Abstract
The phytochemical, antioxidant and mineral composition of hydroalcoholic extract of leaves of Cichorium intybus L., was determined. The leaves were found to possess comparatively higher values of total flavonoids, total phenolic acids. The phytochemical screening confirmed the presence of tannins, saponins, flavonoids, in the leaves of the plant. The leaf extract was found to show comparatively low value of IC50 for 2,2-diphenyl-1-picrylhydrazyl (DPPH) inhibition. The IC50 value of chicory leaves extract was found to be 67.2 ± 2.6 μg/ml. The extracts were found to contain high amount of mineral elements especially Mg and Zn. Due to good phytochemical and antioxidant composition, C. intybus L., leaves would be an important candidate in pharmaceutical formulations and play an important role in improving the human health by participating in the antioxidant defense system against free radical generation.
Keywords: Cichorium intybus L., Phytochemical screening, DPPH, Elemental analysis
1. Introduction
Cichorium intybus L., commonly known as chicory, belongs to family Asteraceae and widely distributed in Asia and Europe (Bais and Ravishankar, 2001). All parts of this plant possess great medicinal importance due to the presence of a number of medicinally important compounds such as alkaloids, inulin, sesquiterpene lactones, coumarins, vitamins, chlorophyll pigments, unsaturated sterols, flavonoids, saponins and tannins (Molan et al., 2003; Nandagopal and Ranjitha kumari, 2007; Muthusamy et al., 2008; Atta et al., 2010). It has been reported that fresh chicory typically contains 68% inulin, 14% sucrose, 5% cellulose, 6% protein, 4% ash, and 3% other compounds, while dried chicory contains approximately 98% inulin and 2% other compounds (Meehye and Shin, 1996). Leaves of chicory are good sources of phenols, vitamins A and C as well as potassium, calcium, and phosphorus (Mulabagal et al., 2009). Furthermore, chicory in rich cichoric acid may stimulate the immune system as well as prevents inflammation and bacterial infections to a limited extent (Nayeemunnisa, 2009). C. intybus has been traditionally used for the treatment of fever, diarrhoea, jaundice and gallstones (Afzal et al., 2009; Abbasi et al., 2009). The studies on rats have shown that C. intybus possesses anti-hepatotoxic and anti-diabetic activities (Saggu et al., 2014). It has been also reported that C. intybus possesses anti-bacterial (Nandagopal and Ranjitha kumari, 2007), anti-inflammatory (Cavin et al., 2005), hyperglycaemic and anti-ulcerogenic activities (Rifat-uz-Zaman et al., 2006). Moreover, C. intybus has been found to be a useful biomonitor of heavy metals such as Pb, Zn, Cu, and Cd (Aksoy, 2008). Forage chicory was used to produce a large quantity of high quality feed in the warm season under favourable conditions. t has been reported that grazing chicory results in reduction of some internal parasites in livestock (Heckendorn et al., 2007) and, therefore, has potential to reduce the use of anthelmintics.
Normal cellular function depends on a balance between the reactive oxygen species (ROS) produced and the antioxidant defense mechanisms available for the cell. This equilibrium is hindered by the ROS upsurge that culminates in oxidative stress (Fidan and Dundar, 2008). They are created during increased metabolic states due to partial reduction of O2. ROS are part and parcel of life. But during chronic or recurrent stress there is increased utilization of energy and more ROS are produced. If the concentration of these ROS exceed the body’s capacity to counteract them, they begin to harm cells and, in cases of chronic stress, our tissues and organs. Many plant extracts and their products have been shown to have significant antioxidant activity which may be an important property of medicinal plants associated with the treatment of several ill-fated diseases including liver toxicity. Thus, herbal plants are considered useful resources to prevent and/or ameliorate certain disorders, such as diabetes, atherosclerosis, hepatotoxicity and other complications.
