Abstract
The pollution of environment by toxic chemicals is a global and chronic problem. Human health risk due to exposure to chemical pollutants is constantly increasing. Pesticides form major toxic chemicals in environment. Scientifically, there is an obviously correlation between the exposure to pesticides and appearance of many diseases. Currently, the significance of natural products for health and medicine has been formidable. The present study investigated the effect of grapeseed oil in male rats exposed to diazinon. The experimental rats were divided into five groups. The rats of the first group were served as control. The experimental animals of the second group were exposed to diazinon (DZN). The animals of the third group were supplemented with grapeseed oil and treated with DZN. The rats of the fourth group were supplemented with grapeseed oil. The experimental rats of the fifth group were supplemented with corn oil. Hematobiochemical and histopathological evaluations were chosen as indicators of DZN toxicity and protective role of grapeseed oil. In rats exposed only to DZN, the levels of serum glucose, triglycerides, cholesterol, low density lipoprotein cholesterol, very low density lipoprotein cholesterol, creatinine, urea nitrogen, uric acid, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, creatine kinase and lactate dehydrogenase were statistically increased, while the level of serum total protein was significantly decreased. Moreover, the histopathological evaluations of the liver, kidney and testis showed that DZN causes several severe alterations. Pretreatment with grapeseed oil exhibited a protective role against DZN toxicity which confirmed by the inhibition of hematobiochemical and histopathological changes due to DZN exposure. Additionally, the present study suggests that the effect of grapeseed oil supplementation against DZN toxicity may be attributed to the antioxidant role of its constituents.
Keywords: Diazinon, Grapeseed oil, Blood, Histopathology, Rats
1. Introduction
The pollution of the environment plays a crucial role in the occurrence of many diseases affecting plants, animals and humans. One of the main factors causing pollution of the environment is the irrational use of organophosphorus pesticides (Al-Haj et al., 2005). Nowadays, contact with organophosphorus pesticides is an important health problem for agricultural workers (Hurtig et al., 2003). Although rapidly metabolized, they are highly toxic for insects and mammals. Due to easy access to organophosphorous pesticides and their higher degree of toxicity, accidental poisonings and also suicides by using them are wide. So, it is one of the toxic materials causing human poisoning and death worldwide annually (Abdollahi et al., 2004).
Diazinon (DZN), C12H21N2O3PS, is a commonly used organophosphorous insecticide. It has been used since 1956 for the control of soil insects and pests, on ornamental plants, and on fruits, vegetables and field crops. Now it is used to control flies around animal facilities, greenhouses, fairgrounds and other businesses and public places where food or animal wastes might be accumulated (Dikshith and Diwan, 2003). DZN can be highly toxic for animals and human kind (Poet et al., 2004; Sarabia et al., 2009). The main mechanism of action of DZN is acetyl-cholinesterase enzyme inhibition (Kamanyire and Karalliedde, 2004). However, it may induce imbalance in the free radicals production/elimination processes with consequent induction of cellular damage (Kamanyire and Karalliedde, 2004; Gokcimen et al., 2007; Roegge et al., 2008; Cakici and Akat, 2013). Additionally, several studies showed that DZN was capable of inducing histopathological, biochemical and physiological alterations (Al-Attar, 2009; Al-Attar and Al-Taisan, 2010; Al-Attar and Abu Zeid, 2013; Boroushaki et al., 2013; Cakici and Akat, 2013; El-Demerdash and Nasr, 2014).
In recent years, a considerable emphasis has been focused on the importance of the naturally available botanicals that can be consumed in an individual’s everyday diet because of their antioxidant and antiinflammatory properties (Nandakumar et al., 2008). Nature has been a source of medicinal treatments for thousands of years and plant-derived products continue to play an essential role in the primary health care of about 80–85% of the world’s population. Despite the trends of molecular biology and chemistry providing fast escalation of synthesized de novo drugs, plants still remain a traditional source of medicinal compounds; up to 40% of modern drugs may directly or indirectly be related to natural compounds (Solyanik et al., 2004). Grape (Vitis vinifera) is one of the world’s largest fruit crops and grape seed extract is a complex matrix containing approximately 40% fiber, 16% oil, 11% proteins, and 7% complex phenols including tannins, in addition to sugars and mineral salts (Shi et al., 2003). Grapeseed oil as an extract of the grape seed has many uses ranging from cooking (as a food additive), cosmetics and in controlling several diseases and wound healing potential (Shivananda et al., 2011). Nowadays, many scientific researchers have revealed that the grapeseed oil has several health benefits and is considered as a good and potent antioxidant compound for its contents of polyphenols, flavonoids, unsaturated fatty acids and vitamin E (El-Ashmawy et al., 2007; Dos Santos Freitas et al., 2008; Hassanein and Abedel-Razek, 2009; Kikalishvili et al., 2011; Hasseeb et al., 2013). Therefore, the present study was aimed to investigate the effect of grapeseed oil supplementation on physiological and histopathological alterations induced by DZN toxicity in male rats.
2. Materials and methods
2.1. Animals
Thirty healthy male albino rats of the Wistar strain (85.4–93.8 g) used in this study were obtained from the Experimental Animal Unit of King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. The experimental animals were housed 6 per cage in a room with 65% humidity, 12:12 h light: dark cycle at ambient temperature of 20 ± 1 °C. Standard diet, commercial feed pellets and tap water were freely available. The principles of laboratory animal care were followed throughout the duration of experiment and instruction given by King Abdulaziz University ethical committee was followed regarding experimental treatments.
2.2. Experimentation
The experimental animals were randomly distributed into five groups of six each. Animals of group 1 were untreated and served as normal control. Rats of group 2 were orally administrated with 50 mg/kg body weight of DZN in corn oil, daily for 3 weeks. Animals of group 3 were orally given grapeseed oil at a dose of 2 g/kg body weight and after 4 h subjected to DZN at the same dose given to group 2, daily for 3 weeks. Rats of group 4 were treated with grapeseed oil at the same dose given to group 3, daily for 3 weeks. Experimental animals of group 5 were supplemented with corn oil at the same dose given to group 2, daily for 3 weeks. At the end of the experimental period, rats were fasted for 10 h, anesthetized using diethyl ether and blood samples were collected from orbital venous plexus in non-heparinized. For obtaining blood serum, collecting blood tubes were centrifuged at 2500 rpm for 15 min. Serum glucose, total protein, triglycerides, cholesterol, high density lipoprotein cholesterol (HDL), creatinine, blood urea nitrogen (BUN), uric acid, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), creatine kinase (CK) and lactate dehydrogenase (LDH) were measured using Dimension Vista® 1500 System, USA. The level of serum low density lipoprotein cholesterol (LDL-C) was estimated according to the equation of Friedewald et al. (1972).
