Abstract
Intravenous fluids (IVFs) represent a basic therapeutic intervention utilized in septic shock. Unfortunately, the optimal method for administering IVFs to maximize patient outcomes is unknown. A meta-analysis of four randomized trials of goal-directed therapy did not demonstrate a significant reduction in mortality (odds ratio 0.609; 95% confidence interval 0.363 to 1.020; P = 0.059), whereas 18 trials with historical controls showed a significant increase in survival (odds ratio 0.580; 95% confidence interval 0.501 to 0.672; P < 0.0001). Based on these data, clinicians should be aware of the potential for harm due to the excessive administration of IVFs to patients with septic shock.
Introduction
Intravenous fluids (IVFs) are one of the most common therapies provided to critically ill patients. IVF administration is largely empiric, although goal-directed approaches have been used in an attempt to optimize resuscitation in unstable patients [1,2]. Excessive use of any therapeutic agent can be associated with potential harm; excessive use of antibiotics [3,4], sedation [5,6], tidal volume [7,8], transfusions [9], and glucose [10] have all been linked to unfavorable patient outcomes. It is now recognized that excessive IVFs may also contribute to new complications and worsening of underlying disease processes, including acute respiratory distress syndrome, abdominal compartment syndrome, coagulopathy, and cerebral edema [11-14]. Unfortunately, a systematic approach for delivery of IVFs in critically ill patients does not exist. This is partly related to the various conditions managed in the ICU setting, as well as the varied approaches to IVF administration and availability of different IVF types (that is, colloids, and balanced and unbalanced crystalloids). Goal-directed therapy (GDT) for IVF administration in the first 6 hours of septic shock is now advocated by the most recent version of the Surviving Sepsis Campaign guidelines with a 1C evidence recommendation [1]. However, not all clinicians and investigators are convinced that this approach is optimal [15,16]. Therefore, the goal of this review and meta-analysis is to assess the evidence in support of IVFs in septic shock, focusing on the quantity of IVFs administered, and to determine if conservative fluid therapy is justifiable in septic patients. Like many other therapeutic areas in critical care, an approach to fluid administration that employs therapeutic minimization may be preferred for the avoidance of complications and optimization of patient outcomes.
Methods
We conducted an English language search of PubMed and Cochrane databases from January 1980 to December 2014 to find human trials of sepsis care bundles in adults (aged ≥18 years) using these search terms: sepsis, septic shock, treatment, guidelines, protocols, GDT, and bundles (Figure 1). The studies that were included had to enroll septic patients, have a control (historical or concurrent), and record mortality rates. The included studies also had to provide targets for their usage of fluids as part of their sepsis intervention or sepsis bundle. Criteria for sepsis or septic shock in patients receiving bundled care had to be consistent with the American College of Chest Physicians and Society of Critical Care Medicine Consensus Conference definitions [17]. Both investigators independently reviewed the included studies by using a standardized data collection form. Discrepancies were resolved by discussion. The Scottish Intercollegiate Guidelines Network checklist for randomized, controlled trials [18] was used to evaluate the methodological quality of the identified studies included in this analysis. A double plus (++) denotes studies very unlikely to have bias, plus (+) studies where bias is unlikely, and minus (−) studies with high risk of bias [18]. Survival was the outcome of interest and tabulated across studies. Conventional forest plots were prepared for survival. A statistical difference between groups was considered to occur if the pooled 95% confidence interval (CI) did not include 1 for the odds ratio (OR). An OR <1 favored bundled GDT when compared with a control group. Two-sided P-values were calculated. A random-effects model was chosen for all analyses. Statistical heterogeneity and inconsistency were assessed by using the Q and I2 tests, respectively [19,20]. When the P-value of the Q-test was <0.10, the I2 was >25%, or both, heterogeneity and inconsistency were considered significant [19,21].
Figure 1.
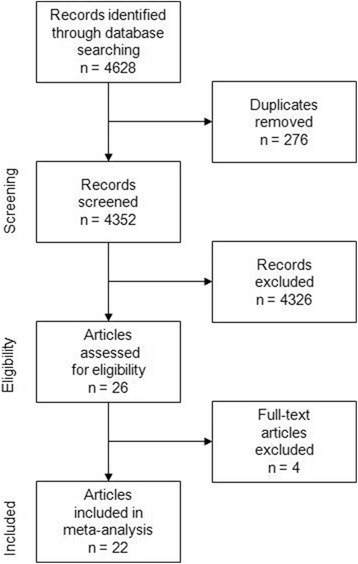
Summary of evidence search and selection.