Phytochemicals, the plant-derived non-nutritive compounds, are one of the different types of the dietary factors which play an important role in various functions of the human body. A huge number of natural compounds present in food materials have been reported to possess antioxidant properties due to the presence of hydroxyl groups in their structure. The antioxidants are the synthetic as well as naturally occurring compounds that avert the oxidative damage to the most important macromolecules such as lipids, proteins and nucleic acids present in human body as well as in food materials by scavenging the free radicals produced in various biochemical processes (Shui and Leong, 2004). The free radicals which are produced due to oxidative stress radicals react with lipids, proteins and nucleic acids and cause stimulation of apoptosis which leads to various neurological, cardiovascular and some other physiological disorders (Uttara et al., 2009). Bioflavonoids, phenolic acids, ascorbic acid and tocopherols are well known subclass of phytochemical compounds which possess antioxidant properties and are used for the treatment of various ailments (Bergman et al., 2001; Barnes, 2001).
A careful review of literature has shown that little data are available on the phytochemical and antioxidant properties of hydroalcoholic extract of leaves of C. intybus. Therefore, the present study was intended to investigate the biochemical, photochemical and antioxidant composition of leaves of C. intybus.
2. Materials and methods
2.1. Extract preparation
The extraction procedure for the hydro-alcoholic extract was carried out as reported by Saggu et al. (2014).
2.2. Phytochemical analysis
Chemical tests for the screening of certain phytochemical compounds were performed on the hydroalcoholic extracts of leaves of C. intybus using standard procedures (Harborne, 1973; Trease and Evans, 1989; Sofowara, 1993) as reported by Shad et al. (2013).
2.2.1. Tannins
Briefly, known amount of sample was boiled in distilled water and then filtered. Few drops of 0.1% ferric chloride solution were added to the filtrate and the change in colour was observed. The appearance of brownish green or a blue-black colour confirmed the presence of tannins.
2.2.2. Saponins
Briefly, known amount of sample was boiled in distilled water in a water bath and filtered. The filtrate was mixed with distilled water and shaken vigorously until a stable persistent froth. The frothing was mixed with olive oil (2 drops) and shaken vigorously. The formation of emulsion indicated the presence of saponins.
2.2.3. Flavonoids
Few drops of 1% aluminium solution were added to a portion of ethanolic extract of each sample. A yellow colouration of the solution indicated the presence of flavonoids.
2.3. Quantitative analysis of phytochemicals
2.3.1. Tannins content
The tannins content from leaves of C. intybus were extracted and estimated by the method described by Shad et al. (2013).
2.3.2. Saponins content
Saponins in different parts of C. intybus were determined by the method as described by Anhawange et al. (2004).The saponins were calculated as g/100 g dry weight.
2.3.3. Total flavonoids content
The total flavonoid contents were measured by a colorimetric assay as reported by Rohman et al. (2010). Absorbance of the mixture, pink in colour, was determined at 510 nm versus a blank containing all reagents except samples of extracts. Rutin was used as standard for the calibration curve. Total flavonoid content of the extracts and fractions were expressed as mg rutin equivalents (RE) per gram of sample (mg/g) and calculated from the calibration curve (R2 = 0.997) (Fig. 1).
Fig. 1.
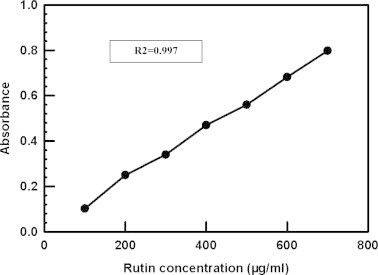
Calibration curve of standard rutin for determination of total flavonoid content.
2.4. Estimation of total phenolic content
Total phenolic content was estimated quantitatively using the method described by Jindal and Singh (1975). A standard curve was prepared by using different concentrations of gallic acid (0.1 mg/ml) and used for the determination of total phenolic compounds content (mg/g gallic acid equivalent). A linear calibration curve of Gallic acid, with coefficient of determination (r2) value of 0.998, was obtained.
