Background: β-Catenin links transmembrane cadherin to actin filaments at cell-cell contacts.
Results: Contractile activation increases the association of β-catenin with N-cadherin, which is regulated by actin polymerization.
Conclusion: Actin polymerization controls the recruitment of β-catenin to N-cadherin, which is essential for smooth muscle contraction.
Significance: The regulated interaction of β-catenin with N-cadherin is a novel mechanism for the control of smooth muscle contraction.
Keywords: Adherens Junction, Cytoskeleton, Excitation-Contraction Coupling (E-C Coupling), Signal Transduction, Smooth Muscle
Abstract
β-Catenin is a key component that connects transmembrane cadherin with the actin cytoskeleton at the cell-cell interface. However, the role of the β-catenin/cadherin interaction in smooth muscle has not been well characterized. Here stimulation with acetylcholine promoted the recruitment of β-catenin to N-cadherin in smooth muscle cells/tissues. Knockdown of β-catenin by lentivirus-mediated shRNA attenuated smooth muscle contraction. Nevertheless, myosin light chain phosphorylation at Ser-19 and actin polymerization in response to contractile activation were not reduced by β-catenin knockdown. In addition, the expression of the β-catenin armadillo domain disrupted the recruitment of β-catenin to N-cadherin. Force development, but not myosin light chain phosphorylation and actin polymerization, was reduced by the expression of the β-catenin armadillo domain. Furthermore, actin polymerization and microtubules have been implicated in intracellular trafficking. In this study, the treatment with the inhibitor latrunculin A diminished the interaction of β-catenin with N-cadherin in smooth muscle. In contrast, the exposure of smooth muscle to the microtubule depolymerizer nocodazole did not affect the protein-protein interaction. Together, these findings suggest that smooth muscle contraction is mediated by the recruitment of β-catenin to N-cadherin, which may facilitate intercellular mechanotransduction. The association of β-catenin with N-cadherin is regulated by actin polymerization during contractile activation.
Introduction
Smooth muscle contraction plays a critical role in regulating bodily functions, including the maintenance of appropriate airway tone. Dysregulation of smooth muscle contraction contributes to the pathogenesis of diseases such as asthma. Despite its importance, the mechanisms that regulate smooth muscle contraction are not fully elucidated.
Upon external stimulation, myosin light chain undergoes phosphorylation at Ser-19, which activates myosin ATPase and initiates the sliding of contractile filaments and smooth muscle contraction (1–4). In addition, contractile stimulation induces actin filament polymerization, which may promote smooth muscle force development by enhancing the transmission of force between the contractile unit and the extracellular matrix and by increasing the contractile unit (5–10). Myosin may serve as an “engine” for smooth muscle contraction, whereas the actin cytoskeleton may function as a “transmission system” in smooth muscle (11, 12). However, other mechanisms for the regulation of smooth muscle contraction may also exist.
β-Catenin, a member of the armadillo family of proteins, is a key component of the cadherin-catenin complex in the plasma membrane (13). β-Catenin is composed of an N-terminal head, an Arm (armadillo)2 domain, and a C-terminal tail. The Arm domain of β-catenin binds to the cytoplasmic domain of cadherins, and the extracellular domain of cadherins interacts with their counterparts of adjacent cells to form cell-cell contacts. The N terminus of β-catenin interacts with actin filaments via linker proteins such as α-catenin, vinculin, and VASP (14, 15). Therefore, β-catenin plays an essential role in connecting actin filaments of cells to the cell-cell interface at adherens junctions (13, 15).
Recent work has shown that the cadherin-catenin complex is a mechanosensive and responsive structure (16). Adherens junctions undergo reorganization in endothelial cells in response to tugging forces and thrombin treatment (16). In keratinocytes, the engagement of adherens junctions occurs upon chemical stimulation. Knockout of β-catenin disrupts the structural change (17). The dynamic change of adherens junctions may allow cells to adapt their mechanical properties and intracellular signaling (16, 17).
β-Catenin has been implicated in mediating mechanical integrity and signal transduction in the epidermis and endothelial cells (16, 17). Loss of β-catenin results in defects of mechanotransduction at the adherens junctions of epithelial cells (17). In addition, β-catenin may have a role in T cell transformation (18) and Wnt signaling (19). However, the functional role of β-catenin/cadherin coupling in smooth muscle contraction is largely unknown.
In this study, we found that contractile activation induces the recruitment of β-catenin to N-cadherin in smooth muscle cells/tissues. Disruption of the recruitment of β-catenin to N-cadherin attenuates smooth muscle contraction. Furthermore, actin polymerization has a role in mediating the coupling of β-catenin with N-cadherin. Therefore, we propose that the recruitment of β-catenin to N-cadherin may promote intercellular mechanotransmission and smooth muscle contractility.
EXPERIMENTAL PROCEDURES
Cell Culture
Human airway smooth muscle (HASM) cells were prepared from human bronchi and adjacent tracheas obtained from the International Institute for Advanced Medicine (20). Human tissues were non-transplantable and consent for research was given. This study was approved by the Albany Medical College Committee on Research Involving Human Subjects. Briefly, muscle tissues were incubated for 20 min with dissociation solution (130 mm NaCl, 5 mm KCl, 1.0 mm CaCl2, 1.0 mm MgCl2, 10 mm Hepes, 0.25 mm EDTA, 10 mm d-glucose, 10 mm taurine (pH 7), 4.5 mg of collagenase (type I), 10 mg of papain (type IV), 1 mg/ml BSA, and 1 mm dithiothreitol). All enzymes were purchased from Sigma-Aldrich. The tissues were then washed with Hepes-buffered saline solution (10 mm Hepes, 130 mm NaCl, 5 mm KCl, 10 mm glucose, 1 mm CaCl2, 1 mm MgCl2, 0.25 mm EDTA, and 10 mm taurine (pH 7)). The cell suspension was mixed with Ham's F12 medium supplemented with 10% (v/v) FBS and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin). Cells were cultured at 37 °C in the presence of 5% CO2 in the same medium. The medium was changed every 3–4 days until cells reached confluence, and confluent cells were passaged with trypsin/EDTA solution (20–23). Smooth muscle cells within passage 5 were used for the studies.
Immunoblot Analysis
Cells were lysed in SDS sample buffer composed of 1.5% dithiothreitol, 2% SDS, 80 mm Tris-HCl (pH 6.8), 10% glycerol, and 0.01% bromphenol blue. The lysates were boiled in the buffer for 5 min and separated by SDS-PAGE. Proteins were transferred to nitrocellulose membranes. The membranes were blocked with bovine serum albumin or milk for 1 h and probed with primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies (Fisher Scientific). Proteins were visualized by enhanced chemiluminescence (Fisher Scientific) using the LAS-4000 Fuji image system. Antibodies against myosin light chain, N-cadherin, β-catenin, and α-tubulin were purchased from Santa Cruz Biotechnology. Phospho-myosin light chain (Ser-19) was purchased from Cell Signaling Technology or Santa Cruz Biotechnology. α-Smooth muscle actin antibody was purchased from Sigma-Aldrich. GAPDH antibody was purchased from Fitzgerald (Acton, MA). The levels of proteins were quantified by scanning densitometry of immunoblots (Fuji Multigauge software). The luminescent signals from all immunoblots were within the linear range.
Coimmunoprecipitation Analysis
Coimmunoprecipitation analysis was used to evaluate protein-protein interactions as described previously (22, 24). Briefly, cell/tissue extracts were incubated overnight with the corresponding antibodies and then incubated for 2–3 h with 125 μl of a 10% suspension of protein A-Sepharose beads. Immunocomplexes were washed four times in buffer containing 50 mm Tris-HCl (pH 7.6), 150 mm NaCl, and 0.1% Triton X-100. The immunoprecipitates were separated by SDS-PAGE followed by transfer to nitrocellulose membranes. The membranes of immunoprecipitates were probed with the corresponding antibodies.