Serum very low density lipoprotein cholesterol (VLDL-C) was evaluated using the following equation:
For histopathological examinations, liver, kidney and testis sections were taken from all groups. The tissues were fixed in 10% neutral formalin, dehydrated with different ethanol solutions and embedded in paraffin, then cut into 4μ thick sections, stained with hematoxylin-eosin and observed under a photomicroscope.
2.3. Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student’s t-test. All data are presented as mean ± standard deviation (SD). Differences below P < 0.05 implies significance.
3. Results
As seen in Tables 1 and 2, the levels of serum glucose (+38.2%), triglycerides (+87.5%), cholesterol (+97.0%), LDL-C (+540.0%), VLDL-C (+90.9%), creatinine (+30.4%), BUN (+89.9%), uric acid (+62.3%), ALT (+71.2%), AST (+70.7%), ALP (+117.8%), CK (+59.7%) and LDH (+61.8%) were statistically increased, while the level of serum total protein (−20.0%) was significantly decreased in rats exposed to DZN compared with control and other treated groups. Moreover, the levels of serum HDL-C were significantly unchanged. In rats exposed to grapeseed oil plus DZN, significant increases in the levels of serum creatinine (+18.4%), AST (+25.0%), ALP (+26.5%) and LDH (+23.1%) were noted, while the levels of other studied parameters (serum glucose, total protein, triglycerides, cholesterol, HDL-C, LDL-C, VLDL-C, BUN, uric acid, ALT and CK) were statistically unchanged compared with control rats (Tables 1 and 2). Significant increases in the levels of serum cholesterol (+25.8%) and HDL-C (+18.8%) were observed in rats treated with corn oil, while the levels of other studied parameters were significantly unchanged compared with control rats. Insignificant alterations in the levels of all studied parameters were noted in rats supplemented with grapeseed oil (Tables 1 and 2).
Table 1.
The levels of serum glucose, total protein, triglycerides, cholesterol, HDL-C, LDL-C and VLDL-C in control, DZN, grapeseed oil plus DZN, grapeseed oil and corn oil treated rats. Percentage changes are included in parentheses.
| Parameters (mmol/L) | Treatments |
||||
|---|---|---|---|---|---|
| Control | DZN | Grapeseed oil + DZN | Grapeseed oil | Corn oil | |
| Glucose | 4.97 ± 0.55 | 6.87 ± 0.67a,b (+38.2) | 4.88 ± 0.31 (−1.8) | 5.02 ± 0.57 (+1.0) | 4.92 ± 9.49 (−1.0) |
| Total protein | 61.00 ± 6.69 | 48.77 ± 4.03a,b (−20.0) | 55.33 ± 7.70 (−0.3) | 58.00 ± 3.46 (−4.9) | 57.17 ± 5.81 (−6.3) |
| Triglycerides | 0.48 ± 0.09 | 0.90 ± 0.15a,b (+87.5) | 0.47 ± 0.10 (−2.1) | 0.46 ± 0.14 (−4.2) | 0.57 ± 0.10 (+18.8) |
| Cholesterol | 0.97 ± 0.08 | 1.94 ± 0.49a,b (+97.0) | 1.15 ± 0.21 (+18.6) | 0.97 ± 0.12 (0) | 1.22 ± 0.07a (+25.8) |
| HDL-C | 1.01 ± 0.07 | 0.98 ± 0. 22 (−3.0) | 1.10 ± 0.06 (+8.9) | 1.01 ± 0.06 (0) | 1.20 ± 0.08a (+18.8) |
| LDL-C | 0.15 ± 0.04 | 0.96 ± 0.41a,b (+540) | 0.20 ± 0.13 (+33.3) | 0.18 ± 0.07 (+20) | 0.16 ± 0.08 (−6.7) |
| VLDL-C | 0.22 ± 0.04 | 0.42 ± 0.07a,b (+90.9) | 0.21 ± 0.04 (−4.6) | 0.21 ± 0.06 (−4.6) | 0.26 ± 0.04 (+18.2) |
P < 0.05: Student’s t-test (significance levels shown for difference between control and treated groups).
P < 0.05: Student’s t-test (significance levels shown for difference between rats exposed to DZN and grapeseed oil plus DZN, grapeseed oil or corn oil).
Table 2.
The levels of serum creatinine, BUN, uric acid, ALT, AST, ALP, CK and LDH in control, DZN, grapeseed oil plus DZN, grapeseed oil and corn oil treated rats. Percentage changes are included in parentheses.
| Parameters | Treatments |
||||
|---|---|---|---|---|---|
| Control | DZN | Grapeseed oil + DZN | Grapeseed oil | Corn oil | |
| Creatinine (μmol/L) | 26.33 ± 3.93 | 34.32 ± 3.27a,b (+30.4) | 31.17 ± 3.49a (+18.4) | 27.83 ± 2.79 (+5.7) | 28.50 ± 3.73 (+8.2) |
| BUN (mmol/L) | 3.65 ± 0.74 | 6.85 ± 1.16a,b (+89.9) | 4.95 ± 1.22 (+35.6) | 3.68 ± 0.62 (+0.8) | 3.32 ± 0.57 (−9.9) |
| Uric acid (μmol/L) | 42.00 ± 5.97 | 68.17 ± 15.41a,b (+62.3) | 46.24 ± 13.00 (+10.1) | 40.33 ± 7.06 (−4.0) | 36.42 ± 6.83 (−13.3) |
| ALT (U/L) | 46.67 ± 5.46 | 79.88 ± 8.2a,b (+71.2) | 51.33 ± 8.80 (+10.0) | 44.66 ± 5.55 (−4.3) | 48.33 ± 2.66 (+3.6) |
| AST (U/L) | 98.83 ± 9.50 | 168.73 ± 24.29a,b(+70.7) | 123.50 ± 19.81a (+25.0) | 106.23 ± 16.72 (+7.5) | 101. 17 ± 10.32 (+2.4) |
| ALP (U/L) | 179.00 ± 15.28 | 389.83 ± 81.14a,b(+117.8) | 226.50 ± 39.56a (+26.5) | 174.83 ± 20.09 (−2.3) | 178.80 ± 14.89 (−0.1) |
| CK (U/L) | 315.50 ± 41.02 | 503.87 ± 74.10a,b (+59.7) | 317.83 ± 50.31 (+0.7) | 297.43 ± 29.03 (−5.7) | 296.72 ± 60.10 (−6.0) |
| LDH (U/L) | 493.33 ± 59.29 | 798.17 ± 75.34a,b (+61.8) | 607.50 ± 51.32a (+23.1) | 504.18 ± 61.62 (+2.2) | 482.31 ± 65.41 (−2.2) |
P < 0.05: Student’s t-test (significance levels shown for difference between control and treated groups).