Randomized controlled trials
The authors of the Surviving Sepsis Campaign recommend the protocolized, quantitative resuscitation of patients with sepsis-induced tissue hypoperfusion (defined as hypotension persisting after initial fluid challenge or blood lactate concentration ≥4 mmol/L) [1]. They recommend initiating this protocol as soon as hypoperfusion is recognized and it should not be delayed pending ICU admission. During the first 6 hours of resuscitation, the recommended resuscitation goals include all of the following: central venous pressure (CVP) 8 to 12 mmHg; mean arterial pressure ≥65 mmHg; urine output ≥0.5 ml kg h−1; superior vena cava oxygen saturation (ScvO2) or mixed venous oxygen saturation 70 or 65%, respectively. The primary rationale for this recommendation comes from a randomized, controlled, single-center study demonstrating that early quantitative resuscitation improved survival for emergency department patients presenting with septic shock [2]. Resuscitation targeting the physiologic goals noted above for the initial 6-hour period was associated with a 15.9% absolute reduction in 28-day mortality (46.5% versus 30.5%, P = 0.009).
Bundled GDT was also evaluated in a multicenter trial of 314 patients with severe sepsis in eight Chinese centers [22]. This trial reported a 17.7% absolute reduction in 28-day mortality (42.5% versus 24.8%, P = 0.001). A single-center randomized controlled study from Taiwan in 224 medical ICU patients using the protocol of Rivers and colleagues [2] also demonstrated a survival advantage with bundled GDT (hospital mortality, 46.3% versus 28.4%, P = 0.006) [23]. Most recently, the multicenter ProCESS trial enrolled 1,341 patients into three treatment groups (protocol-based GDT, protocol-based standard therapy, usual care) [24]. No difference in 60-day mortality was observed between the three groups (protocol-based GDT versus usual care: 21.0% versus 18.9%, P = 0.830).
These four randomized trials included a total of 2,131 patients; 834 (39.1%) in the bundled GDT arm and 851 (39.9%) in the control arm (446 (20.9%) were in the protocol-based standard therapy group of the ProCESS trial). All four studies used the same targeted goals for fluid administration and for the use of vasopressors. The reported total IVF administration for shock varied from 5.0 liters in the control arm of the Taiwanese study to 3.5 liters in the control arm of the Rivers study at 6 hours and 2.3 liters at 6 hours in the control arm of the ProCESS trial (Table 1). Bundled GDT was applied in the emergency department in two studies [2,24] and the ICU in two studies [22,23]. Patients who were treated with bundled GDT did not achieve a significant reduction in mortality compared with those in the control arm (OR 0.609; 95% CI 0.363 to 1.020; P = 0.059; Figure 2). The trials were inconsistent and heterogeneous (I2 = 80%, P = 0.002). With removal of the ProCESS trial, the remaining three trials were without heterogeneity and were consistent (I2 = 0%, P = 0.915), demonstrating a significant reduction in mortality with GDT (OR 0.475; 95% CI 0.353 to 0.639; P < 0.001). However, these three trials combined had fewer patients enrolled compared to the ProCESS trial.
Table 1.
Randomized controlled trials of bundled goal-directed therapy
| Study and country | Sign score | Goal-directed therapy (goals) | Time and fluid quantification (L) | ||
|---|---|---|---|---|---|
| Control | GDT | ||||
| Rivers et al. 2001, USA [2] | + | 500 ml crystalloid bolus every 30 minutes (CVP 8–12 mmHg) | 6 h: | 3.5 ± 2.4 | 5.0 ± 3.0 |
| 7–72 h: | 10.6 ± 6.2 | 8.6 ± 5.2 | |||
| Lin et al. 2006, Taiwan [23] | + | 500 ml crystalloid bolus every 30 minutes (CVP 8–12 mmHg) | Total: | 5.0 ± 2.9 | 5.2 ± 4.0 |
| GDT Collaborative Group of Zhejiang Province 2010, China [22] | - | 500 ml crystalloid bolus every 30 minutes (CVP 8–12 mmHg) | Quantified but not reported | ||
| ProCESS 2014, USA [24] | ++ | 500 ml crystalloid bolus every 30 minutes (CVP 8–12 mmHg) | 6 h: | 2.3 ± 1.9 | 2.8 ± 2.0 |
Double plus signs (++) indicate studies with very unlikely bias, a single plus sign (+) indicates studies with unlikely bias, and a minus sign (−) indicates studies with high risk of bias. CVP, central venous pressure; GDT, goal-directed therapy.
Figure 2.
