Background: System xc− is involved in various pathophysiological conditions, such as neurodegenerative disorders and cancer.
Results: Extracellular cystathionine competitively inhibited cystine uptake and could be exchanged with intracellular glutamate via system xc−.
Conclusion: Cystathionine is exclusively transported into immune tissues as the third physiological substrate of system xc−.
Significance: Cystathionine can be exchanged with glutamate to reduce extracellular glutamate levels.
Keywords: Amino Acid Transport, Exchanger, Glutamate, Oxidative Stress, Substrate Specificity, Cystathionine, Cystine, Glutathione, System xc−
Abstract
The cystine/glutamate transporter, designated as system xc−, is important for maintaining intracellular glutathione levels and extracellular redox balance. The substrate-specific component of system xc−, xCT, is strongly induced by various stimuli, including oxidative stress, whereas it is constitutively expressed only in specific brain regions and immune tissues, such as the thymus and spleen. Although cystine and glutamate are the well established substrates of system xc− and the knockout of xCT leads to alterations of extracellular redox balance, nothing is known about other potential substrates. We thus performed a comparative metabolite analysis of tissues from xCT-deficient and wild-type mice using capillary electrophoresis time-of-flight mass spectrometry. Although most of the analyzed metabolites did not show significant alterations between xCT-deficient and wild-type mice, cystathionine emerged as being absent specifically in the thymus and spleen of xCT-deficient mice. No expression of either cystathionine β-synthase or cystathionine γ-lyase was observed in the thymus and spleen of mice. In embryonic fibroblasts derived from wild-type embryos, cystine uptake was significantly inhibited by cystathionine in a concentration-dependent manner. Wild-type cells showed an intracellular accumulation of cystathionine when incubated in cystathionine-containing buffer, which concomitantly stimulated an increased release of glutamate into the extracellular space. By contrast, none of these effects could be observed in xCT-deficient cells. Remarkably, unlike knock-out cells, wild-type cells could be rescued from cystine deprivation-induced cell death by cystathionine supplementation. We thus conclude that cystathionine is a novel physiological substrate of system xc− and that the accumulation of cystathionine in immune tissues is exclusively mediated by system xc−.
Introduction
In mammalian cultured cells, the activity of the cystine/glutamate transporter, designated as system xc− (1), has been shown to be essential for maintaining intracellular glutathione levels and the extracellular cystine/cysteine redox balance (2). The substrate-specific subunit of this transporter, xCT (3, 4), is strongly induced by various stimuli, such as oxidative stress and electrophile agents, whereupon intracellular glutathione levels are increased. When cells are cultured in cystine-free medium or the cystine uptake via xCT is inhibited, most of cultured cells die within 24 h due to glutathione depletion (5). Thus, system xc− is thought to be one of the adaptive cellular defense systems against oxidative stress.
Constitutive expression of xCT mRNA is observed in specific regions of the brain, such as meninges and circumventricular organs (6), and in immune tissues, such as thymus and spleen (7). We previously generated xCT-deficient mice and analyzed their phenotypes (8). Although mice were healthy in appearance and fertile, the plasma redox balance was shifted to a more oxidative state. We further demonstrated that xCT-deficient mice are more susceptible to the oxidative stress-inducing agent paraquat than wild-type mice and that glutathione levels in lungs of xCT-deficient mice are lower than those of wild-type mice under these conditions (9). These results indicate that xCT plays a protective role against oxidative stress in vivo and contributes to maintaining glutathione levels of tissues exposed to oxidative stress. Hence, the function of xCT is to supply cells with sufficient cysteine as a precursor of intracellular glutathione, which suggests a beneficial and protective role of xCT in cell survival and function.
In recent years, it has been suggested that xCT is involved in cancer development and other diseases (10). For instance, we have demonstrated that increased glutathione via system xc− increases cisplatin resistance of human ovarian cancer (11). Lo et al. (12) have reported that system xc− plays a major role in pancreatic cancer growth and therapy resistance by enhancing glutathione synthesis. In this context, Ishimoto et al. (13), have recently shown that a variant isoform of CD44, one of the cell surface markers associated with cancer stem cells, stabilizes xCT and thereby causes a higher intracellular glutathione level in tumor cells. As a result, the tumor stem cells may thus acquire an increased resistance to chemo- and radiotherapy.
On the other hand, because cystine is taken up into the cell via xCT in exchange for intracellular glutamate with a molar ratio of 1:1 (14), glutamate is inevitably secreted from xCT-expressing cells into the extracellular space. Glutamate released via xCT has been suggested to cause glutamate excitotoxicity and/or oxidative glutamate toxicity in the brain, also known as oxytosis (15). In this context, xCT has been considered to be linked to the pathology of various neurodegenerative disorders, such as Alzheimer disease and Parkinson disease (16, 17). The concentration of extracellular glutamate in the hippocampus seems to be related to the activity of xCT (18). Sontheimer's group (19, 20) provided evidence that inhibition of system xc− restrains tumor growth in the brain and that glutamate released via xCT acts as an essential autocrine/paracrine signal for glioma cell invasion. Savaskan et al. (21) have shown that silencing of xCT by siRNA abrogates glioma-induced neurodegeneration and ameliorates brain edema. In addition to the effects in the brain, glutamate released via xCT by dendritic cells may act as a novel modulator of T cell activation (22). These reports indicate that xCT is also important as a supplier of glutamate into the extracellular space.
In the present study, we sought to investigate the metabolite profile in response to the targeted loss of xCT expression in mice beyond what had already been reported on cystine and glutathione levels in plasma of knock-out mice (8). To this end, we performed an extended, non-targeted analysis of metabolites in several tissues of wild-type and xCT-deficient mice using capillary electrophoresis time-of-flight mass spectrometry. Remarkably, we found that in xCT-deficient mice cystathionine was absent in the thymus and spleen of the xCT-deficient mice as compared with control mice, whereas all other metabolites did not show any substantial differences between wild-type and knock-out animals. Cystathionine is known as an intermediary metabolite in cysteine synthesis from methionine in the transsulfuration pathway, although its function in the immune system has remained enigmatic to date. We therefore investigated the possibility that cystathionine is the third physiological substrate of system xc−.
EXPERIMENTAL PROCEDURES
Materials
l-Cystathionine was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All other chemicals and regents were purchased from Sigma unless otherwise stated.
Metabolome Analysis
C57BL/6J male mice (8–9 weeks old) and xCT-deficient mice (8), which had been back-crossed with C57BL/6J mice for more than 10 generations, were housed at 22 °C with a 12-h alternating light/dark cycle. All mice had free access to standard rodent chow and water. Mice were anesthetized with pentobarbital, and heparinized blood was collected from the inferior vena cava. Collected blood was immediately centrifuged, and 100 μl of plasma was moved into the other tube. In all cases, frozen tissues (∼50 mg) were immediately plunged into methanol (500 μl) containing internal standards (20 μm methionine sulfone and d-camphor-10-sulfonic acid and homogenized for 3 min to inactivate the enzymes. Plasma (50 μl) was added to methanol (450 μl) containing the same internal standards and mixed without homogenizing. Then Milli-Q water (200 μl) (Millipore, Billerica, MA) and chloroform (500 μl) were added, and the mixture was thoroughly mixed. The solution was centrifuged at 4,600 × g for 15 min at 4 °C, and the 450-μl upper aqueous layer was filtered by centrifugation through a Millipore 5 kDa cut-off filter to remove large molecules. Plasma was centrifuged in the same manner but only for 5 min. The filtrate was lyophilized and dissolved in Milli-Q water (50 μl for tissue and 25 μl for plasma) containing a reference compound (200 μm each of 3-aminopyrrolidine and trimesate) prior to capillary electrophoresis-TOF/MS analysis. Capillary electrophoresis-TOF/MS was carried out as described previously (23).
Induction of Experimental Hypercystathionemia
To induce experimental hypercystathionemia, male mice (8–9 weeks) were injected with propargylglycine, a cystathionine γ-lyase (CGL)2 inhibitor, diluted in saline (intraperitoneally; 50 mg/kg) daily for 3 days (24). Control male mice were injected with an equivalent volume of saline (intraperitoneally). After certain treatment periods, blood was collected from inferior vena cava, and the liver, thymus, and spleen were removed. These experiments were approved by the Animal Research Committee of Yamagata University.
