Abstract
The study explored on the commonly available weed plant Commelina nudiflora which has potential in-vitro antioxidant and antimicrobial activity. The different polar solvents such as ethanol, chloroform, dichloromethane, hexane and aqueous were used for the soxhlet extraction. The extracts were identified pharmacologically as important bioactive compounds and their potential free radical scavenging activities, and antimicrobial properties were studied. C. nudiflora extracts were monitored on their in-vitro antioxidant ability by DPPH and ABTS radical scavenging assay. Aqueous extract shows significant free radical scavenging activity of 63.4 mg/GAE and 49.10 mg/g in DPPH and ABTS respectively. Furthermore, the aqueous crude extract was used in antibacterial studies, which shows the highest inhibitory activity against Pseudomonas aeruginosa, Escherichia coli and Salmonella typhi. Among all the extracts, aqueous extract of C. nudiflora has significant control over free radical scavenging activity and inhibition of the growth of food pathogenic bacteria. Also, the aqueous extract contains abundance of phenolics and flavonoids higher than other extracts. This study explored weed plant C. nudiflora as a potential source of antioxidant and antibacterial efficacy and identified various therapeutic value bioactive compounds from GC–MS analysis.
Abbreviations: ABTS, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid); DPPH, 2,2-diphenyl-1-picrylhydrazyl; GAE, gallic acid equivalent; GC–MS, gas chromatography and mass spectrometry
Keywords: Commelina nudiflora L., Antibacterial, Phytochemicals, Free radical scavenging, GC–MS
1. Introduction
Plants naturally are a rich source of secondary metabolites and novel therapeutic compounds to enhance human health with controlled adverse effects (Jin-Ming et al., 2003). Natural products has a vital role in pharmacological and commercial industries, produce a lot of health care and medicinal products such as antimicrobial, anti-tumour agent, anti hepatotoxic, cardio tonic, CNS stimulant, nutraceuticals, sweeteners, food additives and animal feed (Gortzi et al., 2008; Verma et al., 2009). Plants such as herbs, trees, shrubs and climbers are exploited for their various bioactive compounds for human health (Ezzatzadeh et al., 2012). Moreover, plants contain important bioactive clusters such as alkaloids, flavonoids, saponins, steroids, terpenoids, polysaccharides and tannins that are largely contributing to various biological activities in traditional and modern therapeutic principles (Doughari et al., 2012; Mahboubi et al., 2013).
Commonly used medicinal plants have promising biological activity to control various bacterial and fungal diseases (Prasannabalaji et al., 2012). The basis for finding the natural products and their separation using different individual polar solvents is important for extraction of single compounds (Su et al., 2012). Although gradually increasing solvent polarity in sequential extraction such as chloroform, ethyl acetate, ethanol, water and methanol works out well in the separation of functional compounds, overall quality and quantity of extracting compounds from plant species largely are a result of the type of solvents (Ncube et al., 2012). Williamson (2001) reported that crude total extracted compounds showed significant results in disease management than those of a single isolated active fraction or purified specific constituent. The interaction of different groups of active metabolites in the extract might have enhanced the therapeutic effect more than the single ingredients (Mohamed et al., 2010).
Biochemical pathways or cellular mechanisms have been producing free radicals and reactive oxygen species as an end product (Nantitanon et al., 2007). Unstable chemical compounds are dangerous to living cells, it can cause mutation, myocardial infarction, Alzheimer diseases and can be associated with other clinical disorders (Adiguzel et al., 2009; Sugumaran and Raj, 2010). Generally, chemical based antioxidants are used to control free radical activity and they have adverse effects on human health and the development of natural antioxidant from plant resources would be fruitful to humankind (Rajasekar et al., 2011).
Plant C. nudiflora is a perennial herb of neotropical origin which has been distributed in various places in India, China, Malaysia, Africa and Egypt. C. nudiflora belongs to the Commelinaceae family. In principle, Commelina species are used to cure various chronic and acute diseases (Sharma, 1995; Hillocks, 1998). The Chinese used the whole plant of green extract in chilling the blood and performed blood clotting functions (Kaur and Das, 2011). C. nudiflora whole plant extracts were traditionally used to heal various chronic diseases such as diabetes, skin diseases and atherosclerosis (Wendy and Brathwaite, 2007; Ujowundu et al., 2008). Antioxidant and antimicrobial properties of plant metabolites are yet to be documented in detail. The present aim of this study is to identify the chemical constituents of C. nudiflora using different solvents with diversified polarity. Besides, the crude extracts were to be checked for its in vitro free radical scavenging ability and the ability to control food borne pathogenic bacteria.