2.5. DPPH method of total antioxidant capacity assessment
DPPH scavenging activity was measured using the method described by Brand-Williams et al. (1995). This assay is based on the determination of the concentration of 2,2-diphenyl-1-picrylhydrazyl (DPPH) methanolic solution, after adding the antioxidants. DPPH concentration is reduced by the existence of an antioxidant at 515 nm and the absorption gradually disappears with time. Basically, 0.1 ml of the hydroalcoholic extract was added to 0.3 ml of methanolic solution of DPPH radical (660 μM). The mixture was shaken vigorously for 1 min at room temperature. Thereafter 30 min, the absorbance for the sample (Asample) was measured using a spectrophotometer at 515 nm against the methanol blank. All samples were analysed in triplicates and results averaged. A negative control was taken after adding DPPH solution to 0.1 ml of the methanol.
| (1) |
Where
AS- Sample absorbance over 30 min.
AB- Blank absorbance over 30 min.
2.6. Determination of minerals
2.6.1. Wet ashing of plant tissue
Samples (0.5 g) of dried ground plant material were weighed into 100-ml Kjeldahl flasks containing a glass bead. The samples were predigested with 5 ml of HNO3, cooled, and digested to fumes of HC1O4 after adding 5 ml of a mixture of HNO3 and HC1O4 (3 + 1) according to the method reported by Leggett and Westermann (1973). After digestion, about 40 ml of distilled demineralized water was added to each flask and the contents were brought to near boiling to ensure complete dissolution of sample except for silica. Samples were cooled, diluted to 50 ml, and filtered.
2.6.2. Chemical determinations
The metal cations in the extract and digest were determined by atomic absorption spectrometry (model 2380; Perkin Elmer, Norwalk, CT, USA). The solutions were used for determination of calcium (Ca), magnesium (Mg), manganese (Mn), copper (Cu), sodium (Na), iron (Fe), zinc (Zn), and selenium (Se).
2.7. Statistical analysis
All data are presented as means ± SD for at least four replications for each prepared sample. Statistical analysis was performed using one-sample t-test. The results are considered to be significant when P < 0.05. All statistical analyses were performed using SigmaPlot, Systat Software program version 11.
3. Results and discussion
3.1. Quantitative phytochemical and total antioxidant potential analysis
The phytochemical screening of leaves of C. intybus showed the presence of tannins, saponins, flavonoids, and DPPH inhibition. The results of quantitative analysis of phytochemicals are show in Table 1. Tannins and saponins content of C. intybus leaves extract is from 0.59 to 0.18 mg/g (Table 1). These results are somewhat in the agreement as reported by Shad et al. (2013), Tannins, the high molecular weight polyphenolic compounds found naturally in many plants and have been found to play a protective role in plants against micro-organisms, unfavourable climatic conditions and damage by animals. On the other hand, they can form multiple hydrogen bonds with carboxylic groups of dietary proteins and proteolytic enzymes in the gastrointestinal tract which leads to reduced digestibility of proteins and finally the retardation of animal growth (Reed et al., 1990). Saponins are the glycosidic compounds found in most of the plants, possess a bitter taste and foaming properties. Rao and Sung (1995) reported that the saponins have been found to possess anti-carcinogenic and antifungal activity.
Table 1.
Tannins, saponins and total flavonoid contents in chicory leaves extract. Value represents mean ± SD of four determinations.