Immunofluorescence and Fluorescence Analyses
Cells on coverslips were fixed for 15 min in 4% paraformaldehyde and then washed three times in PBS followed by permeabilization with 0.2% Triton X-100 dissolved in PBS for 5 min. These cells were immunofluorescently stained using primary antibodies followed by appropriate secondary antibodies (Invitrogen). The cellular localization of fluorescently labeled proteins was viewed under a high-resolution digital fluorescence microscope (Leica DMI system, ×63 oil objective). The time of image capturing, intensity gaining, and image contrast in each channel were optimally adjusted and kept constant for all experiments to standardize the fluorescence intensity measurements among experiments. National Institutes of Health ImageJ software was used to quantify the fluorescence intensity at the cell-cell contacts.
Virus-mediated RNAi
For β-catenin knockdown (KD), lentiviruses encoding β-catenin shRNA (catalog no. sc-29209-V) or control shRNA (catalog no. sc-108080) were purchased from Santa Cruz Biotechnology. HASM cells were infected with control shRNA lentivirus or β-catenin shRNA lentivirus for 12 h. They were then cultured for 3–4 days. Positive clones expressing shRNAs were selected by puromycin. Immunoblot analysis was used to determine the expression levels of β-catenin in these cells. β-catenin KD cells and cells expressing control shRNA were stable at least five passages after initial infection. The experimental procedures for generating c-Abl KD cells have been described previously (20, 25).
Plasmids and Gene Mutation
pcDNA 3 encoding WT human β-catenin was purchased from Addgene. PCR was used to generate plasmids encoding the Arm domain of β-catenin. The 5-primer sequence was 5′-ATCAAGGGATCCATGTTGATTAACTATCAAGATGA-3′. The 3-primer sequence was 5′ TACTATGCGGCCGCCACCTTCATTCCTAGAGTGAA-3′. The resulting product was double-digested at the BamH I and NotI sites. The enzyme-digested product was then subcloned into pcDNA3 followed by bacterial transformation. Plasmid purification was performed using the Maxiprep kit from Invitrogen.
Analysis of F-actin/G-actin Ratios
The content of F-actin and G-actin in smooth muscle was measured using a method described previously (11, 12, 24). Briefly, smooth muscle cells were treated with F-actin stabilization buffer (50 mm PIPES (pH 6.9), 50 mm NaCl, 5 mm MgCl2, 5 mm EGTA, 5% glycerol, 0.1% Triton X-100, 0.1% Nonidet P40, 0.1% Tween 20, 0.1% β-mercaptoethanol, 1 mm ATP, 1 μg/ml pepstatin, 1 μg/ml leupeptin, and 10 μg/ml benzamidine). The supernatants of protein extracts were collected after centrifugation at 151,000 × g for 60 min at 37 °C. The pellets were resuspended in ice-cold H2O plus 1 μm cytochalasin D and then incubated on ice for 1 h to dissociate F-actin. The resuspended pellets were gently mixed every 15 min. The supernatant of the resuspended pellets was collected after centrifugation at 16,100 × g for 2 min at 4 °C. An equal volume of the first supernatant (G-actin) or second supernatant (F-actin) was subjected to immunoblot analysis using α-actin antibody. The amount of F-actin and G-actin was determined by scanning densitometry.
Measurement of Human Bronchial Ring Contraction
Bronchial rings (diameter, 5 mm) were prepared from human lungs obtained from the International Institutes for Advanced Medicine (see above). Bronchial rings were placed in physiological saline solution at 37 °C in a 25-ml organ bath and attached to a Grass force transducer connected to a computer with an analog-to-digital converter (Grass). For lentivirus-mediated RNAi in tissues, the thin epithelium layer of human bronchial rings was removed using forceps. They were then transduced with lentivirus encoding β-catenin shRNA or control shRNA for 3 days. Force development in response to acetylcholine (ACh) (100 μm, 10 min) activation was compared before and after lentivirus transduction. For biochemical analysis, human tissues were frozen using liquid nitrogen and pulverized as described previously (11, 26, 27).
We used reversible permeabilization (24, 28, 29) to introduce the constructs of WT or mutant β-catenin into human bronchi. Briefly, the contractile responses of human tissues were determined, after which they were placed in 0.5-ml tubes and incubated successively in each of the following solutions: Solution 1 (at 4 °C for 120 min) containing 10 mm EGTA, 5 mm Na2 ATP, 120 mm KCl, 2 mm MgCl2, and 20 mm TES; Solution 2 (at 4 °C overnight) containing 0.1 mm EGTA, 5 mm Na2ATP, 120 mm KCl, 2 mm MgCl2, 20 mm TES, and 10 μg/ml plasmids; Solution 3 (at 4 °C for 30 min) containing 0.1 mm EGTA, 5 mm Na2ATP, 120 mm KCl, 10 mm MgCl2, and 20 mm TES; and Solution 4 (at 22 °C for 60 min) containing 110 mm NaCl, 3.4 mm KCl, 0.8 mm MgSO4, 25.8 mm NaHCO3, 1.2 mm KH2PO4, and 5.6 mm dextrose. Solutions 1–3 were maintained at pH 7.1 and aerated with 100% O2. Solution 4 was maintained at pH 7.4 and was aerated with 95% O2-5% CO2. After 30 min in Solution 4, CaCl2 was added gradually to reach a final concentration of 2.4 mm. The tissues were then incubated in a CO2 incubator at 37 °C for 2 days in DMEM containing 5 mm Na2ATP, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10 μg/ml plasmids.
Statistical Analysis
All statistical analyses were performed using Prism 6 software (GraphPad Software, San Diego, CA). Comparison among multiple groups was performed by one-way analysis of variance followed by Tukey's multiple comparison test. Differences between pairs of groups were analyzed by Student-Newman-Keuls test or Dunn's method. Values of n refer to the number of experiments used to obtain each value. p < 0.05 was considered to be significant.
RESULTS
Contractile Activation Induces the Recruitment of β-Catenin to N-Cadherin in Smooth Muscle
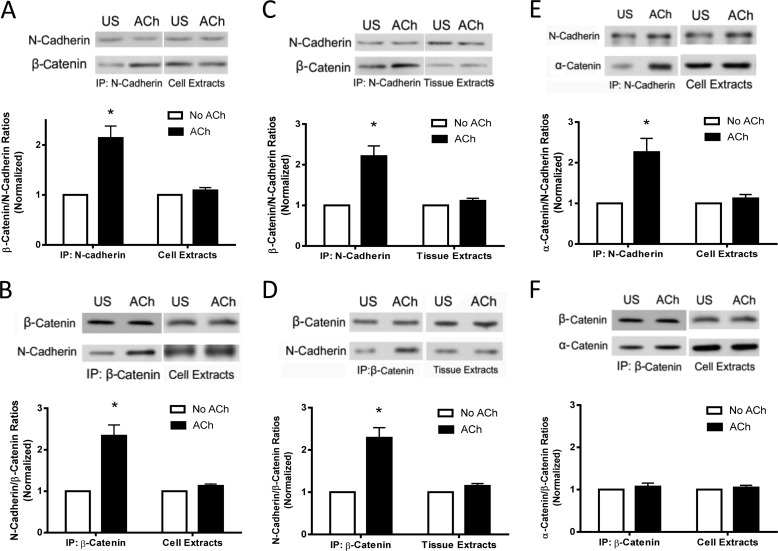
As described earlier, the cytoplasmic tails of N-cadherin bind to β-catenin, which, in turn, connects with actin filaments via linker proteins (13–15). However, the physiological properties of the adherens junctions in smooth muscle under contractile activation are poorly understood. We hypothesized that contractile stimulation may promote the association of β-catenin with N-cadherin, which may enhance the intercellular connection. To test this, HASM cells were stimulated with ACh for 5 min or left unstimulated. Cell extracts were immunoprecipitated with N-cadherin antibody and blotted with antibodies against N-cadherin and β-catenin. The amount of β-catenin in the immunoprecipitates was higher in stimulated cells than in unstimulated cells. The ratios of β-catenin/N-cadherin precipitates were increased in stimulated cells compared with unstimulated cells (Fig. 1A).