2. Materials and methods
2.1. Plant collection and identification
C. nudiflora whole plant was collected in March 2014 from Maran, Pahang, Malaysia. The collected plant materials were transported to the laboratory. The plant was taxonomically identified and authenticated by Assoc. Prof. Dr. Norazian Mohd. Hassan, Kulliyyah of Pharmacy, International Islamic University, Malaysia. The voucher specimen was deposited at the Department herbarium centre and the voucher specimen number is KOPVN2014.
The collected plant materials were washed twice in running tap water to remove clay and grimes, cut into small pieces and shadow dried in normal room temperature at 35 °C 12/12 light and dark cycle. Completely dried plant materials were powdered using mechanical blender and sieved with 40 μm mesh. Different types of polar solvents such as hexane, chloroform, ethyl acetate, ethanol and water have been used for the soxhlet extraction.
2.2. Hot extraction method by soxhlet
10 g of plant powder was extracted in 250 ml of solvent (hexane, chloroform, ethyl acetate, ethanol and water) by soxhlet extraction technique overnight at respective solvent temperatures (Miller, 1972). The obtained extracts were subjected to vacuum evaporator to evaporate excess solvents. After that, the dried crude extract yields were weighed and used for further experimental studies.
2.3. Phytochemical studies
2.3.1. Preliminary characterization of phytochemical constituents
Crude plant extract samples were dissolved in respective solvents used for qualitative confirmation of major phytochemical constituents such as alkaloids, flavonoids, phenolics, saponins, steroids, tannins, carbohydrates and volatile oils (Trease and Evans, 1989; Harborne, 1998).
2.3.1.1. Alkaloids
In a test tube containing 1 ml of extract, a few drops of Dragendorff’s reagent was added and colour development was noticed. Appearance of orange colour indicates the presence of alkaloids.
2.3.1.2. Flavonoids
When 5 ml of 1% hydrochloric acid extract was shaken with sodium hydroxide, a yellow colour appeared indicating the presence of flavonoids.
2.3.1.3. Phenolics
To 1 ml of extract were added 2 ml of distilled water and a few drops of 10% ferric chloride. Appearance of blue or green colour indicates the presence of phenols.
2.3.1.4. Saponins
One ml of the plant under investigation was boiled with 10 ml of water for a few minutes and filtrated. The filtrate was vigorously shaken. The persistent froth (1 cm height) was present for 1 h which indicates the presence of saponins.
2.3.1.5. Steroids
The powder was dissolved in 2 ml of chloroform in a dry test tube. Ten drops of acetic anhydride and 2 drops of concentrated sulphuric acid were added. The solution became red, then blue and finally became bluish which indicates the presence of steroids.
2.3.1.6. Tannins
One drop of ferric chloride was added to 2 ml of the extract, and the appearance of bluish or greenish black colouration indicates the presence of tannins.
2.3.1.7. Carbohydrates
In a test tube, 5 ml of the filtrate was treated with 5 ml of Fehling’s solutions (A & B) and was heated; the appearance of a red precipitate indicates the presence of reducing sugars.
2.3.1.8. Volatile oils
To 2 ml of extract, were added 0.1 ml of diluted sodium hydroxide and a small amount of diluted hydrochloric acid. The formation of a white precipitate indicates volatile oils.
2.3.2. Determination of total phenolic content (TPC)
Total phenolic content of C. nudiflora extracts were determined by using Folin–Ciocalteu phenol reagent method described by Manian et al. (2008). A stock solution of plant extracts was prepared in different aliquots (0.1, 0.2, 0.3, 0.4, 0.5 mg/ml). One ml of plant extracts was taken in test tubes and 0.5 ml of Folin–Ciocalteu phenol reagent was added (FC reagent was dissolved in distilled water with 1:1 ratio). Then 2.5 ml of 20% sodium carbonate was added in each tube and finally the mixture was mixed properly by using vortex and then the test tube was kept in the dark for 40 min. Absorbance spectra were recorded at 725 nm using glass cuvettes. To minimize standard error, the reaction was performed in triplicate and the results were expressed in milligrams of gallic acid equivalent (mg GAE).
2.3.3. Determination of total flavonoid content (TFC) by using calorimetric method
Total flavonoid content of plant extracts was determined by Manian et al. (2008). Concisely, 100 μl of each plant extract (1 mg/ml) was dissolved in corresponding solvents and then extracts were made up to 1 ml by using distilled water followed by the addition of 75 μl of 10% sodium nitrate solution. After a 6 min interval, 150 μl of 5% aluminium chloride solution was added, then 0.5 ml of 1 M NaOH was added in the test tubes. The mixture samples were made up to 2.5 ml by using distilled water and thoroughly mixed. The UV–V absorbance values were read immediately at 510 nm. Results were expressed as mg/g butylated hydroxytoluene (BHT) equivalents.