| Tannins (mg/g) | 0.59 ± 0.04 |
| Saponins (mg/g) | 0.18 ± 0.06 |
| Total flavonoids (TF) (mg/g dry weight) | 6.82 ± 0.07 |
The flavonoids and phenolic acids are known to possess antioxidant activities due to the presence of hydroxyl groups in their structures and their contribution to defence system against the oxidative damage due to endogenous free radicals is extremely important (Saggu et al., 2014). Phenolics or polyphenols are secondary plant metabolites that are ubiquitously present in plants and their products. Many of them have been shown to contain high levels of antioxidant activities (Razali et al., 2008). Due to their redox properties these compounds contribute to the overall antioxidant activities of plants. Usually, the mechanisms of their antioxidant activity are neutralizing lipid free radicals and preventing decomposition of hydroperoxides into free radicals (Javanmardi et al., 2003; Li et al., 2009). The chicory leaves extract used in this study was partially characterized with reference to total flavonoids; total phenolic contents and its total antioxidant capacity (IC50). C. intybus has been found to have great medicinal importance due to the presence of phenolic compounds. The results show that leaves of C. intybus are good source of phenolic compounds. Due to high TF and TPA content, the leaves have been found to possess comparatively good reducing power and DPPH radical scavenging capacity. The extract was found to be rich in high flavonoid content (6.82 mg/g of RE) and phenolic content (85 mg/g of gallic acid equivalent) which may be responsible for its observed antioxidant activity (Table 2). The DPPH test is a widely used method to evaluate the free radical scavenging effect of plant extracts. This method is based on the reduction of methanolic DPPH solution in the presence of antioxidant resulting in the formation of non radical DPPH-H by the reaction. The degree of discolouration shows the scavenging potential of the extract. In this study DPPH radical scavenging based antioxidant potential of the extracts was evaluated using the parameter IC50. Here, IC50 means the concentration of antioxidant required for 50% scavenging of DPPH radicals in the specified time. The extracts had dose-dependent activity, i.e. DPPH scavenging activity increased proportionate to the increase in concentration of the extracts (data not shown). The IC50 value of chicory leaves extract was found to be 67.2 ± 2.6 μg/ml (Table 2). Shad et al. (2013) showed the similar tendency in leaves showing good free radical scavenging capacity due to higher DPPH radical inhibition and lower IC50 value.
Table 2.
Total flavonoids; total phenolics contents and percentage inhibition of antioxidant activity (IC50) in chicory leaves extract. Value represents are mean ± SD of four determinations.
| Parameter | Chicory fruit extracts |
|---|---|
| Total phenolic compound (mg/g gallic acid) | 85 ± 6.23 |
| IC50 (μg/ml) | 67.27 ± 1.17 |
3.2. Elemental analysis
The amount of Ca is 3.50%, Mg 0.28%, Na 0.08%, while Cu is 32 ppm, Zn 47.2 ppm, Mn 71 ppm and Se 0.32 μg/g (Table 3). Trace element plays a crucial role in the medicinal value of a plant, in health and to cure disease. They play a nutritive, catalytic and balancing function in plants (Joyo et al., 1997). Plants take them from the ground and incorporate them into organic compounds that we consume them by eating either the plants or the animals that ate the plants. In the present study Ca, Mg, Na are in large quantity while Cu, Zn and Mn are relatively low compared to the other elements. The Ca is found in large percentage that is (3.5%) Haag and Minami (1998) reported the macro and micro nutrients of leaves of C. intybus from Brazil include 4.39% N, 0.47% P, 2.93% K, 1.0% C, 0.35% Mg, 0.2% S, 59 ppm B, 15 ppm Cu, 2926 ppm Fe, 117 ppm Mn and 80.0 ppm Zn. The main difference which is observed between the two studies is that Ca, Mg and Cu are present in large amount in leaves of C. intybus (Swat) while Zn and Mn are present in lesser amount. Similarly, Kaneez et al. (2000) detected similar elements and showed the same pattern except with slight differences in values. Hence, the plant can be recommended as raw or in addition form for food products.
Table 3.
Elemental analysis of chicory leaves in terms of percentage (%) and ppm.
