Abstract
Background
Babies are frequently exposed to cerebral hypoxia and ischemia (H/I) during the perinatal period as a result of stroke, problems with delivery or post delivery respiratory management. The sole FDA approved treatment for acute stroke is tissue-type plasminogen activator (tPA). Endogenous tPA is upregulated and potentiates impairment of pial artery dilation in response to hypotension after H/I in pigs. Mitogen-activated protein kinase (MAPK), a family of at least 3 kinases, ERK, p38 and JNK, is also upregulated after H/I, with ERK contributing to impaired vasodilation. This study examined the hypothesis that H/I aggravates the vascular response to two important procontractile mediators released during CNS ischemia, endothelin-1 (ET-1) and thromboxane, which is further enhanced by tPA and ERK MAPK.
Methods
Cerebral hypoxia (pO2 35 mmHg for 10 min via inhalation of N2) followed immediately by ischemia (global intracranial pressure elevation for 20 min) was produced in chloralose anesthetized piglets equipped with a closed cranial window.
Results
H/I aggravated pial artery vasconstriction induced by ET-1 and the thromboxane mimetic U 46619. Potentiated vasoconstrictor responses were blocked by EEIIMD, an inhibitor of tPA’s signaling and vascular activities, but unchanged by its inactive analogue EEIIMR. The cerebrospinal fluid concentration of ERK MAPK determined by ELISA was increased by H/I, potentiated by tPA, but blocked by EEIIMD. The ERK MAPK antagonist U 0126 blocked H/I augmented enhancement of ET-1 and U 46619 vasoconstriction.
Conclusions
These data indicate that H/I aggravates ET-1 and thromboxane mediated cerebral vasoconstriction by upregulating endogenous tPA and ERK MAPK.
Keywords: Cerebral ischemia, Pediatric, Signaling pathways, Cerebral circulation, Pial arteries, Endothelin, Prostaglandins
Introduction
Pediatric stroke occurs in as many as 1 in 4,000 births [1], approximately one-third being the result of thrombosis [2]. Maternal and perinatal coagulopathies have been identified as predisposing factors [3, 4], but the etiology and pathogenesis of most cases remains uncertain. Although the incidence of cerebral ischemic events in the pediatric population is relatively low compared to the adult [5], the clinical and social impact is amplified by the potential long-term loss of quality of life years for children with CNS ischemic disorders. The thrombolytic agent tissue-type plasminogen activator (tPA) remains the only approved treatment for acute stroke, but its use in children has been limited and its benefit remains unclear [5, 6]. Indeed, the brief therapeutic window of tPA and the high incidence of post-treatment complications, including intracranial hemorrhage (ICH), have constrained clinical use of tPA to approximately 3–8 % of patients eligible for such therapy [7]. Risk is likely to be higher in neonates with incompletely developed hemostatic and vascular systems. To date there have been no efficacy trials of tPA in the treatment of infants and children with acute stroke. Children, however, are receiving tPA based on the assumption that studies in adults can be generalized to this population [5, 6, 8]. Indeed, the National Institute of Neurological Disorders and Stroke held an international workshop in 2001 to convene experts in the field of perinatal and childhood stroke [9], which recognized that one of the deficiencies in pediatric stroke research was the limited number of animal models for ischemic stroke in the young.
In newborns with stroke, complications such as hypoxic/ischemic (H/I) events are common [10, 11]. One contributor to neurological damage after H/I is loss of cerebrovascular autoregulation caused for example by hypercapnia, a powerful cerebrovasodilator. Using a piglet model, we have shown that pial artery dilation in response to hypotension and hypercapnia is blunted after cerebral H/I [12–16], while administration of tPA potentiates this impairment [17, 18]. Endogenous tPA is also upregulated after cerebral H/I in the piglet [12]. Pretreatment with the plasminogen activator-1-derived peptide, EEIIMD prevents impairment of hypercapnic and hypotensive dilation after H/I and tPA-mediated loss of vascular contractility [13, 17], without inhibiting fibrinolytic activity [19, 20], suggesting that endogenous PA contributes to cerebral hemodynamic outcome post insult.
The intracellular signal transduction events that mediate these changes in cerebral vascular contractility are only partially understood. Mitogen-activated protein kinase (MAPK), a family of at least three kinases (extracellular signal-regulated kinase—ERK, p38, and c-Jun-N-terminal kinase—JNK), is upregulated after cerebral ischemia [21–23]. tPA contributes to impaired stimulus-induced cerebrovascular dilation following cerebral H/I in the newborn pig by upregulating ERK MAPK, which is partially inhibited by the ERK MAPK antagonist U 0126 [12].
Impairment of vasodilation during hypotension and hypercapnia post insult may result from diminished vasodilator activity or enhanced vasoconstrictor activity. While prior studies have carefully delineated the mechanisms underlying diminished vasodilator activity after cerebral hypoxia/ischemia [12–14, 24, 25], little attention has been given to the effects of this insult on the vascular response to vasoconstrictors. Endothelin-1 (ET-1) and the prostaglandin thromboxane A2 (TXA2) are two important vasoconstrictors released during cerebral ischemia [26]. Since TXA2 is metabolically unstable, the analog U 46619 is often used to characterize its vascular activity. Since tPA is administered to treat pediatric stroke [5, 6, 8], this study examined the hypothesis that H/I aggravates the vascular response to two important vasoconstrictors released during CNS ischemia, ET-1 and thromboxane, and that tPA and ERK MAPK exacerbate this response in piglets.