FIGURE 1.
Contractile activation increases the association of β-catenin with N-cadherin in smooth muscle cells/tissues. A, N-cadherin immunoprecipitates (IP) or extracts of HASM cells stimulated with ACh (10−4 m, 5 min) or left unstimulated (US) were separated by SDS-PAGE and blotted with antibodies against N-cadherin and β-catenin. Ratios of β-catenin/N-cadherin in stimulated cells were normalized to corresponding unstimulated cells. The ratios of β-catenin/N-cadherin in immunoprecipitates were significantly higher in stimulated cells than in unstimulated cells (*, p < 0.05). However, the protein ratios in cell extracts are similar in stimulated and unstimulated cells. Data are mean ± S.E. of four independent experiments. B, blots of β-catenin immunoprecipitates or extracts of cells treated with ACh or left untreated were probed with antibodies against N-cadherin and β-catenin. The ratios of N-cadherin/β-catenin in stimulated cells were normalized to corresponding unstimulated cells. Data are mean ± S.E. of four independent experiments. *, p < 0.05. C, N-Cadherin immunoprecipitates or extracts of human bronchial rings treated with ACh or left untreated were analyzed by immunoblotting. The ratios of β-catenin/N-cadherin in stimulated tissues were normalized to corresponding unstimulated tissues. Data are mean ± S.E. of three independent experiments. *, p < 0.05. D, β-catenin precipitates or extracts of human bronchial rings treated with ACh or untreated were evaluated by immunoblot analysis. The ratios of N-cadherin/β-catenin in stimulated tissues were normalized to corresponding unstimulated tissues. Data are mean ± S.E. of three independent experiments. *, p < 0.05. E, N-cadherin immunoprecipitates or extracts of HASM cells treated with ACh or left untreated were analyzed by immunoblotting. The ratios of α-catenin/N-cadherin in stimulated cells were normalized to corresponding unstimulated cells. Data are mean ± S.E. of four independent experiments. *, p < 0.05. F, β-catenin precipitates or extracts of HASM cells treated with ACh or left untreated were evaluated by immunoblot analysis. The ratios of α-catenin/β-catenin in stimulated cells were normalized to corresponding unstimulated cells. Data are mean ± S.E. of four independent experiments.
To verify this, we used reverse coimmunoprecipitation analysis. Cell extracts were immunoprecipitated using β-catenin antibody, and blots of the immunoprecipitates were probed using antibodies against β-catenin and N-cadherin. The ratios of N-cadherin/β-catenin precipitates in stimulated cells were higher compared with unstimulated cells (Fig. 1B).
Furthermore, we evaluated the effects of contractile activation on the interaction of β-catenin with N-cadherin at the tissue level. Human bronchial rings were treated with ACh, and the protein-protein interactions were assessed by coimmunoprecipitation analysis. The ratios of β-catenin/N-cadherin or N-cadherin/β-catenin precipitates were higher in stimulated tissues than in unstimulated tissues (Fig. 1, C and D).
Because α-catenin has also been implicated in connecting actin filaments with N-cadherin (13, 14), we determined whether contractile activation affects the association of α-catenin with N-cadherin and β-catenin. Stimulation with ACh increased the interaction of α-catenin with N-cadherin but not β-catenin (Fig. 1, E and F). These results suggest that contractile activation recruits both α-catenin and β-catenin to N-cadherin, which supports the concept that α-catenin links actin filaments with N-cadherin via β-catenin (13, 14),
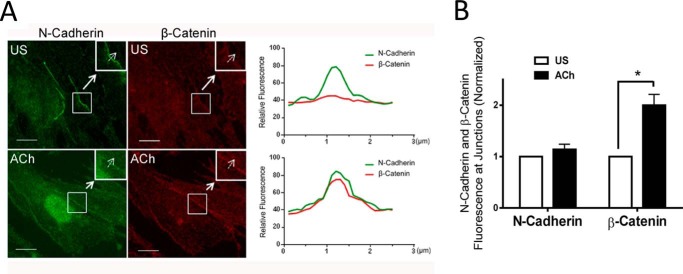
We assessed the spatial localization of β-catenin and N-cadherin in response to contractile activation by immunofluorescent microscopy. N-cadherin was localized in the cell-cell contact in unstimulated cells. ACh stimulation did not affect the spatial distribution of N-cadherin (Fig. 2, A and B). In contrast, the amount of β-catenin at the intercellular junctions of unstimulated cells was relatively lower. The amount of β-catenin at the intercellular contacts increased upon ACh stimulation (Fig. 2, A and B). These results suggest that β-catenin translocates to the intercellular junction in response to contractile stimulation.
FIGURE 2.
Activation with ACh induces the translocation of β-catenin to the intercellular junctions. A, representative micrographs illustrating the effects of ACh (10−4 m, 5 min) on the spatial localization of β-catenin and N-cadherin in HASM cells. The insets are ×1.5 magnifications of the selected areas. Dashed arrows indicate a single line scan to quantify the fluorescence signals at cell-cell junctions. The right panel shows relative fluorescence intensity. Scale bars = 10 μm. US, unstimulated. B, the fluorescence intensity of β-catenin or N-cadherin in stimulated cells was normalized to corresponding unstimulated cells. Data are mean ± S.E. of 10–12 independent experiments. *, p < 0.05.
β-Catenin Is Necessary for Smooth Muscle Contraction but Not Actin Polymerization and Myosin Phosphorylation
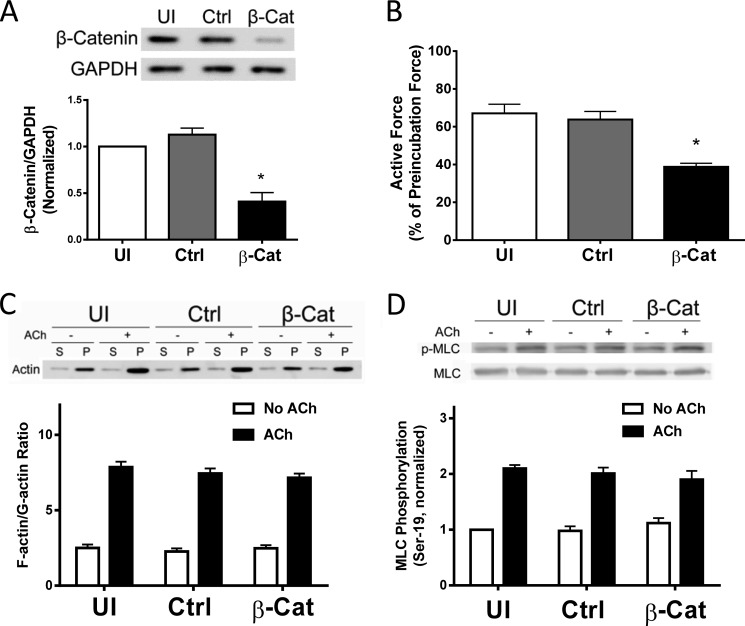
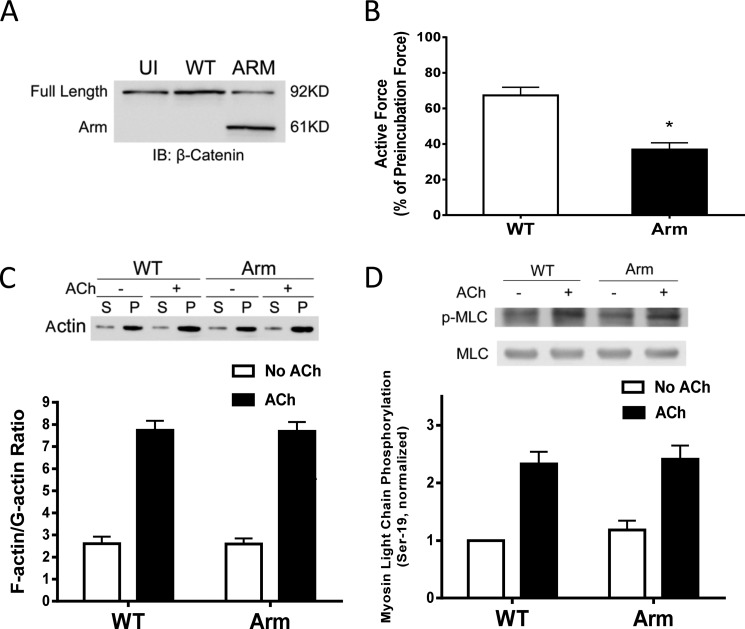
β-Catenin has been implicated in cell adhesion, development, mechanical integrity, and transformation (13, 17, 18). The role of β-catenin in smooth muscle contraction is poorly understood. To determine the role of β-catenin, we utilized a lentivirus-mediated RNAi approach (11, 12) to inhibit the expression of β-catenin. The contractile response of human bronchial rings to ACh was evaluated. Bronchial rings were then transduced with lentiviruses encoding control shRNA or β-catenin shRNA. These tissues were incubated in serum-free medium for 3 days. The contractile force of these tissues was then evaluated. Immunoblot analysis verified the lower expression of β-catenin in tissues transduced with viruses encoding β-catenin shRNA compared with uninfected rings and tissues transduced with viruses for control shRNA (Fig. 3A). More importantly, the contractile responses of human bronchial rings were lower in β-catenin-deficient tissues than in control tissues (Fig. 3B).
