Abstract
A1 adenosine receptor (A1AR) activation within the central nervous system induces torpor, but in obligate hibernators such as the arctic ground squirrel (AGS; Urocitellus parryii), A1AR stimulation induces torpor only during the hibernation season, suggesting a seasonal increase in sensitivity to A1AR signaling. The purpose of this research was to investigate the relationship between body temperature (Tb) and sensitivity to an adenosine A1 receptor agonist in AGS. We tested the hypothesis that increased sensitivity in A1AR signaling would lead to lower Tb in euthermic animals during the hibernation season when compared with the summer season. We further predicted that if a decrease in euthermic Tb reflects increased sensitivity to A1AR activation, then it should likewise predict spontaneous torpor. We used subcutaneous IPTT-300 transponders to monitor Tb in AGS housed under constant ambient conditions (12:12 L:D, 18 °C) for up to 16 months. These animals displayed an obvious rhythm in euthermic Tb that cycled with a period of approximately 8 months. Synchrony in the Tb rhythm within the group was lost after several months of constant L:D conditions; however, individual rhythms in Tb continued to show clear sine wave–like waxing and waning. AGS displayed spontaneous torpor only during troughs in euthermic Tb. To assess sensitivity to A1AR activation, AGS were administered the A1AR agonist N6-cyclohexyladenosine (CHA, 0.1 mg/kg, ip), and subcutaneous Tb was monitored. AGS administered CHA during a seasonal minimum in euthermic Tb showed a greater drug-induced decrease in Tb (1.6 ± 0.3 °C) than did AGS administered CHA during a peak in euthermic Tb (0.4 ± 0.3 °C). These results provide evidence for a circannual rhythm in Tb that is associated with increased sensitivity to A1AR signaling and correlates with the onset of torpor.
Keywords: hibernation, ground squirrel, thermoregulation, seasonal rhythm, metabolic suppression, hypothermia
Hibernation is an adaptation of energy conservation that in the arctic ground squirrel (AGS, Urocitellus parryii) is expressed according to a robust circannual rhythm (Sheriff et al., 2011). We and others have found that stimulation of A1 adenosine receptors (A1AR) within the central nervous system induces torpor onset (Tamura et al., 2005; Jinka et al., 2011; Iliff and Swoap, 2012). We also found that in AGS, A1AR activation induces torpor only during the hibernation season. This finding suggests that a seasonal increase in sensitivity to A1AR signaling underlies the transition into the hibernation phenotype (Jinka et al., 2011). Because stimulation of A1AR decreases body temperature (Tb) in nonhibernating species (Anderson et al., 1994), we hypothesized that a seasonal increase in sensitivity to A1AR would lead to lower Tb in nontorpid animals. A decrease in euthermic Tb precedes onset of torpor in free-ranging AGS (Sheriff et al., 2012), and torpor expression is preceded by a decrease in euthermic Tb and metabolic rate in other hibernating species (Arai et al., 2005; Levesque and Tattersall, 2010). Tonic A1AR activation maintains Tb (Barros et al., 2006), and in rats fed a restricted diet a lower Tb predicts increased sensitivity to an A1AR agonist (Jinka et al., 2010). Thus, Tb in euthermic AGS at rest may reflect sensitivity in A1AR signaling and be functionally linked to seasonal expression of the hibernation phenotype.
Here we test the hypotheses that euthermic Tb in AGS decreases during the hibernation season and that a decrease in daily euthermic Tb reflects increased sensitivity to A1AR activation and predicts torpor onset. We monitored Tb and spontaneous torpor in AGS housed at an ambient temperature of 18 °C throughout an 18-month period. Animals displayed an apparent circannual rhythm in nontorpid (euthermic) Tb where the hibernation season, indicated by spontaneous torpor in AGS housed, was noted by troughs in euthermic Tb. We tested A1AR sensitivity by administering an A1AR agonist during the highs and lows in euthermic Tb and found that animals in a Tb trough displayed greater A1AR agonist sensitivity. We also monitored food intake.
METHODS
Body Temperature and Food Intake Monitoring
Procedures were approved by the UAF Institutional Animal Care and Use Committee. Arctic ground squirrels (AGS; Urocitellus parryii) were captured near 66°38′N, 149°38′W under permit from the Alaska Department of Fish and Game. AGS were housed individually at an ambient temperature of 18 °C on a 12:12 L:D cycle and fed rodent chow ad libitum. To monitor Tb, IPTT-300 transponders (Bio Medic Data Systems, Inc., Seaford DE; calibrated at 30 °C with an accuracy of ±0.5 °C between 24 and 40 °C) were inserted between the scapulae. Others have shown these transponders to provide reliable data (±0.6 °C) over subcutaneous temperature of 21.4 to 36.9 °C in small mammals that do not vary with ambient temperature and indicate when animals are torpid (Wacker et al., 2012).