Materials and Methods
Closed Cranial Window Technique and Cerebral Hypoxia/Ischemia
Newborn pigs (1–5 days, 1.0–1.4 kg) of either sex were studied. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Animals were anesthetized with isoflurane by mask (1–2 %), maintained with a-chloralose (30–50 mg/kg supplemented with 5 mg/kg/h iv). A catheter was inserted into a femoral artery to monitor blood pressure. The trachea was cannulated, and the animals were ventilated with room air. Body temperature was monitored rectally and the piglets were kept warm with a water-circulating heating blanket.
A cranial window was placed in the parietal skull of these anesthetized animals. This window consists of three parts: a stainless steel ring, a circular glass coverslip, and three ports consisting of 17-gauge hypodermic needles attached to three precut holes in the stainless steel ring. For placement, the dura was cut and retracted over the cut bone edge. The cranial window was placed in the opening and cemented in place with dental acrylic. The volume under the window was filled with a solution, similar to CSF, of the following composition (in mM): 3.0 KCl, 1.5 MgCl2, 1.5 CaCl2, 132 NaCl, 6.6 urea, 3.7 dextrose, and 24.6 NaHCO3. This artificial CSF was warmed to 37 °C and had the following chemistry: pH 7.33, pCO2 46 mmHg, and pO2 43 mmHg, which was similar to that of endogenous CSF. Pial arterial vessel diameter was measured with a microscope, a camera, a video output screen, and a video microscaler.
Total cerebral ischemia was accomplished by infusing artificial CSF into a hollow bolt in the cranium to maintain an intracranial pressure 15 mmHg greater than the numerical mean of systolic and diastolic arterial blood pressure [12–18]. Intracranial pressure was monitored via a sidearm of the cranial window. To prevent the arterial pressure from rising inordinately (Cushing response), venous blood was withdrawn as necessary to maintain mean arterial blood pressure at no greater than 100 mmHg. As the cerebral ischemic response subsided, the shed blood (anticoagulated with heparin) was returned to the animal. Cerebral ischemia was maintained for 20 min. Hypoxia (pO2 of approximately 35 mmHg) was produced for 10 min before ischemia by decreasing the inspired O2 via inhalation of N2, which was followed immediately by the total cerebral ischemia. Hypoxia was stopped (ventilation mixture returned to room air) once the ischemic phase started, thus providing reperfusion with normoxic blood.
Protocol
Pial arteries (resting diameter, 120–160 µm) were examined to determine the effects of H/I on vasoconstrictor responses to ET-1 and U 46619. Typically, 2–3 ml of artificial CSF were flushed through the window over a 30-s period, and excess CSF was allowed to run off through one of the needle ports. For sample collection, 300 µl of the total cranial window volume of 500 µl was collected by slowly infusing artificial CSF into one side of the window and allowing the CSF to drip freely into a collection tube on the opposite side.
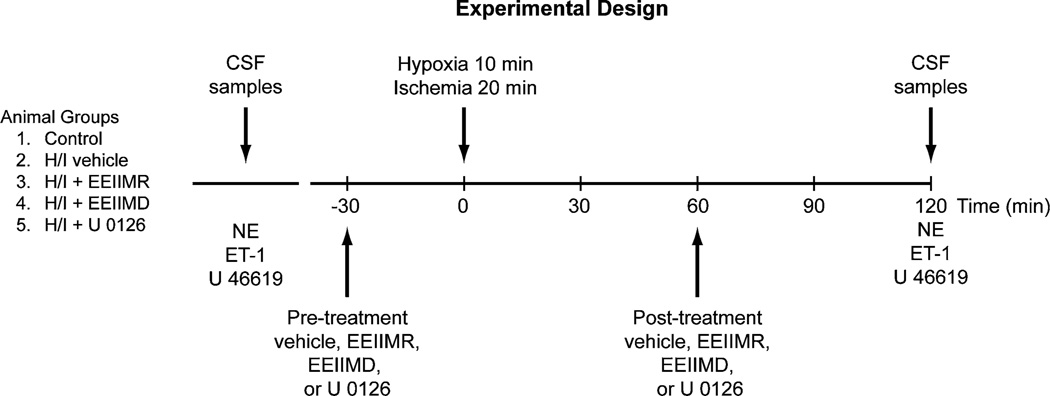
Nine experimental groups of animals were studied (all n = 5, Fig. 1): (1) sham control, vehicle treated; (2) H/I, vehicle pre-treated; (3) H/I, pre-treated with EEIIMD (1 mg/kg iv); (4) H/I, pre-treated with EEIIMR (1 mg/kg iv), the inactive analog of EEIIMD; (5) H/I, pre-treated with the ERK MAPK antagonist, U 0126 (1 mg/kg iv); (6) H/I, post-treated with vehicle; (7) H/I, post-treated with EEIIMD; (8) H/I, post-treated with EEIIMR; and (9) H/I, post-treated with U 0126. The vehicle for all agents was 0.9 % saline, except for the MAPK antagonist, which was DMSO (stock) diluted with saline, with a maximal ratio of 1:1,000. These 2 types of vehicle—CSF controls had no significant effect on pial artery diameter. Figure 1 depicts the animal groups and the timeline of interventions used in this study. In sham control animals, responses to ET-1 (10−10, 10−8 M), the TXA2 mimetic U 46619 (1, 10 ng/ml), and norepinephrine (NE, 10−6, 10−4 M) were obtained initially and again 2 h later in the presence of agent vehicle (Fig. 1). In H/I vehicle treated animals, responses to vasoactive stimuli were obtained initially and then again 2 h post insult in the presence of vehicle (Fig. 1). Where indicated, drugs were administered in a randomized order either 30 min before H/I (pretreatment) or 60 min after H/I (post-treatment) and responses obtained initially and then again 2 h after H/I (Fig. 1).