FIGURE 3.
β-Catenin is required for smooth muscle contraction. A, human bronchial rings were transduced with lentiviruses encoding control shRNA or β-catenin shRNA. These tissues were then incubated in serum-free medium for 3 days. Immunoblot analysis was used to assess protein expression in tissues. UI, uninfected; Ctrl, control shRNA; β-Cat, β-catenin shRNA. *, p < 0.05 (significantly lower protein ratios of β-catenin/GAPDH in tissues transduced with virus encoding β-catenin shRNA than in uninfected tissues and tissues expressing control shRNA). Data are mean ± S.E. of three independent experiments. B, the contraction of human bronchial rings was evaluated, after which they were transduced with lentiviruses as described above. Contractile responses were compared before and after incubation. *, p < 0.05 (significantly lower contractile force in bronchial rings treated with β-catenin shRNA compared with uninfected tissues or tissues infected with viruses encoding control shRNA). Data are mean ± S.E. of three independent experiments. C, uninfected cells and cells expressing control shRNA or β-catenin shRNA were stimulated with ACh (10−4 m, 5 min) or left unstimulated. F/G-actin ratios in the cells were evaluated using a fractionation assay. Data are mean ± S.E. of four independent experiments. (p > 0.05). S, supernatant; P, pellet. D, myosin light chain (MLC) phosphorylation at Ser-19 in uninfected cells and cells transduced with lentivirus encoding control or β-catenin shRNA was assessed by immunoblot analysis. Myosin phosphorylation was similar in uninfected cells, cells expressing control shRNA, or β-catenin shRNA (p > 0.05). Data are mean ± S.E. of four to five independent experiments.
Because actin polymerization and myosin activation are known to regulate smooth muscle contraction (1–3, 5–9), we evaluated the effects of β-catenin knockdown on F/G-actin ratios and myosin light chain phosphorylation at Ser-19. The knockdown of β-catenin did not affect F/G-actin ratios (Fig. 3C) or myosin light chain phosphorylation at Ser-19 (Fig. 3D).
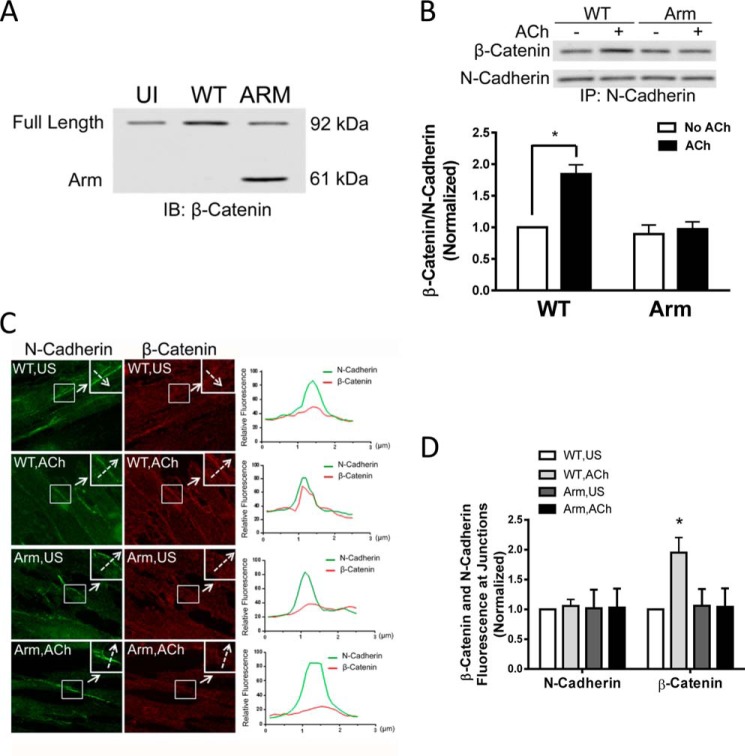
Expression of the β-Catenin Arm Domain Disrupts the Recruitment of β-Catenin to N-Cadherin
β-Catenin is composed of an N-terminal head, an Arm domain, and a C-terminal tail. The Arm domain of β-catenin binds to the cytoplasmic domain of cadherins, whereas the N terminus interacts with actin filaments via linker proteins (14, 15). Therefore, β-catenin is a key element linking transmembrane cadherins to actin filaments at adherens junctions (13, 15). We evaluated the effects of the expression of the Arm domain of β-catenin on the association of β-catenin with N-cadherin in cells. We used β-catenin full-length antibody to assess protein expression in cells transfected with WT or mutant β-catenin. As shown in Fig. 4A, immunoblot analysis verified the expression of WT β-catenin and the Arm domain of β-catenin in these cells. Moreover, expression of the Arm domain inhibited the association of endogenous β-catenin with N-cadherin in cells upon ACh stimulation compared with WT β-catenin, as evidenced by coimmunoprecipitation analysis using antibody against the C terminus of β-catenin (Fig. 4B). Furthermore, expression of the Arm domain attenuated the spatial translocation of endogenous β-catenin (Fig. 4, C and D). These results suggest that the Arm domain of β-catenin is able to compete with endogenous β-catenin for N-cadherin binding. Therefore, expression of the Arm domain inhibits the interaction of endogenous β-catenin with N-cadherin and the spatial redistribution of endogenous β-catenin to the intercellular junction upon contractile activation.
FIGURE 4.
Expression of the Arm domain of β-catenin attenuates the recruitment of β-catenin to N-cadherin. A, representative immunoblots illustrating the expression of WT β-catenin or the Arm domain of β-catenin in cells. Extracts of cells transfected with plasmids encoding WT β-catenin or the Arm domain of β-catenin were immunoblotted (IB) with β-catenin full-length antibody. The Arm domain with a molecular mass of 60 kDa was detected in the extracts of cells transfected with the plasmid for Arm domain but not in untransfected cells (UI) or in cells transfected with WT β-catenin plasmid, indicating the effective expression of the Arm domain of β-catenin in these cells. The blots are representative of four identical experiments. B, cells expressing WT β-catenin or the Arm domain of β-catenin were stimulated with 10−4 m ACh for 5 min or left unstimulated. The interaction of N-cadherin with β-catenin was evaluated by coimmunoprecipitation (IP) analysis using antibody against the β-catenin C terminus and antibody against N-cadherin. The ratios of β-catenin/N-cadherin in stimulated cells were normalized to corresponding unstimulated cells. *, p < 0.05 (significantly lower ACh-induced β-catenin/N-cadherin ratios in cells expressing the Arm domain of β-catenin compared with cells expressing WT β-catenin). Data are mean ± S.E. of four independent experiments. C, representative images illustrating the effects of the expression of the Arm domain of β-catenin on the localization of β-catenin and N-cadherin at the adherens junctions in response to ACh activation. Cells were immunostained with antibodies against the β-catenin C terminus and N-cadherin. The insets are ×1.5 magnifications of the selected areas. Dashed arrows indicate a single line scan to quantify the signals at cell-cell junctions. The right panel shows the relative fluorescence intensity. Scale bars = 10 μm. D, the fluorescence intensity of β-catenin or N-cadherin in stimulated cells was normalized to corresponding unstimulated (US) WT cells. Data are mean ± S.E. of 10–12 independent experiments. *, p < 0.05.