2.4. Instrumentation characterization
2.4.1. GC–MS studies
Basic organic and inorganic chemical profiles of plant and other environmental samples were focused on using gas chromatography and mass spectral studies as one of the main established diagnostic tools. GC–MS analysis of the plant extract was performed by using Agilent GC–MS built with bonded-phase fused silica capillary column (30 mm × 0.25 mm ID; df = 0.25) (J&W Scientific, Folsom, CA, year) and mass spectrum for identification of the corresponding metabolites with correlation by known spectra. Instrumentation of GC–MS operating key procedure for volatile and semi volatile organic compounds are as follows. Column flow used highly reactive helium as a carrier gas at 1.5 ml/min. The splitless injection was maintained at 260 °C. The splitless injection mode was used with a split ratio of 40:1. The transfer line temperature was set at 260 °C. The mass analyser (mz) was set at 60 eV, electron impact source temperature at 200 °C, electron-multiplier voltage at 1588 mV and solvents delay at 2 min. All scanned data were obtained by the full-scan mass spectra within the scan range of 50–400 amu. The oven temperature programme was as follows: increased from 190 to 250 °C at a rate of 220 °C/min, and from 200 to 260 °C at a rate of 1 °C/min. Finally, the acquired spectrum of plant extracts was compared with the standard known database in the NIST library and confirmed as scrupulous compound (Lisec et al., 2006).
2.5. Antioxidant evaluation
2.5.1. DPPH assay
Free radical scavenging ability was determined by using the stable radical DPPH using the method of Siddhuraju and Becker (2003). Crude extract samples were prepared at 100 μg/ml, the plant extract of 2 ml was taken in test tubes and was added 3 ml of 0.3 mM methanolic solution of DPPH, mixed well and allowed to incubate at 30 °C for 20 min. The absorbance value of the sample was measured at 517 nm. The percentage of DPPH radical scavenging activity was theoretically calculated by the following formula:
2.5.2. ABTS assay
The 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) assay is used for the determination of total antioxidant activity of a plant sample. Inactive form of ABTS+ free radical cation is converted to the active form by reacting 7 mM ABTS aqueous solution with 2.4 mM potassium persulphate in the dark for 12–16 h at room temperature. The solution was further diluted with ethanol (1:89 v/v) and equilibrated at 30 °C. Afterwards, 1 ml of ABTS solution was added with 100 μl of plant extract, and after an initial mixing of 30 min the absorbance value was measured at 734 nm. The percentage of scavenging ABTS activity was calculated using the following formula (Manian et al., 2008):
2.6. Antibacterial activity
2.6.1. Agar disc diffusion method using C. nudiflora extracts
Aqueous C. nudiflora extract was checked for its potential biomedical application of antibacterial activity using the agar disc diffusion method. Gram positive and gram negative human pathogenic bacteria were used for the experiment and cultures were purchased from ATCC. Bacterial cultures of Escherichia coli (G−) (ATCC 25922), Staphylococcus aureus (G+) (ATCC 29213), Salmonella typhi (G+) (ATCC 14028), Klebsiella pneumoniae (G−) (ATCC BAA 2473), Pseudomonas aeruginosa (G−) (ATCC 15442) were used. Authentic bacterial cultures were sub cultured in nutrient broth. According to Hi media manufacturer’s specification, the MHA nutrient agar was prepared by using distilled water. The medium was autoclaved at 121 °C and at a pressure of 15 lbs for 20 min. The sterile medium was poured into autoclaved Petri plates and allowed to dry for a few minutes. Then, the cultured bacteria were swabbed onto agar plates. The surface of the agar medium is placed on a prepared standard disc (streptomycin 50 μg/ml) as control. Stock plant extract was prepared in 100 mg/ml. After that, the crude plant extracts were prepared at a different concentration of 50 μl, 75 μl and 100 μl and discs were soaked in the extracts overnight. After that, the disc was placed into bacterial swabbed agar plates and incubated for 24 h at 37 °C. Subsequently, incubation zone of inhibition was examined with the naked eye. Results were observed and the zone of inhibition (ZOI) was measured by zone reader. Experiments were repeated thrice to get results without error.
2.6.2. Minimal inhibitory concentration
Minimum inhibitory concentration (MIC) was determined by the microdilution method of Hammer et al. (1999) with minor modifications. MIC is defined as the lowest concentration of drug which controls microbial population growth. In this study, we prepared different concentrations of aqueous plant extracts (100, 50, 25, 12.5 and 6.5 mg/ml) to find the effective concentration for inhibition of bacterial growth. The MIC assay was used in 96 well plates, filled with 50 μl of nutrient agar broth and 30 μl of bacterial culture, and then for treatment 30 μl of plant extract (each prepared concentration) was added. Plates were incubated at 37 °C for 24 h. Incubated plates were read at 560 nm in a microplate reader and values are tabulated.