Fig. 1.
Experimental design for this study
ELISA
Commercially available ELISA Kits (Calbiochem) were used to quantity CSF total and phospho ERK MAPK isoform concentration. Phosphorylated MAPK enzyme values were normalized to total form and then expressed as percent of the control condition. The lower limit of detection was 10 pg/ml after subtraction of non-specific background and cross selectivity paradigms using respective MAPK isoform antagonists confirmed specificity (e.g., ERK elevation after H/I unchanged by the p38 inhibitor SB 203580 or the JNK antagonist SP 600125).
Statistical Analysis
Pial artery diameter and CSF MAPK values were analyzed using ANOVA for repeated measures. If the value was significant, the data were then analyzed by Fishers protected least significant difference test. The sample populations studied were normally distributed and the power was 0.86. An α level of p < 0.05 was considered significant in all statistical tests. Values are represented as mean ± SEM of the absolute value or as percentage changes from control value.
Results
H/I Aggravates Pial Artery Vasoconstriction Induced by ET-1 and the TXA2 Mimetic U 46619
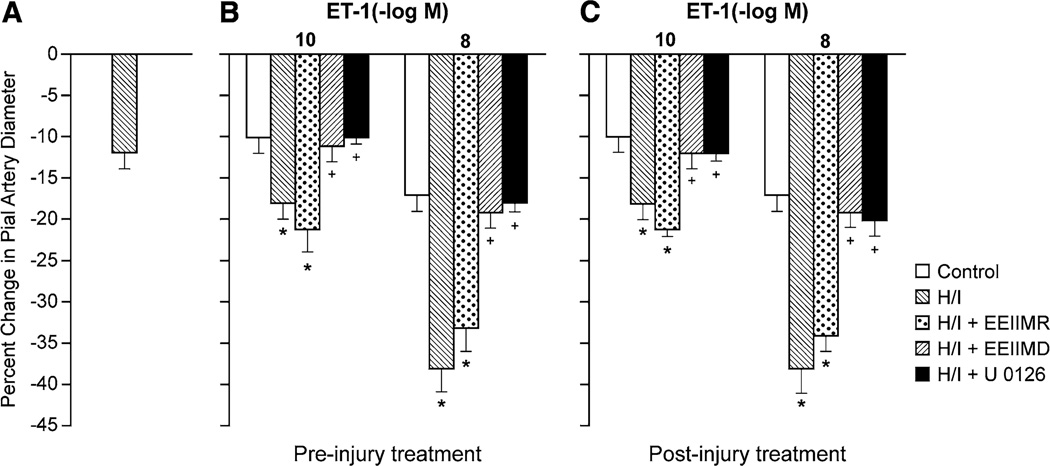
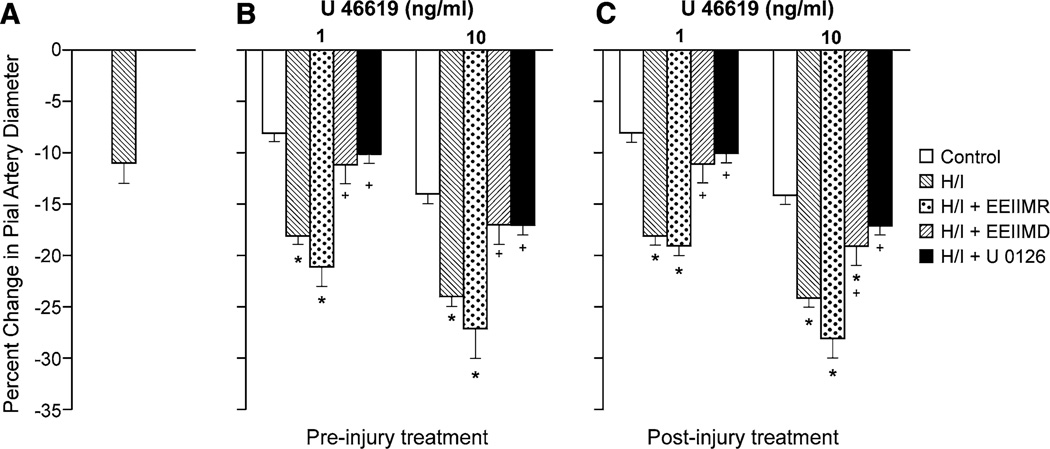
H/I alone produced pial artery vasoconstriction, which was augmented in the presence of ET-1 and U 46619 (Figs. 2, 3). Topically applied ET-1 (10−10, 10−8 M) and U 46619 (1, 10 ng/ml) elicted reproducible pial artery vasoconstriction (time control), as shown previously [26, 27], which was aggravated by H/I (Figs. 2, 3). Animals were treated with antagonists 30 min prior to (pre-injury, proof of principle) or 60 min post H/I (post injury and translationally relevant). Pre- and post-injury treatment with EEIIMD (1 mg/kg iv) blocked aggravation of pial artery vasoconstriction in response to ET-1 and U 46619 (Figs. 2, 3), whereas the inactive analog EEIIMR (1 mg/kg iv) had no effect (Figs. 2, 3). In the absence of injury (sham control), EEIIMD had no effect on baseline pial artery diameter or on ET-1- and U 46619-induced pial artery vasoconstriction (−10 ± 1 and −17 ± 2 vs −11 ± 2 and −18 ± 2 % for ET-1 10−10, 10−8 M, before and after EEIIMD, respectively, n = 5). Topical NE (10−6, 10−4 M) elicited reproducible pial artery vasoconstriction, which was unchanged after H/I (−9 ± 1, −19 ± 1 vs −10 ± 1, −18 ± 2 %, respectively, for the two concentrations of NE before and after H/I), similar to that observed previously [16].