Subcutaneous Tb was measured between 1030 and 1130 h daily in a total of 24 AGS (12 male and 12 female); 16 AGS were monitored for 16 months from 24 September 2008 until 1 February 2010, and 8 AGS were monitored for 9 to 13 months beginning on 24 September 2008. Animals were noted to be torpid if they were unresponsive to touch and displayed a Tb less than 30 °C. Daily mass of food intake was measured from 2 June 2009 to 18 December 2009 in 16 of the 24 AGS.
Experimental Design
Seasonal changes in Tb and food intake were monitored as described above. To determine whether seasonal lows in euthermic Tb predicted increased sensitivity to Tb-lowering effects of A1AR activation, the A1AR agonist N6-cyclohexyladenosine (CHA) or vehicle (phosphate buffered saline [PBS]) was administered to 22 AGS on 2 separate occasions.
AGS were handled and weighed the day before treatment. This handling induced arousal if animals were torpid. CHA was dissolved in PBS and filtered through 0.22-μm syringe filters into a sterile BD vacutainer. Baseline Tb was collected for 1 h before the drug was administered. CHA (0.1 mg/kg, ip) or vehicle (PBS) was injected using 25-gauge, 1.5-inch needles. Tb was recorded every 30 min for 4 h following the injection and again at 24 h post injection.
Data Analysis
Seasonal fluctuation in Tb and food intake was assessed as present or absent from graphs of individual animals. In cases where a rhythm was evident during the 6-month observation period in both Tb and food intake, the temporal relationship between the waxing and waning of Tb and that of food intake varied. In some animals Tb decreased before food intake, and in others Tb and food intake decreased at approximately the same time. No animals showed a clear decrease in food intake before a decrease in Tb. We compared the frequency of animals that decreased Tb before or at approximately the same time as food intake to an expected random (50-50) distribution using a chi-square goodness-of-fit test.
Each individual animal's euthermic Tb cycle was assessed and the position assigned as a trough, a peak, or an intermediate position by a person unaware of the drug response. A 2-tailed t test was used to compare drug-induced change in Tb between AGS tested during a trough and those animals tested during a peak in Tb. Regression analysis was performed to assess the relationship between euthermic Tb and magnitude of drug response in all animals tested (MSExcel). Significance was set at p < 0.05. Data shown are mean ± SEM.
RESULTS
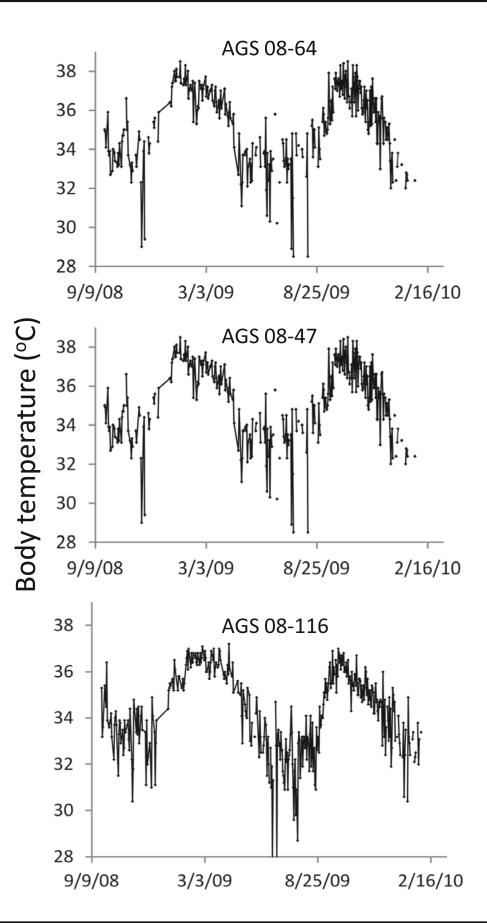
All 24 animals showed a waxing and waning in euthermic Tb that ranged between 30 and 40 °C and fluctuated with a circannual period between 7 and 9 months. Group synchrony was noted in the rhythm during the first 8 months but was lost in later months. Tb rhythms for individual animals persisted under constant L:D conditions (Fig. 1). Torpor, illustrated by a precipitous drop in Tb, was observed only during troughs in euthermic Tb (Fig. 1).
Figure 1.
Spontaneous torpor, noted by a large drop in Tb, only occurs during a trough in the euthermic Tb cycle. Representative graphs show daily Tb in 3 AGS from september 2008 to February 2010. Graph titles indicate animal ID number.