Measurement of Cystathionine Levels in Plasma, Liver, Thymus, and Spleen
Collected blood was immediately centrifuged, and 100 μl of plasma was transferred into another tube containing 10 μl of 50% sulfosalicylic acid. After 30 min in an ice bath, the mixture was stored at −20 °C until further processing. The samples were thawed and centrifuged at 10,000 × g for 15 min, and the supernatant was removed. Isolated liver, thymus, and spleen were weighed and immediately homogenized in 5% trichloroacetic acid and then treated with ether to remove the acid. Then, samples were adjusted to pH 2.2 with 1 m LiOH. Cystathionine levels were analyzed using an amino acid analyzer (Hitachi model L-8800, Tokyo, Japan).
Reverse Transcription-PCR (RT-PCR)
Total RNA from tissues and cultured cells was isolated using Isogen (Nippon Gene, Toyama, Japan) by following the manufacturer's instructions. First-strand cDNA synthesis and quantitative PCR were performed using the PrimeScript RT reagent kit (Takara Bio, Shiga, Japan) and SYBR® Premix Ex TaqTM (Takara Bio, Shiga, Japan) according to the manufacturer's instructions. The primer sets for mouse xCT, mouse Cgl, mouse cystathionine β-synthase (Cbs), and mouse glyceraldehyde-3-phosphate dehydrogenase (Gapdh) used for quantitative RT-PCR were 5′-CTCGTGACAGCTGTGGGCAT-3′ and 5′-GGCACTAGACTCAAGAACTGTG-3′, 5′-TGGATCGAGCTTTGAAGGCAGC-3′ and 5′-CAGTTCTGCGTATGCTCCGTAA-3′, 5′-GATTGGCTACGACTTCATCC-3′ and 5′-AGTCCTTCCTGTGCGATGAG-3′, and 5′-GACCCCTTCATTGACCT-3′ and 5′-CCACCACCCTGTTGCTGT-3′, respectively.
Cell Culture
Mouse embryonic fibroblasts (MEF) isolated from wild-type and xCT-deficient mice (8) and xCT-overexpressing MEF (25) were cultured routinely in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2-mercaptoethanol (50 μm), insulin-transferrin-selenium A (Life Technologies, Inc.), penicillin (50 units/ml), and streptomycin (50 μg/ml) at 37 °C in 5% CO2 and 95% air. The addition of 2-mercaptoethanol is necessary for xCT-deficient MEF to grow even under routine cell culture conditions (8, 26). For measurement of cell survival, cells were cultured in cystine-free medium (without 2-mercaptoethanol) with and without 0.1 mm cystathionine for the time periods indicated. The number of viable cells was measured by trypan blue exclusion.
Determination of Intracellular Total Glutathione (GSH and the Oxidized Form of GSH (GSSG))
Cells were seeded on 35-mm dishes (2 × 105 cells/dish) and cultured for 24 h in the routine culture medium. Then cells were cultured in cystine-free medium with and without 0.1 mm cystathionine for the time periods indicated. Cells were washed three times with ice-cold PBS, extracted with 5% trichloroacetic acid, and then treated with ether to remove trichloroacetic acid. Total glutathione content in the aqueous layer was measured using an enzymatic method, which is based on the catalytic action of glutathione in the reduction of 5,5′-dithiobis(2-nitrobenzoic acid) by the glutathione reductase system (27).
Cystine Uptake
Cells were seeded on 35-mm dishes (2 × 105 cells/dish) and cultured for 24 h in the routine culture medium. Then the cells were cultured with or without 50 μm diethyl maleate for an additional 24 h. Cells were washed three times in prewarmed Na+-free PBS(+)G (10 mm phosphate-buffered saline (137 mm choline chloride, 3 mm KCl), pH 7.4, containing 0.01% CaCl2, 0.01% MgCl2·6H2O, and 0.1% glucose) and then incubated in 0.5 ml of prewarmed uptake medium at 37 °C for 2 min. This uptake medium contained 20 μm cystine plus [14C]cystine (0.2 μCi/ml) in Na+-free PBS(+)G. l-Cystathionine, l-glutamate, l-leucine, l-serine, or l-arginine each at final concentrations of 200 μm were added to the uptake medium as inhibitors. Uptake was terminated by rapidly rinsing the cells three times with ice-cold PBS, and the radioactivity in the cells was counted.
Determination of Intracellular and Extracellular Cystathionine and Glutamate Levels
Cells were seeded on 35-mm dishes (2 × 105 cells/dish) and cultured for 24 h in the routine culture medium. Then the cells were cultured with or without 50 μm diethyl maleate for an additional 24 h. Cells were washed three times with prewarmed PBS(+)G (10 mm phosphate-buffered saline (137 mm NaCl, 3 mm KCl), pH 7.4, containing 0.01% CaCl2, 0.01% MgCl2·6H2O, and 0.1% glucose) and then incubated in 0.5 ml of prewarmed PBS(+)G containing 0.1 mm cystathionine or cystine at 37 °C for 15 min. PBS(+)G was collected, dried using a rotary evaporator, and dissolved in 100 μl of amino acid analysis buffer (pH 2.2) for measuring extracellular amino acids. Cells were washed three times with ice-cold PBS, extracted with 5% trichloroacetic acid, treated with ether to remove trichloroacetic acid, and used for measuring intracellular amino acids. Extracellular and intracellular amino acids were analyzed using the amino acid analyzer.
Statistical Analyses
Statistical significances of the differences were determined by Student's t test (*, p < 0.05; **, p < 0.01).
RESULTS
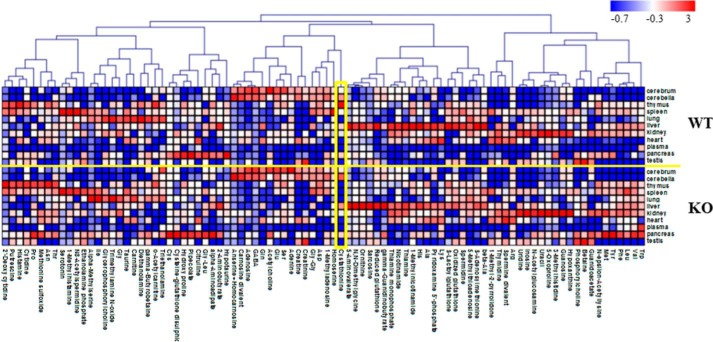
Previously, we showed that mice lacking xCT revealed perturbations in extracellular cystine and GSH levels but otherwise appeared healthy and fully viable (8). Here, we performed a detailed analysis of metabolites in cerebrum, cerebellum, thymus, spleen, lung, liver, kidney, heart, pancreas, testis, and plasma of wild-type (WT) and xCT-deficient (KO) mice using capillary electrophoresis time-of-flight mass spectrometry so that all possible metabolite peaks were profiled. Although most of the metabolite peaks showed no significant differences between WT and KO mice, cystathionine was not detectable in the thymus and spleen of KO mice (Fig. 1 and Table 1). However, significant amounts of cystathionine were detected in the same tissues of WT mice (Table 1), indicating that cystathionine might be a novel substrate for system xc−.
FIGURE 1.
Heat map showing relative metabolite concentrations. In total, 153 metabolites were identified and quantified by metabolomics analysis. Of these, 90 metabolites detected in multiple samples (≥3) from WT or KO mice as well as multiple tissues (≥3) were used here. Each metabolite concentration was transferred to a Z-score and assigned the corresponding color. Pearson correlation was used for clustering. MeV TM4 (47) was used for the analysis and the visualization. The concentrations of cystathionine (indicated with a yellow box) are also given in Table 1.
TABLE 1.