2.7. Statistical analysis
The raw data of antioxidant and antimicrobial values were entered into statistical analysis using statistical Package for the Social Sciences (SPSS) 16.0 version, (SPSS Inc., Headquarters, Chicago, IL). Experimental values were expressed as mean ± SD and comparisons were performed by a one way ANOVA followed by Student’s t-test. A value of p ⩽ 0.05 was considered statistically significant.
3. Results and discussion
3.1. Identification of secondary metabolites from C. nudiflora extracts
Table 1 shows the identified phytoconstituents from C. nudiflora extracts. Preliminary phytochemical analysis was carried out on select solvents such as hexane, ethyl acetate, chloroform, ethanol and aqueous. These crude extracts show positive results of alkaloids, flavonoids, terpenoids and phenolics compounds in chloroform, ethanol and aqueous extracts. Saponin is present in all solvent extracts. On the other hand, terpenoids is absent in the chloroform extract, however it is present in other solvent extracts. Different phytochemicals such as alkaloids, flavonoids, saponins, carbohydrates, phenolics, tannins, terpenoids and volatile oils were detected in the plant extracts. Furthermore, total phenolic content in aqueous extract has been found to be the highest (63.4 mg GAE/g) when compared with other extracts. Similarly, hexane possessed significant activity (59.7 mg GAE/g) and ethanol (31.6 mg GAE/g) in the DPPH antioxidant assay. Total flavonoid content of ethanol extract was calculated as 55.3 mg/g. Water and ethyl acetate extracts had moderate activity in total flavonoid content of 53.4 mg/g and 43.1 mg/g respectively (Table 2).
Table 1.
Preliminary identification of phytochemical constituents from C. nudiflora plant extracts.
| Secondary metabolites | Chloroform | Ethanol | Hexane | Aqueous | Ethyl acetate |
|---|---|---|---|---|---|
| Alkaloids | + | + | − | + | − |
| Flavonoids | + | + | + | + | + |
| Saponins | + | + | + | + | + |
| Sterols | − | − | − | − | + |
| Terpenoids | + | + | − | + | + |
| Volatile oils | + | − | + | − | + |
| Tannins | + | + | + | − | + |
| Phenolics | + | + | + | + | + |
| Carbohydrates | − | + | − | + | − |
+ = Present, − = Absent.
Table 2.
Quantitative analysis of phenolic and flavonoid contents from C. nudiflora crude extracts.
| Solvent extracts | Total phenolic content (mg/GAE)a,b | Total flavonoids content (mg/g)a,b |
|---|---|---|
| Water | 63.4 ± 0.05 | 53.40 ± 0.06 |
| Hexane | 59.7 ± 0.11 | 43.15 ± 0.04 |
| Ethyl acetate | 14.6 ± 0.05 | 23.40 ± 0.11 |
| Chloroform | 18.2 ± 0.08 | 24.56 ± 0.02 |
| Ethanol | 31.6 ± 0.05 | 55.30 ± 0.05 |
| Standard | 74.4 ± 0.04⁎ | 78.1 ± 0.05# |
Gallic acid.
α-BHT.
Mean values (n = 3) with significant difference at P < 0.05.
Percentage of activity due to extract concentration of 100 μg/ml.
The identified chemical constituents have pharmaceutical importance to defend against pathogenic bacteria, fungus and virus. This ethno pharmacological importance of plant extracts has a wide range of activity to control microbial infections (Ahameethunisa and Hopper, 2012). For instance, chemically synthesized antibiotics have some disadvantages for human beings such as low activity at target sites and harmful to distinct organs. The development of natural antimicrobial agents has active site action and induces the primary immune system and metabolic function in the human body.
3.2. GC–MS characterization of C. nudiflora crude extracts
Figs. 1 and 2 show GC–MS chromatogram of C. nudiflora extracts. Different classes of organic chemical constituents were identified by GC–MS studies. The GC–MS result shows medicinally valued phytochemicals are present in the plant extracts. These compounds are well known as plant derived antimicrobial agents (Table 3).
Figure 1.
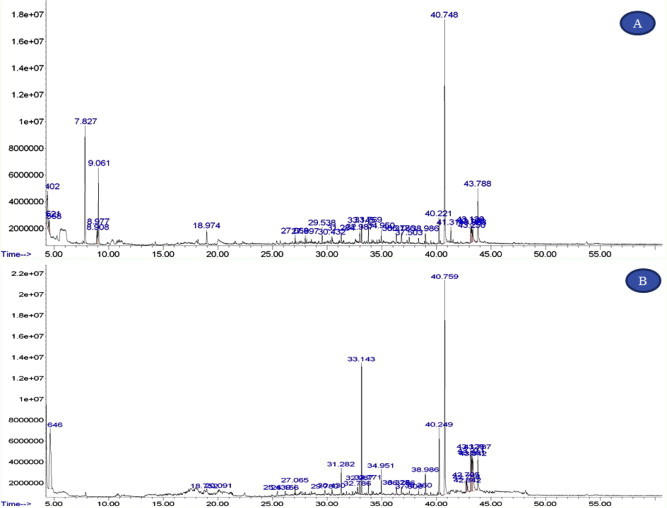
GC–MS chromatogram of different solvent extracts from Commelina nudiflora (A) Ethanol (B) Chloroform (C) Dichloromethane.