The Expression of the Arm Domain of β-Catenin Affects Smooth Muscle Contraction, But Not Actin Polymerization and Myosin Light Chain Phosphorylation
To determine the functional role of the β-catenin/N-cadherin interaction, we assessed the effects of the expression of the Arm domain on smooth muscle contraction. The contractile response of human bronchial rings to ACh was evaluated. Plasmids encoding WT or mutant β-catenin were introduced into bronchial rings by reversible permeabilization (24, 28, 29). Tissues were then incubated in the medium for 3 days. Immunoblot analysis confirmed the expression of the recombinant proteins (Fig. 5A). The contractile force of these tissues was compared before and after incubation. The contractile force was reduced in tissues expressing the Arm domain of β-catenin compared with tissues transfected with WT β-catenin (Fig. 5B).
FIGURE 5.
Expression of the Arm domain of β-Catenin inhibits smooth muscle contraction. A, representative immunoblots showing the expression of β-catenin and its mutant in tissues. Extracts of human bronchial rings transduced with plasmids encoding WT β-catenin or the Arm domain of β-catenin were immunoblotted (IB) with antibodies against full-length β-catenin. The blots are representative of three identical experiments. UI, uninfected. B, the contraction of human bronchial rings was evaluated, after which they were transduced with plasmids as described under “Experimental Procedures.” Contractile responses were compared before and after incubation. *, p < 0.05 (significantly lower contractile force in bronchial rings expressing the Arm domain of β-catenin compared with tissues transfected with WT β-catenin). Data are mean ± S.E. of three independent experiments. C, cells expressing WT or mutant β-catenin were stimulated with ACh or left unstimulated. F/G-actin ratios in the cells were evaluated using a fractionation assay. Data are mean ± S.E. of four independent experiments (p > 0.05). S, supernatant; P, pellet. D, myosin light chain (MLC) phosphorylation at Ser-19 in uninfected cells and cells transduced with lentivirus encoding control or β-catenin shRNA was assessed by immunoblot analysis. Myosin phosphorylation was similar in uninfected cells and cells expressing control shRNA or β-catenin shRNA (p > 0.05). Data are mean ± S.E. of four to five independent experiments.
We then determined whether the disruption of the β-catenin/N-cadherin coupling by the Arm domain affects actin polymerization and myosin phosphorylation. F/G-actin ratios and myosin light chain phosphorylation at Ser-19 were comparable in cells expressing WT or mutant β-catenin (Fig. 5, C and D).
Actin Polymerization Regulates the Recruitment of β-Catenin to N-Cadherin upon Contractile Activation
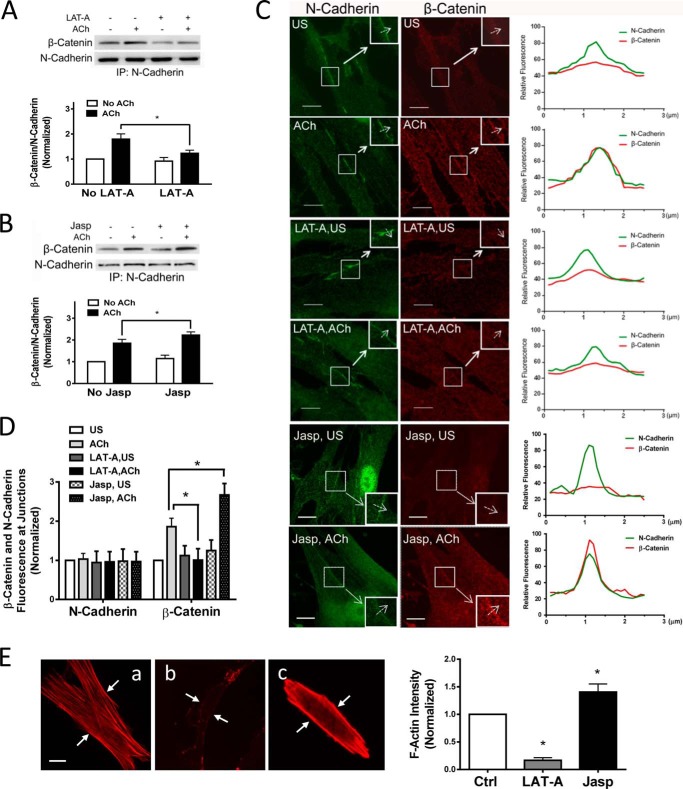
Actin polymerization has been implicated in regulating intracellular trafficking of the glucose transporter GLUT4 in adipocytes and striated muscle cells (30). Because actin polymerization occurs in smooth muscle upon contractile activation, we questioned whether the actin cytoskeleton affects the recruitment of β-catenin to N-cadherin. Smooth muscle cells were pretreated with the actin polymerization inhibitor latrunculin A (31) for 15 min. They were then stimulated with ACh or left unstimulated. Coimmunoprecipitation analysis was used to assess the protein-protein interaction. The amount of β-catenin in N-cadherin immunoprecipitates in response to ACh stimulation was reduced in cells treated with latrunculin A compared with cells not treated with the inhibitor. The ACh-induced increase in β-catenin/N-cadherin coupling was reduced in cells treated with latrunculin A (Fig. 6A). Furthermore, treatment with jasplakinolide (an inducer of actin polymerization and stabilization, 3 μm, 30 min) increased the association of β-catenin with N-cadherin upon ACh stimulation (Fig. 6B).
FIGURE 6.
Actin polymerization regulates the recruitment of β-catenin to N-cadherin upon contractile activation. A, cells were pretreated with 1 μm latrunculin A (LAT-A) for 15 min. They were then stimulated with 10−4 m ACh for 5 min or left unstimulated. The protein-protein interaction was evaluated by coimmunoprecipitation (IP). Ratios of β-catenin/N-cadherin under various treatments were normalized to unstimulated cells not treated with latrunculin A. Data are mean ± S.E. of four independent experiments. *, p < 0.05. B, cells were pretreated with 3 μm jasplakinolide (Jasp) for 30 min. The protein-protein interaction in unstimulated and stimulated cells was evaluated by coimmunoprecipitation. The ratios of β-catenin/N-cadherin under various treatments were normalized to unstimulated cells not treated with jasplakinolide. Error bars indicate S.E. *, p < 0.05; n = 4. C, cells pretreated with latrunculin A or jasplakinolide were stimulated with ACh or left unstimulated (US). Cells were then immunostained with antibodies against the β-catenin C terminus and N-cadherin. The insets are ×1.5 magnifications of the selected areas. Dashed arrows indicate a single line scan to quantify the fluorescence signals at cell-cell junctions. The right panel shows relative fluorescence intensity. Scale bar = 10 μm. D, the fluorescence intensity of β-catenin or N-cadherin in stimulated cells was normalized to corresponding unstimulated and untreated cells. Data are mean ± S.E. of 10–12 independent experiments. *, p < 0.05. E, untreated HASM cells (a) or cells treated with latrunculin A (1 μm, 15 min) (b) or jasplakinolide (3 μm, 30 min) (c) were stained with rhodamine-phalloidin to visualize F-actin. National Institutes of Health ImageJ software was used to quantify the fluorescence intensity of the total cell areas. Arrows point to the cell edges. Data are mean ± S.E. *, p < 0.05 versus control; n = 38–40 cells. Scale bars = 10 μm.