Content of cystathionine in the tissues of WT and xCT-deficient (KO) mice
Cystathionine in each tissue was determined as described under “Materials and Methods” (n = 3–4). All other metabolites in the wild-type mice are shown in Ref. 23. ND, not detected.
| Cystathionine |
||
|---|---|---|
| WT | KO | |
| nmol/g wet weight | ||
| Thymus | 98 ± 43.1 | ND |
| Spleen | 15 ± 3.7 | ND |
| Cerebrum | 15 ± 4.6 | 20 ± 2.7 |
| Cerebellum | 46 ± 4.1 | 67 ± 7.0 |
| Kidney | 10 ± 1.1 | 10 ± 1.3 |
| Liver | 27 ± 11.3 | 46 ± 12.6 |
| Pancreas | 27 ± 16.4 | 26 ± 18.2 |
| Heart | 1 ± 1.6 | 1 ± 1.6 |
| Lung | 1 ± 2.6 | ND |
| Testis | 2 ± 1.3 | ND |
| Plasma | ND | ND |
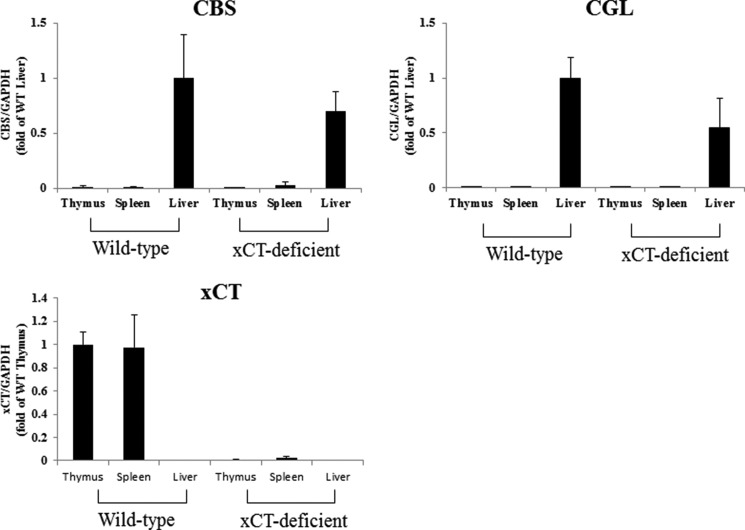
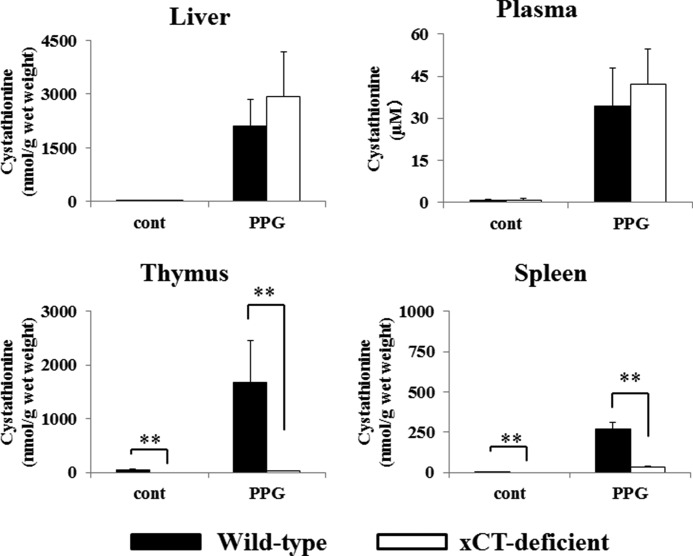
Therefore, we first examined expression of key enzymes involved in cystathionine biosynthesis, CBS and CGL. As shown in Fig. 2, the expression of Cbs and Cgl mRNA were hardly detectable in the thymus and spleen of both WT and KO mice, whereas they were markedly expressed in the livers of these mice. In WT mice, significant expression of xCT mRNA could be detected in the thymus and spleen, whereas no xCT expression could be observed in liver tissue. Although the amount of cystathionine in the thymus of WT mice was completely different from that of KO mice, histological differences in the thymus were not apparent between these mice (data not shown). To induce experimental hypercystathionemia, WT and KO mice were treated with propargylglycine, a CGL inhibitor. Three days after treatment, cystathionine levels in liver, plasma, thymus, and spleen were measured by the amino acid analyzer (Fig. 3). In plasma and liver of xCT-deficient and wild-type mice, cystathionine was markedly increased like in the thymus and spleen of wild-type mice. By stark contrast, it was barely detectable in the thymus and spleen of xCT-deficient mice. These results suggest that in the thymus and spleen, cystathionine is not synthesized but transported via system xc−, whereupon it accumulates in these tissues. Therefore, we have further addressed the possibility that cystathionine is a yet unrecognized physiological substrate of system xc−.
FIGURE 2.
Expression of Cbs, Cgl, and xCT mRNA in the thymus, spleen, and liver of WT and xCT-deficient (KO) mice. Thymus, spleen, and liver were collected from male xCT WT and KO mice. Total RNA was extracted, and Cbs, Cgl, and xCT mRNA expression was determined by quantitative RT-PCR as described under “Experimental Procedures.” GAPDH was used as the internal standard. Cbs and Cgl mRNA expression is represented as -fold of that of liver of wild type, and xCT mRNA expression is represented as -fold of that of thymus of wild-type (n = 3). Error bars, S.D.
FIGURE 3.
Cystathionine levels in liver, plasma, and thymus of WT and xCT-deficient (KO) mice treated with dl-propargylglycine (PPG). Male xCT WT and KO mice were injected with propargylglycine (intraperitoneally; 50 mg/kg) daily for 3 days. Control male mice were injected with an equivalent volume of saline. After treatment periods, blood, liver, thymus, and spleen were collected. Plasma was collected from blood by centrifuge immediately. Cystathionine levels were determined as described under “Experimental Procedures.” Values are means ± S.D. (error bars) (n = 3–4).
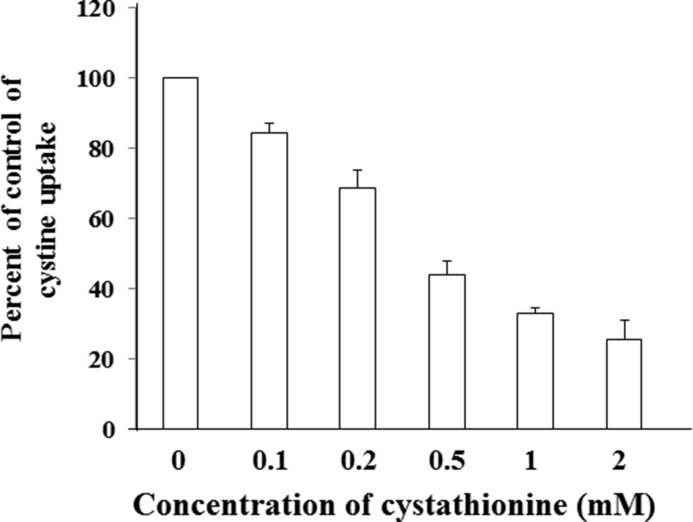
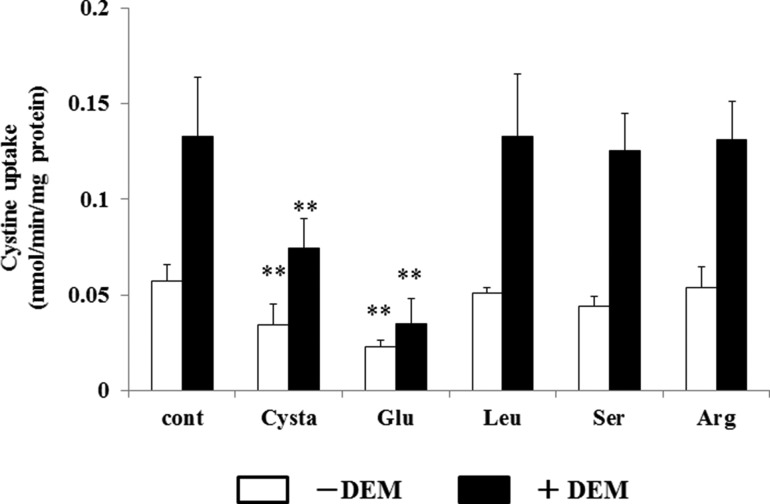
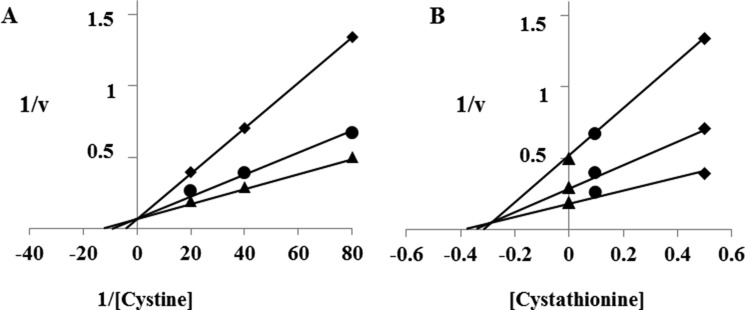
To this end, we measured the activity of cystine uptake in the presence of various concentrations of cystathionine in MEF derived from WT mice (WT MEF) (Fig. 4). The activity of cystine uptake was substantially inhibited by cystathionine in a concentration-dependent manner. As shown in Fig. 5, cystine uptake in WT MEF was significantly inhibited by cystathionine and glutamate, one of the two established physiological substrates of system xc−, but not by leucine, serine, or arginine. When cells were incubated with diethyl maleate, a strong inducer of xCT (3), the activity of cystine uptake was significantly increased. Under these conditions, more prominent inhibition of cystine uptake by cystathionine was observed. Hence, these results strongly suggest that cystathionine is a novel substrate of system xc−. Because radiolabeled cystathionine is commercially not available, we could not perform the kinetic analysis of cystathionine uptake. Instead, the rates of the uptake of cystine at various concentrations were measured in the absence or presence of 0.1 and 0.5 mm cystathionine, and the Michaelis-Menten parameter and Ki value were determined by graphing the data as a Lineweaver-Burk plot and Dixon plot, respectively (Fig. 6). Because the activity of cystine uptake in WT MEF is too low to perform kinetic analyses, we used MEF in which xCT expression was manipulated. These cells stably express xCT in xCT KO MEF under the control of the strong synthetic CAG (chicken β-actin and CMV) promoter (25). The apparent Ki value of cystathionine for cystine uptake was 0.23 ± 0.04 mm.