P < 0.05: Student’s t-test (significance levels shown for difference between rats exposed to DZN and grapeseed oil plus DZN, grapeseed oil or corn oil).
Histological examinations of the liver of control rats showed the normal structure (Fig. 1 A). Histopathological influences of DZN on the liver of treated rats are presented in Fig. 1B–D. Rats treated with DZN showed many severe histopathological alterations including a damage of liver structure along with disarrangement of hepatic strands. Several cells also show histological features of necrosis. Moreover, an enlargement of the sinusoids and vacuole formations in hepatocytes, dilation and a large droplet of glycogen were noted in the liver of rats exposed to DZN (Fig. 1B–D). Grapeseed oil treated brought back the cellular arrangement around the central vein and reduced necrosis (Fig. 1E). Mild enlargement in the sinusoids, vacuole formations in hepatocytes, leukocytic infiltrations and dilation were observed in rats treated with grapeseed oil plus DZN compared with DZN treated rats and control. Additionally, no detectable histological differences are observed by the light microscope between livers of control rats and rats supplemented with grapeseed oil (Fig. 1F). Areas of renal cortex containing renal corpuscles and associated tubules were showed more pronounced changes in treated animals compared with control. Therefore, these areas were selected for histological examination with the light microscope. The normal renal corpuscle consists of a tuft of capillaries, the glomerulus, surrounded by a double walled epithelial capsule called Bowman’s capsule. Between the two layers of the capsule is the urinary or Bowman’s space (Fig. 2A). In rats treated with DZN there were pronounced alterations in the structure of renal corpuscle including a highly degeneration and necrosis of glomeruli, Bowman’s capsules and associated tubules structure (Fig. 2B). The structure of renal corpuscles in rats treated with grapeseed oil plus DZN showed a normal appearance with some necrosis (Fig. 3B) and the most changes were noted in the structure of some glomeruli (Fig. 3B). In comparison with control rats, the renal sections from rats supplemented with grapeseed showed normal structure (Fig. 3C). Normal histology of the testis in control rats is shown in Fig. 3 A. Seminiferous tubules appear as rounded or oval structures, each surrounded by a thin basal membrane and contains in its wall several layers of cell representing spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids and spermatozoa, which are connected to cells of Sertoli. The intertubular tissue, which is formed of connective tissue holding the seminiferous tubules with each other and contains blood vessels. It also contains Leydig (interstitial) cells, cells of endocrine secretion. In rats treated with DZN, the structure of the testis showed histopathological alterations including the loss of the intertubular tissue and an increase of distances between the seminiferous tubules (Fig. 3B). Supplementation with grapeseed oil showed a marked recovery from severe damages induced by DZN toxicity (Fig. 3B). The histological structure of the testis in rats treated with corn oil showed a normal appearance compared to control rats (Fig. 3C). It is worth mentioning that the histological structures of the liver, kidney and testis (not included) in rats treated with corn oil (group 5) showed normal appearances compared to the control group.
Figure 1.
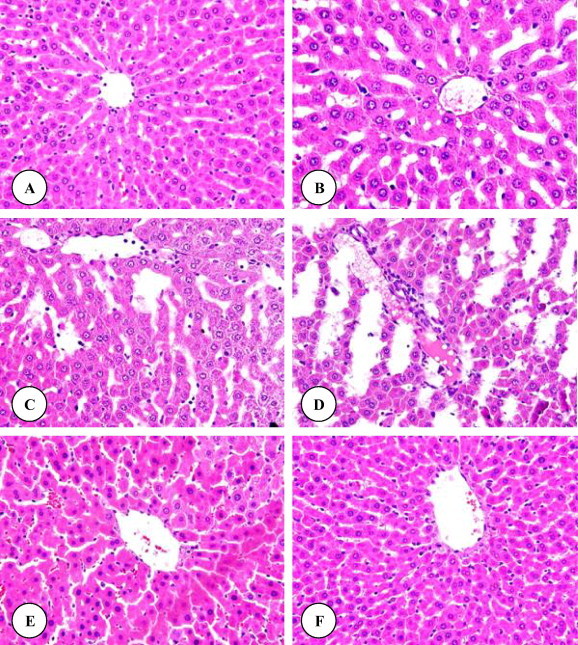
(A–F) Liver micrographs of control (A), DZN (B–D), grapeseed oil plus DZN (E) and grapeseed oil (F) treated rats. Original magnification ×400.
Figure 2.
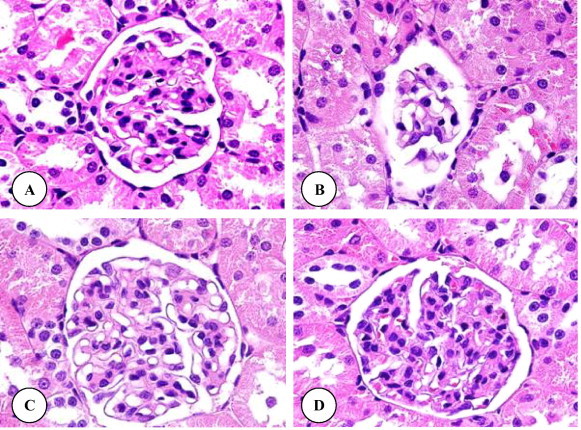
(A–D) Renal corpuscle micrographs of control (A), DZN (B), grapeseed oil plus DZN (C) and grapeseed oil (D) treated rats. Original magnification ×1000.
Figure 3.
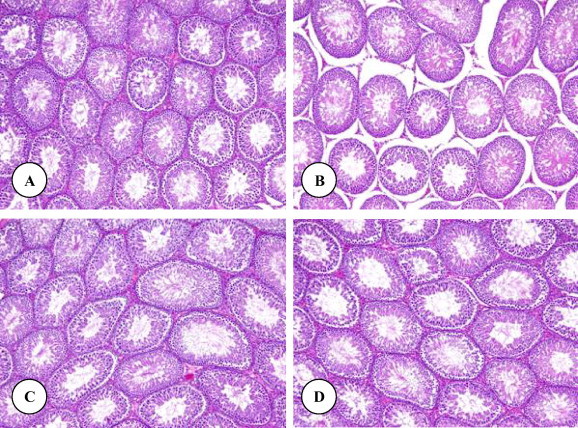
(A–D) Testis micrographs of control (A), DZN (B), grapeseed oil plus DZN (C) and grapeseed oil (D) treated rats. Original magnification ×100.
