Abstract
Human exposure to ionizing radiation from medical procedures has increased sharply in the last three decades. Recent epidemiological studies suggest a direct relationship between exposure to ionizing radiation and health problems, including cancer incidence. Therefore, minimizing the impact of radiation exposure in patients has become a priority in the development of future clinical practices. Crucial players in radiation-induced DNA damage include reactive oxygen species (ROS), but the sources of these have remained elusive. To the best of our knowledge, we show here for the first time that two members of the ROS-generating NADPH oxidase family (NOXs), NOX4 and NOX5, are involved in radiation-induced DNA damage. Depleting these two NOXs in human primary fibroblasts resulted in reduced levels of DNA damage as measured by levels of radiation-induced foci, a marker of DNA double-strand breaks (DSBs) and the comet assay coupled with increased cell survival. NOX involvement was substantiated with fulvene-5, a NOXs-specific inhibitor. Moreover, fulvene-5 mitigated radiation-induced DNA damage in human peripheral blood mononuclear cells ex vivo. Our results provide evidence that the inactivation of NOXs protects cells from radiation-induced DNA damage and cell death. These findings suggest that NOXs inhibition may be considered as a future pharmacological target to help minimize the negative effects of radiation exposure for millions of patients each year.
INTRODUCTION
Exposure to ionizing radiation leads to a wide range of DNA lesions including single-strand breaks (SSBs), double-strand breaks (DSBs), and the chemical modification of the DNA bases and sugar backbone (1). If the great majority of these DNA lesions are not repaired in a timely manner, accrued and/or chronic exposure may lead to misrepaired lesions and ultimately result in severe health problems including cancer (2). The use of ionizing radiation has increased sharply in the last few decades with the development of instrumentation for computed tomography, radiotherapy and angioplasty, among others, leading to increasing concern about long-term health issues (3). Thus, new clinical studies are now aimed at limiting the impact of radiation exposure in patients by limiting doses, increasing shielding and/or increasing the sensitivity of radiation detection (4–6).
A substantial portion of DNA damage is due to reactive oxygen species (ROS). While ROS are normally present in cells, their levels are elevated when ionizing radiation interacts with water molecules and produces additional ROS. Lowering endogenous ROS levels with antioxidants before exposure to radiation has been shown to protect cells from its harmful effects both in vitro and in vivo (7–9). Additionally, other studies support a role for chronic oxidative stress in radiation-induced DNA damage and subsequent cellular radiosensitivity (2, 10, 11).
However, the sources of cellular endogenous ROS remain unclear. One possible source is mitochondrial deregulation, but there is little evidence supporting crucial and/or unique roles of mitochondria-derived (ROS) in radiation-induced DNA damage. Another possibility is the family of nicotinamide adenosine dinucleotide phosphate (NADPH) oxidases, known as the NOX/DUOX family. Consisting of 7 homologs, NOX1 through NOX5, DUOX1 and DUOX2, these NADPH oxidase complexes transfer an electron from NADPH to molecular oxygen, thus producing superoxide anion, a precursor for other reactive oxygen and nitrogen species (12). ROS production is part of the primary function of these NOXs/DUOXs in a large variety of cell types (12, 13). Recently, a chronic oxidative stress response ascribed to NOXs activity has been detected in mouse hematopoietic cells after exposure to radiation (14).
These findings suggested that decreasing NOX activity may alleviate radiation-induced DNA damage and cell death. Utilizing human primary fibroblasts (HPFs) in this study, we show that NOX4 and/or NOX5 inactivation results in significant reductions in both DSB frequency in and cellular mortality of these cultures. Two independent techniques for decreasing NOX activity in HPFs were used: decreasing NOX4 and NOX5 protein levels by genespecific small interfering RNAs and direct enzyme inhibition with fulvene-5. We further show that treatment of human peripheral blood mononuclear cells (PBMCs) ex vivo with the NOX specific inhibitor also reduced radiation-induced DNA damage while improving cell survival. Thus, our study identifies a critical role for NOX4 and NOX5 in radiation-induced DNA damage and cell death in HPFs. We suggest that NOX proteins may be useful targets for protecting cells from radiation.
MATERIALS AND METHODS
Cell Culture
Human primary fibroblasts (GM03652 and BJ foreskin fibroblasts) were grown at 37°C with 5% CO2 in Dulbecco’s modified Eagle medium (DMEM) (Invitrogen, Carlsbad) supplemented with 15% fetal bovine serum (FBS; Atlanta® Biologicals, Norcross, GA). All media were supplemented with 2 mM glutamine, penicillin and streptomycin (Invitrogen™, Carlsbad, CA). Fulvene-5 was kindly provided by Dr. J. L. Arbiser (Department of Dermatology and Atlanta Veterans Administration Medical Center, Emory University, Atlanta, GA).
siRNA Transfection
Small interfering RNA (siRNA) transfection experiments were performed with DharmaFECT® (Thermo Fisher Scientific Inc., Hudson, NH). A SMARTpool consisting of four short sequences of siRNA specific for NOX4 or NOX5 (Dharmacon; Thermo Fisher Scientific Inc.) along with scrambled control (nontargeting siRNA) were used under conditions specified by the manufacturer.
Real-Time PCR
Total RNA from cells was extracted using TRIzol® reagent (Invitrogen) according to the manufacturer’s instructions. Quality of RNA preparation, based on the 28S/18S ribosomal RNAs ratio, was assessed using the RNA 6000 Nano lab-on-a-chip (Agilent Technologies, Palo Alto, CA). Reverse transcription and real-time PCR were performed as previously described (15). Oligonucleotides were purchased from Life Technologies (Grand Island, NY) and their sequences are available on demand.
Clonogenic Assay
Cell survival was assessed by colony formation assay. Cells were trypsinized and identical numbers of fibroblasts were plated on 35 mm dishes. Six hours after seeding, cells were irradiated with doses ranging from 1–8 Gy in a Mark-1 gamma-irradiator (JL Shepherd & Associates, San Fernando, CA) at a dose rate of 2.2 Gy/min. After 10 days of incubation, the colonies were fixed with methanol for 10 min, then stained with Coomassie blue. Colonies with >50 cells were counted under a dissection microscope. Clonogenic survival curves were constructed from at least three independent experiments.