FIGURE 4.
Effect of cystathionine on cystine uptake in MEF derived from WT mice. WT MEF were cultured for 24 h in the routine culture medium, and then l-[14C]cystine (0.02 mm) uptake was measured in the absence or presence of 0.1–2 mm cystathionine under Na+-free conditions. Each point represents the mean ± S.D. (error bars) (n = 3–4).
FIGURE 5.
Effects of cystathionine and various amino acids on the cystine uptake in MEF derived from WT mice. WT MEF were cultured for 24 h in the routine culture medium and then cultured further for 24 h with or without 50 μm diethyl maleate (DEM). l-[14C]Cystine (0.02 mm) uptake was measured in the absence or presence of 0.2 mm cystathionine, glutamate, leucine, serine, or arginine under Na+-free conditions. Each point represents the mean ± S.D. (error bars) (n = 6). **, p < 0.01 compared with control.
FIGURE 6.
Lineweaver-Burk plot (A) and Dixon plot (B) for the inhibition of l-cystine uptake by cystathionine. xCT-overexpressing MEF were cultured for 24 h in the routine culture medium and then cultured further for 24 h. l-[14C]cystine (0.0125, 0.025, and 0.05 mm) uptake was measured in the absence (▴) or presence of 0.1 mm (●) or 0.5 mm (♦) cystathionine under Na+-containing conditions. The data points are the means of duplicate determinations from one experiment representative of three similar experiments.
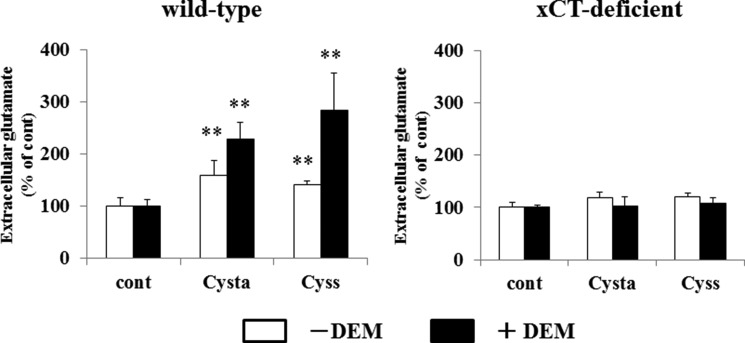
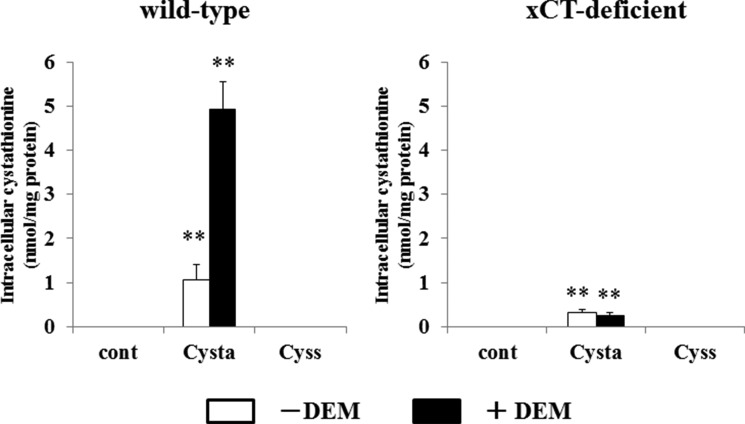
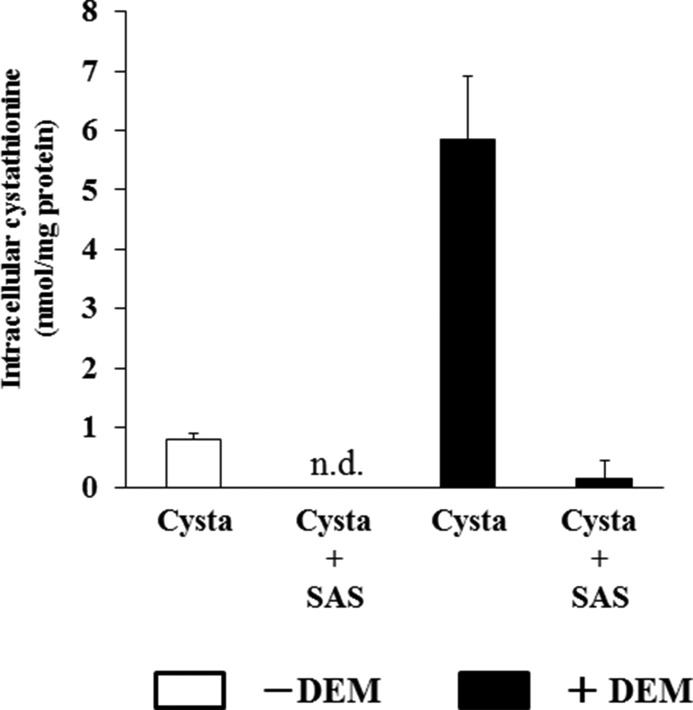
We then studied whether cystathionine can be exchanged for intracellular glutamate. In WT MEF, glutamate was significantly released when the cells were incubated with cystathionine (Fig. 7). When cells were pretreated with diethyl maleate, cystathionine accelerated the release of glutamate into the extracellular space. In contrast to these results, no increase in the release of glutamate was observed in KO MEF when cells were incubated with cystathionine or with cystine. These results indicate that extracellular cystathionine can be exchanged for intracellular glutamate via system xc−. As a direct measurement, Fig. 8 shows that cystathionine indeed accumulated in WT MEF, when cells were incubated with cystathionine. Pretreatment of the cells with diethyl maleate caused a marked accumulation of cystathionine in WT cells in stark contrast to KO MEF. Here, cystathionine was only slightly detectable when cultured with cystathionine, which might be due to nonspecific entry of cystathionine not mediated by system xc−. To test whether cystathionine is transported by system xc−, we have measured the accumulation of cystathionine in the presence of sulfasalazine, a well established system xc− inhibitor (28). As expected from our previous results, the accumulation of cystathionine in WT MEF was completely inhibited in the presence of sulfasalazine (Fig. 9).