Fig. 2.
a Influence of cerebral hypoxia/ischemia (H/I) on pial artery diameter in the absence of endothelin-1 (ET-1). Influence of ET-1 (10−10, 10−8 M) on pial artery diameter in newborn pigs before (control), after H/I, or H/I treated with EEIIMD (PAI-1-derived peptide), EEIIMR (inactive analog), or U 0126 (ERK MAPK antagonist) (all 1 mg/kg iv), n = 5, b pretreatment 30 min before H/I, c post-treatment 60 min after H/I. Baseline pial artery diameters were 139 ± 14, 120 ± 11, 117 ± 10, 120 ± 13, and 115 ± 11 µm for control, H/I, H/I + EEIIMR, H/I + EEIIMD, and H/I + U 0126 treated, respectively. The new baseline diameter was used to calculate percent changes in diameter after H/I. *p < 0.05 versus corresponding control value +p < 0.05 versus corresponding non-treated H/I value
Fig. 3.
a Influence of cerebral hypoxia/ischemia (H/I) on pial artery diameter in the absence of U 46619. Influence of U 46619 (1, 10 ng/ml) on pial artery diameter in newborn pigs before (control), after H/I, or H/I treated with EEIIMD (PAI-1-derived peptide), EEIIMR (inactive analog), or U 0126 (ERK MAPK antagonist) (all 1 mg/kg iv), n = 5, b pretreatment 30 min before H/I, c post-treatment 60 min after H/I. Baseline pial artery diameters were 140 ± 15, 122 ± 13, 116 ± 10, 122 ± 13, and 113 ± 11 µm for control, H/I, H/I + EEIIMR, H/I + EEIIMD, and H/I + U 0126 treated respectively. The new baseline diameter was used to calculate percent changes in diameter after H/I. *p < 0.05 versus corresponding control value +p < 0.05 versus corresponding non-treated H/I value
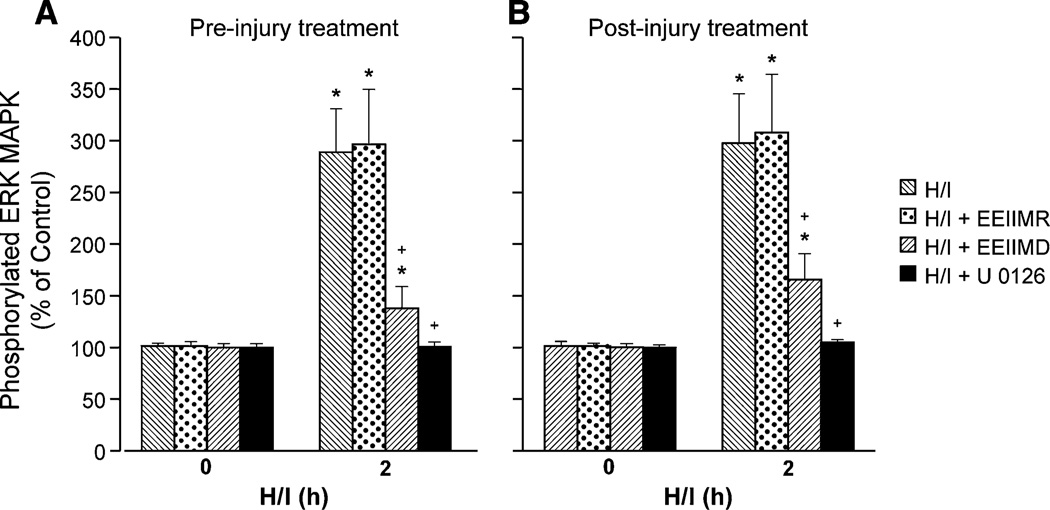
CSF ERK MAPK Upregulation After H/I is Blunted by EEIIMD
Both total and phosphorylated isoforms of CSF ERK MAPK were increased within 2 h after cerebral H/I, which was aggravated by exogenous tPA (2 mg/kg iv) [18], and data are reported in terms of percent change from total form to normalize these data (Fig. 4). Pre- and post-injury administration of EEIIMD blunted the elevation of CSF ERK MAPK after H/I, whereas EEIIMR had no effect (Fig. 4). The ERK MAPK antagonist U 0126 blocked elevations of CSF ERK MAPK and also blocked aggravation of pial artery vasoconstriction in response to ET-1 and U 46619 post insult (Figs. 2, 3 and 4). U 0126 had no effect on baseline pial artery diameter. U 0126 also had no effect on vasoconstriction induced by ET-1 and U 46619 in sham control animals.