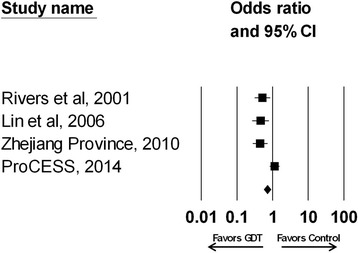
Effect of bundled goal-directed therapy (GDT) on the odds ratio of survival (95% confidence interval (CI)) for the four randomized trials analyzed (odds ratio 0.609; 95% CI 0.363 to 1.020; P = 0.059; I 2 = 80% , P = 0.002). Rivers et al. 2001 [2]; Lin et al. 2006 [23]; Zhejiang province 2010 [22]; ProCESS 2014 [24].
Observational trials
Eighteen observational trials of bundled GDT were identified [25-42] (Table 2). Seven of these trials were assessed in an earlier analysis demonstrating that a bundled care protocol for septic shock that included the elements of GDT was associated with a significant improvement in hospital survival [15]. Across the 18 studies, the effect of bundled care on survival was heterogeneous and inconsistent (I2 = 32%, P = 0.091; Figure 3). However, when the study by Ferrer and colleagues [33] was removed, the remaining 17 studies were without heterogeneity and were consistent (I2 = 0%, P = 0.796). Overall, there was a statistically significant increase in the odds of surviving with bundled care compared with controls when all studies were examined (OR 0.580; 95% CI 0.501 to 0.672; P < 0.0001). Statistical significance was maintained when the Ferrer study was removed from the analysis (OR 0.561; 95% CI 0.499 to 0.631; P < 0.0001).
Table 2.
Observational controlled trials of bundled goal-directed therapy
| Study and country | Sign score | Goal-directed therapy (goals) | Time and fluid quantification (L) a | ||
|---|---|---|---|---|---|
| Control | Bundled GDT | ||||
| Cardoso et al. 2010, Portugal [42] | (−) | 500 ml to 1000 ml crystalloid bolus, or 300 ml to 500 ml colloid bolus to achieve CVP ≥12 mmHg | Quantified but not reported. | ||
| Castellanos-Ortega et al. 2010, Spain [35] | (−) | 500-1000 ml crystalloid bolus, additional fluid to achieve CVP ≥8 mmHg | Quantified but not reported. | ||
| El Solh et al. 2008, USA [31] | (−) | 500 ml crystalloid bolus, repeat until CVP 8–12 mmHg | 6 h: | 2.5 ± 1.0 | 3.9 ± 2.0 |
| 24 h: | 3.2 ± 1.3 | 4.9 ± 2.5 | |||
| Ferrer et al. 2008, Spain [33] | (−) | 500 ml crystalloid bolus, repeat until CVP 8–12 mmHg | Quantified but not reported | ||
| Gao et al. 2005, UK [36] | (−) | Immediate fluid bolus of 0.5 L | Quantified but not reported | ||
| Girardis et al. 2009, Italy [34] | (−) | Fluids targeting CVP >6 mmHg or a global end-diastolic volume by trans-pulmonary thermodilution >700 ml/m2 | Quantified but not reported | ||
| Heppner et al. 2012, Germany [39] | (−) | 500 ml crystalloid bolus, repeat until CVP 8–12 mmHg | Quantified but not reported. | ||
| Jones et al. 2007, USA [30] | (−) | 500 ml crystalloid bolus, repeat until CVP 8–12 mmHg | 6 h: | 2.5 ± 2.4 | 4.7 ± 1.8 |
| Kortgen et al. 2006, Germany [27] | (−) | CVP 8–12 mmHg or intrathoracic blood volume index 850–1000 ml/m2 | 6 h: | 2.8 [1.8,3.8] | 2.5 [1.6,3.9] |
| Lefrant et al. 2010, France [41] | (−) | ≥20 ml/kg crystalloids or colloids within 6 hours | 6 h crystalloid: | 1.0 [0.5,2.0] | 1.5 [0.5,2.0] |
| 6 h colloid: | 0.5 [0.5,1.0] | 1.0 [0.5,1.1] | |||
| Micek et al. 2006, USA [25] | (−) | 500 ml crystalloid bolus, repeat until CVP 8–12 mmHg | In ED: | 2.8 ± 1.6 | 3.8 ± 1.7 |
| Miller et al. 2013, USA [38] | (−) | 500 ml crystalloid bolus, repeat until CVP 8–12 mmHg | Quantified but not reported. | ||
| Na et al. 2012, Asia [40]b | (−) | Fluid bolus to achieve CVP >8 mmHg by 6 hours | In ED: | 1.5 [1.0,2.5] | 1.5 [0.9,2.7] |
| In ICU: | 7.8 [5.2,11.6] | 5.6 [3.3,9.2] | |||
| Nguyen et al. 2007, USA [29] | (−) | CVP ≥8 mmHg | In ED: | 2.8 ± 1.5 | 2.8 ± 2.1 |
| 72 h: | 7.8 ± 5.2 | 7.9 ± 6.1 | |||
| Pestaña et al. 2010, Spain [37] | (−) | Crystalloid within 6 hours to achieve CVP ≥8 mmHg or global end-diastolic volume index ≥680 ml/m2 | Quantified but not reported. | ||