FIGURE 7.
Effects of cystathionine on glutamate release in MEF derived from WT and xCT-deficient (KO) mice. WT and KO MEF were cultured for 24 h in the routine culture medium and then cultured further for 24 h with or without 50 μm diethyl maleate (DEM). Then the cells were incubated for 15 min in 1 ml of PBS(+)G (PBS containing 0.1% glucose, 0.01% Ca2+, and 0.01% Mg2+) with or without 0.1 mm cystathionine or 0.1 mm cystine. After a 15-min incubation, the solution was collected, and the glutamate level in the PBS(+)G was determined as described under “Experimental Procedures.” Each point represents the mean ± S.D. (error bars) and is expressed as percentage of control (n = 3–8). **, p < 0.01 compared with control.
FIGURE 8.
Intracellular cystathionine levels in MEF derived from WT and xCT-deficient (KO) mice. WT and KO MEF were cultured for 24 h in the routine culture medium, and then cultured further for 24 h with or without 50 μm diethyl maleate (DEM). Then the cells were incubated for 15 min in 1 ml of PBS(+)G (PBS containing 0.1% glucose, 0.01% Ca2+, and 0.01% Mg2+) with or without 0.1 mm cystathionine or 0.1 mm cystine. After a 15-min incubation, intracellular cystathionine levels were determined as described under “Experimental Procedures.” Each point represents the mean ± S.D. (error bars) (n = 3–8). **, p < 0.01 compared with control.
FIGURE 9.
Intracellular cystathionine levels in MEF derived from WT embryos exposed to sulfasalazine. WT MEF were cultured for 24 h in the routine culture medium and then cultured further for 24 h with or without 50 μm diethyl maleate (DEM). Then, the cells were incubated for 15 min in 1 ml of PBS(+)G (PBS containing 0.1% glucose, 0.01% Ca2+, and 0.01% Mg2+) with or without 0.1 mm sulfasalazine (+SAS) in the presence of 0.1 mm cystathionine. After a 15-min incubation, intracellular cystathionine levels were determined as described under “Experimental Procedures.” Each point represents the mean ± S.D. (error bars) (n = 4; n.d., not detectable).
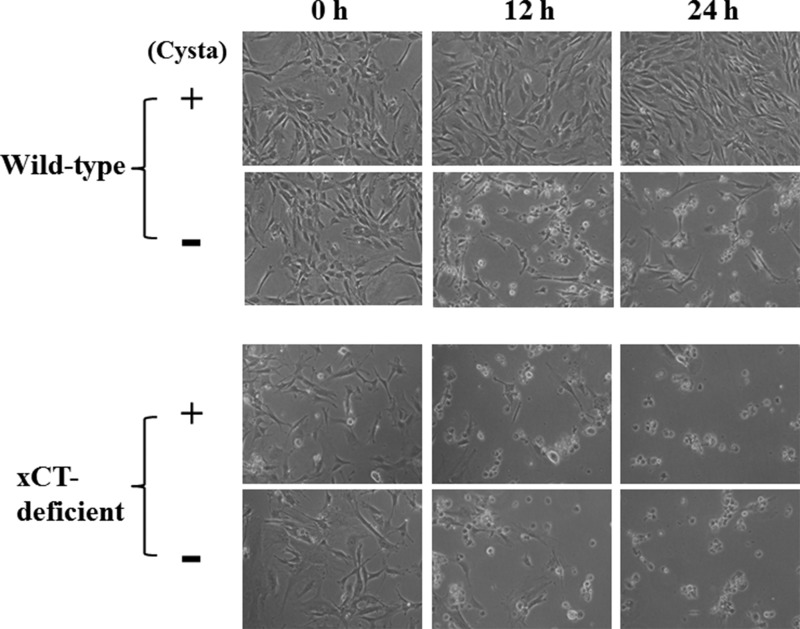
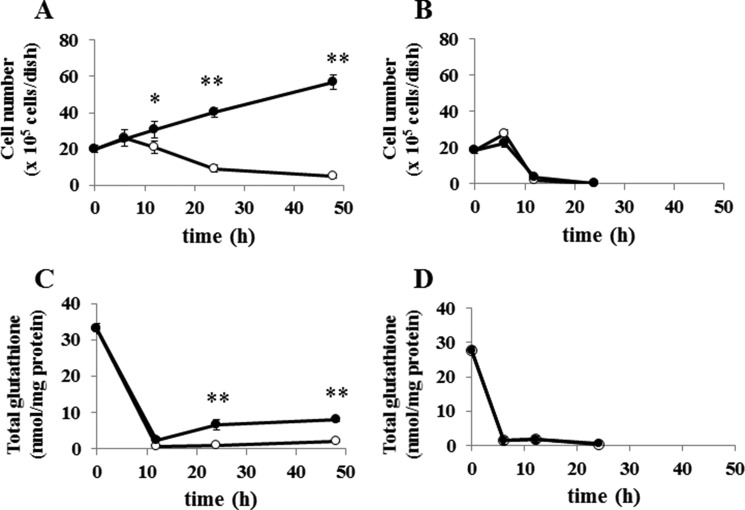
When WT MEF were cultured in cystine-free medium, they failed to proliferate and died within 24 h as expected (Figs. 10 and 11A). However, they survived and even proliferated by adding 0.1 mm cystathionine to cystine-free medium. On the contrary, KO MEF died within 24 h in cystine-free medium regardless of cystathionine (0.1 mm) supplementation (Figs. 10 and 11B). Although total glutathione in WT MEF was rapidly decreased by culturing cells in cystine-free medium, it was significantly restored by culturing the cells in cystine-free medium containing cystathionine (Fig. 11C). In contrast, the intracellular glutathione levels were not increased in KO MEF by culturing in the cystine-free medium with cystathionine (Fig. 11D). These data indicate that cystathionine is able to sustain intracellular glutathione levels even in the absence of exogenous cystine in WT cells but not in KO cells.
FIGURE 10.
Effect of cystathionine on cell viability in MEF derived from WT and xCT-deficient (KO) mice. WT and KO MEF were cultured for 24 h in the routine culture medium and then cultured in cystine-free medium with or without 0.1 mm cystathionine for the indicated time periods in the absence of 2-mercaptoethanol.
FIGURE 11.
Effect of cystathionine on cell viability and intracellular glutathione levels in MEF derived from WT and xCT-deficient (KO) mice. WT and KO MEF were cultured for 24 h in the routine culture medium and then cultured in the cystine-free medium with (●) or without (○) 0.1 mm cystathionine for the indicated time periods in the absence of 2-mercaptoethanol. A and B, cell viability of WT MEF and KO MEF, respectively; C and D, intracellular glutathione levels of WT and KO MEF, respectively. Each point represents the mean ± S.D. (error bars) (n = 4–8). *, p < 0.05; **, p < 0.01 compared with control (without cystathionine).