Fig. 4.
Phosphorylation of ERK MAPK in CSF prior to cerebral hypoxia/ischemia (H/I) (0 min) and as a function of time (hour) after H/I in vehicle (H/I), or treated with EEIIMD (PAI-1 derived peptide), EEIIMR (inactive analog), or U 0126 (ERK MAPK antagonist) (all 1 mg/kg iv), n = 5. Data expressed as percent of control by ELISA determination of phosphorylated MAPK and total MAPK isoform and subsequent normalization to total form. a Pretreatment 30 min before H/I, b post-treatment 60 min after H/I, *p < 0.05 versus corresponding 0 time value +p < 0.05 versus corresponding H/I nontreated value H/I b post-treatment 1 h after H/I
Blood Chemistry, Mean Arterial Blood Pressure, and Temperature
Blood chemistry values were collected before and after all experiments. There were no statistical differences between sham control, H/I, and H/I drug-treated animals. Hypoxia decreased pO2 to 34 ± 4 mmHg while pCO2 was unchanged. Mean arterial blood pressure was modestly decreased at 1 h post H/I (66 ± 9 and 52 ± 8 mmHg for control and 1 h post H/I, respectively), but the changes did not reach statistical significance. Similarly, body temperature was no different at the end compared to the beginning of the experiment (37.6 ± 0.5 and 37.8 ± 0.6 °C).
Discussion
There are two principal new findings to be derived from this study. First, these data show that vasoconstriction in response to ET-1 and the thromboxane mimetic U 46619 is aggravated by H/I. While it is well known that ET-1 and constrictor prostaglandins such as TXA2 are upregulated and likely contribute to vascular spasm during cerebral ischemic events [26], this is, to our knowledge, the first study that characterized the influence of induction of cerebral ischemia on the vascular response to these substances. Thus, it is not just the release of a vasoconstrictor that contributes to hypoperfusion post insult, but the nature of the response to the released agent (aggravated vasoconstriction) that ultimately bears on outcome. In this context, only the withdrawal of a dilator influence, such as impairment of autoregulation during hypotension, had been previously seen as contributing to altered cerebral hemodynamics after H/I in the piglet [12–15, 17, 18]. In light of the present observations, this may be a relatively simplistic view of the dynamic relationship between vasodilator and vasoconstrictor influences that integrate with one another to lead to the ultimate disposition of physiological cerebrovascular tone and/or in pathological settings. The ability of cerebral ischemia to aggravate vasoconstriction induced by ET-1 and U 46619 appears specific in that vasoconstriction to norepinephrine is unchanged after this insult, similar to that observed previously [16]. The concentrations of ET-1 and thromboxane used in this study were chosen based on those measured in CSF under physiologic and pathologic conditions [27–29]. An experimental caveat is that, while the concentration of thromboxane in the CSF post ischemia is known for the pig [29], comparable values for ET-1 in the pig have not been investigated and our studies were based on values measured following fluid percussion brain injury in the piglet [27].
The second key finding of this study relates to the mechanism underlying the aggravated vasoconstriction in response to ET-1 and the TXA2 mimetic U 46619. Previously, we had reported that endogenous CSF tPA is elevated after cerebral H/I in the piglet [12]. New data show the PAI-1-derived peptide EEIIMD given post injury prevents the augmented vasoconstriction that occurs in response to ET-1 and U 46619. This means that upregulation of tPA contributes to the potentiated vasoconstrictor responses in the setting of cerebral H/I. The protection afforded by EEIIMD appears selective since the peptide analog EEIIMR failed to prevent augmented vasoconstriction post insult. Mechanistically, we have previously observed that ERK MAPK is upregulated after H/I, upregulation is potentiated by administration of tPA exogenously, and that the ERK MAPK inhibitor U 0126 partially prevents impaired reactivity to hypercapnia and hypotension [12, 14]. These data indicate that upregulation of this MAPK isoform likely contributes to H/I-induced impairment of vasodilation [12]. New data show that U 0126 blocked enhanced vasoconstriction in response to ET-1 and U 46619. These results indicate that upregulation of endogenous tPA aggravates vasoconstriction in response to these two procontractile substances in an ERK MAPK-dependent manner in the setting of cerebral H/I. Thus tPA and ERK MAPK upregulation can be viewed as pivotal mediators of hypoperfusion after H/I both through by impairing responsiveness to vasodilators and by enhancing responsiveness to vasoconstrictors. While pre-injury administration of EEIIMD is a proof of principle paradigm, protection afforded by post-injury administration of EEIIMD and U 0126 is more translationally relevant.