| Ca (%) | Mg (%) | Na (%) | Cu (ppm) | Zn (ppm) | Mn (ppm) | Se (μg/g) |
|---|---|---|---|---|---|---|
| 3.5 | 0.28 | 0.08 | 32.00 | 47.2 | 71.0 | 0.32 |
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbasi A.M., Khan M.A., Ahmad M., Zafar M., Khan H., Muhammad N., Sultana S. Medicinal plants used for the treatment of jaundice and hepatitis based on socio-economic documentation. Afr. J. Biotechnol. 2009;8(8):1643–1650. [Google Scholar]
- Afzal S., Afzal N., Awan M.R., Khan T.S., Gilani A., Khanum R., Tariq S. Ethno-botanical studies from Northern Pakistan. J. Ayub Med. Coll. Abbotabad. 2009;21(1):52–57. [PubMed] [Google Scholar]
- Aksoy A. Chicory (Cichorium inthybus L.): possible biomonitor of metal pollution. Pak. J. Bot. 2008;40(2):791–797. [Google Scholar]
- Anhawange A.A., Ajibola V.O., Oniye S.J. Chemical studies of seeds of moronga oleifera (Lam) and detarium microcarpum (Guill and Sperr) J. Biol. Sci. 2004;4(6):711–715. [Google Scholar]
- Atta A.H., Elkoly T.A., Mouneir S.M., Kamel G., Alwabel N.A., Zaher S. Hepatoprotective effect of methanolic extracts of Zingiber officinale and Cichorium intybus. Indian J. Pharm. Sci. 2010;72(5):564–570. doi: 10.4103/0250-474X.78521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais H.P., Ravishankar G.A. Cichorium intybus L. cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. J. Sci. Food Agric. 2001;81:467–484. [Google Scholar]
- Barnes S. Role of phytochemicals in prevention and treatment of prostate cancer. Epidemiol. Rev. 2001;23(1):201–205. doi: 10.1093/oxfordjournals.epirev.a000773. [DOI] [PubMed] [Google Scholar]
- Bergman M., Varshavsky L., Gottlieb H.E., Grossman S. The antioxidant activity of aqueous spinach extract: chemical identification of active fractions. Phytochem. 2001;58:143–152. doi: 10.1016/s0031-9422(01)00137-6. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995;28:25–30. [Google Scholar]
- Cavin C., Delannoy M., Malnoe A., Debefve E., Touche A., Courtois D., Schilter B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem. Biophys. Res. Commun. 2005;327(3):742–749. doi: 10.1016/j.bbrc.2004.12.061. [DOI] [PubMed] [Google Scholar]
- Fidan A.F., Dundar Y. The effects of Yucca schidigera and Quillaja saponaria on DNA damage, protein oxidation, lipid peroxidation and some biochemical parameters in streptozotocin-induced diabetic rats. J. Diabetes Complications. 2008;22:348–356. doi: 10.1016/j.jdiacomp.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Haag H.P., Mianami K. Mineral nutrition of vegetable crops. Absorption of nutrients by chicory crop. Anaisda-Escola-Superior-de-Agricultura. Luiz-de-Queiroz. 1998;45(2):257–603. [Google Scholar]
- Harborne J.B. Chapman and Hall Ltd; London: 1973. Phytochemical Methods. [Google Scholar]
- Heckendorn F., Haring D.A., Maurer V., Senn M., Hertzberg H. Individual administration of three tanniferous forage plants to lambs artificially infected with Haemonchus contortus and Cooperia curticei. Vet. Parasitol. 2007;146:123–134. doi: 10.1016/j.vetpar.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Javanmardi J., Stushnoff C., Locke E., Vivanco J.M. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003;83:547–550. [Google Scholar]
- Jindal K.K., Singh R.N. Phenolic content in male and female carica papaya: a possible physiological marker for sex identification of vegetable seedlings. Physiol. Plant. 1975;33:104–107. [Google Scholar]
- Joyo M., Ali S.S., Kazi T., Kazi G.H. Determination of trace elements in Helotropium europaeum L. Hamdard Medicus. 1997;40(4):50–53. [Google Scholar]
- Kaneez R.A., Shirin K.M., Qadiruddin M.A.K., Badar Y. Essential elements in different parts of Kasni (Cichorium intybus) Pak. J. Sci. Ind. Res. 2000;43(5):283–284. [Google Scholar]
- Leggett G.E., Westermann D.T. Determination of mineral elements in plant tissues using trichloroacetic acid extraction. J. Agric. Food Chem. 1973;21(1):65–69. [Google Scholar]