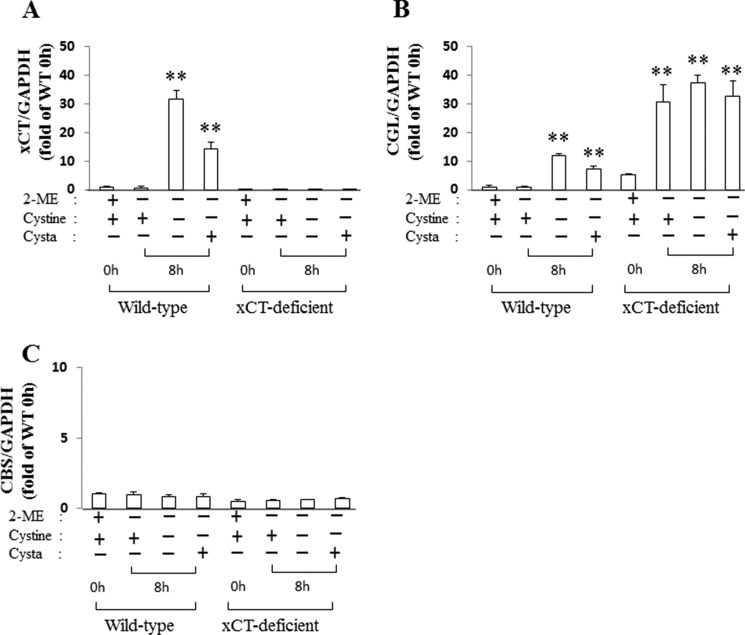
When WT MEF were cultured for 8 h in cystine-free medium with or without cystathionine, the expression of xCT mRNA was strongly induced, compared with cells cultured in the routine culture medium (Fig. 12A). Under these conditions, Cgl mRNA was also markedly induced (Fig. 12B). On the other hand, Cbs mRNA was unchanged by culturing the cells in cystine-free medium with or without cystathionine (Fig. 12C). It is noteworthy that Cgl mRNA in KO MEF was significantly induced even in the routine culture medium (Fig. 12B). The addition of 2-mercaptoethanol is necessary for KO MEF to grow even in the routine culture medium. In Fig. 12B, 2-mercaptoethanol was removed when culturing the cells in cystine-free medium started. Because KO MEF cannot take up extracellular cystine, it is likely that Cgl mRNA was induced in response to the decrease of intracellular cysteine by the removal of 2-mercaptoethanol in these cells.
FIGURE 12.
Expression of xCT, Cgl, and Cbs mRNA in MEF derived from xCT-deficient (KO) and WT mice. WT and KO MEF were cultured for 24 h in the routine culture medium with 50 μm 2-mercaptoethanol and then in cystine-free medium with or without 0.1 mm cystine or cystathionine followed by another 8 h without 2-mercaptoethanol (2-ME). Total RNA was extracted, and xCT (A), Cgl (B), and Cbs (C) mRNA expression was determined by quantitative RT-PCR as described under “Experimental Procedures.” Gapdh was used as an internal standard (n = 3). **, p < 0.01 compared with control (0 h). Error bars, S.D.
DISCUSSION
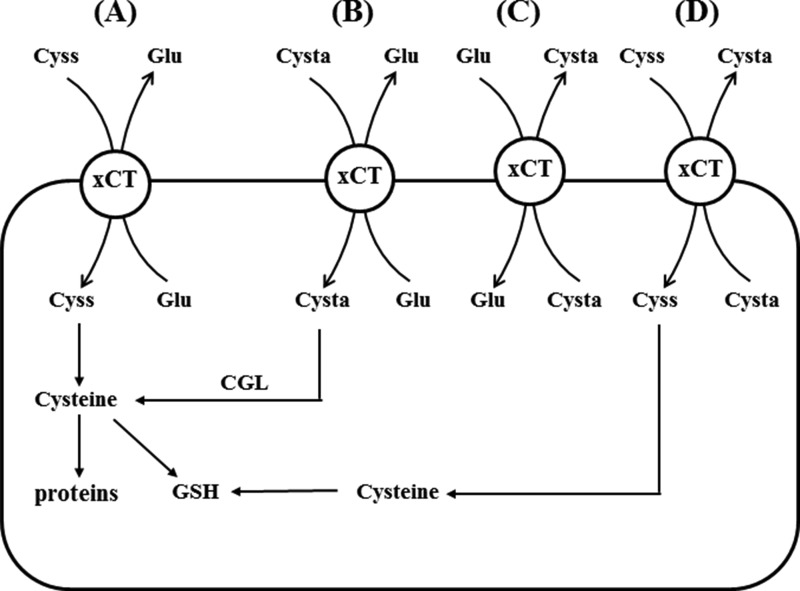
In the present study, we have found that cystathionine is absent in the thymus and spleen of xCT-deficient mice, although it was significantly detected in the same tissues of wild-type mice. As reported previously (7), the expression of xCT mRNA is constitutively expressed in the thymus and spleen. Cbs and Cgl mRNA are not expressed in these tissues of xCT-deficient and wild-type mice (Fig. 2). Our present study thus demonstrates that cystathionine is a novel substrate of system xc− and transported via system xc− in exchange for glutamate in MEF (Fig. 13). From this, we conclude that cystathionine is exclusively transported via system xc− from the extracellular space in the thymus and spleen. Recently, we have observed that xCT mRNA is constitutively expressed in Payer's patch, mesenteric and inguinal lymph nodes, and bone marrow.3 It is thus likely that occurrence of cystathionine in the immune tissues is dependent on the transport activity of system xc−.
FIGURE 13.
Physiological substrates for system xc−. Several exchange transport scenarios for (novel) system xc− substrates are depicted. A, the well established exchange of cystine (Cyss) and glutamate via system xc− is shown. Cystine transported into cells is reduced to cysteine and used for GSH synthesis and protein synthesis. B, exchange of extracellular cystathionine (Cysta) for intracellular glutamate occurs in cells such as WT MEF, expressing CGL, and thus providing cysteine for GSH synthesis. C, exchange of extracellular glutamate for intracellular cystathionine may occur in cells in the thymus, spleen, and brain to regulate extracellular glutamate concentrations. D, exchange of extracellular cystine for intracellular cystathionine may occur especially in the brain to secure intracellular cysteine levels without increasing extracellular glutamate concentrations.
Patel et al. (29) have analyzed the differences of substrate and non-substrate inhibitors of system xc− and revealed that potent inhibitors such as l-quisqualate and (S)-4-carboxyphenylglycine were not always substrates for system xc−. They also showed that cystathionine significantly inhibits the activity of glutamate uptake, although it has remained unclear whether it is a direct substrate for system xc−. The present study now clearly shows that cystathionine is a physiological substrate of system xc− and that it can be exchanged with intracellular glutamate. In general, some portion of cystine exists as a tripolar (monocationic and dianionic) form at pH 7.4, which occupies 19.2% of total cystine molecule (pKa3 = 8.02). System xc− only recognizes the tripolar form of cystine, and this causes the exchange transport with intracellular glutamate (30). Calculating from the pKa values (pKa3 = 8.54) of cystathionine (31), 6.8% of cystathionine exists as a tripolar molecule at pH 7.4; thus, only a small part of cystathionine can be a substrate for system xc−. As illustrated in Fig. 6, cystathionine was indeed shown to inhibit cystine uptake to a lesser extent than glutamate, which might be due to the fact that only the tripolar form of the cystathionine molecule can inhibit cystine uptake. The apparent Ki value of cystathionine for cystine uptake was 0.23 ± 0.04 mm, which is similar to the calculated Km value of glutamate uptake (0.20–0.30 mm) via system xc− in human fibroblasts (1, 32). Therefore, under these conditions, less than 10% of cystathionine is able to inhibit cystine uptake. It is likely that cystathionine with the tripolar form has even higher affinity to xCT than glutamate. In mammalian cells, cystine is transported also by a Na+-independent amino acid transporter called b0,+AT, which was first described in blastocysts and is mainly expressed in kidney and intestine (33, 34). Therefore, the tetrapolar form of cystathionine may also be transported by this transporter, but because cystathionine was not detected in the thymus and spleen of xCT-deficient mice, b0,+AT is probably not expressed in these tissues. Recently, a plasma membrane cystine-specific transporter, CgCYN1, in Candida glabrata was reported (35). Cystine uptake via this transporter is strongly inhibited by cystathionine; however, this transporter shows no similarity to the hitherto known plasma membrane cystine transporters, including xCT. Nonetheless, CgCYN1 may recognize the tetrapolar form of cystine and cystathionine. Recently, a small molecule inhibitor specific for xCT, erastin, has been reported (36, 37), a compound that might prove useful for studying xCT function and other cystine transporters in the future.