Intracellular ROS Detection
Intracellular ROS measurements were performed using dihydroethidium (DHE). Briefly, cells treated with 10 µM fulvene-5 for 30 min were washed before trypsinization, harvested and resuspended in 500 µL Hanks’ balanced salt solution (HBSS) medium containing 10 µM DHE. Cells were then incubated for 30 min at 37°C and used for analysis by flow cytometry (BD Biosciences, San Jose, CA).
Alkaline Comet Assay for Analysis of DNA Damage
Cell samples were handled under dimmed or yellow light to prevent DNA damage from ultraviolet light. Briefly, cells were harvested with trypsin, washed once with phosphate-buffered saline (PBS) and resuspended in 1× Tris-EDTA buffer. Cells were then mixed with 37°C prewarmed/melted 1.6% low melting agarose to make a final agarose concentration of 0.85%. The mixture was spread over a sample area on FLARE™ slides (Trevigen®, Gaithersburg, MD). After lysis, slides were transferred in alkaline solution (pH >13) for 1 h at room temperature in the dark, then electrophoresis was performed (0.7 V/cm/300 mA) for 1 h at room temperature in the dark. After electrophoresis, the slides were washed 3 times for a total of 20 min with water, after which they were washed in chilled 70% alcohol for 15 min and air-dried overnight in the dark. Each slide was stained with 50 µL of 1:10,000 diluted of a SYBR® Green solution (Trevigen). Individual cells with or without comet were visualized using an Olympus fluorescence microscope. Analysis was performed by scoring at least 50 comets per sample using the CometScore software package (TriTek Corp., Sumerduck, VA). The results are shown as means ± standard error.
Isolation of Human Peripheral Blood Mononuclear Cells
Isolation of human PBMCs was performed using previously described methods (16). Blood samples collected by venipuncture were diluted into anticoagulant tubes with 1 volume of PBS. One volume of the blood–PBS mix was layered on the top of Ficoll-Paque™ medium in a centrifuge tube. Centrifugation was then performed at 700g for 25 min at room temperature (the breaking function of the centrifuge was deactivated for this step). The collected layer containing PBMCs was washed 3 times with 10 mL of PBS by centrifuging at 600g for 5 min at room temperature. PBMCs were then fixed with 2% of paraformaldehyde, washed 3 times with PBS and diluted to 4 × 106 cells/mL. Samples were spotted onto slides by cytospinning 200–300 µL of cell suspension for 4 min at 800 rpm (Shandon™ Cytospin™ 3 Cytocentrifuge, Thermo Fisher Scientific Inc., Boston, MA) at room temperature prior to immunofluorescence staining.
Immunofluorescence
Cells were fixed for 20 min with freshly prepared 2% paraformaldehyde in PBS, after which they were permeabilized for 10 min in 0.1% SDS, then blocked with 10% FBS for 30 min and incubated for 2 h at room temperature with primary antibody γ-H2AX (05–636; Millipore, Billerica, MA). After the cells were washed in PBS/0.1% BSA, cells were stained with Alexa Fluor® 488 for 1 h at room temperature. Finally, the cells were washed in PBS and coverslips were mounted for analysis. Fluorescent images were captured using a confocal microscope (Nikon PCM 2000™).
Western Blots
Cells were washed twice with PBS, directly solubilized in denaturing sample buffer and then subjected to SDS-PAGE. Proteins were electrotransferred to 0.2 µm Protran BA83 nitrocellulose sheets (Invitrogen) for immunodetection with primary antibodies NOX4 and NOX5 (cat. no. ab133303 and ab110400, respectively; Abcam®, Cambridge, MA). Immune complexes were detected with horseradish peroxidase coupled anti-rabbit IgG antibody (Amersham™, GE Healthcare Bio-Sciences, Pittsburgh, PA).
Statistical Analysis
Statistical analyses were performed using Microsoft Excel software. P values were calculated by Student’s t test with the level of significance set at P < 0.05. #Nonsignificant; *P < 0.05; **P < 0.001.
RESULTS
NOX4 and NOX5 Inactivation Mitigates Radiation-Induced DNA Damage and Cell Mortality
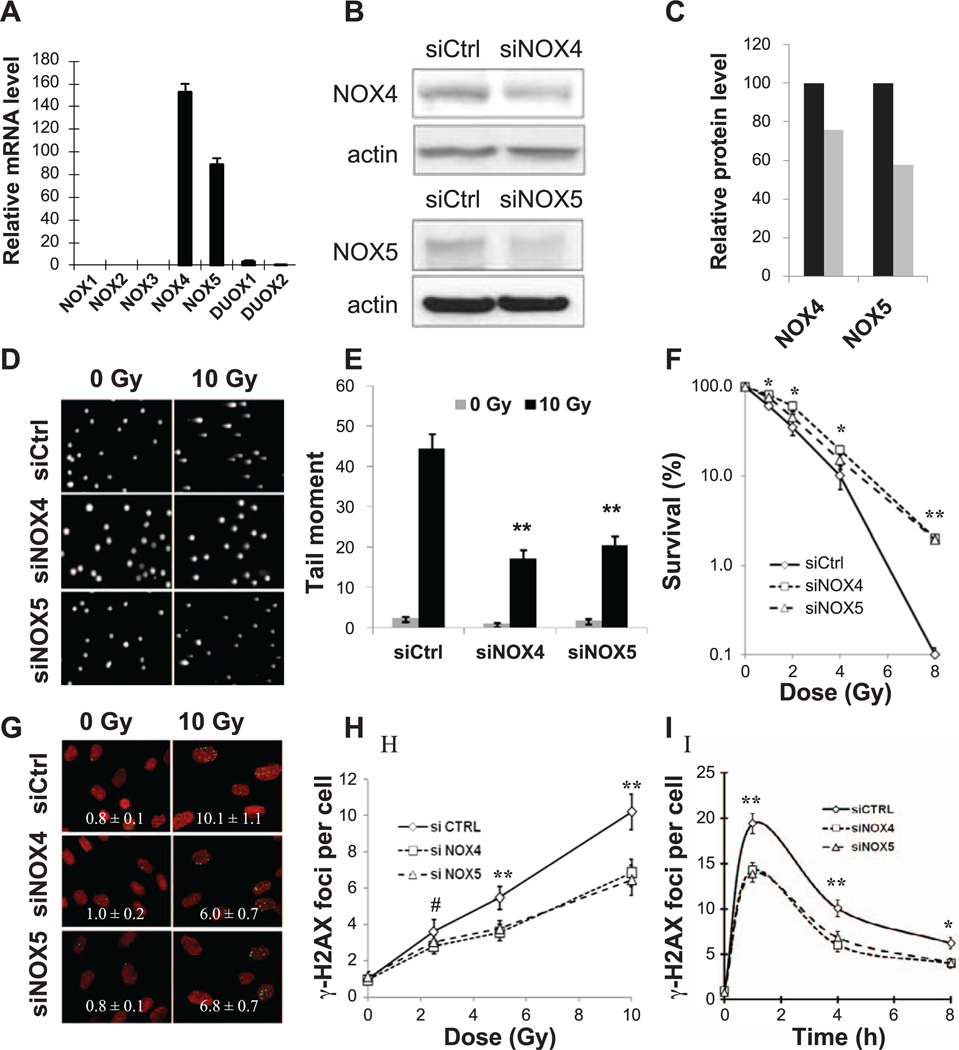
The first step was to determine which of the NOX/DUOX family members were present in HPFs. When expression levels in HPF cultures were measured by real-time PCR, NOX4 and NOX5 were found at high gene expression levels (Fig. 1A). Two others, DUOX1 and DUOX2, had detectible but 30 times lower expression levels. The expression of NOX1, NOX2 and NOX3 was undetectable (17). Immunoblots verified that NOX4 and NOX5 proteins were present in HPFs (Fig. 1B).