4. Discussion
The undesired effects of pesticides have been recognized as a serious public health concern during the past decades. The introduction of novel, more toxic and rapidly disseminating pesticides into the environment has necessitated an accurate identification of their potential hazards to human health. Although these toxic chemicals have become an integral part of the ecosystem, many of them remain extremely toxic to mammals and other non-target creatures. The present results showed that the administration of diazinon caused statistical increases in the levels of serum glucose, triglycerides, cholesterol, LDL-C and VLDL-C, while the level of serum total protein was significantly decreased. These results indicate that the exposure to DZN caused a severe disturbance of carbohydrates, lipids and proteins metabolism. The progressive accumulation of blood glucose revealed that mice became hyperglycemic due to diazinon intoxication. This case may be due to the enhancement of the activities of the enzymes involved in gluconeogenesis leading to the formation of glucose from non-carbohydrate sources coupled with inhibition of liver glycogenolysis or stimulating glycogenolysis processes to increase the level of blood glucose from the liver as a main source of carbohydrates in the body. Salih (2010) reported that the level of serum glucose was statistically increased in rabbits exposed to dimethoate and DZN. Al-Attar and Abu Zeid (2013) showed that the level of serum glucose was significantly increased in mice exposed to DZN. The present disturbances in lipids profile are in agreement with previous studies which indicated that the exposure to DZN and other pesticides caused severe alterations in values of serum lipids parameters (Zari and Al-Attar, 2011; Al-Attar and Abu Zeid, 2013; Abd Elmonem, 2014). These alterations in lipid profile may be attributed to the increased lipolysis and fatty acid formation in the blood. Hypertriglyceridemia in combination with abnormally low concentrations of HDL-C is one of the most common atherogenic profiles of lipid metabolism. Agbor et al. (2005) reported that the accumulation of blood triglycerides may be a result of an imbalance between the rate of synthesis and the rate of release of triglycerides by the parenchyma cells into the systemic circulation. The present hypercholesterolemia may be due to the inhibition of liver cytochrome P-450 enzymes. It is well documented that a low level of HDL-C is indicative of high risk for cardiovascular disease, an increase in HDL-C level could potentially contribute to antiatherogenicity (Assmann and Nofer, 2003). HDL-C may hasten the removal of cholesterol from peripheral tissue to the liver for catabolism and excretion. Also, high level of HDL may compete with LDL receptor sites on arterial smooth muscle cells and thus partially inhibit uptake and degradation of LDL. Also, HDL-C could protect LDL-C against oxidation in vivo, because the lipids in HDL-C are preferentially oxidized before those in LDL-C (Bowry et al., 1992). The present decrease in serum total protein in the DZN-treated group may be due to the liver dysfunctions and disturbance in the biosynthesis of protein. However, several investigators reported that the levels of blood total protein were decreased in experimental animals exposed to DZN and other pesticides (Al-Attar, 2010; Zari and Al-Attar, 2011; Al-Attar and Abu Zeid, 2013; Abd Elmonem, 2014).
Administering DZN to rats resulted in statistically significant increases of serum ALT, AST, ALP, creatinine, BUN and uric acid. Liver and kidney histopathological examinations also confirm these results. Damage of hepatocytes is reflected by an elevation in the levels of hepato specific enzymes (ALT, AST and ALP), these are cytoplasmic in lection and are released into circulation after cellular damage (Sallie et al., 1991). However, these results were in accordance with the findings of many studies showing elevations of these enzymes and liver histopathological alterations in experimental animals exposed to DZN (Al-Attar, 2009; Al-Attar and Al-Taisan, 2010; Sarhan and Al-Sahhaf, 2011; Al-Attar and Abu Zeid, 2013; El-Demerdash and Nasr, 2014).
The present results showed that rats treated with DZN display a pronounced impairment in renal function which is confirmed by the increase of serum creatinine, BUN and uric acid levels, and histopathological alterations. Moreover, the histopathological examinations demonstrated that the cortex is more affected than the medulla due to exposure to DZN. This could be partly due to uneven distribution of DZN and its metabolites in the tissue of the kidney where about 90% of the total renal blood flow enters the cortex via the bloodstream. Accordingly, a relatively high concentration of DZN and its metabolites might reach the cortex via the bloodstream than that would enter the medulla. However, several investigations showed a significant enhancement of blood creatinine, urea and uric acid levels, and renal histological changes in experimental animals exposed to DZN (Yehia et al., 2007; Al-Attar and Al-Taisan, 2010; Salih, 2010; Sarhan and Al-Sahhaf, 2011; Al-Attar and Abu Zeid, 2013).
The serum level of muscle enzymes is a marker of the functional status of muscle tissue and varies widely in both pathological and physiological conditions. An increase in these enzymes may represent an index of cellular necrosis and tissue damage following acute and chronic muscle injuries (Mokuno et al., 1987; Szumilak et al., 1998). Serum CK was first used as a diagnostic aid in progressive muscular dystrophy by Ebashi et al. (1959). It has since become an important clinical marker for muscle damage. The serum CK level in healthy individuals depends on age, race, lean body mass and physical activity (Rosalki, 1970; Meltezer, 1971). LDH is an intracellular enzyme capable of reversible formation of pyruvate and lactate in all eukaryotic and prokaryotic cells. LDH is found particularly in the kidney, heart, liver, lungs and skeletal muscle (Onyeneke et al., 2007). In a cell metabolic level of pyruvate is normally utilized through one of three pathways, such as (1) conversion of pyruvate to lactate by LDH, (2) generation of glucose through gluconeogenesis and (3) formation of acetyl-coA and its reutilization in the tricarboxylic acid (TCA) cycle. In contrast, lactate is released into the blood stream by red blood cells (RBC) and skeletal muscle cells, for converting it to glucose (Plummer, 1999). Hence, modulations in the cellular status of pyruvate or lactate will affect metabolic pathways involving pyruvate in a coordinated manner. The measurement of serum LDH has therefore been used as a diagnostic tool for the clinical elevation of subjects (McKenzie and Henderson, 1983; Drent et al., 1996). When disease or injury affects tissues containing LDH, cells release LDH into the blood stream, where it is identified in higher than normal levels. For example, when a person has a heart attack, the LDH level begins to rise about 12 h after the attack and usually returns to normal within 5–10 days (Sabouni et al., 2007). Increased serum level of LDH is usually found in cellular death and/or leakage from cells or in some cases it is a useful marker of myocardial or pulmonary infarction (Onyeneke et al., 2007). However, the present high activity of serum CK and LDH demonstrated that the cellular membranes integrity of myocardial tissues may be disturbed. Furthermore, several investigations showed that the exposure to DZN led to cardiotoxicity accompanied with an increase of serum CK and LDH levels in rats and mice (Al-Attar, 2009; Al-Attar and Al-Taisan, 2010; Al-Attar and Abu Zeid, 2013; El-Demerdash and Nasr, 2014).