We then determined whether the actin cytoskeleton affects the cellular localization of β-catenin. Although treatment with latrunculin A did not affect the cellular localization of N-cadherin, the ACh-induced increase in the fluorescent intensity of β-catenin at the cell-cell contact was reduced in cells treated with latrunculin A (Fig. 6, C and D). In contrast, the treatment with jasplakinolide enhanced the spatial redistribution of β-catenin (Fig. 6, C and D). Fluorescence microscopy verified the efficacy of latrunculin A and jasplakinolide in the cells (Fig. 6E).
Effects of the Myosin II Inhibitor Blebbistatin on the Interaction of β-Catenin with N-Cadherin
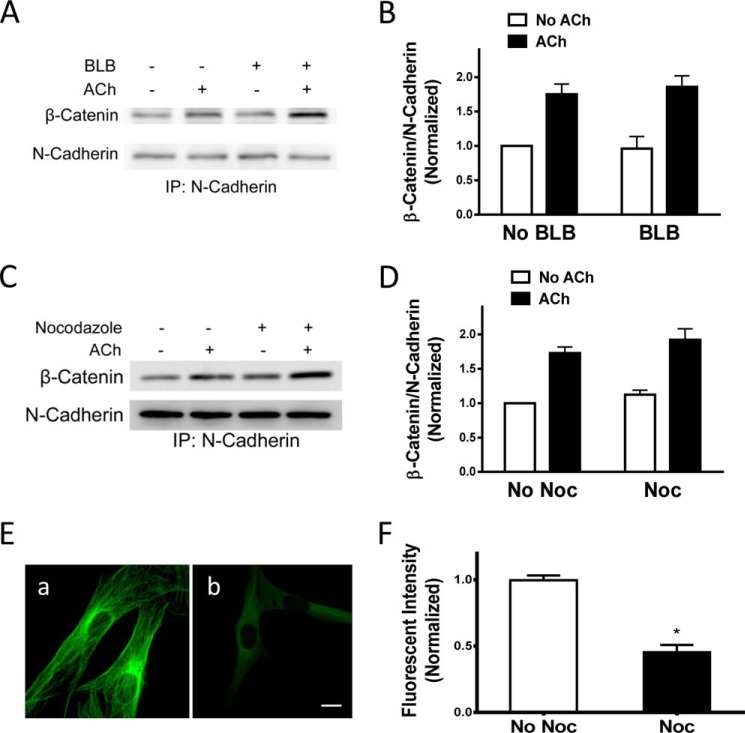
Because actin polymerization affects tension in smooth muscle (5–8), the effects of latrunculin A on the association of β-catenin with N-cadherin may be attributed to actin polymerization and/or changes in tension. To distinguish this, we evaluated the effects of blebbistatin on the protein-protein interaction. Treatment with blebbistatin did not affect the interaction of β-catenin with N-cadherin (Fig. 7, A and B). The results suggest that actin polymerization, but not tension, regulates the recruitment of β-catenin with N-cadherin in smooth muscle upon contractile activation.
FIGURE 7.
Tension and microtubules do not regulate the interaction of β-catenin with N-cadherin upon contractile activation. A, representative immunoblots illustrating the effects of blebbistatin (BLB) on the interaction of β-catenin with N-cadherin. Cells were pretreated with 30 μm blebbistatin for 15 min. They were then stimulated with 10−4 m ACh for 5 min or left unstimulated. β-Catenin/N-cadherin coupling was evaluated by coimmunoprecipitation (IP). B, β-catenin/N-cadherin ratios were normalized to the ratios in unstimulated cells not treated with blebbistatin. Data are mean ± S.E. of four independent experiments. C, representative immunoblots illustrating the effects of nocodazole on the interaction of β-catenin/N-cadherin. Cells were pretreated with 1 μm nocodazole for 15 min. They were then stimulated with 10−4 m ACh for 5 min or left unstimulated. β-Catenin/N-cadherin coupling was evaluated by coimmunoprecipitation. D, β-catenin/N-cadherin ratios were normalized to the ratios in unstimulated cells not treated with nocodazole (Noc). Data are mean ± S.E. of four independent experiments. E, untreated cells and HASM cells treated with nocodazole (1 μm, 15 min) were stained with α-tubulin antibody. a, untreated cells display a well defined microtubule structure. b, treated cells have a less defined filamentous structure. F, the fluorescence intensity in treated cells was normalized to untreated cells. Data are mean ± S.E. of 37–41 independent experiments. *, p < 0.05.
Microtubules Do Not Modulate the Association of β-Catenin with N-Cadherin upon ACh Stimulation
Because microtubules have been implicated in regulating the movement of intracellular vesicles and molecules in neurons (32), we also evaluated the effects of the microtubule depolymerizer nocodazole on the protein-protein interaction. The ratios of β-catenin/N-cadherin in cells pretreated with nocodazole were similar to cells not pretreated with nocodazole (Fig. 7, C and D). Furthermore, treatment with nocodazole decreased the fluorescence intensity of microtubules in the cells (Fig. 7, E and F).
DISCUSSION
β-Catenin is a key element of adherens junctions that plays an essential role in cell-cell contacts (13–15). The intercellular junctions provide the structural basis for maintaining multicellular integrity as well as the endothelial/epidermal barrier (13, 14, 16, 33). In addition, external stress imposed on a cell is able to be sensed by adjacent cells via adherens junctions (13, 16). In this study, knockdown of β-catenin attenuated human smooth muscle contraction. The results suggest that β-catenin is required for human smooth muscle contraction, which is supported by a previous study by others (34).
There is evidence to suggest that adherens junctions undergo reorganization in endothelial cells and the epidermis in response to tugging force and chemical stimulation (13, 16, 17). Here we found that contractile activation induced the coupling of β-catenin with N-cadherin and the spatial redistribution of β-catenin in smooth muscle cells. More importantly, the disruption of the recruitment of β-catenin to N-cadherin by the Arm domain of β-catenin attenuated smooth muscle contraction. To the best of our knowledge, this is the first evidence to suggest that the interaction of β-catenin with N-cadherin is dynamic and that the recruitment of β-catenin to N-cadherin is critical for smooth muscle contraction.
The adherens junctions in nonmuscle cells have been implicated in cell signaling (33, 35, 36). Engagement of N-cadherin in MC3T3E1 preosteoblasts promotes the activation of PI3K, which is reduced by an N-cadherin-blocking antibody (35). E-Cadherin is required for the activation of PI3K in epithelial ovarian cancer cells (36). PI3K is able to activate Tiam-1 and VAV-2 (guanine nucleotide exchange factors), which may subsequently regulate Rac/Cdc42 activation and actin polymerization (33, 37). Here the knockdown of β-catenin or the disruption of β-catenin with N-cadherin did not affect actin polymerization upon contractile activation. Therefore, cadherin-regulated actin filament assembly is not mediated by β-catenin in smooth muscle during contractile activation. Cadherin may directly interact with pleckstrin homology domains on Tiam-1 or VAV-2, which activates Rac/Cdc42 and actin polymerization (33, 37).
E-Cadherin is required for the activation of the small GTPase Rho in epithelial cells (38, 39). Rho is known to regulate myosin light chain phosphorylation in smooth muscle (4). In this study, the silencing of β-catenin or the disruption of the β-catenin/N-cadherin association did not affect myosin light chain phosphorylation at Ser-19 in response to contractile activation. Therefore, the interaction of β-catenin with N-cadherin does not regulate myosin light chain phosphorylation in smooth muscle.
Because the recruitment of β-catenin to cadherin does not regulate actin polymerization and myosin phosphorylation, it is likely that the coupling of β-catenin with N-cadherin may enhance the linkage of actin filaments to N-cadherin, which, in turn, promotes intercellular force transmission and smooth muscle contraction (13, 14, 16, 17).