Figure 1.
Silencing of NOX4 and NOX5 in human primary fibroblasts (HPFs) reduces radiation-induced DNA damage and cell death. Panel A: Expression pattern of NADPH oxidases (NOX/DUOX) in HPFs. HPFs (GM03652) were cultured in 15% FBS for 3 days, and total RNAs were extracted for reverse transcription. Transcript levels were analyzed by real-time PCR. Expression values are relative fold change for NOXs transcripts normalized to 18S RNA (NOX/18S ratio) and are displayed on base 2 logarithmic scales. Error bars indicate standard deviations (n = 3). Panels B and C: Silencing of NOX4 and NOX5 in HPFs decreases their protein levels. Panel B shows Western blot, and panel C shows quantitation (black box, siCtrl; gray box, siNOX). Panels D and E: Silencing of NOX4 and NOX5 in HPFs reduces radiation-induced DNA fragmentation. Human primary fibroblasts (GM03652) were transfected with small interference RNAs (siRNAs) against NOX4 or NOX5 or a scrambled control sequence 3 days prior to irradiation. Cells were 0 or 10 Gy irradiated and incubated at 37°C for 30 min before preparation for comet assay as described in Materials and Methods. Panel D shows representative images, and panel E shows quantitation. The comet tail of 50 cells for each experimental condition was quantified using CometScore software. Error bars indicate standard errors (n = 50) of a representative experiment. Panel F: Silencing of NOX4 and NOX5 protects HPFs from radiation-induced cell death. Clonogenic survival of HPFs transfected with scrambled sequence siRNA (siCtrl) or siRNAs prepared against NOX4 (siNOX4) or NOX5 (siNOX5). Three days after transfection, cells were harvested and seeded at 500 cells per 35 mm dish, then exposed to increasing doses of gamma radiation. Plates were incubated at 37°C for 10 days prior to Coomassie staining and colony counts. Percentage cell survival was calculated relative to nonirradiated cell cultures. Error bars indicate standard deviations (n = 3). Panels G–I: Silencing of NOX4 and NOX5 reduces the incidence of radiation-induced γ-H2AX foci in HPFs. Images shown are representative of those analyzed by FociCounter software, with the values of average numbers of γ-H2AX foci per cell shown in each image (panel G). Foci per cell values as a function of radiation dose. HPF cultures were 0, 2.5, 5 and 10 Gy irradiated and incubated at 37°C for 24 h before fixation (panel H). Foci per cell values as a function of time. HPF cultures were irradiated with 2 Gy and incubated at 37°C for various lengths of time before preparation for γ-H2AX detection (panel I). Error bars indicate standard deviations (n = 3). Green: γ-H2AX foci; red: DNA stained with propidium iodide.
To investigate whether the presence of NOX4 and NOX5 affects the level of radiation-induced DNA damage, we decreased their expression with specific interference RNA (siRNA) species in HPF cultures. When HPFs were incubated with the siRNA species for 3 days before irradiation, the efficiency of NOX4 and NOX5 silencing was found to be 25 and 40%, respectively (Fig. 1B and C).
We next investigated the effects of silencing NOX4 and NOX5 in HPF cultures on radiation-induced DNA damage with the alkaline comet assay, which measures both SSBs and DSBs (Fig. 1D and E). Tail moments were increased 40–45 times in cells exposed to radiation (10 Gy) compared to nonirradiated cells, and were reduced by 45–50% when NOX4 and NOX5 were silenced. Taken together, these results indicate that the silencing of NADPH oxidases NOX4 or NOX5 protects HPFs from radiation-induced DNA damage.
An important question is whether decreased DNA damage levels in HPFs with silenced NOX4 and NOX5 after radiation exposure are associated with any cell benefits such as decreased cell mortality. HPF cultures were incubated with siRNA to NOX4, NOX5, or a scrambled control for 3 days prior to radiation exposure (Fig. 1F). HPF cultures with silenced NOX4 or NOX5 exhibited decreased mortality, indicating that this treatment had a protective effect on the HPFs.
While the alkaline comet assay measures both DNA SSBs and DSBs, the latter make up a small minority of radiation-induced DNA lesions. However, DSBs are of great importance because their presence in chromosomes leads to fragmentation, translocations and mutations. DSBs can be visualized by staining for γ-H2AX foci, which rapidly form at the sites of nascent DSBs.
Before radiation exposure, HPFs exhibited less than 1 γ-H2AX focus per cell (Fig. 1G). Exposure to increasing doses of radiation resulted in significant increases in the numbers of γ-H2AX foci, as measured 24 h postirradiation (Fig. 1H). Inactivation of NOX4 or NOX5 was accompanied by a ~ 35% reduction in γ-H2AX foci numbers. A similar difference was found when HPF cultures were exposed to 2 Gy, followed by various recovery periods (Fig. 1I). However, the kinetics of γ-H2AX focal disappearance was virtually identical between control and silenced HPF cultures. Thus, these results show that silencing NOX4 or NOX5 by siRNA in HPFs led to reduced levels of γ-H2AX foci and presumably in DNA DSBs, it did not alter the DSB repair rates.