| Sebat et al. 2005, USA [32] | (−) | 1000 ml crystalloid in ED, 600 ml increments per MAP & UO protocol | Quantified but not reported. | ||
| Shapiro et al. 2006, USA [28] | (−) | 500 ml crystalloid bolus, repeat until CVP 8–12 mmHg | 6 h: | 2.9 ± 1.8 | 4.1 ± 2.6 |
| 24 h: | 6.5 ± 4.5 | 7.6 ± 3.9 | |||
| Trzeciak et al. 2006, USA [26] | (−) | 250-1000 ml crystalloid bolus until CVP ≥8 mmHg | ED: | 3.5 ± 2.3 | 5.7 ± 3.0 |
| ICU 24 h: | 5.5 ± 4.9 | 2.8 ± 1.7 | |||
| ED and ICU 24 h: | 9.1 ± 5.1 | 7.9 ± 3.4 | |||
Double plus signs (++) indicate studies with very unlikely bias, a plus sign (+) indicates studies with unlikely bias, and a minus sign (−) indicates studies with high risk of bias. aValues expressed as mean ± standard deviation or median [interquartile range]. bChina, India, Taiwan, Singapore, Korea. CVP, central venous pressure; ED, emergency department; GDT, goal-directed therapy; MAP, mean arterial pressure; UO, urine output.
Figure 3.
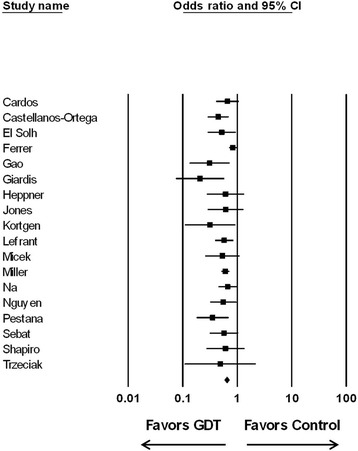
Effect of bundled goal-directed therapy (GDT) on the odds ratio of survival (95% confidence interval (CI)) for the 18 observational trials analyzed. All 18 studies: odds ratio, 0.580; 95% CI 0.501 to 0.672; P < 0.0001 (I 2 = 32%, P = 0.091). With the Ferrer study [33] removed: odds ratio 0.561; 95% CI 0.499 to 0.631; P < 0.0001 (I 2 = 0%, P = 0.796).
Limitations of the bundled goal-directed therapy studies
One of the proposed main limitations of the pivotal Rivers trial is the lack of generalizability of the study. For example, Ho and colleagues [43] examined 4,784 emergency department patients and found only 40 (0.8%) were candidates for bundled GDT. Moreover, these investigators found patient mortality to be 26.0%, much lower than the mortality observed in the control arm of the Rivers study, which was 46.5%, and the control arm mortality in the other two older randomized trials of bundled GDT [2,22,23,43]. Variability in mortality was also observed in the 18 observational studies examined where the mortality of the control arms ranged from 15.5% to 67.6% [25-42]. Interestingly, the ProCESS trial had one of the lowest observed mortality rates in their control arm at 18.9%. Other limitations of these trials include resuscitation protocol complexity, potential risks associated with elements of the protocol (especially the use of dobutamine and red blood cell transfusions), and financial and infrastructure implications necessary to carry out these protocols. For example, in the Rivers trial the bundled GDT patients had a dedicated investigator to ensure compliance with the study protocol and had central venous catheters placed to continuously assess ScvO2.
The observational studies of bundled GDT have a number of other important limitations, foremost being their lack of scientific rigor (Table 2). It is also important to note that the earlier meta-analysis of seven of these trials found that only time to antibiotic administration (in hours) between bundle and control patients was consistent between studies, whereas crystalloids, vasopressors, inotropes, packed red blood cell transfusion, corticosteroids, and drotrecogin alfa (activated) exhibited significant heterogeneity between the studies [15]. The overall importance of timely antibiotic therapy was further supported by the analysis of the Spanish study that examined 2,319 patients showing a reduction in mortality with bundled GDT [33]. In a subsequent analysis of their data, the Spanish investigators reported on compliance with four therapeutic goals and four treatments employed in their bundle [44]. Only timely administration of antibiotics and drotrecogin alfa (activated) for multiorgan failure were associated with significantly lower mortality [44].