In an unrelated model of CNS injury characterized by hypoperfusion and impaired responsiveness to vasodilatory stimuli, lateral fluid percussion brain injury (which simulates injury associated with concussion and shaken impact in the pediatric population) [30], also aggravated ET-1- and U 46619-induced pial artery vasoconstriction post insult [27, 28]. In the latter studies, upregulation of the opioid nociceptin/orphanin FQ was observed to contribute to aggravated vasoconstriction to ET-1 and thromboxane [28]. Interestingly, nociceptin/orphanin FQ impairs cerebral hemodynamics following cerebral H/I and fluid percussion brain injury in the piglet by signaling through ERK MAPK [24, 25]. Therefore, future studies will be designed to investigate if this opioid interacts with tPA to contribute to aggravated vasoconstriction to ET-1 and thromboxane via ERK MAPK in the setting of cerebral H/I.
There are two important limitations to our study. First, neonatal strokes occur most commonly in utero, but some occur in otherwise healthy normal infants and follow otherwise uneventful deliveries [31, 32]. Stroke may lead to asphyxia, hypoxia/ischemia, and coagulopathy [10, 11, 32] thereby providing a clinically relevant, albeit uncommon, model for studying the pathophysiology of H/I. H/I, which may occur simultaneously or in sequence [32], remains an important clinical problem [31] that can occur in antepartum, intrapartum, or postpartum life [32, 33], may lead to leukomalacia [31], cerebral palsy [34], and cortical infarction [31].
Second, because most experimental studies of cerebral hypoxia/ischemia involve laboratory animals, several caveats need to be considered. First, the extent of neurological damage will depend on the maturity of the brain [31, 35, 36], supported by the observation that tolerance to anoxia decreases with maturity [31, 35]. The perinatal growth spurt suggests that, in comparison with human brains at birth, guinea pig, sheep, and rhesus monkey brains are more mature, pig brains are of similar maturity, and rat and rabbit brains are less mature [31, 37]. Based on parameters of maturation, such as myelination, suture closure, development of functional and electrical activity, overall brain size, and others, the full-term human brain is probably comparable with that of the newborn pig [31]. Other important variables that bear on the relevance of a model include the direct neuroprotective effect of anesthesia or its indirect effect of lowering body temperature (hypothermia), lissencephalic versus gyrencephalic brain architecture, white/gray matter ratio, stress of the surgical procedure, recovery period with recirculation–reperfusion, ipsilateral versus contralateral hemisphere outcome effects, umbra versus penumbra, specific brain region (e.g., cortex, hippocampus, etc.), cell type, timeframe, and the specific end point measured.
There are many models of cerebral hypoxia/ischemia that are used in animal experiments [31]. For example, the following are used in different species: uterine artery clamping which causes enlarged cerebral ventricles, unilateral or bilateral carotid artery ligation with and without concomitant hypoxia and/or asphyxia, bilateral carotid ligation, umbilical artery occlusion, elevation of ICP to produce ischemia, carotid artery ligation + hypotension and hypoxia, hypoxia alone, hypoxia + ischemia via elevated ICP, and inflation of neck cuff to produce ischemia and removal of venous blood to accentuate hypotension.
As a direct model of human neonatal hypoxic/ischemic brain injury, the focal acute injury (e.g., carotid artery ligation) is flawed since this type of injury is rare in humans, wherein injury is more generalized and protracted [31]. In terms of choice of species, only in the rhesus monkey, dog, and pig models white matter infarction comparable to periventricular leukomalacia has been shown to occur [31]. This condition is most often met in the gyrencephalic brain found in the pig; rodents and rabbits, which are lissencephalic make poor models of white matter destruction by hypoxia/ischemia. Monkeys are too expensive and impractical and dogs present animal welfare issues, leaving pigs as a reasonable experimental model to study hypoxia/ischemia. However, notwithstanding numerous modifications, no single animal model replicates with fidelity the neuropathologic findings reported in the human fetus or infant [35]. Nevertheless, despite their shortcomings and because of their availability and reproducibility, animal models have provided and will continue to provide insight into the pathology of hypoxia/ischemia, particularly at the cellular/molecular level [35]. It is hoped that these investigations will lead to more efficacious therapeutic interventions.
We also note a limitation of the closed cranial window technique to quantify CSF MAPK concentration, that the cellular site of origin cannot be determined. Potential sources include neurons, glia, vascular smooth muscle, and endothelial sources, which are the subject of ongoing study.