The present study indicated that the exposure to DZN produced testicular damage, which led to spermatogenic arrest. Similar observations were noted in experimental animals treated with DZN and other pesticides (Adamkovičová et al., 2010; Jorsaraei et al., 2010; Zari and Al-Attar, 2011). Organophosphate compounds are proved to have toxic effects on reproductive tract in male rats and histologically they induce severe focal necrosis and/or degeneration of the germ cells in the seminiferous tubules associated with remarkable tubular atrophy (Hatjian et al., 2000; Aluigi et al., 2005). Fattahi et al. (2009) investigated the effects of DZN on the structure of testis and levels of sex hormones in adult male mice. A significant reduction was observed in diameter and weight of testes after DZN administration. Furthermore, DZN brought about significant reduction in sperm counts and spermatogenic, Leydig and Sertoli cells and a decrease in serum testosterone concentration. Histopathological examination of the testes showed degenerative changes in seminiferous tubules. The levels of luteinizing hormone (LH) and follicle stimulating hormone (FSH) were increased in DZN groups compared to the control. The damage may have occurred by direct toxic effects of DZN on cells or tissue, but it might also occur because of imbalanced hormone levels. DZN may directly affect the testis tissue or by entering into the pituitary gland which could cause changes of gonadotropins to arise. Moreover, Ali et al. (2011) demonstrated that the rats which received DZN showed highly degenerated testes with remarkable atrophy and edema in seminiferous tubules and interstitial connective tissue as well. They suggested that the direct effects of the DZN induced sever apoptosis in the germinal cells and remarkable germinal cells degeneration lowered the sperm quality and quantity. Therefore high content of pressured germinal cells, elevated abnormal, immature, death sperms and high infiltration of immune cells. There are several possible mechanisms for the antigonadal actions of organophosphates; they may exert a direct inhibitory action on the testis; they may affect the pituitary, causing changes in gonadotropins concentrations and thus subsequent spermatogenic impairment; or they may change the concentration of neurotransmitter (Tamura et al., 2001). Antiandrogens can disrupt male differentiation by several mechanisms, including antagonism of receptor binding, or by inhibition of the production, transport, or metabolism of androgens (Chattopadhyay et al., 2005).
The results of this study showed that grapeseed oil supplementation attenuated the extensive changes in biochemical and histopathological profiles in diazinon-treated rats. Organophosphate toxicity is based on the inhibition of the enzyme acetylcholinesterase which cleaves the neurotransmitter acetylcholine. Inhibition of acetylcholinesterase by organophosphate insecticides, such as diazinon, interferes with proper neurotransmission in cholinergic synapses and neuromuscular junctions. Moreover, this mechanism involves the inhibition of acetylcholinesterase and other non-specific esterases through phosphorylation at–OH serine in the esterase center of the enzyme. This mechanism is the same for all insecticides of the group, irrespective of differences in their chemical structure (Lotti, 2001). The inhibition of the activity of cholinesterase enzymes causes an increase in the level of endogenous acetylcholine in the organism and results in its binding to muscarinic and nicotinic receptors in both the peripheral and central nervous systems (CNS). This increase in the CNS disturbs the balance between neurotransmitters and causes the onset of acute intoxication symptoms (Lotti, 2001). The symptoms of acute intoxication with organophosphates have been well described, while the effects of chronic exposure to these compounds are not completely clear. Many authors postulate that they may have an effect on redox processes in a number of organs, thus leading to disturbances in these processes and causing enhancement of lipid peroxidation, both in acute and chronic intoxication by these compounds (Abdollahi et al., 2004; Sharma et al., 2005; Costa, 2006; Fortunato et al., 2006). As increased generation of reactive oxygen species and lipid peroxidation induced by these species underlies many diseases, it is extremely important to determine the effect of organophosphate insecticides on lipid peroxidation processes (Yagi, 1987; Ueda et al., 1997; Matés et al., 1999). It has been previously suggested that several pesticides exert their biological effects mainly through electrophilic attack of cellular constituents with simultaneous generation of reactive oxygen species (ROS). ROS may, therefore, be involved in the toxicity of various pesticides (Gültekin et al., 2000). Pesticide chemicals may induce oxidative stress leading to generation of free radicals and alteration in antioxidants or oxygen free radical (OFR) scavenging enzyme systems (Banerjee et al., 1999). However, several studies showed that diazinon exposure led to an increase in lipid peroxidation with tissue specific alterations in the liver, kidney, heart, testis and brain (Akturk et al., 2006; Yilmaz et al., 2012; Boroushaki et al., 2013; Lari et al., 2013; Oksay et al., 2013; Razavi et al., 2013; El-Demerdash and Nasr, 2014).