Another important limitation of both the randomized trials and the observational studies examining bundled GDT is that multiple interventions occurred that could potentially influence patient outcome. This is supported by the observation that statistical differences in the use of vasopressors, red blood cell transfusions, corticosteroids, and timely administration of antibiotics existed between study arms when the trials reporting specific interventions were combined for analysis (Table 3). Moreover, across the 12 studies reporting on IVF administration, most showed greater fluids administered to patients receiving bundled GDT (median (interquartile range): 3,875 ml (2,638 ml, 4,901 ml) versus 2,779 ml (2,332 ml, 3,342 ml); P = 0.143) (Tables 1 and 2). However, the studies were inconsistent and significant heterogeneity existed between studies for the difference in IVFs administered by treatment group (I2 = 90%, P < 0.001) (Figure 4). Removal of any one study failed to significantly reduce heterogeneity (I2 remained 87% to 91%, with P < 0.001).
Table 3.
Comparison of specific interventions employed in trials of bundled goal-directed therapy
| Vasopressors | Inotropes | PRBC | Corticosteroids | rhAPC | Appropriate antibiotics | Timely antibiotics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Control | Bundled care | Control | Bundled care | Control | Bundled care | Control | Bundled care | Control | Bundled care | Control | Bundled care | Control | Bundled care |
| ProCESS [24] | 201/456 | 241/439 | 4/456 | 35/439 | 34/456 | 63/439 | 37/456 | 54/439 | 0/456 | 1/439 | 442/456 | 428/439 | NA | NA |
| Rivers et al. [2] | 40/133 | 36/130 | 1/133 | 18/130 | 25/133 | 83/130 | NA | NA | NA | NA | 125/133 | 126/130 | 123/133 | 112/130 |
| Lin et al. [23] | 81/116 | 80/108 | 16/116 | 13/108 | 43/116 | 39/108 | 25/116 | 32/108 | NA | NA | 107/116 | 102/108 | NA | NA |
| El Solh et al. [31] | NA | NA | NA | NA | 11/87 | 12/87 | 14/87 | 83/87 | 2/87 | 11/87 | 73/87 | 84/87 | 79/87 | 83/87 |
| Ferrer et al. [33] | 329/854 | 630/1,465 | NA | NA | NA | NA | 311/854 | 611/1,465 | 51/854 | 74/1,465 | 568/854 | 1,009/1,465 | NA | NA |
| Jones et al. [30] | 27/79 | 53/77 | 1/79 | 2/77 | 1/79 | 4/77 | 5/79 | 31/77 | 3/79 | 3/77 | NA | NA | NA | NA |
| Kortgen et al. [27] | NA | NA | 0/30 | 6/30 | 5/30 | 5/30 | 13/30 | 30/30 | 0/30 | 7/30 | 28/30 | 28/30 | 30/30 | 30/30 |
| Lefrant et al. [41] | NA | NA | 40/230 | 20/215 | 24/230 | 34/215 | 91/230 | 122/215 | 0/230 | 4/215 | NA | NA | 141/230 | 145/215 |
| Micek et al. [25] | 60/60 | 43/60 | NA | NA | 4/60 | 12/60 | 30/60 | 13/60 | 7/60 | 2/60 | 43/60 | 52/60 | 36/60 | 52/60 |
| Na et al. [40] | 171/364 | 135/192 | 144/364 | 78/192 | 17/364 | 4/192 | NA | NA | NA | NA | NA | NA | NA | NA |
| Nguyen et al. [29] | 39/77 | 111/253 | 18/77 | 67/253 | 11/77 | 32/253 | 23/77 | 41/253 | 6/77 | 4/253 | NA | NA | 77/77 | 227/253 |
| Shapiro et al. [28] | 23/51 | 63/79 | 1/51 | 6/79 | 3/51 | 8/79 | 12/51 | 23/79 | 0/51 | 3/79 | 45/51 | 77/79 | 48/51 | 78/79 |
| Trezeciak et al. [26] | 7/16 | 13/22 | 0/16 | 2/22 | 0/16 | 3/22 | 5/16 | 8/22 | 2/16 | 7/22 | NA | NA | NA | NA |
| Totals | 978/2206 (44.3) | 1,405/2,825 (49.7) | 225/1,552 (14.5) | 247/1,545 (16.0) | 178/1,699 (10.5) | 299/1,692 (17.7) | 566/2,056 (27.7) | 1,048/2,835 (37.0) | 71/1940 (3.7) | 116/ 2,727 (4.3) | 1,431/1,787 (80.1) | 1,906/2,398 (79.5) | 534/668 (79.9) | 727/854 (85.1) |
| P-valuesa | <0.001 | 0.249 | <0.001 | <0.001 | 0.308 | 0.636 | 0.008 | |||||||
a P-values for comparison between control and bundled care groups for each intervention. NA, not available; PRBC, packed red blood cells; rhAPC, recombinant human activated protein C.