Conclusions
Data in the present study indicate that H/I aggravates ET-1-and thromboxane-mediated cerebral vasoconstriction by upregulating tPA and ERK MAPK. The ability of cerebral ischemia to aggravate vasoconstriction induced by ET-1 and U 46619 appears specific in that vasoconstriction to norepinephrine is unchanged after this insult. These data suggest that tPA, whether of endogenous origin and increased in response to cerebral ischemia or exogenously administered as thrombolytic therapy, may dysregulate cerebrovascular hemodynamics, in part, by augmenting vasoconstriction induced by procontractile mediators released contemporaneously from ischemic tissue.
Acknowledgments
This research was funded by Grants from the National Institutes of Health, NS53410 and HD57355 (WMA), HL82545 (AARH), HL76406, CA83121, HL76206, HL07971, and HL81864 (DBC).
Footnotes
Conflict of interest William M. Armstead, John Riley, Abd Al-Roof Higazi, and Douglas B. Cines declare that they have no conflict of interest.
Contributor Information
William M. Armstead, Email: armsteaw@uphs.upenn.edu, Department of Anesthesiology and Critical Care, University of Pennsylvania, 3620 Hamilton Walk, JM3, Philadelphia, PA l9l04, USA; Department of Pharmacology, University of Pennsylvania, Philadelphia, PA l9l04, USA.
John Riley, Department of Anesthesiology and Critical Care, University of Pennsylvania, 3620 Hamilton Walk, JM3, Philadelphia, PA l9l04, USA.
Douglas B. Cines, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA
Abd Al-Roof Higazi, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Department of Clinical Biochemistry, Hebrew University-Hadassah Medical School, Jerusalem, Israel.
References
- 1.Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150–158. doi: 10.1016/S1474-4422(04)00679-9. [DOI] [PubMed] [Google Scholar]
- 2.DeVeber G, Andrew M. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–423. doi: 10.1056/NEJM200108093450604. [DOI] [PubMed] [Google Scholar]
- 3.Gunther G, Junker R, Strater R, Schobess R, Kurnik K, Kosch A, Nowak-Gottl U. Symptomatic ischemic stroke in full-term neonates. Stroke. 2000;31:2437–2441. doi: 10.1161/01.str.31.10.2437. [DOI] [PubMed] [Google Scholar]
- 4.Kraus FT, Acheen VI. Fetal thrombotic vasculopathy in the placenta: cerebral thrombi and infarcts, coagulopathies, and cerebral palsy. Hum Pathol. 1999;30:759–769. doi: 10.1016/s0046-8177(99)90136-3. [DOI] [PubMed] [Google Scholar]
- 5.Janjua N, Nasar A, Lynch JK, Qureshi AI. Thrombolysis for ischemic stroke in children: data from the nationwide inpatient sample. Stroke. 2007;38:1850–1854. doi: 10.1161/STROKEAHA.106.473983. [DOI] [PubMed] [Google Scholar]
- 6.Benedict SL, Ni OK, Schloesser P, White KS, Bale JF. Intra-arterial thrombolysis in a 2-year old with cardioembolic stroke. J Child Neurol. 2007;22:225–227. doi: 10.1177/0883073807300296. [DOI] [PubMed] [Google Scholar]
- 7.Lapchak PA. Hemorrhagic transformation following ischemic stroke: significance, causes and relationship to therapy and treatment. Curr Neurol Neurosci Rep. 2002;2:1–6. doi: 10.1007/s11910-002-0051-0. [DOI] [PubMed] [Google Scholar]
- 8.Cremer S, Berliner Y, Warren D, Jones AE. Successful treatment of pediatric stroke with recombinant tissue plasminogen activator (rt-PA): a case report and review of the literature. CJEM. 2008;10:575–578. [PubMed] [Google Scholar]
- 9.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 10.Ferriero DM. Neonatal brain injury. New Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 11.Volpe JJ. Brain injury in the premature infant: overview of clinical aspects, neuropathology and pathogenesis. Semin Pediatr Neurol. 1998;5:135–151. doi: 10.1016/s1071-9091(98)80030-2. [DOI] [PubMed] [Google Scholar]
- 12.Armstead WM, Cines DB, Bdeir K, Kulikovskaya I, Stein SC, Higazi A. uPA impairs cerebrovasodilation after hypoxia/ischemia through LRP and ERK MAPK. Brain Res. 2008;1231:121–131. doi: 10.1016/j.brainres.2008.06.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstead WM, Riley J, Kiessling JW, Cines DB, Higazi AAR. PAI-1 derived peptide EEIIMD prevents impairment of cerebrovasodilation by augmenting p38 MAPK upregulation after cerebral hypoxia/ischemia. AJP. 2010;299:H76–H80. doi: 10.1152/ajpheart.00185.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jagolino AL, Armstead WM. PTK MAPK, and NOC/oFQ impair hypercapnic cerebrovasodilation after hypoxia/ischemia. Am J Physiol. 2003;284:H101–H107. doi: 10.1152/ajpheart.00457.2002. [DOI] [PubMed] [Google Scholar]