Figure 2.
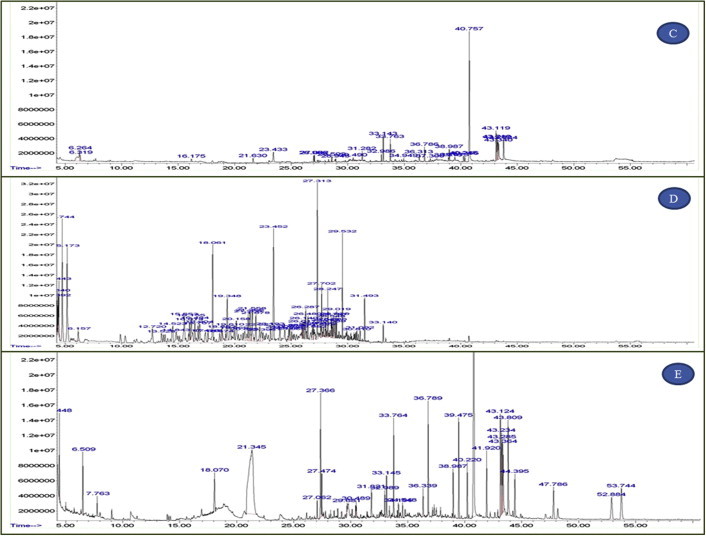
(continued) GC–MS chromatogram (D) Hexane (E) Aqueous extracts of C. nudiflora.
Table 3.
Organic constituent’s characterization by GC–MS from C. nudiflora extracts.
| S. no | Solvent system | Retention time | Area % | Chemical constituents |
|---|---|---|---|---|
| 1 | Ethyl acetate extract | 33.144 | 1.16 | Benzoic acid |
| 33.763 | 3.04 | Ethanedioic acid | ||
| 36.339 | 0.97 | 2-pyrimidine | ||
| 43.125 | 4.14 | Phytol | ||
| 43.808 | 3.89 | n-Decanoic acid | ||
| 53.743 | 2.78 | Phthalic acid | ||
| 2 | Chloroform extract | 4.402 | 7.13 | Acetic acid |
| 4.522 | 1.52 | 1-Propanol | ||
| 7.827 | 14.88 | Ethane | ||
| 8.976 | 2.08 | Oxalic acid | ||
| 33.769 | 1.98 | Phosphonic acid | ||
| 31.283 | 1.00 | Muscimol | ||
| 3 | Aqueous extract | 31.281 | 2.41 | 2-H-azepin-2-one |
| 32.785 | 0.44 | Difluorobenzoic acid | ||
| 33.771 | 1.22 | 1-Butanamine | ||
| 36.786 | 0.81 | N-ethyl formamide | ||
| 40.250 | 6.19 | Hexadecanoic acid | ||
| 4 | Ethanol extract | 4.522 | 0.40 | Carbonic acid |
| 30.408 | 0.57 | Ethanedioic acid | ||
| 34.549 | 0.27 | Cystamine | ||
| 40.718 | 0.28 | Isobutylamine | ||
| 41.796 | 20.34 | Tridecanoic acid | ||
| 39.476 | 0.29 | 3,8 dioxa-2,9 disilade-5-ene | ||
| 5 | Hexane extract | 4.339 | 0.56 | Cyclopentane |
| 4.743 | 5.76 | Heptane | ||
| 14.524 | 1.79 | Toluene | ||
| 17.644 | 0.47 | Octane | ||
| 23.452 | 7.05 | Oxalic acid | ||
| 61.57 | 0.69 | Undecane | ||
3.3. Free radical scavenging activity on C. nudiflora extracts
DPPH and ABTS radical scavenging activities of solvent extracts are shown in Table 4. Aqueous extracts of C. nudiflora showed significant activity (43.4%) compared with gallic acid standard. Other extracts such as hexane and ethanol have moderate scavenging properties of 31.63% and 33.29% respectively. In addition, ethyl acetate has the lowest radical scavenging activity at 22.79% in this study. Among these extracts, aqueous extract of C. nudiflora has more DPPH radical scavenging activity. ABTS free radical scavenging activity was analysed using BHT as standard. Aqueous plant extracts revealed significant ABTS radical scavenging activity (46.64%), followed by chloroform (34.8%) and hexane (30.14%) respectively. In the ABTS test, aqueous extracts were a potential candidate to control free radical formation.
