) Keap1 wild-type and knockout cells were mock treated or treated with puromycin (10 µg/ml) alone, or co-treated with puromycin and MG132 (2 µM) for 4 hours. Cell numbers were counted 2 days after treatments in a Trypan blue assay. There data were summarized from three different experiments.
In Keap1 wild-type MEFs, both the number and size of protein aggregates decreased upon puromycin treatment compared to that in Keap1 knockout MEFs (Fig. 5C, part a). To confirm that these protein aggregates are indeed degraded through autophagy, we treated these cells with chloroquine (CQ), a compound that raises the pH of lysosomes and blocks autophagy. CQ treatment significantly increased the size of ubiquitin aggregates (Fig. 5C, part b), suggesting that protein aggregates grow bigger when the degradation pathway is blocked. In Keap1 knockout MEF cells, ubiquitin positive protein aggregates upon puromycin treatment were larger than those in wild-type cells (Fig. 5C, part c), and the size of these puncta did not undergo any dramatic change upon CQ treatment (Fig. 5C, part d), suggesting that the degradation of these protein aggregates is likely blocked in Keap1 ablated cells.
Protein aggregate accumulation often leads to cytotoxicity. Keap1 knockout cells treated with puromycin sustained a much lower survival rate than that of wild-type cells (Fig. 5D). Normally, protein aggregates are efficiently degraded through the ubiquitin proteasome system and the autophagy lysosome pathway.34 To evaluate the contribution of autophagy to protein aggregate clearance, we blocked the ubiquitin proteasome system with a proteasome inhibitor MG132. MG132 treatment sickened the already puromycin-stressed Keap1 knockout cells, since significantly less surviving cells were detected in Keap1 knockout cells treated with puromycin and MG132 (Fig. 5D). This phenotype suggests that Keap1 ablation severely compromises the cellular ability to clear protein aggregates via autophagy and confers greater sensitivity to misfolded protein response upon these cells.
Keap1 affects LC3 lipidation and autophagosome formation
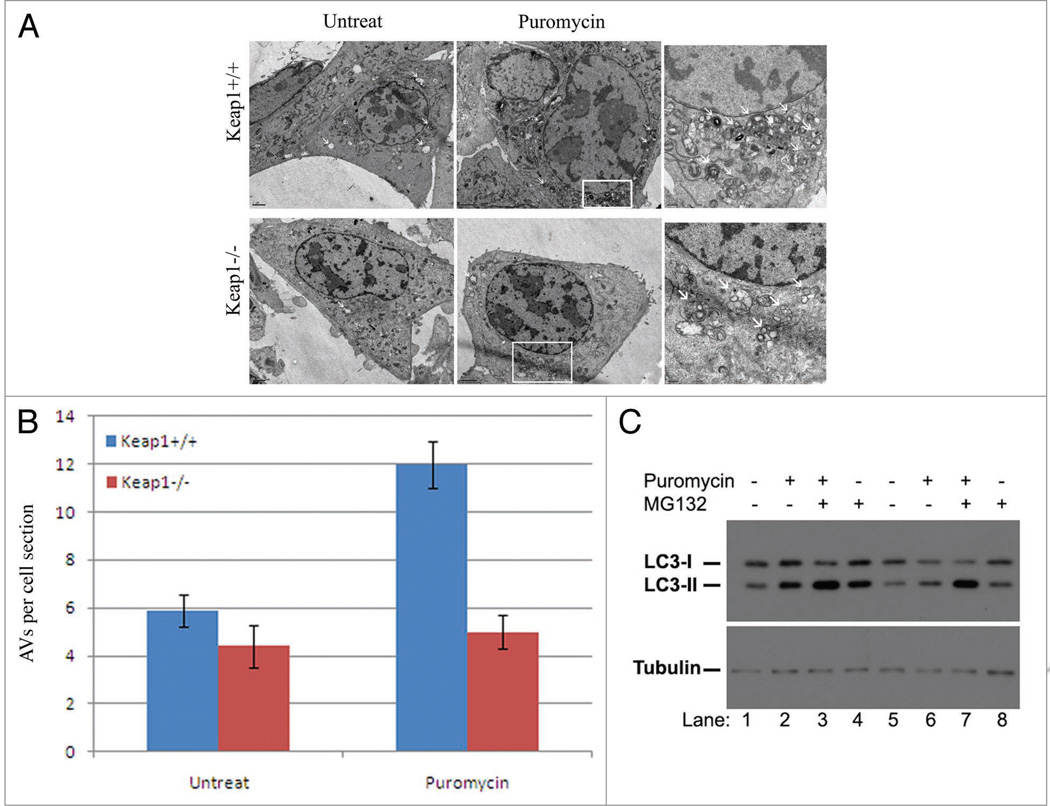
We propose that Keap1 is required for the selective autophagic pathway that eliminates ubiquitinated protein aggregates. The impairment of ubiquitin aggregate clearance in Keap1 knockout cells is likely due to a defect in autophagy. Keap1 knockout cells and wild-type cells were treated with puromycin and observed under transmission electron microscopy, from which we could directly visualize autophagic vacuoles in both Keap1 wild-type and knockout cells. The formation of autophagic vacuoles following puromycin treatment was dramatically compromised in Keap1 knockout cells compared to that in Keap1 wild-type cells (Fig. 6A and B). Hence, Keap1 is required for the autophagy pathway that is activated to clear ubiquitin aggregates.