NOX4 and NOX5 Inhibition with Fulvene-5 in HPFs Mitigates Radiation-Induced DNA Damage and Cell Mortality
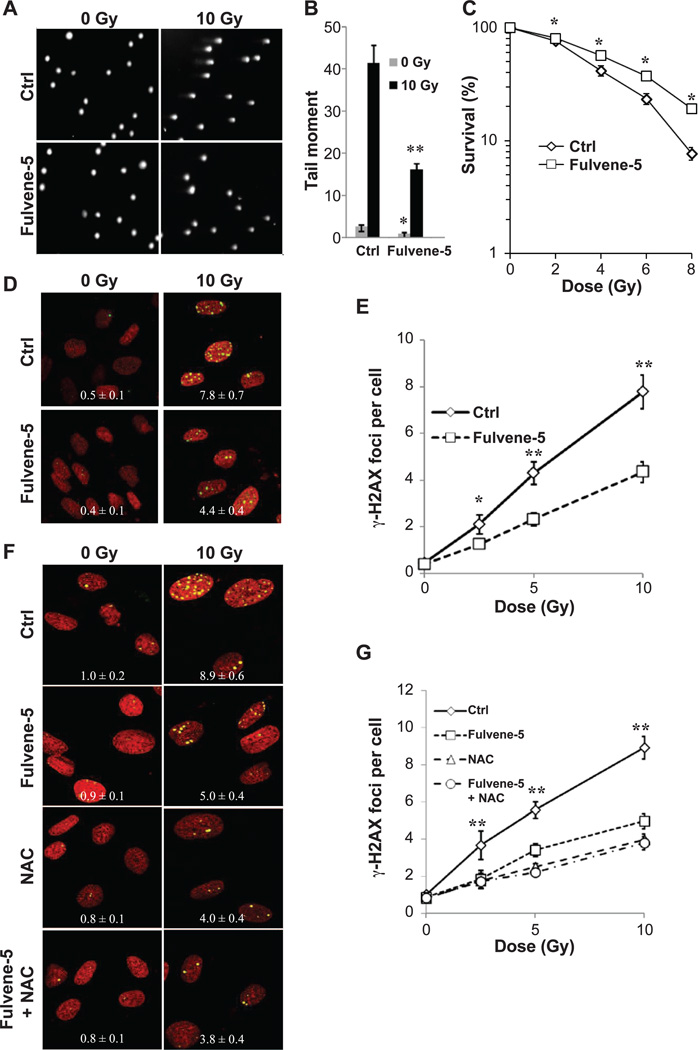
Fulvene-5 is a diphenyleneiodonium (DPI) analog, which has recently been shown to exhibit NOX2/NOX4-specific inhibiting activity unlike the broad scale flavoprotein-targeting activity of DPI. Based on its structural similarity to DPI, fulvene-5 appears to target NADPH oxidases by abstracting an electron from the flavin adenine dinucleotide (FAD) to create a radical, which in turn forms covalent adducts with the FAD motif of the enzyme (18). This mechanism confers a NOX-inhibiting capacity to fulvene-5 as opposed to an antioxidant capacity. Fulvene-5 has been shown to inhibit NOX4 in vitro and to retard the growth of NOX4-dependent endothelial tumors in mice (19).
When DNA damage levels were measured in HPFs with the comet assay, fulvene-5 treatment resulted in a greater than 50% decrease in tail moments after 10 Gy of gamma radiation (Fig. 2A and B). The same results were obtained with the lower dose of 5 Gy gamma radiation. However, there was no significant increase in DNA damage with the lower dose of 2 Gy. The latter observation may be due to the possibility that the limit of detection for the comet assay was reached (Supplementary Fig. S1; http://dx.doi.org/10.1667/RR13799.1.S1). In addition, clonal survival of HPFs increased significantly when treated with fulvene-5 (Fig. 2C). Fulvene-5 was found to protect HPF cultures from radiation-induced γ-H2AX foci formation when they were treated 1 h prior to radiation exposure (Fig. 2D and E). Similar results were obtained with BJ foreskin fibroblasts, suggesting that the role of NOXs in radiation-induced DNA damage and cell death may be broadly conserved in HPFs (Supplementary Fig. S2; http://dx.doi.org/10.1667/RR13799.1.S1).
Figure 2.
Pharmacologic inhibition of NOXs in human primary fibroblasts (HPFs) reduces radiation-induced DNA damage and cell death. Panels A and B: Treatment of HPFs with fulvene-5 reduces radiation-induced DNA fragmentation. HPF cultures were incubated with DMSO or fulvene-5 for 1 h, irradiated with 0 or 10 Gy, further incubated at 37°C for 30 min, and prepared for the comet assay as described in Materials and Methods. Panel A shows representative images, and panel B shows quantitation. Comet tails of 50 cells were analyzed for each experimental condition using the CometScore software program. Error bars indicate standard errors (n = 50) of a representative experiment. Panel C: Clonogenic survival of HPFs treated with DMSO or fulvene-5. HPF cultures were incubated with DMSO or 10 µM fulvene-5 for 1 h before irradiation with zero, 2, 4, 6 or 8 Gy. After a 24 h recovery period, the cultures were harvested, seeded at 500 cells per 35 mm dish and incubated at 37°C for 10 days. Cells were stained with Coomassie blue and colonies counted. Percentage survival was calculated relative to the nonirradiated cell cultures. Error bars indicate standard deviations (n = 3). Panels D and E: Treatment of HPFs with fulvene-5 reduces radiation-induced γ-H2AX foci formation. DMSO or fulvene-5 (10 µM) was added 1 h prior to 0, 2.5, 5 or 10 Gy irradiation. After a 24 h recovery period, cells were fixed and treated for γ-H2AX foci detection. Panel D shows representative images with foci per cell values calculated using FociCounter software. Panel E shows a graph of relationship of foci numbers to dose. Values are averages of γ-H2AX foci per cell. Error bars indicate standard deviations (n = 3). Panels F and G: Treatment of HPFs with fulvene-5 and N-acetyl-cysteine on radiation-induced γ-H2AX foci formation. DMSO, fulvene-5 (10 µM) or N-acetyl-cysteine (10 mM) was added 1 h prior to 0, 2.5, 5 and 10 Gy irradiation. After a 24 h recovery period, cells were fixed and treated for γ-H2AX detection. Panel F shows representative images with values calculated using the FociCounter software. Panel G shows a graph of the relationship of foci numbers to dose. Values are averages of γ-H2AX foci per cell. Error bars indicate standard deviations (n = 3).