Actin polymerization may mediate intracellular trafficking of certain molecules. In adipocytes and striated muscle cells, GLUT4 undergoes spatial translocation to the plasma membrane from the cytoplasm upon insulin activation, which may promote glucose uptake. Inhibition of actin polymerization by molecular approaches attenuates the intracellular trafficking of GLUT4 during insulin activation (30). In addition, Rac may promote adherens junction assembly by binding to IQ motif containing GTPase activating protein (a scaffold protein), which enhances the linkage of actin filaments to β-catenin (33), and by promoting actin polymerization (8, 37, 39). Furthermore, actin polymerization regulates Raf-1 translocation in smooth muscle cells (20).
In this study, treatment with latrunculin A attenuated the agonist-induced association of β-catenin with N-cadherin. Exposure to jasplakinolide enhanced the interaction of β-catenin with N-cadherin. Furthermore, treatment with the myosin II inhibitor blebbistatin did not affect protein-protein interaction. In vitro biochemical studies have shown that blebbistatin is less effective on turkey smooth muscle myosin than it is on cytoplasmic myosin (40). Moreover, there is evidence that treatment with 10–30 μm blebbistatin effectively inhibits the contraction of smooth muscle tissues (41, 42). Therefore, it is likely that actin polymerization, not tension, is necessary for the translocation of β-catenin and the recruitment of β-catenin to N-cadherin. It is possible that actin polymerization may create a local environment to allow β-catenin to have better access to the cytoplasmic domain of N-cadherin (13, 30, 43).
Actin polymerization may facilitate force development by enhancing the linkage of actin filaments to integrins and strengthening the transmission of mechanical force between the contractile unit and the extracellular matrix (5, 8, 44, 45). In addition, actin filament assembly may also increase the numbers of contractile units and the length of actin filaments, providing more and efficient contractile elements for force development (46). In this study, we discovered that actin polymerization also promotes the recruitment of β-catenin to N-cadherin, which may facilitate cell-to-cell force transmission.
β-Catenin has also been implicated in regulating the TGF-β-regulated gene expression in airway smooth muscle cells (47). TGF-β was not present under our experimental conditions, and, therefore, the effects of β-catenin knockdown or mutant expression on TGF-β-regulated gene expression was minimal in these cells/tissues. However, it is possible that β-catenin knockdown or mutant expression might affect other cellular processes in smooth muscle cells/tissues. Future studies are needed to investigate the possibility.
Microtubules may regulate the movement of intracellular vesicles and molecules in neurons (32). In addition, the microtubule-associated protein dynein binds to β-catenin and may tether microtubules to adherens junctions (48). In this study, disruption of microtubules did not affect the coupling of β-catenin with N-cadherin. The results suggest that microtubules are not required for the recruitment of β-catenin to N-cadherin.
We unveiled a novel mechanism that is necessary for smooth muscle contraction. In response to contractile activation, β-catenin is recruited to N-cadherin, which may promote the intercellular transmission of contractile force. In addition, the recruitment of β-catenin to N-cadherin in smooth muscle is regulated by actin polymerization upon contractile activation (Fig. 8).
FIGURE 8.
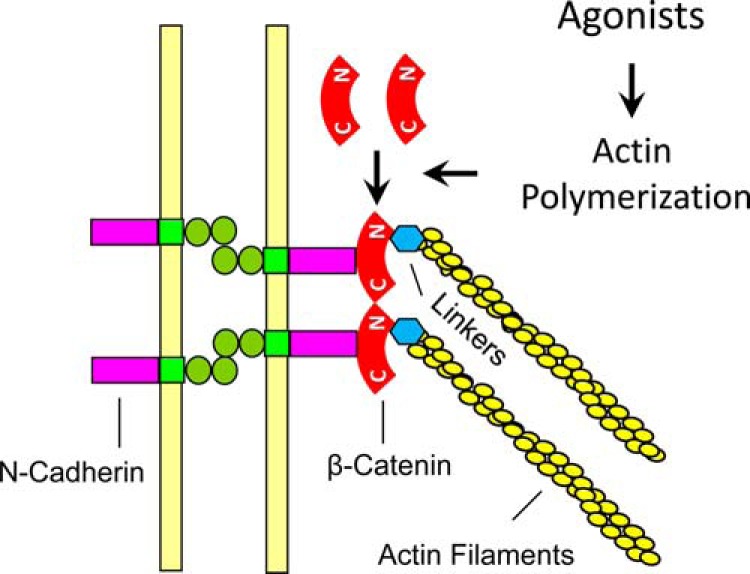
Proposed mechanism for intercellular force transmission. In addition to myosin activation, contractile agonists induce actin polymerization, which promotes the recruitment of β-catenin to N-cadherin. The increase in protein-protein interaction may enhance linkage of actin filaments to adherens junctions and promote intercellular force transmission and smooth muscle contraction. C, C terminus; N, N terminus; Linkers, linker proteins such as α-catenin, vinculin, and VASP.
This work was supported, in whole or in part, by NHLBI/National Institutes of Health Grants HL-110951 and HL-113208 (to D. D. T.).
- Arm
- armadillo
- VASP
- vasodilator-stimulated phosphoprotein
- HASM
- human airway smooth muscle
- KD
- knockdown
- ACh
- acetylcholine
- TES
- 2-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}ethanesulfonic acid.
REFERENCES
- 1. Kamm K. E., Stull J. T. (1989) Regulation of smooth muscle contractile elements by second messengers. Annu. Rev. Physiol 51, 299–313 [DOI] [PubMed] [Google Scholar]
- 2. Morgan K. G. (2008) Contractility in health and disease. J. Cell Mol. Med. 12, 2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amrani Y., Panettieri R. A. (2003) Airway smooth muscle: contraction and beyond. Int. J. Biochem. Cell Biol. 35, 272–276 [DOI] [PubMed] [Google Scholar]
- 4. Somlyo A. V., Khromov A. S., Webb M. R., Ferenczi M. A., Trentham D. R., He Z. H., Sheng S., Shao Z., Somlyo A. P. (2004) Smooth muscle myosin: regulation and properties. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 359, 1921–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunst S. J., Zhang W. (2008) Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am. J. Physiol. Cell Physiol. 295, C576–C587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim H. R., Gallant C., Leavis P. C., Gunst S. J., Morgan K. G. (2008) Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am. J. Physiol. Cell Physiol. 295, C768–C778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rembold C. M., Tejani A. D., Ripley M. L., Han S. (2007) Paxillin phosphorylation, actin polymerization, noise temperature, and the sustained phase of swine carotid artery contraction. Am. J. Physiol. Cell Physiol. 293, C993–C1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang D. D., Anfinogenova Y. (2008) Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J. Cardiovasc. Pharmacol. Ther. 13, 130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang D. D. (2009) p130 Crk-associated substrate (CAS) in vascular smooth muscle. J. Cardiovasc. Pharmacol. Ther. 14, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang T., Cleary R. A., Wang R., Tang D. D. (2014) Glia maturation factor-γ phosphorylation at Tyr-104 Regulates actin dynamics and contraction in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 51, 652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang T., Cleary R. A., Wang R., Tang D. D. (2013) Role of the adapter protein Abi1 in Actin-associated signaling and smooth muscle contraction. J. Biol. Chem. 288, 20713–20722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang R., Cleary R. A., Wang T., Li J., Tang D. D. (2014) The association of cortactin with profilin-1 is critical for smooth muscle contraction. J. Biol. Chem. 289, 14157–14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pokutta S., Weis W. I. (2007) Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu. Rev. Cell Dev. Biol. 23, 237–261 [DOI] [PubMed] [Google Scholar]
- 14. Jamora C., Fuchs E. (2002) Intercellular adhesion, signalling and the cytoskeleton. Nat. Cell Biol. 4, E101–E108 [DOI] [PubMed] [Google Scholar]
- 15. Castaño J., Raurell I., Piedra J. A., Miravet S., Duñach M., García de Herreros A. (2002) β-catenin N- and C-terminal tails modulate the coordinated binding of adherens junction proteins to β-catenin. J. Biol. Chem. 277, 31541–31550 [DOI] [PubMed] [Google Scholar]