Figure 6.
Genetic ablation of Keap1 leads to defective autophagy activation. (A) Electron microscopy analysis of Keap1+/+ and Keap1−/− cells. Puromycin (10 µg/ml, 4 hours) treated and untreated cells were analyzed under transmission electron microscope. The right panel is the enlarged image in the white frame. Scale bar, regular image: 2 µm, enlarge image: 0.5 µm. These experiments have been repeated at least three times. (B) The number of autophagic vesicles per cell cross section was calculated and quantified in this chart. There data were summarized from at least three different experiments. (C) LC3 conjugation was examined in Keap1 wildtype (Lanes 1–4) and knockout (Lanes 5–8) upon treatment of puromycin in combination with MG132 by western blotting. These experiments have been repeated at least three times.
Another faithful marker of autophagy activity is LC3 conjugation with PE,10 which is strongly induced by autophagic stimuli. Upon puromycin or MG132 treatment, Keap1 knockout cells displayed a compromised LC3 lipid conjugation compared to that of wild-type cells (Fig. 6C). When both puromycin and MG132 drugs were applied, the two cell types exhibited only a slight difference, since the combination of puromycin and MG132 could induce LC3 lipidation strongly in both Keap1 wild-type and knockout cells (Fig. 6C). These data further confirmed that Keap1 plays a positive role in autophagy regulation.
Discussion
In this study, we have revealed an unexpected positive function of Keap1 in autophagy regulation. Keap1 interacts with LC3 in a stress-inducible manner, and this interaction is mediated by p62. Keap1 is involved in a selective autophagic degradation pathway that degrades ubiquitinated protein aggregates, which is supported by multiple lines of evidence. First, Keap1 colocalizes with p62 and LC3 in the ubiquitin aggregates. Secondly, ubiquitinated protein aggregates accumulate in the Keap1 knockout cells. Lastly, autophagic vacuole formation and LC3 lipidation are defective in Keap1 mutant cells. All these results support a positive role of Keap1 in stress-induced autophagy (Fig. 7).
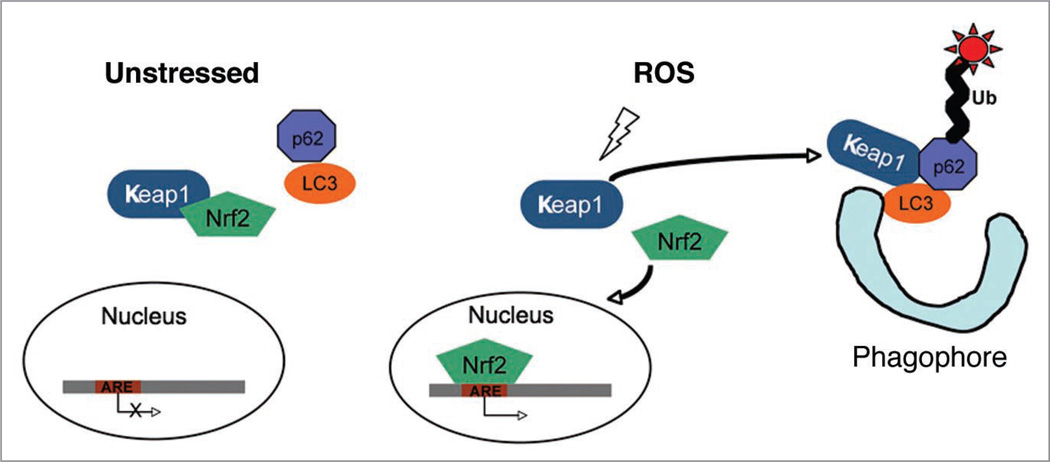
Figure 7.