The present study further suggests that the supply of cysteine from cystathionine is negligible in the thymus and spleen. There has been no known function for cystathionine other than serving as an intermediate in the transsulfuration pathway. Maclean et al. (38) have recently found that cystathionine is capable of blocking the induction of hepatic steatosis, kidney injury, and apoptotic cell death by mitigating endoplasmic reticulum stress. Thymus is known to undergo profound age-associated atrophy (i.e. thymus involution), which results in less efficient T-cell development and decreased emigration of naive T cells to the periphery (39). During the involution, the thymic epithelial space (cortex and medulla), in which T-cell development or thymopoiesis occurs, is gradually replaced with adipocytes. The present study shows that even if the transsulfuration pathway terminates, cystathionine can exist in the cell only when xCT is expressed. We have observed that the average weight of the thymus of xCT-deficient mice at the age of 8–9 weeks old is greater than that of wild-type mice.3 It seems an appealing hypothesis that cystathionine transported via system xc− plays an important role in preventing steatosis in the thymus. We have previously demonstrated that xCT is induced by endoplasmic reticulum stress caused by amino acid deprivation or the endoplasmic reticulum stress-inducing agent tunicamycin and that the induction is mediated by a genomic cis-element termed the amino acid response element and a transcription factor, activating transcription factor 4 (ATF4) (40). Recently, Dickhout et al. (41) have demonstrated that Cgl is up-regulated by the ATF4 pathway under endoplasmic reticulum stress conditions. In ATF4-deficient MEF, xCT is down-regulated, and intracellular GSH is significantly lower than in wild-type MEF (41). Because ATF4 seems to be one of the important regulatory factors for the expression of xCT (42, 43), the importance of ATF4 for the regulation of gene expression involved in thiol metabolism deserves further investigation.
Glutamate is the major excitatory amino acid neurotransmitter in the mammalian central nervous system, whose function is mediated by several glutamate receptors. In addition, several glutamate transporters play an important role in regulating extracellular glutamate levels in the central nervous system. Recently, the importance of glutamate receptors in non-neural tissues has been recognized (44). Especially, in the immune system, several glutamate receptors are expressed in T cells, and several glutamate transporters are expressed in antigen-presenting cells, such as dendritic cells and macrophages (45). Pacheco et al. (22) have demonstrated that glutamate released via system xc− by dendritic cells modulates T cell activation. Intracellular cystine transported via system xc− is rapidly converted to cysteine, and thus it is hardly detectable in the cell. If cystathionine accumulates in dendritic cells, macrophages, or thymic stromal cells, it can be exchanged with extracellular glutamate, thereby possibly contributing to the regulation of extracellular glutamate levels also in immune tissues (Fig. 13C). It is noteworthy that a substantial amount of cystathionine accumulates in human brain (46). These observations may result from an imbalance between the relative activities of CBS and CGL. The accumulation of cystathionine in the brain has been mainly considered to be a reservoir of cysteine for glutathione synthesis until now; however, it is conceivable that cystathionine might play a role also in exchanging with glutamate to reduce and equilibrate extracellular glutamate levels in the brain (Fig. 13C). In cases where CGL is not fully expressed in some parts of the brain, it is possible that accumulated cystathionine becomes exchanged for cystine, which is then reduced to cysteine and can be used for glutathione synthesis more efficiently than cystathionine (Fig. 13D). Under oxidative stress conditions in the brain, such as ischemia/reperfusion, it is likely that xCT is induced, and cystine is actively transported into cells at the exchange for cystathionine to boost intracellular glutathione concentrations.
Conclusively, our data presented here identify cystathionine as a novel substrate of system xc− and imply that cystathionine may play an important role not only in the immune system (e.g. in thymopoiesis), but also in regulating extracellular glutamate homeostasis in brain through system xc− activity.
This work was supported, in part, by the Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation from the Japan Society for the Promotion of Sciences; a research grant from the Naito Foundation; and Collaborative Research Program of the Institute for Chemical Research, Kyoto University, Grant 2013-43.
S. Kobayashi, S. Azumi, M. Conrad, S. Bannai, and H. Sato, unpublished data.
- CGL
- cystathionine γ-lyase
- CBS
- cystathionine β-synthase
- MEF
- mouse embryonic fibroblast(s)
- ATF4
- activating transcription factor 4.
REFERENCES
- 1. Bannai S., Kitamura E. (1980) Transport interaction of l-cystine and l-glutamate in human diploid fibroblasts in culture. J. Biol. Chem. 255, 2372–2376 [PubMed] [Google Scholar]
- 2. Bannai S., Tateishi N. (1986) Role of membrane transport in metabolism and function of glutathione in mammals. J. Membr. Biol. 89, 1–8 [DOI] [PubMed] [Google Scholar]
- 3. Sato H., Tamba M., Ishii T., Bannai S. (1999) Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 274, 11455–11458 [DOI] [PubMed] [Google Scholar]
- 4. Sato H., Tamba M., Kuriyama-Matsumura K., Okuno S., Bannai S. (2000) Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc. Antioxid. Redox. Signal 2, 665–671 [DOI] [PubMed] [Google Scholar]
- 5. Conrad M., Sato H. (2012) The oxidative stress-inducible cystine/glutamate antiporter, system x(c)(−): cystine supplier and beyond. Amino Acids 42, 231–246 [DOI] [PubMed] [Google Scholar]
- 6. Sato H., Tamba M., Okuno S., Sato K., Keino-Masu K., Masu M., Bannai S. (2002) Distribution of cystine/glutamate exchange transporter, system x(c)−, in the mouse brain. J. Neurosci. 22, 8028–8033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taguchi K., Tamba M., Bannai S., Sato H. (2007) Induction of cystine/glutamate transporter in bacterial lipopolysaccharide induced endotoxemia in mice. J. Inflamm. (Lond.) 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sato H., Shiiya A., Kimata M., Maebara K., Tamba M., Sakakura Y., Makino N., Sugiyama F., Yagami K., Moriguchi T., Takahashi S., Bannai S. (2005) Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem. 280, 37423–37429 [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi S., Kuwata K., Sugimoto T., Igarashi K., Osaki M., Okada F., Fujii J., Bannai S., Sato H. (2012) Enhanced expression of cystine/glutamate transporter in the lung caused by the oxidative-stress-inducing agent paraquat. Free Radic. Biol. Med. 53, 2197–2203 [DOI] [PubMed] [Google Scholar]
- 10. Lo M., Wang Y. Z., Gout P. W. (2008) The x(c)− cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J. Cell Physiol. 215, 593–602 [DOI] [PubMed] [Google Scholar]
- 11. Okuno S., Sato H., Kuriyama-Matsumura K., Tamba M., Wang H., Sohda S., Hamada H., Yoshikawa H., Kondo T., Bannai S. (2003) Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines. Br. J. Cancer 88, 951–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lo M., Ling V., Wang Y. Z., Gout P. W. (2008) The xc- cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br. J. Cancer 99, 464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishimoto T., Nagano O., Yae T., Tamada M., Motohara T., Oshima H., Oshima M., Ikeda T., Asaba R., Yagi H., Masuko T., Shimizu T., Ishikawa T., Kai K., Takahashi E., Imamura Y., Baba Y., Ohmura M., Suematsu M., Baba H., Saya H. (2011) CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell 19, 387–400 [DOI] [PubMed] [Google Scholar]
- 14. Bannai S. (1986) Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J. Biol. Chem. 261, 2256–2263 [PubMed] [Google Scholar]