Fulvene-5 and the Antioxidant N-Acetyl-Cysteine
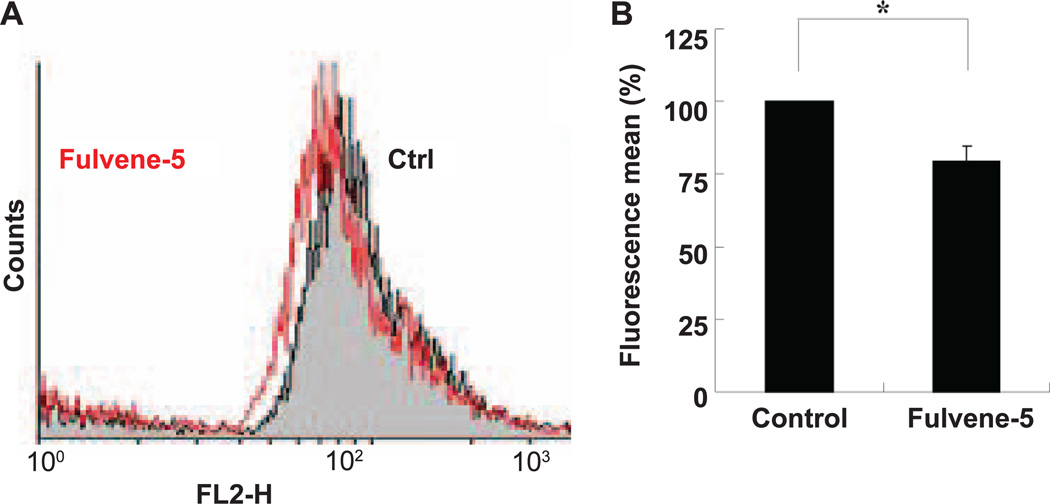
Antioxidants such as N-acetylcysteine (NAC) protect cells from radiation-induced DNA damage and cell death. The contribution of NOX4 and NOX5 to the total cellular ROS level was estimated by comparing γ-H2AX foci formation in HPFs incubated with fulvene-5, with NAC, as well as there combination (Fig. 2F and G). NAC treatment lowered γ-H2AX formation by 55% while fulvene-5 treatment lowered it by 44%. Treatment of HPFs with both compounds did not lower the ROS levels further. Thus it appears that NOX4 and NOX5 may be responsible for a major portion of total cellular ROS levels. Direct measurement of ROS levels in HPF cells using DHE as a general indicator verified that incubation with fulvene-5 lowers ROS levels (Fig. 3A and B). Furthermore, treatment of HPFs with both NAC and fulvene-5 resulted in the same range of protection from radiation-induced cell death as fulvene-5 alone, with no additional protective effects by NAC (Supplementary Fig. S3; http://dx.doi.org/10.1667/RR13799.1.S1). These results are consistent with a previous finding that NAC is protective against radiation-induced genotoxicity, but not cytotoxicity (20).
Figure 3.
Treatment of HPFs with fulvene-5 reduces intracellular ROS levels. HPF cultures were treated with 10 µM fulvene-5 for 1 h, washed, trypsinized and resuspended in 500 µL HBSS medium containing 10 µM dihydroethidium (DHE). The HPF suspensions were incubated for 30 min at 37°C and analyzed by flow cytometry. Panel A shows the data and panel B shows the quantitation.
NOXs inhibition with fulvene-5 at 24 h postirradiation led to a decrease in ROS levels, indicating a chronic NOXs activity induced by ionizing radiation (Supplementary Fig. S4; http://dx.doi.org/10.1667/RR13799.1.S1). These data are in agreement with previous observations that NOX4 may be induced long after radiation exposure (21, 22), and open up new lines of investigation into the molecular mechanisms underlying the chronic activation of NOXs by ionizing radiation.
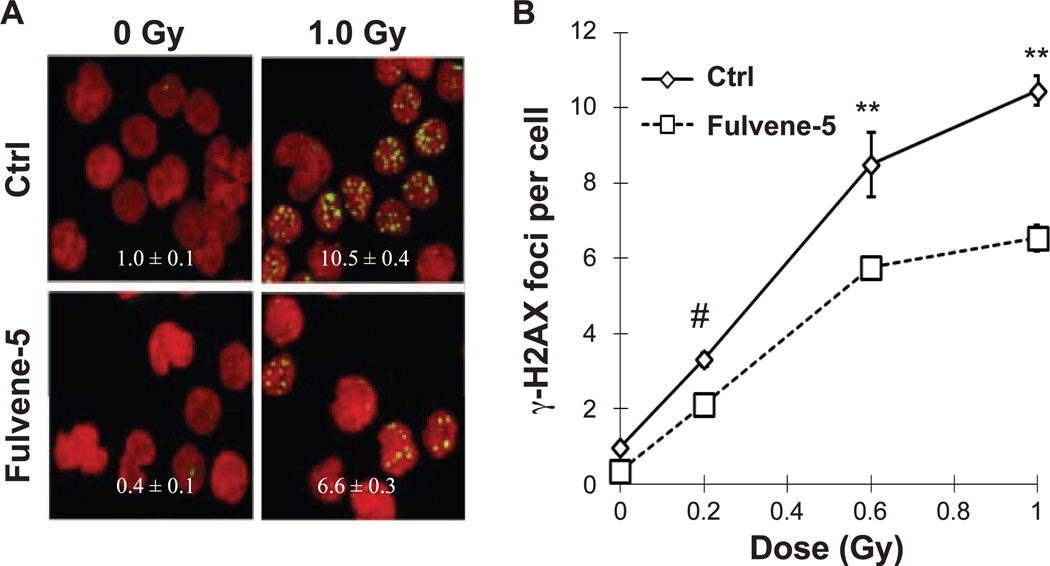
Treatment of Blood Ex Vivo with Fulvene-5 Mitigates Radiation-Induced DNA Damage in PBMCs
To evaluate the action of fulvene-5 ex vivo, we next investigated the effect of the drug in blood samples. Human blood samples were incubated 1 h with or without fulvene-5 prior to ex vivo irradiation (Fig. 4A and B). Fulvene-5 treatment was accompanied by ~35% reduction in the incidence of γ-H2AX foci.