- 16. Liu Z., Tan J. L., Cohen D. M., Yang M. T., Sniadecki N. J., Ruiz S. A., Nelson C. M., Chen C. S. (2010) Mechanical tugging force regulates the size of cell-cell junctions. Proc. Natl. Acad. Sci. U.S.A. 107, 9944–9949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ray S., Foote H. P., Lechler T. (2013) β-Catenin protects the epidermis from mechanical stresses. J. Cell Biol. 202, 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dose M., Emmanuel A. O., Chaumeil J., Zhang J., Sun T., Germar K., Aghajani K., Davis E. M., Keerthivasan S., Bredemeyer A. L., Sleckman B. P., Rosen S. T., Skok J. A., Le Beau M. M., Georgopoulos K., Gounari F. (2014) β-Catenin induces T-cell transformation by promoting genomic instability. Proc. Natl. Acad. Sci. U.S.A. 111, 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosenbluh J., Wang X., Hahn W. C. (2014) Genomic insights into WNT/β-catenin signaling. Trends Pharmacol Sci. 35, 103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang R., Mercaitis O. P., Jia L., Panettieri R. A., Tang D. D. (2013) Raf-1, Actin dynamics and Abl in human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 48, 172–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Q. F., Spinelli A. M., Tang D. D. (2009) Cdc42GAP, reactive oxygen species, and the vimentin network. Am. J. Physiol. Cell Physiol. 297, C299-C309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Q. F., Spinelli A. M., Wang R., Anfinogenova Y., Singer H. A., Tang D. D. (2006) Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J. Biol. Chem. 281, 34716–34724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cleary R. A., Wang R., Waqar O., Singer H. A., Tang D. D. (2014) Role of c-Abl tyrosine kinase in smooth muscle cell migration. Am. J. Physiol. Cell Physiol. 306, C753–C761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anfinogenova Y., Wang R., Li Q. F., Spinelli A. M., Tang D. D. (2007) Abl silencing inhibits CAS-Mediated process and constriction in resistance arteries. Circ. Res. 101, 420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jia L., Wang R., Tang D. D. (2012) Abl regulates smooth muscle cell proliferation by modulating actin dynamics and ERK1/2 activation. Am. J. Physiol. Cell Physiol. 302, C1026–C1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang R., Li Q. F., Anfinogenova Y., Tang D. D. (2007) Dissociation of Crk-associated substrate from the vimentin network is regulated by p21-activated kinase on ACh activation of airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L240–L248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang R., Li Q., Tang D. D. (2006) Role of vimentin in smooth muscle force development. Am. J. Physiol. Cell Physiol. 291, C483–C489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang Y., Zhang W., Gunst S. J. (2011) Activation of vinculin induced by cholinergic stimulation regulates contraction of tracheal smooth muscle tissue. J. Biol. Chem. 286, 3630–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao R., Du L., Huang Y., Wu Y., Gunst S. J. (2008) Actin depolymerization factor/cofilin activation regulates actin polymerization and tension development in canine tracheal smooth muscle. J. Biol. Chem. 283, 36522–36531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Balamatsias D., Kong A. M., Waters J. E., Sriratana A., Gurung R., Bailey C. G., Rasko J. E., Tiganis T., Macaulay S. L., Mitchell C. A. (2011) Identification of P-Rex1 as a novel Rac1-guanine nucleotide exchange factor (GEF) that promotes actin remodeling and GLUT4 protein trafficking in adipocytes. J. Biol. Chem. 286, 43229–43240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coué M., Brenner S. L., Spector I., Korn E. D. (1987) Inhibition of actin polymerization by latrunculin A. FEBS Lett. 213, 316–318 [DOI] [PubMed] [Google Scholar]
- 32. Hirokawa N., Takemura R. (2005) Molecular motors and mechanisms of directional transport in neurons. Nat. Rev. Neurosci. 6, 201–214 [DOI] [PubMed] [Google Scholar]
- 33. Erez N., Bershadsky A., Geiger B. (2005) Signaling from adherens-type junctions. Eur. J. Cell Biol. 84, 235–244 [DOI] [PubMed] [Google Scholar]
- 34. Jansen S. R., Van Ziel A. M., Baarsma H. A., Gosens R. (2010) β-Catenin regulates airway smooth muscle contraction. Am. J. Physiol. Lung Cell Mol. Physiol. 299, L204–L214 [DOI] [PubMed] [Google Scholar]
- 35. Guntur A. R., Rosen C. J., Naski M. C. (2012) N-cadherin adherens junctions mediate osteogenesis through PI3K signaling. Bone 50, 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Santis G., Miotti S., Mazzi M., Canevari S., Tomassetti A. (2009) E-cadherin directly contributes to PI3K/AKT activation by engaging the PI3K-p85 regulatory subunit to adherens junctions of ovarian carcinoma cells. Oncogene 28, 1206–1217 [DOI] [PubMed] [Google Scholar]
- 37. Demarco R. S., Struckhoff E. C., Lundquist E. A. (2012) The Rac GTP exchange factor TIAM-1 acts with CDC-42 and the guidance receptor UNC-40/DCC in neuronal protrusion and axon guidance. PLoS Genet. 8, e1002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Priya R., Yap A. S., Gomez G. A. (2013) E-cadherin supports steady-state Rho signaling at the epithelial zonula adherens. Differentiation 86, 133–140 [DOI] [PubMed] [Google Scholar]
- 39. Ratheesh A., Priya R., Yap A. S. (2013) Coordinating Rho and Rac: the regulation of Rho GTPase signaling and cadherin junctions. Prog. Mol. Biol. Transl. Sci. 116, 49–68 [DOI] [PubMed] [Google Scholar]
- 40. Limouze J., Straight A. F., Mitchison T., Sellers J. R. (2004) Specificity of blebbistatin, an inhibitor of myosin II. J. Muscle Res. Cell Motil. 25, 337–341 [DOI] [PubMed] [Google Scholar]
- 41. Eddinger T. J., Meer D. P., Miner A. S., Meehl J., Rovner A. S., Ratz P. H. (2007) Potent inhibition of arterial smooth muscle tonic contractions by the selective myosin II inhibitor, blebbistatin. J. Pharmacol. Exp. Ther. 320, 865–870 [DOI] [PubMed] [Google Scholar]
- 42. Zhang W. C., Peng Y. J., Zhang G. S., He W. Q., Qiao Y. N., Dong Y. Y., Gao Y. Q., Chen C., Zhang C. H., Li W., Shen H. H., Ning W., Kamm K. E., Stull J. T., Gao X., Zhu M. S. (2010) Myosin light chain kinase is necessary for tonic airway smooth muscle contraction. J. Biol. Chem. 285, 5522–5531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meng W., Takeichi M. (2009) Adherens junction: molecular architecture and regulation. Cold Spring Harb. Perspect. Biol. 1, a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gunst S. J., Tang D. D. (2000) The contractile apparatus and mechanical properties of airway smooth muscle. Eur. Respir. J. 15, 600–616 [DOI] [PubMed] [Google Scholar]
- 45. Gerthoffer W. T., Gunst S. J. (2001) Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J. Appl. Physiol 91, 963–972 [DOI] [PubMed] [Google Scholar]
- 46. Herrera A. M., Martinez E. C., Seow C. Y. (2004) Electron microscopic study of actin polymerization in airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 286, L1161–L1168 [DOI] [PubMed] [Google Scholar]
- 47. Kumawat K., Menzen M. H., Slegtenhorst R. M., Halayko A. J., Schmidt M., Gosens R. (2014) TGF-β-activated kinase 1 (TAK1) signaling regulates TGF-β-induced WNT-5A expression in airway smooth muscle cells via Sp1 and β-catenin. Plos ONE 9, e94801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ligon L. A., Karki S., Tokito M., Holzbaur E. L. (2001) Dynein binds to β-catenin and may tether microtubules at adherens junctions. Nat. Cell Biol. 3, 913–917 [DOI] [PubMed] [Google Scholar]