- 15. Lewerenz J., Hewett S. J., Huang Y., Lambros M., Gout P. W., Kalivas P. W., Massie A., Smolders I., Methner A., Pergande M., Smith S. B., Ganapathy V., Maher P. (2013) The cystine/glutamate antiporter system x(c)(−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox. Signal. 18, 522–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin S., Colin C., Hinners I., Gervais A., Cheret C., Mallat M. (2006) System Xc- and apolipoprotein E expressed by microglia have opposite effects on the neurotoxicity of amyloid-β peptide 1–40. J. Neurosci. 26, 3345–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Massie A., Schallier A., Kim S. W., Fernando R., Kobayashi S., Beck H., De Bundel D., Vermoesen K., Bannai S., Smolders I., Conrad M., Plesnila N., Sato H., Michotte Y. (2011) Dopaminergic neurons of system x(c)(−)-deficient mice are highly protected against 6-hydroxydopamine-induced toxicity. FASEB J. 25, 1359–1369 [DOI] [PubMed] [Google Scholar]
- 18. De Bundel D., Schallier A., Loyens E., Fernando R., Miyashita H., Van Liefferinge J., Vermoesen K., Bannai S., Sato H., Michotte Y., Smolders I., Massie A. (2011) Loss of system x(c)- does not induce oxidative stress but decreases extracellular glutamate in hippocampus and influences spatial working memory and limbic seizure susceptibility. J. Neurosci. 31, 5792–5803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung W. J., Lyons S. A., Nelson G. M., Hamza H., Gladson C. L., Gillespie G. Y., Sontheimer H. (2005) Inhibition of cystine uptake disrupts the growth of primary brain tumors. J. Neurosci. 25, 7101–7110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lyons S. A., Chung W. J., Weaver A. K., Ogunrinu T., Sontheimer H. (2007) Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 67, 9463–9471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Savaskan N. E., Heckel A., Hahnen E., Engelhorn T., Doerfler A., Ganslandt O., Nimsky C., Buchfelder M., Eyüpoglu I. Y. (2008) Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat. Med. 14, 629–632 [DOI] [PubMed] [Google Scholar]
- 22. Pacheco R., Oliva H., Martinez-Navío J. M., Climent N., Ciruela F., Gatell J. M., Gallart T., Mallol J., Lluis C., Franco R. (2006) Glutamate released by dendritic cells as a novel modulator of T cell activation. J. Immunol. 177, 6695–6704 [DOI] [PubMed] [Google Scholar]
- 23. Sugimoto M., Ikeda S., Niigata K., Tomita M., Sato H., Soga T. (2012) MMMDB: Mouse Multiple Tissue Metabolome Database. Nucleic Acids Res. 40, D809–D814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barber T., Triguero A., Martínez-López I., Torres L., García C., Miralles V. J., Viña J. R. (1999) Elevated expression of liver γ-cystathionase is required for the maintenance of lactation in rats. J. Nutr. 129, 928–933 [DOI] [PubMed] [Google Scholar]
- 25. Lewerenz J., Baxter P., Kassubek R., Albrecht P., Van Liefferinge J., Westhoff M. A., Halatsch M. E., Karpel-Massler G., Meakin P. J., Hayes J. D., Aronica E., Smolders I., Ludolph A. C., Methner A., Conrad M., Massie A., Hardingham G. E., Maher P. (2014) Phosphoinositide 3-kinases upregulate system xc(−) via eukaryotic initiation factor 2α and activating transcription factor 4: a pathway active in glioblastomas and epilepsy. Antioxid. Redox. Signal. 20, 2907–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ishii T., Bannai S., Sugita Y. (1981) Mechanism of growth stimulation of L1210 cells by 2-mercaptoethanol in vitro: role of the mixed disulfide of 2-mercaptoethanol and cysteine. J. Biol. Chem. 256, 12387–12392 [PubMed] [Google Scholar]
- 27. Tietze F. (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. 27, 502–522 [DOI] [PubMed] [Google Scholar]
- 28. Gout P. W., Buckley A. R., Simms C. R., Bruchovsky N. (2001) Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia 15, 1633–1640 [DOI] [PubMed] [Google Scholar]
- 29. Patel S. A., Warren B. A., Rhoderick J. F., Bridges R. J. (2004) Differentiation of substrate and non-substrate inhibitors of transport system xc(−): an obligate exchanger of l-glutamate and l-cystine. Neuropharmacology 46, 273–284 [DOI] [PubMed] [Google Scholar]
- 30. Bannai S., Kitamura E. (1981) Role of proton dissociation in the transport of cystine and glutamate in human diploid fibroblasts in culture. J. Biol. Chem. 256, 5770–5772 [PubMed] [Google Scholar]
- 31. Aitken S. M., Kirsch J. F. (2003) Kinetics of the yeast cystathionine β-synthase forward and reverse reactions: continuous assays and the equilibrium constant for the reaction. Biochemistry 42, 571–578 [DOI] [PubMed] [Google Scholar]
- 32. Bannai S. (1984) Induction of cystine and glutamate transport activity in human fibroblasts by diethyl maleate and other electrophilic agents. J. Biol. Chem. 259, 2435–2440 [PubMed] [Google Scholar]
- 33. Van Winkle L. J., Campione A. L., Gorman J. M. (1988) Na+-independent transport of basic and zwitterionic amino acids in mouse blastocysts by a shared system and by processes which distinguish between these substrates. J. Biol. Chem. 263, 3150–3163 [PubMed] [Google Scholar]
- 34. Lee W. S., Wells R. G., Sabbag R. V., Mohandas T. K., Hediger M. A. (1993) Cloning and chromosomal localization of a human kidney cDNA involved in cystine, dibasic, and neutral amino acid transport. J. Clin. Invest. 91, 1959–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yadav A. K., Bachhawat A. K. (2011) CgCYN1, a plasma membrane cystine-specific transporter of Candida glabrata with orthologues prevalent among pathogenic yeast and fungi. J. Biol. Chem. 286, 19714–19723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dixon S. J., Lemberg K. M., Lamprecht M. R., Skouta R., Zaitsev E. M., Gleason C. E., Patel D. N., Bauer A. J., Cantley A. M., Yang W. S., Morrison B., 3rd, Stockwell B. R. (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dixon S. J., Patel D. N., Welsch M., Skouta R., Lee E. D., Hayano M., Thomas A. G., Gleason C. E., Tatonetti N. P., Slusher B. S., Stockwell B. R. (2014) Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3, e02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maclean K. N., Greiner L. S., Evans J. R., Sood S. K., Lhotak S., Markham N. E., Stabler S. P., Allen R. H., Austin R. C., Balasubramaniam V., Jiang H. (2012) Cystathionine protects against endoplasmic reticulum stress-induced lipid accumulation, tissue injury, and apoptotic cell death. J. Biol. Chem. 287, 31994–32005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lynch H. E., Goldberg G. L., Chidgey A., Van den Brink M. R., Boyd R., Sempowski G. D. (2009) Thymic involution and immune reconstitution. Trends Immunol. 30, 366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sato H., Nomura S., Maebara K., Sato K., Tamba M., Bannai S. (2004) Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem. Biophys. Res. Commun. 325, 109–116 [DOI] [PubMed] [Google Scholar]
- 41. Dickhout J. G., Carlisle R. E., Jerome D. E., Mohammed-Ali Z., Jiang H., Yang G., Mani S., Garg S. K., Banerjee R., Kaufman R. J., Maclean K. N., Wang R., Austin R. C. (2012) Integrated stress response modulates cellular redox state via induction of cystathionine γ-lyase: cross-talk between integrated stress response and thiol metabolism. J. Biol. Chem. 287, 7603–7614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lewerenz J., Maher P. (2009) Basal levels of eIF2α phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J. Biol. Chem. 284, 1106–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lewerenz J., Sato H., Albrecht P., Henke N., Noack R., Methner A., Maher P. (2012) Mutation of ATF4 mediates resistance of neuronal cell lines against oxidative stress by inducing xCT expression. Cell Death Differ. 19, 847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Julio-Pieper M., Flor P. J., Dinan T. G., Cryan J. F. (2011) Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol. Rev. 63, 35–58 [DOI] [PubMed] [Google Scholar]
- 45. Pacheco R., Gallart T., Lluis C., Franco R. (2007) Role of glutamate on T-cell mediated immunity. J. Neuroimmunol. 185, 9–19 [DOI] [PubMed] [Google Scholar]
- 46. Tallan H. H., Moore S., Stein W. H. (1958) l-Cystathionine in human brain. J. Biol. Chem. 230, 707–716 [PubMed] [Google Scholar]
- 47. Saeed A. I., Sharov V., White J., Li J., Liang W., Bhagabati N., Braisted J., Klapa M., Currier T., Thiagarajan M., Sturn A., Snuffin M., Rezantsev A., Popov D., Ryltsov A., Kostukovich E., Borisovsky I., Liu Z., Vinsavich A., Trush V., Quackenbush J. (2003) TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34, 374–378 [DOI] [PubMed] [Google Scholar]