The results of this study showed that grapeseed oil supplementation alleviated the extensive changes in physiological and histopathological profiles in rats exposed to DZN. Maheswari and Rao (2005) studied the effect of oral administration of grapeseed oil against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats. They reported that grapeseed oil has protected the liver from CCl4 damage. Probable mechanism of action may be due to the protection against oxidative damage produced by CCl4. Abd El-Rahim and Hafiz (2009) evaluated the protection conferred by grape seed oil and linseed oil against cyclophosphamide induced bone marrow chromosomal aberrations and sperm abnormalities as well as DNA fragmentation in adult Swiss albino mice. Cyclophosphamide induced genotoxicity indicated by increased number of aberrant cells and different types of structural chromosomal aberrations (gap, break, fragment and deletion) and numerical aberrations (hypoploidy and hyperploidy). In sperm morphology cyclophosphamide induced sperm abnormalities for both head and tail abnormalities. Pretreatment with grapeseed oil or linseed oil prior to an interperitoneal dose of cyclophosphamide reduced the number of chromosomal aberrations and sperm abnormalities and also reduced the percentage of DNA fragmentation caused by cyclophosphamide. It could be concluded that each of grapeseed oil and linseed oil acts as a potent antioxidant that prevented genotoxicity of bone marrow cells and sperm abnormalities as well as DNA fragmentation. Khalifa et al. (2011) assessed the antioxidant role of wheat germ oil and grapeseed oil in an organophosphorus insecticide chlorpyrifos-induced oxidative stress, biochemical and histological changes in the liver in male albino rats. Wheat germ oil and grape seed oil supplementation significantly reduce the toxic effects of chlorpyrifos-induced oxidative stress and caused significant improvement in different biochemical parameters. Naguib (2011) investigated the beneficial effects of grapeseed oil on gamma radiation-induced oxidative stress in the irradiated rat eyes. The results lead to the conclusion that administration of grapeseed oil prior to radiation exposure may be a promising attempt in attenuating the extent of oxidative damage accompanying radiotherapy. Additionally, Hasseeb et al. (2013) evaluated the ameliorating effect of grapeseed oil on the lesions of experimental acrylamide intoxication in male rat genital organs. They concluded that the acrylamide induced lesions in male rats were obviously seen in the testes and epididymis, while less change was seen in the prostate glands and the seminal vesicles. The testicular changes for the impairment of the spermatogenesis sure lead to infertility of the exposed males. The comparative microscopic evaluation, for the concurrent administration of the grapeseed oil led to main conclusion for the positive impact and ameliorating effect of the grapeseed oil (especially in high levels) against the induced lesions of the acrylamide in the male reproductive organs of the rats, so it could be used in human diet as a food supplement to improve the fertility, nutritive and protective value. However, the present results suggest that grapeseed oil has a protective role against the toxicity of DZN. Moreover, the antioxidative effects of grapeseed oil components may play an important role in cell protection from enhancement of peroxidative injuries. Considering the present results, it can be concluded that this study shows for the first time that the grapeseed oil supplementation is beneficial in lowering the physiological and histopathological alterations induced by DZN exposure in rats. Finally, further investigations are needed to explore the mechanism action of grapeseed oil against DZN toxicity.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd Elmonem H.A. Assessment the effect of pomegranate molasses against diazinon toxicity in male rats. J. Environ. Sci. Toxicol. Food Technol. 2014;8:135–141. [Google Scholar]
- Abd El-Rahim A.H., Hafiz N.A. Investigation on the protective effect of grape seed and linseed oils against cyclophosphamide induced genotoxicity in mice. Global Vet. 2009;3:377–382. [Google Scholar]
- Abdollahi M., Mostafalou S., Pournourmohammadi S., Shadnia S. Oxidative stress and cholinesterase inhibition in saliva and plasma of rats following subchronic exposure to malathion. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2004;137:29–34. doi: 10.1016/j.cca.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Adamkovičová M., Toman R., Cabaj M. Diazinon and cadmium acute testicular toxicity in rats examined by histopathological and morphometrical methods. Slovak J. Anim. Sci. 2010;43:134–140. [Google Scholar]
- Agbor G.A., Oben J.E., Nkegoum B., Takla J.P., Ngogang J.Y. Hepatoprotective activity of Hibiscus cannabinus (Linn.) against carbon tetrachloride and paracetamol induced liver damage in rats. Pak. J. Biol. Sci. 2005;8:1397–1401. [Google Scholar]
- Akturk O., Demirin H., Sutcu R., Yilmaz N., Koylu H., Altuntas I. The effects of diazinon on lipid peroxidation and antioxidant enzymes in rat heart and ameliorating role of vitamin E and vitamin C. Cell Biol. Toxicol. 2006;22:455–461. doi: 10.1007/s10565-006-0138-5. [DOI] [PubMed] [Google Scholar]
- Al-Attar A.M. The ameliorative role of β-carotene pretreatment on diazinon-induced enzymological and histopathological changes in Wistar male rats. Global J. Pharmacol. 2009;3:171–177. [Google Scholar]
- Al-Attar A.M. Physiological and histopathological investigations on the effects of α-lipoic acid in rats exposed to malathion. J. Biomed. Biotechnol. 2010;2010:1–8. doi: 10.1155/2010/203503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attar A.M., Abu Zeid I.M. Effect of tea (Camellia sinensis) and olive (Olea europaea L.) leaves extracts on male mice exposed to diazinon. BioMed Res. Int. 2013;2013:1–6. doi: 10.1155/2013/461415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attar A.M., Al-Taisan W.A. Preventive effects of black seed (Nigella sativa) extract on Sprague Dawley rats exposed to diazinon. Aust. J. Basic Appl. Sci. 2010;4:957–968. [Google Scholar]
- Al-Haj M., Nasser A., Anis A. Survey of pesticides used in Qat cultivation in Dhale and Yafe and their adverse effects. J. Nat. Appl. Sci. 2005;9:103–110. [Google Scholar]