Proposed model of the function of Keap1 in oxidative stress response and autophagy. In the unstressed cells, Keap1 sequesters Nrf2 in the cytosol. Keap1 is oxidized by reactive oxygen species (ROS), and Keap1 oxidation leads to Nrf2 dissociation. Nrf2 translocates to the nucleus and activates antioxidant response element (ARE)-containing genes. ROS also promotes the formation of Keap1-p62-LC3 complex that delivers ubiquitinated protein aggregates to the forming autophagosomes (phagophore).
This study also provides an interesting link to autophagy induced by oxidative stress. Oxidative stress induces autophagy through a largely unknown molecular mechanism.35 It has been suggested that oxidative stress might oxidize Atg4 at Cysteine residues to regulate LC3 processing.36 Here, we provide strong evidence that Keap1, the major oxidative sensor and regulator, directly regulates autophagy through its interaction with LC3 and p62.
Oxidative stress can cause a variety of damaging effects on DNA, proteins and lipids. One of the pathological outcomes is the promotion of protein aggregates that are highly enriched in ubiquitin chains. The inclusion bodies of protein aggregates have been implicated in multiple human pathological conditions, including neurodegenerative disease, diabetes and liver diseases.9,37 The Keap1-Nrf2 pathway serves as the major cytoprotective pathway against oxidative stress in the mammalian cells.26,27,38 Upon oxidative stress, Keap1 is oxidized, which leads to its dissociation with Nrf2. The released Nrf2 translocates to nuclei and activates anti-oxidant response element (ARE) containing proteins.39–46 In the screening of Nrf2-induced anti-oxidative stress responsive genes, p62 was listed as a top target that was activated by Nrf2.47 It is likely that the induced expression of p62 might facilitate the interaction between Keap1 and LC3, thereby stimulating autophagy to accelerate protein aggregate clearance. Keap1 not only binds to Nrf2 but also serves as a ubiquitin ligase to degrade Nrf2 in 26S proteasomes.39,40,48,49 Whether Keap1 also mediates LC3 and p62 ubiquitination and degradation needs to be further investigated. Unlike p62, Keap1 is not an autophagic substrate, since Keap1 did not accumulate in autophagy mutant cells (Fig. 4C) and neither did it degrade upon autophagic stimuli (Fig. 4D).
During the course of this work, a paper by Komatsu et al. was published describing the interaction between Keap1 and p62.50 According to this study, p62 interacts with the Nrf2 binding site on Keap1, acting as a competitor for the Nrf2-Keap1 association. This competition leads to stabilization and transcriptional activation of Nrf2 target genes. However, no function of Keap1 in selective autophagy has been characterized in this study. Here we provide substantial evidence demonstrating that Keap1 positively regulates p62-mediated ubiquitinated protein aggregates’ clearance via autophagy. Hence, the Keap1-p62-LC3 interaction is likely the major interaction that leads to the response to oxidative stress and the accumulation of protein aggregates. On the one hand, p62 competes with Keap1 for Nrf2 to activate the antioxidative response, which is shown by Komatsu et al.50 On the other hand, according to our study, Keap1 complexes with p62 and LC3 to facilitate the efficient removal of protein aggregates via autophagy in a stress-inducible manner.
In summary, we link the oxidative stress response, clearance of ubiquitin aggregates, and autophagy together via the Keap1 association with LC3 and p62 (Fig. 7). Further investigation into the biochemical mechanisms will yield a more thorough understanding of how human cells can work under environmental stress using a complex network orchestrated by multiple cellular pathways. More importantly, we should be able to identify the pivotal turning points that might have therapeutic values in human disease caused by oxidative damages.