Figure 4.
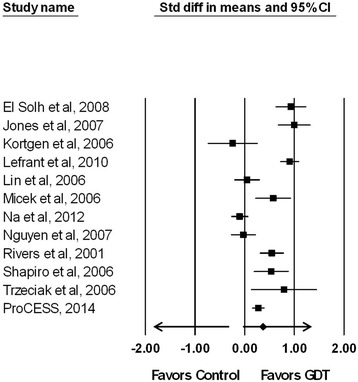
Effect of bundled goal-directed therapy (GDT) on the standardized paired difference (Std diff) of the means for intravenous fluid use based on study defined resuscitation goals ( I 2 = 90% , P < 0.001). CI, confidence interval.
For completeness, one additional observational study was identified but not included in our analysis due to its makeup. This was a multicenter quality improvement study conducted in the United States, Europe, and South America utilizing a multifaceted intervention to facilitate compliance with selected guideline recommendations for the management of severe sepsis [45]. This intervention included the use of a CVP greater than 8 mmHg to target fluid administration. Data on 15,022 patients at 165 hospitals were included in the analysis. Compliance with the initial 6-hour bundle targets increased linearly from 10.9% of subjects in the first quarter to 31.3% by the end of 2 years of the quality improvement campaign. This was associated with a significant reduction in mortality over the same time period (37.0% in the first quarter in the campaign to 30.8% by 2 years, P = 0.001). After adjustment for baseline characteristics, administration of broad-spectrum antibiotics, obtaining blood cultures before antibiotic initiation, administration of drotrecogin alfa (activated) in the first 24 hours, achieving plateau pressure control, and maintaining blood glucose control were all associated with lower hospital mortality. In those with septic shock, there was no association between mortality and the ability to achieve a CVP ≥8 mmHg or demonstration of ScvO2 ≥ 70% [45].
The limitations of the available clinical studies of bundled GDT, to include the inconsistent results regarding IVF administration, have served as the major impetus for the conduct of three multicenter trials examining the elements of GDT (ARISE in Australasia, ProMISe in the United Kingdom, and ProCESS in the United States) [24,43,46]. These trials have similar structures, interventions, and patient entry criteria that will allow the trial investigators to collaboratively conduct a prospective individual patient data meta-analysis, using the raw data from each trial [46]. With over 4,000 subjects combined, these trials are powered to find smaller effects on outcome and to better explore subgroups. The ProCESS trial has already been published and showed that protocolized GDT did not reduce mortality compared with usual care [24]. However, patients in the usual care arm received significantly less IVF at 6 hours and had a numerically lower mortality compared to the protocolized GDT arm.
Conclusion
Clinicians caring for critically ill patients must always weigh the benefits and risks of administered therapies to include the use of IVFs. Our meta-analysis supports the findings of an earlier analysis [15] demonstrating that IVF volume was not consistently altered by the use of GDT bundles, and thus firm recommendations regarding their quantitative use cannot be made. Clinicians should at least be aware of the potential for harm due to the excessive administration of IVFs to patients with septic shock.
Abbreviations
- CI
Confidence interval
- CVP
Central venous pressure
- GDT
Goal-directed therapy
- IVF
Intravenous fluid
- OR
Odds ratio
- ScvO2
Superior vena cava oxygen saturation
Footnotes
Competing interests
MHK’s effort was supported by the Barnes-Jewish Hospital Foundation. The authors have no conflicts or competing interests to report in relation to this manuscript.
Contributor Information
Catherine Chen, Email: cxchen@dom.wustl.edu.
Marin H Kollef, Email: mkollef@dom.wustl.edu.