- Ali K., Reza N.G., Syamak S., Aref H., Ehsan H., Leila R., Mohammad B., Ali N., Davoud K., Esmaiel G. Protective effect of selenium on diazinon induced determination impact on the testes in mature male rats. Global Vet. 2011;7:370–380. [Google Scholar]
- Aluigi M.G., Angelini C., Falugi C., Fossa R., Genever P., Gallus L., Layer P.G., Prestipino G., Rakonczay Z., Sgro M., Thielecke H., Trombino S. Interaction between organophosphate compounds and cholinergic functions during development. Chem. Biol. Interact. 2005;157:305–316. doi: 10.1016/j.cbi.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Assmann G., Nofer J.-R. Atheroprotective effects of high-density lipoproteins. Annu. Rev. Med. 2003;54:321–341. doi: 10.1146/annurev.med.54.101601.152409. [DOI] [PubMed] [Google Scholar]
- Banerjee B.D., Seth V., Bhattacharya A., Pahsa S.T., Chacraborty A.K. Biochemical effects of some pesticides on lipid peroxidation and free radical scavengers. Toxicol. Lett. 1999;107:33–47. doi: 10.1016/s0378-4274(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Boroushaki M.T., Arshadi D., Jalili-Rasti H., Asadpour E., Hosseini A. Protective effect of pomegranate seed oil against acute toxicity of diazinon in rat kidney. Iran. J. Pharm. Res. 2013;12:821–827. [PMC free article] [PubMed] [Google Scholar]
- Bowry V.W., Stanley K.K., Stocker R. High density lipoprotein is the major of lipid hydroperoxides in human blood plasma from fasting donors. Proc. Natl. Acad. Sci. 1992;89:1316–1320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakici O., Akat E. Effects of oral exposure to diazinon on mice liver and kidney tissues: biometric analyses of histopathologic changes. Anal. Quant. Cytol. Histol. 2013;35:7–16. [PubMed] [Google Scholar]
- Chattopadhyay A., Sarkar M., Biswas N.M. Dose-dependent effect of copper chloride on male reproductive function in immature rats. Kathmandu Univ. Med. J. (KUMJ) 2005;3:392–400. [PubMed] [Google Scholar]
- Costa L.G. Current issues in organophosphate toxicology. Clin. Chim. Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Dikshith T.S.S., Diwan P.V. Wiley-IEEE; 2003. Industrial Guide to Chemical and Drug Safety. pp.189–191. [Google Scholar]
- Dos Santos Freitas L., Jacques R.A., Richter M.F., Silva A.L., Caramão E.B. Pressurized liquid extraction of vitamin E from Brazilian grape seed oil. J. Chromatogr. A. 2008;1200:80–83. doi: 10.1016/j.chroma.2008.02.067. [DOI] [PubMed] [Google Scholar]
- Drent M., Cobben N.A.M., Henderson R.F., Wouters E.F.M., van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur. Respir. J. 1996;9:1736–1742. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Toyokura T., Momoi H., Sugita H. High creatine phosphokinase activity of sera of progressive muscular dystrophy patients. Biochem. J. Tokyo. 1959;46:103. [Google Scholar]
- El-Ashmawy I.M., Amal S., Osama M.S. Effects of marjoram volatile oil and grape seed extract on ethanol toxicity in male rats. Basic Clin. Pharmacol. Toxicol. 2007;101:320–327. doi: 10.1111/j.1742-7835.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- El-Demerdash F.M., Nasr H.M. Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J. Trace Elem. Med Biol. 2014;28:89–93. doi: 10.1016/j.jtemb.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Fattahi E., Parivar K., Jorsaraei S.G.A., Moghadamnia A.A. The effects of diazinon on testosterone, FSH and LH levels and testicular tissue in mice. Iran. J. Reprod. Med. 2009;7:59–64. [Google Scholar]
- Fortunato J.J., Agostinho F.R., Reus G.Z., Petronilho F.C., Dal-Pizzol F., Quevedo J. Lipid peroxidative damage on malathion exposure in rats. Neurotox. Res. 2006;9:23–28. doi: 10.1007/BF03033304. [DOI] [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.I., Donald S., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Gokcimen A., Gulle K., Demirin H., Bayram D., Kocak A., Altuntas I. Effects of diazinon at different doses on rat liver and pancreas tissues. Pestic. Biochem. Physiol. 2007;87:103–108. [Google Scholar]
- Gültekin F., Öztürk M., Akdoğan M. The effect of organophosphate insecticide chlorpyrifos-ethyl on lipid peroxidation and antioxidant enzymes (in vitro) Arch. Toxicol. 2000;74:533–538. doi: 10.1007/s002040000167. [DOI] [PubMed] [Google Scholar]
- Hassanein M.M., Abedel-Razek A.G. Chromatographic quantitation of some bioactive minor components in oils of wheat germ and grape seeds produced as by-products. J. Oleo Sci. 2009;58:227–233. doi: 10.5650/jos.58.227. [DOI] [PubMed] [Google Scholar]
- Hasseeb M.M., Al-Hizab F.A., Hamouda M.A. Impacts of grape seed oil supplementation against the acrylamide induced lesions in male genital organs of rats. Pak. Vet. J. 2013;33:282–286. [Google Scholar]
- Hatjian B.A., Mutch E., Williams F.M., Blain P.G., Edwards J.W. Cytogenetic response without changes in peripheral cholinesterase enzymes following exposure to a sheep dip containing diazinon in vivo and in vitro. Mut. Res. 2000;427:85–92. doi: 10.1016/s1383-5718(00)00131-5. [DOI] [PubMed] [Google Scholar]
- Hurtig A.K., San Sebastian M., Soto A., Shingre A., Zambrano D., Guerrero W. Pesticide use among farmers in the Amazon basin of Ecuador. Arch. Environ. Health. 2003;58:223–228. doi: 10.3200/AEOH.58.4.223-228. [DOI] [PubMed] [Google Scholar]
- Jorsaraei S.G.A., Firoozjaee A., Pasha Y.Y., Marzony E.T., Sarabi E. Histopathological effects of single dose treatment of diazinon on testes structure in rat. Yakhteh Med. J. 2010;12:39–42. [Google Scholar]
- Kamanyire R., Karalliedde L. Organophosphate toxicity and occupational exposure. Occup. Med. 2004;54:69–75. doi: 10.1093/occmed/kqh018. [DOI] [PubMed] [Google Scholar]
- Khalifa F.K., Khalil F.A., Barakat H.A., Hassan M.M. Protective role of wheat germ and grape seed oils in chlorpyrifos-induced oxidative stress, biochemical and histological alterations in liver of rats. Aust. J. Basic Appl. Sci. 2011;5:54–66. [Google Scholar]
- Kikalishvili B., Zurabashvili D., Nikolaishvili M., Zurabashvili Z., Giorgobiani I. The most biological important constances of Rkatsiteli grape oil and its effect as a 5% and 10% food-additive. Georgian Med. News. 2011;195:85–87. [PubMed] [Google Scholar]
- Lari, P., Abnous, K., Imenshahidi, M., Rashedinia, M., Razavi, M., Hosseinzadeh, H., 2013. Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol. Ind. Health (in press). [DOI] [PubMed]
- Lotti, M., 2001. Clinical toxicology of anticholinesterase agents in humans. Handbook of Pesticide Toxicology, 2nd Ed., Academic Press, USA, p. 1043.