Figure 4.
Fulvene-5 treatment of human blood reduces radiation-induced γ-H2AX formation in PBMCs. Blood samples from healthy patients were incubated with DMSO or 10 µM fulvene-5 for 1 h prior to 0, 0.2, 0.6 or 1 Gy irradiation. After recovery at 37°C for 30 min, PBMCs were isolated and stained for γ-H2AX foci detection. Panel A shows representative images with foci per cell values calculated by FociCounter software. Panel B shows a graph of the relationship of foci numbers to dose. Values are averages of γ-H2AX foci per cell. Error bars indicate standard deviations (n = 3).
DISCUSSION
Taken together, these data provide evidence that lowering cellular ROS levels by either inhibiting or silencing NOX4 or NOX5 protects against radiation-induced DNA damage and subsequent cell death, and point to fulvene-5 as a novel radioprotector.
The physiological purpose of NADPH oxidases appears to be linked to the generation of ROS, reactive species that are involved in a broad range of cell functions such as immunity, angiogenesis and cell metabolism, among others (13). NOXs deregulation can lead to ROS overproduction, and in turn, to increased oxidative-induced DNA damage, which is associated with inflammation-related diseases and cancer (12, 23). Our results suggest that two NADPH oxidases, NOX4 and NOX5, may play a significant part in radiation-induced DNA damage. These observations correlate with an increasing number of studies that point to the likely role of NOXs in the detrimental, long-term effects of ionizing radiation (22, 24), and to some extent, the redox signaling pathways induced by radiation in bystander cells (25). In one recent study, it was shown that NOX2 and NOX4 inhibition were thought to contribute to the relief of radiation-induced bone marrow suppression (14). However, the authors of that study believe that NOX4 is the critical factor involved in bone marrow cell injury after radiation exposure.
It has been previously shown that only NOX4 and NOX5 are abundantly expressed in HPFs (17). Using the γ-H2AX assay (DSB detection) and the alkaline comet assay (SSB and DSB detection), the current study shows that silencing of NOX4 or NOX5 in HPFs with specific interference RNA (siRNA) mitigated radiation-induced damage (detected by both γ-H2AX and comet assays) while increasing survival. Furthermore, inhibiting NOXs with the NOX2/NOX4 inhibitor fulvene-5 (19) in primary fibroblasts led to a decreased amount of radiation-induced DNA damage in a same range as NOX4 silencing. As HPFs exhibited no detectable NOX2 expression level, our results are consistent with the findings that fulvene-5 potently inhibits NOX4 (19) and point to fulvene-5 as a potent radioprotector. Inactivation of NOX4 or NOX5 by siRNA significantly reduced the overall amount of DSBs visualized by γ-H2AX foci, an observation that correlated with a decrease in other DNA breaks, i.e., SSBs detected by the comet assay. Our findings are consistent with the observation that a nonselective inhibition of NADPH oxidases attenuated chromosomal aberrations in human hematopoietic cells after total body irradiation (26). Conversely, the overexpression of another NADPH oxidase, NOX1, was shown to increase DNA fragmentation in mammalian cells, indicating the critical importance of NADPH oxidase-induced ROS on DNA integrity (27). However, inactivating both NOX4 and NOX5 did not lead to further protection from radiation-induced DNA damage compared to single inhibition of NOX4 or NOX5. Investigating the interplay between NOX4 and NOX5 enzymatic activities in endogenous ROS production will help to clarify this lack of additive effects on radiation-induced DNA damage.
Interestingly, γ-H2AX kinetics were not altered in cells after NOX4 or NOX5 silencing, suggesting that NOXs depletion did not alter the repair capacity of cells exposed to radiation. Finally, the fact that the incubation of fulvene-5 with human blood protects human PBMCs from radiation-induced DNA damage raises the possibility that this drug may be functional as a radioprotector in vivo. Nevertheless, since the tissue distribution of NOX isoform transcripts shows great discrepancy between tissues (13), further attention may be given to the development of NOX isoform-specific drugs, especially when a specific organ is targeted by irradiation. For example, Tateishi et al. showed that a specific silencing of NOX1 was optimal to protect salivary gland acinar cells from radiation-induced ROS and apoptosis (28). In such cases, drug specificity may also decrease radiation side effects.
Thus, our study shows that NOXs silencing or inhibition partly protects cells from both radiation-induced DNA damage and cell death, suggesting that the inhibition of NOXs reduces the radiosensitivity of normal cells. Because NOXs function is the production of ROS, our observations support the hypothesis that oxidative stress is at least partly causative for radiation-induced DNA damage (2, 21, 29). We therefore propose a model suggesting that the endogenous ROS produced by NADPH oxidases may lead to accrued DNA damage during cell irradiation. In this model, chronic oxidative DNA lesions produced by NOXs-derived ROS and DNA lesions produced by radiation exposure would have a cumulative effect (Fig. 5). In our model, scavenging ROS with the fulvene-5 inhibitor or decreasing intracellular ROS by depleting the cellular NOX enzyme pool by siRNA would partially protect cells from radiation by lowering the interaction between radiation-induced DNA damage and ROS-induced DNA damage (for example, ROS-induced SSBs generated in close proximity to radiation-induced SSBs may generate additional DSBs). Because NOX4 is highly expressed in human fibroblasts (17), localized to the immediate environment of nucleus (30–33) and constitutively generates ROS (34), NOX4 isoform may be a main contributor in radiation-induced DNA damage in this particular cell type, although the protection exhibited by silencing either NOX4 or NOX5 was similar. Thus, the exact mechanisms of NOXs inhibition on the detrimental effects of radiation still require further investigation.