Table 4.
Antioxidants activity of C. nudiflora plant extracts.
| Antioxidant assays | Aqueous | Hexane | Ethyl acetate | Chloroform | Ethanol | Standard |
|---|---|---|---|---|---|---|
| DPPH assaya,b | 43.40 ± 0.05 | 31.63 ± 0.04 | 22.79 ± 0.02 | 32.70 ± 0.05 | 33.29 ± 0.05 | 67.29 ± 0.04⁎ |
| ABTS assaya,b | 46.64 ± 0.03 | 30.14 ± 0.03 | 29.46 ± 0.04 | 34.80 ± 0.05 | 26.20 ± 0.05 | 69.23 ± 0.05⁎ |
Ascorbic acid.
Mean values (n = 3) with significant difference at P < 0.05.
Percentage of inhibition due to extract concentration of 100 μg/ml.
Excess free radical formation caused cellular damage and induced many dysfunctions like atherosclerosis, myocardial infarction, cancer and neurogenerative disorders in human beings. But, natural antioxidant compounds are useful in repairing free radical formation in cells and manage various chronic diseases (Fakruddin et al., 2012). For instance, C. heptaphylla plant extract plays a significant role in DPPH radical scavenging activity with IC50 values of 3.11 μg/ml. Plant extracts were detected to have three major types of natural compounds which are saponin, flavonoids and iridoid glycosides and, steroid and spermine. The iridoid phenylethanoid glycosides are highly quantified in the verbascum genus. These chemical groups are mainly participating in the antioxidant activities (Ozcan et al., 2011). Yildirim et al. (2001) studied the percentage of DPPH scavenging activities of Rumex crispus ethanolic and water extracts. The antioxidant activity depends on the high concentration of extracts. The amount of phenolic content and related antioxidant compounds increases the DPPH free radical scavenging activity. R. crispus seed extracts are prepared at different concentrations of 50–400 μg, which exposed better scavenging activities in the highest concentration. Also, it is correlated with the amount of phenolic content in the extract and DPPH scavenging levels.
Bazzaz et al. (2011) proved that antioxidant capacity is based on the composition of different phenolic contents present in the Scutellaria litwinowii extracts. Antioxidant tests are highly specific and sensitive to temperature and incubation period. Physiochemical properties of the sample are very important for analysing antioxidant properties. Similarly, our results expressed different solvent extracts such as aqueous, hexane, ethyl acetate, chloroform and ethanol among aqueous extracts which have significant antioxidant activity observed using DPPH and ABTS methods. Hence, the higher activity shows the relationship between abundant quantities of phenolic and flavonoids which are 63.4 mg/GAE and 49.1 mg/g respectively.
3.4. Antibacterial activities of C. nudiflora extracts
Antimicrobial efficacy of aqueous extracts of C. nudiflora is shown in Fig. 3. Aqueous plant extracts showed higher inhibitory activity against P. aeruginosa (13 mm) followed by E. coli and S. typhi. The least activity was measured by Bacillus subtilis and S. aureus (11 mm and 9 mm respectively). In contrast, aqueous plant extracts showed less activity against S. aureus (7 mm). Overall, aqueous C. nudiflora extract showed effective antibacterial activity against food borne pathogenic bacteria. MIC range was calculated to be 12.25 mg/ml as the lowest concentration of aqueous plant extract active against P. aeruginosa.
Figure 3.
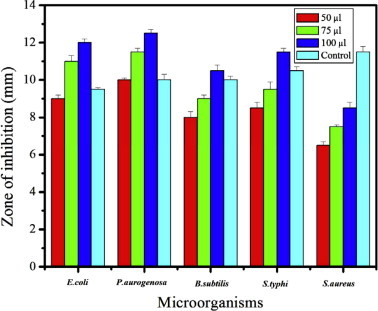
Antimicrobial activity of aqueous extract of C. nudiflora.
Recently, the increasing resistance rates of bacterial strains and control of the growth of pathogens are a big challenge. Developing more potent antibacterial agents using plant extracts is important in inhibiting growth rate of pathogens. Multidrug resistance pathogens have been found to be sensitive to the crude plant extracts. Clinically challenging S. aureus strains are a major cause of community and hospital-associated infections with an estimated mortality rate of around 7–10% (Tamokou et al., 2012). Similarly, antibacterial property of Hippophae rhamnoides extracts was used to control food borne microorganisms. Methanolic plant extracts exhibited higher activity in gram positive bacteria B. subtilis and S. aureus. Plants contain bioflavonoids and phenolics, which are marker compounds for antimicrobial and antioxidant activities. H. rhamnoides extracts have lesser activity than synthetic antibiotics such as tetracycline and microbe-driven (Jeong et al., 2010). Mohamed et al. (2013) revealed high polarity of solvents in Syzygium cumini extracts has been more effective in controlling free radical scavenging activity than the non-polar solvent extracts. S. cumini contains adequate amounts of essential oils and other active metabolites, where it can participate in antioxidant mechanisms to prevent cellular damages. Moreover, S. cumini extracts act as natural preservative agents for different food products. On the other hand, aqueous C. nudiflora extracts were observed to have major in vitro antimicrobial/antioxidant activities. Our results successfully argue for the commonly available weed plants which are effective in the treatment of food pathogenic microorganisms and efficient in controlling oxidative damage.