References
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 2.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 3.Carlet J, Jarlier V, Harbarth S, Voss A, Goossens H, Pittet D. Participants of the 3rd World Healthcare-Associated Infections Forum: Ready for a world without antibiotics? The Pensières Antibiotic Resistance Call to Action Antimicrobial Resistance and Infection Control. Antimicrob Resist Infect Control. 2012;1:11. doi: 10.1186/2047-2994-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368:299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arroliga AC, Thompson BT, Ancukiewicz M, Gonzales JP, Guntupalli KK, Park PK, Wiedemann HP, Anzueto A, Acute Respiratory Distress Syndrome Network Use of sedatives, opioids, and neuromuscular blocking agents in patients with acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2008;36:1083–1088. doi: 10.1097/CCM.0B013E3181653895. [DOI] [PubMed] [Google Scholar]
- 6.Mehta S, Burry L, Fischer S, Martinez-Motta JC, Hallett D, Bowman D, Wong C, Meade MO, Stewart TE, Cook DJ, Canadian Critical Care Trials Group Canadian survey of the use of sedatives, analgesics, and neuromuscular blocking agents in critically ill patients. Crit Care Med. 2006;34:374–380. doi: 10.1097/01.CCM.0000196830.61965.F1. [DOI] [PubMed] [Google Scholar]
- 7.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 8.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 9.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 10.Finfer S, Wernerman J, Preiser JC, Cass T, Desaive T, Hovorka R, Joseph JI, Kosiborod M, Krinsley J, Mackenzie I, Mesotten D, Schultz MJ, Scott MG, Slingerland R, Van den Berghe G, Van Herpe T. Clinical review: Consensus recommendations on measurement of blood glucose and reporting glycemic control in critically ill adults. Crit Care. 2013;17:229. doi: 10.1186/cc12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balogh Z, Moore FA, Moore EE, Biffl WL. Secondary abdominal compartment syndrome: a potential threat for all trauma clinicians. Injury. 2007;38:272–279. doi: 10.1016/j.injury.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Coats TJ, Brazil E, Heron M, MacCallum PK. Impairment of coagulation by commonly used resuscitation fluids in human volunteers. Emerg Med J. 2006;23:846–849. doi: 10.1136/emj.2006.036574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krausz MM. Initial resuscitation of hemorrhagic shock. World J Emerg Surg. 2006;1:14. doi: 10.1186/1749-7922-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heart N, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, DeBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 15.Barochia AV, Cui X, Vitberg D, Suffredini AF, O'Grady NP, Banks SM, Minneci P, Kern SJ, Danner RL, Natanson C, Eichacker PQ. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med. 2010;38:668–678. doi: 10.1097/CCM.0b013e3181cb0ddf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 17.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis, The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 18.Scottish Intercollegiate Guidelines Network (SIGN) 50: A Guideline Developer's Handbook.http://www.sign.ac.uk/guidelines/fulltext/50/index.html.
- 19.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deeks JJ, Higgins JPT, Altman DG. Analysing data undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1. Sussex: The Cochrane Collaboration; 2008. pp. 244–265. [Google Scholar]
- 22.Early Goal-Directed Therapy Collaborative Group of Zhejiang Province The effect of early goal-directed therapy on treatment of critical patients with severe sepsis/septic shock: a multi-center, prospective, randomized, controlled study. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22:331–334. [PubMed] [Google Scholar]
- 23.Lin SM, Huang CD, Lin HC, Liu CY, Wang CH, Kuo HP. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock. 2006;26:551–557. doi: 10.1097/01.shk.0000232271.09440.8f. [DOI] [PubMed] [Google Scholar]
- 24.The ProCESS Investigators A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Micek ST, Roubinian N, Heuring T, Bode M, Williams J, Harrison C, Murphy T, Prentice D, Ruoff BE, Kollef MH. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2006;34:2707–2713. doi: 10.1097/01.CCM.0000241151.25426.D7. [DOI] [PubMed] [Google Scholar]
- 26.Trzeciak S, Dellinger RP, Abate NL, Cowan RM, Stauss M, Kilgannon JH, Zanotti S, Parrillo JE. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest. 2006;129:225–232. doi: 10.1378/chest.129.2.225. [DOI] [PubMed] [Google Scholar]
- 27.Kortgen A, Niederprum P, Bauer M. Implementation of an evidence-based “standard operating procedure” and outcome in septic shock. Crit Care Med. 2006;34:943–949. doi: 10.1097/01.CCM.0000206112.32673.D4. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro NI, Howell MD, Talmor D, Lahey D, Ngo L, Buras J, Wolfe RE, Weiss JW, Lisbon A. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med. 2006;34:1025–1032. doi: 10.1097/01.CCM.0000206104.18647.A8. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen HB, Corbett SW, Steele R, Banta J, Clark RT, Hayes SR, Edwards J, Cho TW, Wittlake WA. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35:1105–1112. doi: 10.1097/01.CCM.0000259463.33848.3D. [DOI] [PubMed] [Google Scholar]