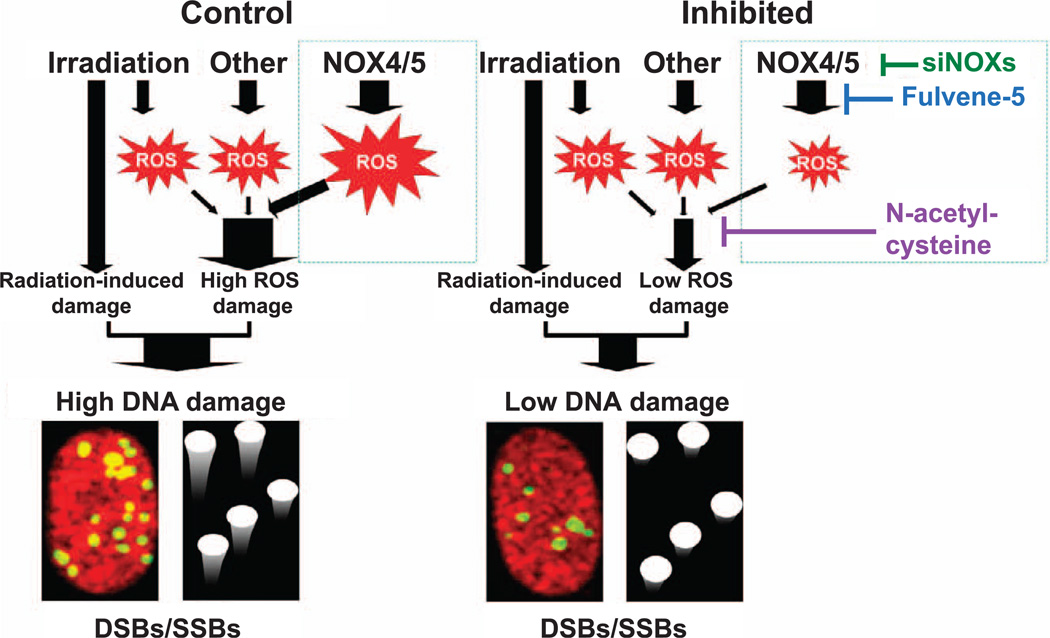
Figure 5.
Model for NADPH oxidase involvement in radiation-induced DNA damage. NOX4/5 produce reactive oxygen species (ROS), which can diffuse into nuclei and generate oxidative lesions in the DNA (ROS damage) resulting in damaged base and sugar residues as well as single- and double-strand breaks (SSBs and DSBs). ROS production can be partially reduced by downregulating NOX4/5 or inhibiting NOXs activity with fulvene-5. Once formed, ROS can also be neutralized with antioxidants such as NAC (compare the right panel with the left control panel). DNA damage from radiation exposure arises from both direct and indirect effects. Direct-effect damage occurs from ionization of the DNA while indirect-effect damage involves the production of hydroxyl radical, another type of ROS. Direct and indirect radiation-induced DNA lesions add to the DNA lesions induced by ROS of intracellular origins as detected by the comet assay and the γ-H2AX focus assay (high DNA damage).
In conclusion, our findings provide evidence that targeting NOXs with the use of NOX-specific drugs may help minimize the negative impacts of radiation. We believe that the development of such drugs, if effective, may benefit millions of patients who undergo diagnostic and therapeutic clinical procedures using ionizing radiation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Allergy and Infectious Diseases, Radiation/Nuclear Countermeasures Program and the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, National Institutes of Health.
Footnotes
The online version of this article (DOI: 10.1667/RR13799.1) contains supplementary information that is available to all authorized users.
Fig. S1. Inhibition of NOXs with fulvene-5 reduces radiation-induced DNA fragmentation. Human primary fibroblasts (GM03652) were transfected with small interference RNAs (siRNAs) against NOX4 or NOX5 or a scrambled control sequence 3 days prior to irradiation. Cells were irradiated with 0, 2 or 5 Gy and incubated at 37°C for 30 min before preparation for comet assay as described in Materials and Methods. Panel A: Representative images. Panel B: Quantitation. The comet tail of 50 cells for each experimental condition was quantified using CometScore software. Error bars indicate standard errors (n = 50) of a representative experiment. #Nonsignificant; **P < 0.001.
Fig. S2. Pharmacologic inhibition of NOXs in human BJ cells reduces radiation-induced DNA damage and cell death. Panels A and B: DMSO or fulvene-5 (10 µM) was added 1 h prior to 0, 2.5, 5 or 10 Gy γ radiation exposure. After a 24 h recovery period, cells were fixed and treated for γ-H2AX foci detection. Panel A: Representative images. Panel B: Quantitation. Panel C: Clonogenic survival of human BJ cells treated with DMSO or fulvene-5. Cells cultures were incubated with DMSO or 10 µM fulvene-5 for 1 h before irradiation with 0, 2, 4, 6 or 8 Gy. After a 24 h recovery period, the cultures were harvested, seeded at 500 cells per 35 mm dish, and incubated at 37°C for 10 days. Cells were stained with Coomassie blue and colonies counted. Percentage survival was calculated relative to the nonirradiated cell cultures. Error bars indicate standard deviations (n = 3). *P <0.05; **P < 0.001.
Fig. S3. Effect of fulvene-5 and the antioxidant N-acetylcysteine on cell survival. Human primary fibroblast (HPF) cultures were incubated with N-acetyl-cysteine (10 mM) alone, or with fulvene-5 (10 µM)/N-acetyl-cysteine (10 mM) for 1 h before 0, 2, 4, 6 or 8 Gy irradiation. After a 24 h recovery period, the cultures were harvested, seeded at 500 cells per 35 mm dish and incubated at 37°C for 10 days. Cells were stained with Coomassie blue and colonies counted. Panel A: Data for N-acetyl-cysteine alone. Panel B: Data for fulvene-5/N-acetyl-cysteine. Percentage survival was calculated relative to the nonirradiated cell cultures. Error bars indicate standard deviations (n=3). *P < 0.05; **P < 0.001.
Fig. S4. Silencing or inhibition of NOX4 and NOX5 with fulvene-5 reduces intracellular ROS levels 24 h postirradiation. Human primary fibroblasts (GM03652) were transfected with small interference RNAs (siRNAs) against NOX4 or NOX5 or a scrambled control sequence 2 days prior to irradiation. Cells were 0 or 10 Gy gamma irradiated and incubated at 37°C for 24 h before analysis of ROS levels by flow cytometry. For the drug treatment, cell cultures were incubated with 10 µM fulvene-5 for 1 h before ROS detection. For both conditions, cells were washed, trypsinized and resuspended in 500 µL HBSS medium containing 10 µM dihydroethidium (DHE). The HPF suspensions were incubated for 30 min at 37°C and analyzed by flow cytometry. Panels A and B show data for NOX4 and NOX5 silencing by siRNA and quantitation, respectively. Panels C and D show data for fulvene-5 and quantitation, respectively. *P < 0.05.