4. Conclusion
Edible weed plant C. nudiflora possesses novel bioactive compounds in various solvent extracts. Besides, C. nudiflora extracts controlled free radicals and food borne pathogens such as E. coli and P. aeruginosa. Aqueous extracts of C. nudiflora showed significant antioxidant and antimicrobial activity. Moreover, the ethanol > ethyl acetate > chloroform > hexane have been found to have moderate radical scavenging and antibacterial activities. Furthermore, we are now focusing on saponin purification from C. nudiflora extracts and possible structural elucidation of compounds and their anticancer properties.
Conflict of interest
We (authors) have declared that there is no conflict of interests in the study.
Acknowledgements
The authors would like to acknowledge Universiti Malaysia Pahang, for its support through PGRS research Grant No. GRS 130336. PK also appreciates IPS for the financial assistance in the form of DSS.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adiguzel A., Ozer H., Sokmen M., Gulluce M., Sokmen A., Kilic H., Sahin F., Baris O. Antimicrobial and antioxidant activity of essential oil and methanol extract of Nepeta cataria. Pol. J. Microbiol. 2009;58:69–76. [PubMed] [Google Scholar]
- Ahameethunisa A.R., Hopper W. In vitro antimicrobial activity on clinical microbial strains and antioxidant properties of Artemisia parviflora. Ann. Clin. Microbiol. Antimicrob. 2012;11:30. doi: 10.1186/1476-0711-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzaz B.S.F., Khayat M.H., Emamic S.A., Asili J., Sahebkara A., Neishabory E.J. Antioxidant and antimicrobial activity of methanol, dichloromethane, and ethyl acetate extracts of Scutellaria litwinowii. Sci. Asia. 2011;37:327–334. [Google Scholar]
- Doughari J.H., Ndakidemi P.A., Human I.S., Benade S. Antioxidant, antimicrobial and antiverotoxic potentials of extracts of Curtisia dentata. J. Ethnopharmacol. 2012;141:1041–1050. doi: 10.1016/j.jep.2012.03.051. [DOI] [PubMed] [Google Scholar]
- Ezzatzadeh E., Farjam M.H., Rustaiyan A. Comparative evaluation of antioxidant and antimicrobial activity of crude extract and secondary metabolites isolated from Artemisia kulbadica. Asian Pac. J. Trop. Dis. 2012;2:S431–S434. [Google Scholar]
- Fakruddin M., Mannan K.S., Mazumdar R.M., Afroz H. Antibacterial, antifungal and antioxidant activities of the ethanol extract of the stem bark of Clausena heptaphylla. BMC Complement. Altern. Med. 2012;12:232. doi: 10.1186/1472-6882-12-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gortzi O., Lalas S., Chinou I., Tsaknis J. Reevaluation of bioactivity and antioxidant activity of Myrtus communis extract before and after encapsulation in liposomes. Eur. Food Res. Technol. 2008;226:583–590. [Google Scholar]
- Hammer K.A., Carson C.F., Riley T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- Harborne J.B. Chapman and Hall Publication; London: 1998. Phytochemical Methods. pp. 34–88. [Google Scholar]
- Hillocks R.J. The potential benefits of weeds with reference to small holder agriculture in Africa. Integrated Pest Manag. Rev. 1998;3:155–167. [Google Scholar]
- Jeong J.H., Lee J.W., Kim K.S., Kim J.-S., Han S.N., Yu C.Y., Lee J.K., Kwon Y.S., Kim M.J. Antioxidant and antimicrobial activities of extracts from a medicinal plant sea buckthorn. J. Korean Soc. Appl. Biol. Chem. 2010;53:33–38. [Google Scholar]
- Jin-Ming K., Ngoh-Khang G., Lian-Sai C., Tet-Fatt C. Recent advances in traditional plant drugs and orchids. Acta Pharmacol. Sin. 2003;24:7–21. [PubMed] [Google Scholar]
- Kaur S., Das M. Functional foods: an overview. Food Sci. Biotechnol. 2011;20:861–875. [Google Scholar]
- Lisec J., Schauer N., Kopka J., Willmitzer L., Fernie A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006;1:387–396. doi: 10.1038/nprot.2006.59. [DOI] [PubMed] [Google Scholar]
- Mahboubi M., Haghi G., Kazempour N., Hatemi A.R. Total phenolic content, antioxidant and antimicrobial activities of Blepharis edulis extracts. Songklanakarin J. Sci. Technol. 2013;35:11–16. [Google Scholar]