Materials and Methods
Reagents and cell culture
HEK293T, U2OS and MEF cells were cultured in DMEM with 10% FBS and 1% penicillin and streptomycin in 5% CO2 incubator. Lipofectamine 2000 (Invitrogen, 11668-027) was used for mammalian cell transfection. Cells were incubated in OPTI medium (GIBCO, 51985091) for one hour before transfection. 2 µg plasmid was used for each 150 mm tissue culture dish (1 µg for 100 mm TCD). Ubiquitinated protein aggregates were induced by 10 µg/ml puromycin for 4 hours. U2OS and HEK293 and Myc-LC3 stably expressed cells were described before.23 Keap1+/+ and Keap1−/− MEF cells were kind gifts from Masayuki Yamamoto’s group at Tohoku University. p62+/+ and p62−/− MEFs, as well as Atg3+/+ and Atg3−/− MEFs, were kind gifts from Masaaki Komatsu’s group at Tokyo Metropolitan Institute of Medical Science. Atg5+/+ and Atg5−/− MEFs, were kind gifts from Noboru Mizushima’s group at Tokyo Medical and Dental University. DsRED-Keap1 was purchased from Addgene (Addgene, 21551).49 Keap1 was cloned into HrGFP by primers: p1: GCA AGC TTC GCA GCC AGA TCC CAG GCC TAG and p2: GCG GAT CCT CAA CAG GTA CAG TTC TGC T. p62 was cloned into pcdna4-HA by p1: GGA ATT CTA TGG CGT CGC TCA CCG TGA A, p2: GAG TGC GGC CGC GCA ACG GCG GGG GAT GCT TTG. The following antibodies were also used in this study: anti-Myc (Santa Cruz, 9E10, sc-40), anti-HA (Roche, 3F10, 11867423001), anti-FLAG (Sigma, M2, A8592), anti-LC3 (Sigma, L7543), anti-p62 (MBL International, M162-3), and anti-conjugated ubiquitin FK2 antibody (Biomol Res, PW8810).
LC3 tandem affinity purification (TAP)
LC3 was cloned into pcDNA5/FRT with double N terminal tags ZZ and FLAG, and U2OS cells that inducibly express ZZ-FLAG-LC3 were built with Flp-ln system as described.23 An extremely low dose (10 ng/ml) doxycycline was used to induce LC3 expression. The procedure of tandem affinity purification was performed as described before.23 In short, cells were induced with doxycycline for 48 hours before harvested. Cell pellets were then lysed in TAP lysis buffer (20 mM Tris-Hcl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 1 mM NaVO3, and freshly added protease inhibitors). Cell lysate was collected after centrifugation 20 min at 10,000 g. The LC3 complex was pulled down sequentially with IgG beads and anti-FLAG M2 beads. FLAG peptide was used for the final elution. The eluate was separated by SDS-PAGE and visualized by silver staining. Protein bands were cut out and identified by mass spectrometry.
Immunofluorescence
Cells grown on cover slips were subjected to different treatments followed by fixation in 4% paraformaldehyde for 20 min, then they were permeabilized by 0.4% Triton X-100 for 20 min at room temperature. Antibodies were diluted in DPBS with 10% goat serum and 0.1% Tween 20. FITC goat anti-mouse and Rhodamine red mouse anti-rabbit fluorescent secondary antibodies were used. Images were taken by Zeiss LSM 510 Meta laser scanning confocal microscope.
Transmission electron microscopy
Cells were fixed with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 1 h followed by 1% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.2) for 2 hours. Samples were enblocked with 0.5% aqueous uranyl acetate overnight and treated by low-temperature dehydration and infiltration with a graded series of Epon/Araldite, followed by the embedment in 100% Epon/Araldite. Thin sections (70 nM) were cut and stained with Reynalds lead citrate and analyzed by a FEI Tecnai 12 Transmission electron microscope.
Autophagy analysis
Autophagy was evaluated upon two autophagic stresses: starvation and rapamycin treatments. For starvation, cells were washed three times with PBS and incubated in EBSS for 1–2 h at 37°C. For rapamycin treatment, cells were incubated in complete medium containing 500 nM rapamycin (Fisher, NC9163747) for 12 hours. Autophagy activation was assessed by LC3 conjugation. For the detection of endogenous LC3 conjugation, cells with different treatments were sonicated in 1× SDS loading buffer. Cell lysates were heated and then subjected to immunoblotting analysis with an antibody against LC3 (Sigma, L7543).
Acknowledgements
We thank all Zhong lab members for helpful discussion, Keling Chen for the preliminary experiments and Irena Tan for the critical reading of the manuscript. We thank Masayuki Yamamoto, Terje Johansen, Noboru Mizushima and Masaaki Komatsu for reagents. We thank Cell Signaling and Abgent Company for Keap1 antibody production. The work is supported in part by a New Investigator Award for Aging from the Ellison Medical Foundation to Q.Z.