- 15.Leffler CW, Busija DW, Armstead WM, Mirro R, Beasley DG. Ischemia alters cerebral vascular responses to hypercapnia and acetylcholine in piglets. Pediatr Res. 1989;25:180–183. doi: 10.1203/00006450-198902000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Leffler CW, Busija DW, Beasley DG, Armstead WM, Mirro R. Post-ischemic micro-vascular cerebral responses to norepinephrine and hypotension in newborn pigs. Stroke. 1989;20:541–546. doi: 10.1161/01.str.20.4.541. [DOI] [PubMed] [Google Scholar]
- 17.Armstead WM, Cines DB, Higazi AA. Plasminogen activators contribute to impairment of hypercapnic and hypotensive cerebrovasodilation after cerebral hypoxia/ischemia in the newborn pig. Stroke. 2005;36:2265–2269. doi: 10.1161/01.STR.0000181078.74698.b0. [DOI] [PubMed] [Google Scholar]
- 18.Armstead WM, Ganguly K, Kiessling JW, et al. RBC-coupled tPA prevents impairment of cerebral vasodilatory responses and tissue injury in pediatric cerebral hypoxia ischemia through inhibition of ERK MAPK. J Cereb Blood Flow Metab. 2009;29:1463–1474. doi: 10.1038/jcbfm.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstead WM, Cines DB, Higazi AA. Plasminogen activators contribute to age dependent impairment of NMDA cerebrovasodilation after brain injury. Dev Brain Res. 2005;156:139–146. doi: 10.1016/j.devbrainres.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Nassar T, Haj-Yehia A, Akkawi S, et al. Binding of urokinase to low density lipoprotein-related receptor (LRP) regulates vascular smooth muscle cell contraction. J Biol Chem. 2002;277:40499–40504. doi: 10.1074/jbc.M207172200. [DOI] [PubMed] [Google Scholar]
- 21.Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Nat Acad Sci. 1999;96:12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi T, Sakai K, Sasaki C, Zhang WR, Warita H, Abe K. c-Jun-N-terminal kinase (JNK) and JNK interacting protein response in rat brain after transient middle cerebral artery occlusion. Neuroscience Lett. 2000;284:195–199. doi: 10.1016/s0304-3940(00)01024-7. [DOI] [PubMed] [Google Scholar]
- 23.Laher I, Zhang JH. Protein kinase C and cerebral vasospasm. J Cerebral Blood Flow Metab. 2001;21:887–906. doi: 10.1097/00004647-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Armstead WM. NOC/oFQ and NMDA contribute to piglet hypoxic ischemic hypotensive cerebrovasodilation impairment. Pediatr Res. 2002;51:586–591. doi: 10.1203/00006450-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Armstead WM. Nociceptin/Orphanin FQ and cardiovacular disease. Cardiovasc Ther. 2011;29:23–28. doi: 10.1111/j.1755-5922.2010.00241.x. [DOI] [PubMed] [Google Scholar]
- 26.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 27.Armstead WM. Role of endothelin in pial artery vasoconstriction and altered responses to vasopressin following brain injury. J Neurosurg. 1996;85:901–907. doi: 10.3171/jns.1996.85.5.0901. [DOI] [PubMed] [Google Scholar]
- 28.Ford J, Armstead WM. Nociceptin/Orphanin FQ alters prostaglandin cerebrovascular action following brain injury. J Neurotrauma. 2004;21:187–193. doi: 10.1089/089771504322778640. [DOI] [PubMed] [Google Scholar]
- 29.Leffler CW, Mirro R, Armstead WM, Busija DW. Prostanoid synthesis and vascular responses to exogenous arachidonic acid following cerebral ischemia in piglets. Prostaglandins. 1990;40:241–248. doi: 10.1016/0090-6980(90)90012-k. [DOI] [PubMed] [Google Scholar]
- 30.Gennarelli TA. Animate models of human head injury. J Neurotrauma. 1994;11:357–368. doi: 10.1089/neu.1994.11.357. [DOI] [PubMed] [Google Scholar]
- 31.Tuor UI, Del Bigio MR, Chumas PD. Brain damage due to cerebral hypoxia/ischemia in the neonate: pathology and pharmacological modification. Cerebrovasc Brain Metab Rev. 1996;8:159–193. [PubMed] [Google Scholar]
- 32.Rivkin MJ, Volpe JJ. Hypoxic-Ischemic brain injury in the newborn. Semin Neurol. 1993;13:30–39. doi: 10.1055/s-2008-1041104. [DOI] [PubMed] [Google Scholar]
- 33.Volpe JJ. Neurology of the newborn. 2nd ed. Philadelphia: WB Saunders; 1987. p. 168. [Google Scholar]
- 34.Kuban KCK, Leviton A. Cerebral palsy. N Engl J Med. 1994;330:188–195. doi: 10.1056/NEJM199401203300308. [DOI] [PubMed] [Google Scholar]
- 35.Longo LD, Packianathan S. Hypoxia-ischemia and the developing brain: hypotheses regarding the pathophysiology of fetal-neonatal brain damage. Br J Obstet Gynaecol. 1997;104:652–662. doi: 10.1111/j.1471-0528.1997.tb11974.x. [DOI] [PubMed] [Google Scholar]
- 36.Raju TNK. Some animal models for the study of perinatal asphyxia. Biol Neonate. 1992;62:202–214. doi: 10.1159/000243873. [DOI] [PubMed] [Google Scholar]
- 37.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Human Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]