REFERENCES
- 1.Georgakilas AG, O’Neill P, Stewart RD. Induction and repair of clustered DNA lesions: what do we know so far? Radiat Res. 2013;180:100–109. doi: 10.1667/RR3041.1. [DOI] [PubMed] [Google Scholar]
- 2.Sedelnikova OA, Redon CE, Dickey JS, Nakamura AJ, Georgakilas AG, Bonner WM. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat Res. 2010;704:152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redon CE, Weyemi U, Parekh PR, Huang D, Burrell AS, Bonner WM. Gamma-H2AX and other histone post-translational modifications in the clinic. Acta Biochim Biophys. 2012;1819:743–756. doi: 10.1016/j.bbagrm.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nambiar S, Yeow JT. Polymer-composite materials for radiation protection. ACS Appl Mater Interfaces. 2012;4:5717–5726. doi: 10.1021/am300783d. [DOI] [PubMed] [Google Scholar]
- 5.Chambers CE, Fetterly KA, Holzer R, Lin PJ, Blankenship JC, Balter S, et al. Radiation safety program for the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2011;77:546–556. doi: 10.1002/ccd.22867. [DOI] [PubMed] [Google Scholar]
- 6.Bartelink H, Keus RB. Progress in radiotherapy by treatment tailoring in head and neck cancer. Curr Opin Oncol. 1991;3:523–528. doi: 10.1097/00001622-199106000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Kuefner MA, Brand M, Ehrlich J, Braga L, Uder M, Semelka RC. Effect of antioxidants on X-ray-induced gamma-H2AX foci in human blood lymphocytes: preliminary observations. Radiology. 2012;264:59–67. doi: 10.1148/radiol.12111730. [DOI] [PubMed] [Google Scholar]
- 8.Huang XJ, Song CX, Zhong CQ, Wang FS. Research progress in the radioprotective effect of superoxide dismutase. Drug Discov Ther. 2012;6:169–177. [PubMed] [Google Scholar]
- 9.Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010;15:360–371. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neill P, Wardman P. Radiation chemistry comes before radiation biology. Int J Radiat Biol. 2009;85:9–25. doi: 10.1080/09553000802640401. [DOI] [PubMed] [Google Scholar]
- 11.Weyemi U, Dupuy C. The emerging role of ROS-generating NADPH oxidase NOX4 in DNA-damage responses. Mutat Res. 2012;751:77–81. doi: 10.1016/j.mrrev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Weyemi U, Redon CE, Parekh PR, Dupuy C, Bonner WM. NADPH Oxidases NOXs and DUOXs as putative targets for cancer therapy. Anticancer Agents Med Chem. 2013;13:502–514. [PMC free article] [PubMed] [Google Scholar]
- 13.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48:348–356. doi: 10.1016/j.freeradbiomed.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weyemi U, Caillou B, Talbot M, Ameziane-El-Hassani R, Lacroix L, Lagent-Chevallier O, et al. Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr Relat Cancer. 2010;17:27–37. doi: 10.1677/ERC-09-0175. [DOI] [PubMed] [Google Scholar]
- 16.Redon CE, Nakamura AJ, Sordet O, Dickey JS, Gouliaeva K, Tabb B, et al. gamma-H2AX detection in peripheral blood lymphocytes, splenocytes, bone marrow, xenografts, and skin. Meth Mol Biol. 2011;682:249–270. doi: 10.1007/978-1-60327-409-8_18. [DOI] [PubMed] [Google Scholar]
- 17.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290(Pt 1):41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, et al. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Investig. 2009;119:2359–2365. doi: 10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reliene R, Pollard JM, Sobol Z, Trouiller B, Gatti RA, Schiestl RH. N-acetyl cysteine protects against ionizing radiation-induced DNA damage but not against cell killing in yeast and mammals. Mutat Res. 2009;665:37–43. doi: 10.1016/j.mrfmmm.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Collins-Underwood JR, Zhao W, Sharpe JG, Robbins ME. NADPH oxidase mediates radiation-induced oxidative stress in rat brain microvascular endothelial cells. Free Radic Biol Med. 2008;45:929–938. doi: 10.1016/j.freeradbiomed.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S, Ahn JY, Lim MJ, Kim MH, Yun YS, Jeong G, et al. Sustained expression of NADPH oxidase 4 by p38 MAPK-Akt signaling potentiates radiation-induced differentiation of lung fibroblasts. J Mol Med (Berl) 2010;88:807–816. doi: 10.1007/s00109-010-0622-5. [DOI] [PubMed] [Google Scholar]
- 23.Meitzler JL, Antony S, Wu Y, Juhasz A, Liu H, Jiang G, et al. NADPH Oxidases: A perspective on reactive oxygen species production in tumor biology. Antioxid Redox Signal. 2014;20:2873–2889. doi: 10.1089/ars.2013.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Zhai Z, Wang Y, Zhang J, Wu H, Li C, et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med. 2013;54:40–50. doi: 10.1016/j.freeradbiomed.2012.10.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azzam EI, De Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 26.Pazhanisamy SK, Li H, Wang Y, Batinic-Haberle I, Zhou D. NADPH oxidase inhibition attenuates total body irradiationinduced haematopoietic genomic instability. Mutagenesis. 2011;26:431–435. doi: 10.1093/mutage/ger001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiera F, Meccia E, Degan P, Aquilina G, Pietraforte D, Minetti M, et al. Overexpression of human NOX1 complex induces genome instability in mammalian cells. Free Radic Biol Med. 2008;44:332–342. doi: 10.1016/j.freeradbiomed.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Tateishi Y, Sasabe E, Ueta E, Yamamoto T. Ionizing irradiation induces apoptotic damage of salivary gland acinar cells via NADPH oxidase 1-dependent superoxide generation. Biochem Biophys Res Commun. 2008;366:301–307. doi: 10.1016/j.bbrc.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 29.Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997;57:3963–3971. [PubMed] [Google Scholar]
- 30.Matsushima S, Kuroda J, Ago T, Zhai P, Park JY, Xie LH, et al. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ Res. 2013;112:651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weyemi U, Lagente-Chevallier O, Boufraqech M, Prenois F, Courtin F, Caillou B, et al. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene. 2012;31:1117–1129. doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer NY, Yan Z, Boudreau RL, Zhang Y, Luo M, Li Q, et al. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. J Biol Chem. 2011;286:8977–8987. doi: 10.1074/jbc.M110.193821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CF, Qiao M, Schroder K, Zhao Q, Asmis R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ Res. 2010;106:1489–1497. doi: 10.1161/CIRCRESAHA.109.215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.