References
- 1.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 6.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 7.Kaniuk NA, Kiraly M, Bates H, Vranic M, Volchuk A, Brumell JH. Ubiquitinated-protein aggregates form in pancreatic beta-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes. 2007;56:930–939. doi: 10.2337/db06-1160. [DOI] [PubMed] [Google Scholar]
- 8.Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 10.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott SV, Guan J, Hutchins MU, Kim J, Klionsky DJ. Cvt19 is a receptor for the cytoplasm-to-vacuole targeting pathway. Mol Cell. 2001;7:1131–1141. doi: 10.1016/s1097-2765(01)00263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shintani T, Huang WP, Stromhaug PE, Klionsky DJ. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev Cell. 2002;3:825–837. doi: 10.1016/s1534-5807(02)00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Kamada Y, Stromhaug PE, Guan J, Hefner-Gravink A, Baba M, et al. Cvt9/Gsa9 functions in sequestering selective cytosolic cargo destined for the vacuole. J Cell Biol. 2001;153:381–396. doi: 10.1083/jcb.153.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yorimitsu T, Klionsky DJ. Atg11 links cargo to the vesicle-forming machinery in the cytoplasm to vacuole targeting pathway. Mol Biol Cell. 2005;16:1593–1605. doi: 10.1091/mbc.E04-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanki T, Wang K, Cao Y, Baba M, Klionsky DJ. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev Cell. 2009;17:98–109. doi: 10.1016/j.devcel.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto K, Kondo-Okamoto N, Ohsumi Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev Cell. 2009;17:87–97. doi: 10.1016/j.devcel.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Kirkin V, Lamark T, Sou YS, Bjorkoy G, Nunn JL, Bruun JA, et al. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell. 2009;33:505–516. doi: 10.1016/j.molcel.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Clausen TH, Lamark T, Isakson P, Finley K, Larsen KB, Brech A, et al. p62/SQSTM1 and ALFY interact to facilitate the formation of p62 bodies/ALIS and their degradation by autophagy. Autophagy. 2010;6:330–344. doi: 10.4161/auto.6.3.11226. [DOI] [PubMed] [Google Scholar]
- 19.Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Noda NN, Kumeta H, Nakatogawa H, Satoo K, Adachi W, Ishii J, et al. Structural basis of target recognition by Atg8/LC3 during selective autophagy. Genes Cells. 2008;13:1211–1218. doi: 10.1111/j.1365-2443.2008.01238.x. [DOI] [PubMed] [Google Scholar]
- 22.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Q, Fan W, Chen K, Ding X, Chen S, Zhong Q. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 2008;105:19211–19216. doi: 10.1073/pnas.0810452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Q, Fan W, Zhong Q. Regulation of Beclin 1 in autophagy. Autophagy. 2009;5:713–716. doi: 10.4161/auto.5.5.8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, Overvatn A, et al. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J Cell Biol. 2010;188:253–269. doi: 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Ann Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 28.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 29.Lelouard H, Ferrand V, Marguet D, Bania J, Camosseto V, David A, et al. Dendritic cell aggresome-like induced structures are dedicated areas for ubiquitination and storage of newly synthesized defective proteins. J Cell Biol. 2004;164:667–675. doi: 10.1083/jcb.200312073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szeto J, Kaniuk NA, Canadien V, Nisman R, Mizushima N, Yoshimori T, et al. ALIS are stress-induced protein storage compartments for substrates of the proteasome and autophagy. Autophagy. 2006;2:189–199. doi: 10.4161/auto.2731. [DOI] [PubMed] [Google Scholar]
- 31.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 32.Sou YS, Waguri S, Iwata J, Ueno T, Fujimura T, Hara T, et al. The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Mol Biol Cell. 2008;19:4762–4775. doi: 10.1091/mbc.E08-03-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 34.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 35.Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci USA. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 39.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, et al. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motohashi H, Katsuoka F, Engel JD, Yamamoto M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc Natl Acad Sci USA. 2004;101:6379–6384. doi: 10.1073/pnas.0305902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Kern JT, Walker JR, Johnson JA, Schultz PG, Luesch H. A genomic screen for activators of the antioxidant response element. Proc Natl Acad Sci USA. 2007;104:5205–5210. doi: 10.1073/pnas.0700898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]