- Li H., Hao Z., Wang X., Huang L., Li J. Antioxidant activities of extracts and fractions from Lysimachia foenum-graecum Hance. Bioresour. Technol. 2009;100:970–974. doi: 10.1016/j.biortech.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Meehye K., Shin H.K. The water-soluble extract of chicory reduces glucose uptake from the perfused jejunum in rats. J. Nutr. 1996;126(9):2236–2242. doi: 10.1093/jn/126.9.2236. [DOI] [PubMed] [Google Scholar]
- Molan A.L., Duncan A.J., Barryand T.N., McNabb W.C. Effect of condensed tannins and sesquiterpene lactones extracted from chicory on the motility of larvae of deer lungworm and gastrointestinal nematodes. Parasitol. Int. 2003;52:209–218. doi: 10.1016/s1383-5769(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Mulabagal V., Wang H., Ngouajio M., Nair M.G. Characterization and quantification of health beneficial anthocyanins in leaf chicory (Cichorium intybus) varieties. Eur. Food Res. Technol. 2009;230:47–53. [Google Scholar]
- Muthusamy V.S., Anand S., Sangeetha K.N., Sujatha S., Arun B., Lakshami B.S. Tannins present in Cichorium intybus enhance glucose uptake and inhibit adipogenesis in 3T3-L1 adipocytes through PTP1B inhibition. Chem. Biol. Interact. 2008;174(1):69–78. doi: 10.1016/j.cbi.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Nandagopal S., Ranjitha kumari B.D. Phytochemical and antibacterial studies of chicory (Cichorium intybus L.) – a multipurpose medicinal plant. Adv. Biol. Res. 2007;1(1-2):17–21. [Google Scholar]
- Nayeemunnisa A. Alloxan diabetes-induced oxidative stress and impairment of oxidative defense system in rat brain: neuroprotective effects of Cichorium intybus. Int. J. Diabetes Metabol. 2009;17:105–109. [Google Scholar]
- Rao A.V., Sung M.K. Saponins as anticarcinogens. J. Nutr. 1995;125(3):717–724. doi: 10.1093/jn/125.3_Suppl.717S. [DOI] [PubMed] [Google Scholar]
- Razali N., Razab R., Mat Junit S., Abdul Aziz A. Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale) Food Chem. 2008;111:38–44. [Google Scholar]
- Reed J.D., Soller H., Woodward A. Fodder tree and straw diets for sheep: intake, growth, digestibility and the effect of phenolics on nitrogen utilization. Anim. Feed Sci. Technol. 1990;30:39–50. [Google Scholar]
- Rifat-uz-Zaman, Akhtar M.S., Khan M.S. In vitro antibacterial screening of Anethum graveolens L. Fruit, Cichorium intybus L. leaf, Plantago ovata L. seed husk and Polygonum viviparum L. root extracts against helicobacter pylori. Int. J. Pharmacol. 2006;2:674–677. [Google Scholar]
- Rohman A., Riyanto S., Yuniarti N., Saputra W.R., Utami R., Mulatsih W. Antioxidant activity, total phenolic, and total flavaonoid of extracts and fractions of red fruit (pandanus conoideus lam) Int. Food Res. J. 2010;17:97–106. [Google Scholar]
- Saggu S., Sakeran M.I., Zidan N., Tousson E., Mohan A., Rehman H. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food Chem. Toxicol. 2014;72C:138–146. doi: 10.1016/j.fct.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Shad M.A., Nawaz H., Rehman T., Ikram N. Determionation of some biochemicals, phytochemicals and antioxidant properties of different parts of Cichorium intybus L: a comparative study. J. Anim. Plant Sci. 2013;23(4):1060–1066. [Google Scholar]
- Shui G.H., Leong L.P. Analysis of polyphenolic antioxidants in star fruit using liquid chromatography and mass spectrometry. J. Chromatogr. A. 2004;1022:67–75. doi: 10.1016/j.chroma.2003.09.055. [DOI] [PubMed] [Google Scholar]
- Sofowara A. Spectrum Books Ltd; Ibadan (Nigeria): 1993. Medicinal Plants and Traditional Medicine in Africa; p. 289. [Google Scholar]
- Trease G.E., Evans W.C. 11th ed. Macmillian Publishers; Brailliar, Tiridel Can: 1989. Pharmacognsy. [Google Scholar]
- Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]