- 30.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425–432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Solh AA, Akinnusi ME, Alsawalha LN, Pineda LA. Outcome of septic shock in older adults after implementation of the sepsis “bundle”. J Am Geriatr Soc. 2008;56:272–278. doi: 10.1111/j.1532-5415.2007.01529.x. [DOI] [PubMed] [Google Scholar]
- 32.Sebat F, Johnson D, Musthafa AA, Watnik M, Moore S, Henry K, Saari M. A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients. Chest. 2005;127:1729–1743. doi: 10.1378/chest.127.5.1729. [DOI] [PubMed] [Google Scholar]
- 33.Ferrer R, Artigas A, Levy MM, Blanco J, González-Díaz G, Garnacho-Montero J, Ibáñez J, Palencia E, Quintana M, De la Torre-Prados MV, Edusepsis Study Group Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299:2294–2303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
- 34.Girardis M, Rinaldi L, Donno L, Marietta M, Codeluppi M, Marchegiano P, Venturelli C, Sopravvivere alla Sepsi Group of the Modena-University Hospital Effects on management and outcome of severe sepsis and septic shock patients admitted to the intensive care unit after implementation of a sepsis program: a pilot study. Crit Care. 2009;13:R143. doi: 10.1186/cc8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castellanos-Ortega A, Suberviola B, García-Astudillo LA, Holanda MS, Ortiz F, Llorca J, Delgado-Rodríguez M. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med. 2010;38:1036–1043. doi: 10.1097/CCM.0b013e3181d455b6. [DOI] [PubMed] [Google Scholar]
- 36.Gao F, Melody T, Daniels DF, Giles S, Fox S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study. Crit Care. 2005;9:R764–R770. doi: 10.1186/cc3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pestaña D, Espinosa E, Sangüesa-Molina JR, Ramos R, Pérez-Fernández E, Duque M, Martínez-Casanova E, REASEP Sepsis Study Group Compliance with a sepsis bundle and its effect on intensive care unit mortality in surgical septic shock patients. J Trauma. 2010;69:1282–1287. doi: 10.1097/TA.0b013e3181c4539f. [DOI] [PubMed] [Google Scholar]
- 38.Miller RR, 3rd, Dong L, Nelson NC, Brown SM, Kuttler KG, Probst DR, Allen TL, Clemmer TP, Intermountain Healthcare Intensive Medicine Clinical Program Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188:77–82. doi: 10.1164/rccm.201212-2199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heppner HJ, Singler K, Kwetkat A, Popp S, Esslinger AS, Bahrmann P, Kaiser M, Bertsch T, Sieber CC, Christ M. Do clinical guidelines improve management of sepsis in critically ill elderly patients? A before-and-after study of the implementation of a sepsis protocol. Wien Klin Wochenschr. 2012;124:692–698. doi: 10.1007/s00508-012-0229-7. [DOI] [PubMed] [Google Scholar]
- 40.Na S, Kuan WS, Mahadevan M, Li CH, Shrikhande P, Ray S, Batech M, Nguyen HB, ATLAS Investigators Implementation of early goal-directed therapy and the surviving sepsis campaign resuscitation bundle in Asia. Int J Qual Health Care. 2012;24:452–462. doi: 10.1093/intqhc/mzs045. [DOI] [PubMed] [Google Scholar]
- 41.Lefrant JY, Muller L, Raillard A, Jung B, Beaudroit L, Favier L, Masson B, Dingemans G, Thévenot F, Selcer D, Jonquet O, Capdevila X, Fabbro-Peray P, Jaber S, Sepsi d'Oc study Group in the AzuRéa Group Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: a multicenter study. Ann Fr Anesth Reanim. 2010;29:621–628. doi: 10.1016/j.annfar.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Cardoso T, Carneiro AH, Ribeiro O, Teixeira-Pinto A, Costa-Pereira A. Reducing mortality in severe sepsis with the implementation of a core 6-hour bundle: results from the Portuguese community-acquired sepsis study (SACiUCI study) Crit Care. 2010;14:R83. doi: 10.1186/cc9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho BC, Bellomo R, McGain F, Jones D, Naka T, Wan L, Braitberg G. The incidence and outcome of septic shock patients in the absence of early-goal directed therapy. Crit Care. 2006;10:R80. doi: 10.1186/cc4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, Pérez XL, Sirvent JM, Edusepsis Study Group Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009;180:861–866. doi: 10.1164/rccm.200812-1912OC. [DOI] [PubMed] [Google Scholar]
- 45.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC, Surviving Sepsis Campaign The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 46.ProCESS/ARISE/ProMISe Methodology Writing Committee. Huang DT, Angus DC, Barnato A, Gunn SR, Kellum JA, Stapleton DK, Weissfeld LA, Yealy DM, Peake SL, Delaney A, Bellomo R, Cameron P, Higgins A, Holdgate A, Howe B, Webb SA, Williams P, Osborn TM, Mouncey PR, Harrison DA, Harvey SE, Rowan KM. Harmonizing international trials of early goal-directed resuscitation for severe sepsis and septic shock: methodology of ProCESS, ARISE, and ProMISe. Intensive Care Med. 2013;39:1760–1775. doi: 10.1007/s00134-013-2933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