- Manian R., Anusuya N., Siddhuraja P., Manian S. The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem. 2008;107:1000–1007. [Google Scholar]
- Miller G.L. Wiley Eastern Ltd; New Delhi: 1972. Biochemical methods for agricultural sciences. pp. 6–100. [Google Scholar]
- Mohamed A.A., Ali S.I., El-Baz F.K. Antioxidant and antibacterial activities of crude extracts and essential oils of syzygium cumini leaves. PLoS One. 2013;8:e60269. doi: 10.1371/journal.pone.0060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A.A., Khalil A.A., El-Beltagi H. Antioxidant and antimicrobial properties of kaff Maryam (Anastatica hierochuntica) and doum palm (Hyphaene thebaica) Grasas Aceites. 2010;61:67–75. [Google Scholar]
- Nantitanon W., Chowwanapoonpohnm S., Onogi O.K.S. Antioxidant and antimicrobial activities of Hyptis suaveolens essential oil. Sci. Pharm. 2007;75:35–46. [Google Scholar]
- Ncube B., Finnie J.F., Staden J.V. In vitro antimicrobial synergism within plant extracts combinations from there South African medicinal bulbs. J. Ethnopharmacol. 2012;139:81–89. doi: 10.1016/j.jep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Ozcan B., Esen M., Caliskan M., Mothana R.A., Cihan A.C., Yolcu H. Antimicrobial and antioxidant activities of the various extracts of Verbascum pinetorum Boiss. O. Kuntze (Scrophulariaceae) Eur. Rev. Med. Pharmacol. 2011;15:900–905. [PubMed] [Google Scholar]
- Prasannabalaji N., Muralitharan G., Sivanandan R.N., Kumaran S., Pugazhvendan S.R. Antibacterial activities of some Indian traditional plant extracts. Asian Pac. J. Trop. Dis. 2012;2:S291–S295. [Google Scholar]
- Rajasekar M., Jegadeesh R., Raaman N., Das T.M. Studies on the synthesis and the antimicrobial and antioxidant activities of a novel class of fluorescein-based glycosides. Carbohydr. Res. 2011;346:2362–2367. doi: 10.1016/j.carres.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Sharma A.K. Cytology of some of the members of Commelinaceae and its bearing on the interpretation of phylogeny. Genetica. 1995;27:323–363. [Google Scholar]
- Siddhuraju P., Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Su B.L., Zeng R., Chen J.Y., Chen C.Y., Guo J.H., Huang C.G. Antioxidant and antimicrobial properties of various solvent extracts from Impatiens balsamina L. stems. J. Food Sci. 2012;77:C614–C619. doi: 10.1111/j.1750-3841.2012.02709.x. [DOI] [PubMed] [Google Scholar]
- Sugumaran S., Raj A.D.S. Medicinal plants of sacred groves in Kanyakumari district south Western Ghats. Indian J. Traditional Knowledge. 2010;9:291–299. [Google Scholar]
- Tamokou J.D., Mpetga D.J.S., Lunga P.K., Tene M., Tane P., Kuiate J.R. Antioxidant and antimicrobial activities of ethyl acetate extract, fractions and compounds from stem bark of Albizia adianthifolia (Mimosoideae) BMC Complement. Altern. Med. 2012;12:99. doi: 10.1186/1472-6882-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trease G.E., Evans W.C. Bailliere Tindall Ltd; London: 1989. Pharmacology. pp. 60–75. [Google Scholar]
- Ujowundu C.O., Igwe C.U., Enemor V.H.A., Nwaogu L.A., Okafor D.E. Nutritive and anti nutritive properties of Boerhavia diffusa and Commelina nudiflora leaves. Pak. J. Nutr. 2008;7:90–92. [Google Scholar]
- Verma P.S., Sreevidya N., Verma R.S. Antibacterial and antioxidant activity of methanol extract of Evolvulus nummularius. Indian J. Pharmacol. 2009;41:233–236. doi: 10.4103/0253-7613.58514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendy A.P.I., Brathwaite R.A.I. Commelina species – A review of its weed status and possibilities for alternative weed management in the tropics. Agro Thesis. 2007;5:3–18. [Google Scholar]
- Williamson E.M. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8:401–409. doi: 10.1078/0944-7113-00060. [DOI] [PubMed] [Google Scholar]
- Yildirim A., Mavi A., Kara A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agri. Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]