- Maheswari M.U., Rao P.G.M. Antihepatotoxic effect of grape seed oil in rat. Indian J. Pharmacol. 2005;37:179–182. [Google Scholar]
- Matés J.M., Pérez-Gómez C., Núñez de Castro I. Antioxidant enzymes and human diseases. Clin. Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- McKenzie D., Henderson A.R. Electrophoresis of lactate dehydrogenase isoenzymes. Clin. Chem. 1983;29:189–195. [PubMed] [Google Scholar]
- Meltezer H.Y. Factors affecting creatine phosphokinase levels in the general population. The role of race, activity and age. Clin. Chem. Acta. 1971;33:165–172. doi: 10.1016/0009-8981(71)90264-6. [DOI] [PubMed] [Google Scholar]
- Mokuno K., Riku S., Sugimura K., Takahashi A., Kato K., Osugi S. Serum creatine kinase isoenzymes in Duchenne muscular dystrophy determined by sensitive enzyme immunoassay methods. Muscle Nerve. 1987;10:459–463. doi: 10.1002/mus.880100513. [DOI] [PubMed] [Google Scholar]
- Naguib N.I. Grape seed oil extract protects against radiation-induced oxidative damage in rat’s eye. Isotope Rad. Res. 2011;43:1255–1264. [Google Scholar]
- Nandakumar V., Singh T., Katiyar S.K. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008;269:378–387. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksay T., Nazıroğlu M., Ergün O., Doğan S., Özatik O., Armağan A., Özorak A., Çelik Ö. N-acetyl cysteine attenuates diazinon exposure-induced oxidative stress in rat testis. Andrologia. 2013;45:171–177. doi: 10.1111/j.1439-0272.2012.01329.x. [DOI] [PubMed] [Google Scholar]
- Onyeneke E.C., Oluba O.M., Adeyemi O., Eriyamremu G.E., Ojeaburu S.I., Adebisi K.E., Adeyemi O., Aboluwoye C.O. Effects of soy protein on serum level of cardiovascular disease diagnostic enzymes in cholesterol-fed rats. Internet J. Nutr. Wellness. 2007;4:1. [Google Scholar]
- Plummer D.T. third ed. McGraw Hill Co.; New York, USA: 1999. An Introduction to Practical Biochemistry to the Molecular Basis of Life. [Google Scholar]
- Poet T.S., Kousba A.A., Dennison S.L., Timchalk C. Physiologically base pharmacokinetic/pharmacodynamic model for the organophosphorus pesticide diazinon. Neurotoxicology. 2004;25:1013–1030. doi: 10.1016/j.neuro.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Razavi B.M., Hosseinzadeh H., Movassaghi A.R., Imenshahidi M., Abnous K. Protective effect of crocin on diazinon induced cardiotoxicity in rats in subchronic exposure. Chem. Biol. Interact. 2013;203:547–555. doi: 10.1016/j.cbi.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Roegge C.S., Timofeeva O.A., Seidler F.J., Slotkin T.A., Levin E.D. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res. Bull. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosalki S.B. Enzyme assays in diseases of the heart and skeletal muscle. J. Clin. Pathol. 1970;24:60–70. doi: 10.1136/jcp.s1-4.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabouni F., Siadati A., Soriyash Farpoor S., Sabouni F. Elevated serum lactate dehydrogenase values in children with multiorgan involvements and severe febrile illness. Iran. J. Pediatr. Soc. 2007;1:31–35. [Google Scholar]
- Salih E.M.A. Toxic effect of dimethoate and diazinon on the biochemical and hematological parameters in male rabbits. Jordan J. Biol. Sci. 2010;3:77–82. [Google Scholar]
- Sallie R., Tredger J.M., William R. Drug and liver. Biopharm. Drug Dispos. 1991;12:251–259. doi: 10.1002/bdd.2510120403. [DOI] [PubMed] [Google Scholar]
- Sarabia L., Maurer I., Bustos-Obregón E. Melatonin prevents damage elicited by the organophosphorous pesticide diazinon on the mouse testis. Ecotoxicol. Environ. Saf. 2009;72:938–942. doi: 10.1016/j.ecoenv.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Sarhan O.M.M., Al-Sahhaf Z.Y. Histological and biochemical effects of diazinon on liver and kidney of rabbits. Life Sci. J. 2011;8:1183–1189. [Google Scholar]
- Sharma Y., Bashir S., Irshad M., Gupta S.D., Dogra T.D. Effects of acute dimethoate administration on antioxidant status of liver and brain of experimental rats. Toxicology. 2005;206:49–57. doi: 10.1016/j.tox.2004.06.062. [DOI] [PubMed] [Google Scholar]
- Shi J., Jianmel Y., Joseph E.P., Yukio K. Polyphenolics in grape seeds biochemistry and functionality. J. Med. Food. 2003;6:291–299. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- Shivananda Nayak B., Dan Ramdath D., Marshall J.R., Isitor G., Xue S., Shi J. Wound-healing properties of the oils of Vitis vinifera and Vaccinium macrocarpon. Phytother. Res. 2011;25:1201–1208. doi: 10.1002/ptr.3363. [DOI] [PubMed] [Google Scholar]
- Solyanik G.I., Fedorchuk A.G., Pyaskovskaya O.N., Dasyukevitch O.I., Khranovskaya N.N., Aksenov G.N., Sobetsky V.V. Anticancer activity of aconitine-containing herbal extract BC1. Exp. Oncol. 2004;26:307–311. [PubMed] [Google Scholar]
- Szumilak D., Sulowicz W., Walatek B. Rhabdomyolysis: clinical features, causes, complications and treatment. Przegl. Lek. 1998;55:274–279. [PubMed] [Google Scholar]
- Tamura H., Maness S.C., Reischmann K., Dorman D.C., Gray L.E., Gaido K.W. Androgen receptor antagonism by the organophosphate insecticide fenitrothion. Toxicol. Sci. 2001;60:56–62. doi: 10.1093/toxsci/60.1.56. [DOI] [PubMed] [Google Scholar]
- Ueda K., Hara S.S., Yagami T., Asakur A.K., Kawasaki K. Amyloid β protein potentiates Ca2+ influx through L-type voltage-sensitive Ca2+ channels: possible involvement of free radicals. J. Neurochem. 1997;68:265–271. doi: 10.1046/j.1471-4159.1997.68010265.x. [DOI] [PubMed] [Google Scholar]
- Yagi K. Lipid peroxides in human disease. Chem. Phys. Lipids. 1987;45:337–351. doi: 10.1016/0009-3084(87)90071-5. [DOI] [PubMed] [Google Scholar]
- Yehia M.A., El-Banna S.G., Okab A.B. Diazinon toxicity affects histophysiological and biochemical parameters in rabbits. Exp. Toxicol. Pathol. 2007;59:215–225. doi: 10.1016/j.etp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Yilmaz N., Yilmaz M., Altuntas I. Diazinon-induced brain toxicity and protection by vitamins E plus C. Toxicol. Ind. Health. 2012;28:51–57. doi: 10.1177/0748233711404035. [DOI] [PubMed] [Google Scholar]
- Zari T.A., Al-Attar A.M. Therapeutic effects of olive leaves extract on rats treated with a sublethal concentration of carbendazim. Eur. Rev. Med. Pharmacol. Sci. 2011;15:413–426. [PubMed] [Google Scholar]