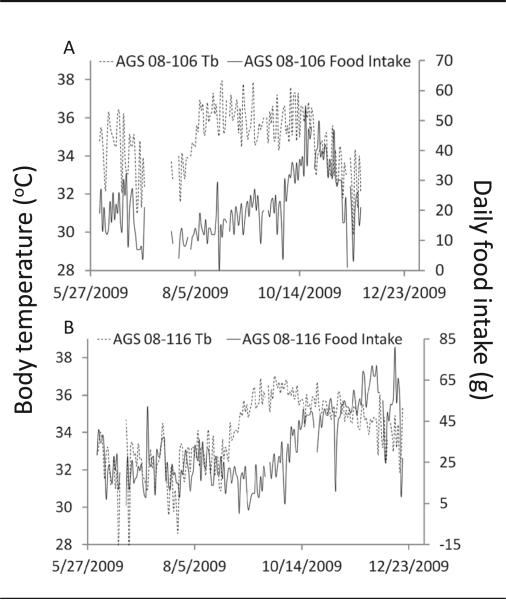
To test the hypothesis that a decrease in food intake produced the decrease in Tb, as described for rats fed a restricted diet (Jinka et al., 2010), we compared the temporal patterns of food intake and Tb. Food intake varied between 0 and 80 g/day, including days when animals were torpid. None of the AGS showed a decrease in food intake that preceded a decrease in Tb. Fourteen of the 16 AGS showed a clear waxing and waning in food intake and Tb during the 6-month monitoring period. In 6 of these 14 AGS, Tb began to decrease before food intake (Fig. 2B), and in 7 of these 14 AGS, Tb and food intake decreased at about the same time (Fig. 2A). The distribution of occasions when food intake decreased (1) before Tb decreased, or (2) after or at the same time as Tb decreased, was different from an expected 50:50 distribution χ2(1,14) = 0.0002].
Figure 2.
Representative examples illustrate Tb and food intake decreasing at approximately the same time (A) and Tb decreasing prior to food intake. (B). No animals showed a decrease in food intake prior to a decrease in Tb.
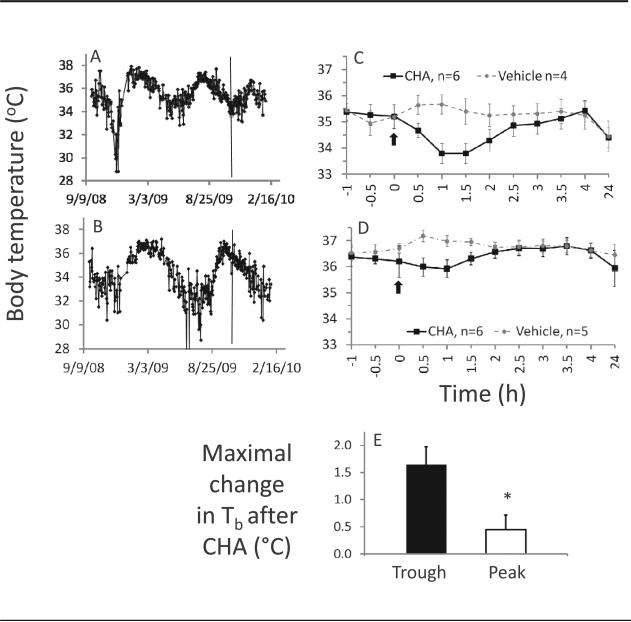
AGS tested with the A1AR agonist CHA during their trough in euthermic Tb showed a greater decrease in Tb (1.6 ± 0.3 °C) than AGS tested during their peak in euthermic Tb (0.4 ± 0.3 °C; t test; p < 0.05 trough vs. peak, n = 6 CHA tests during a trough and 6 tests during a peak in a total of 8 AGS; 4 males and 4 females). The magnitude of CHA-induced decline in euthermic Tb was not predicted by predrug Tb. Regression analysis of euthermic Tb and CHA-induced change in Tb in all animals tested with CHA (n = 22) was not statistically significant. By contrast, the position within an individual animal's rhythm in Tb predicted response to CHA (Fig. 3). Vehicle had no effect on euthermic Tb when administered during a trough or a peak (n = 4 vehicle tests during a trough and 5 tests during a peak in a total of 6 AGS, 3 males and 3 females).
Figure 3.
The drug-induced change in Tb depends on the position of AGS in their circannual Tb cycle. Timing of drug administration indicated by vertical lines was identified as a trough (A) or a peak (B). C and D show CHA (0.1 mg/kg)–induced effects on Tb for AGS tested in a Tb trough (C) or a peak (D); arrows indicate time of drug administration. The drug-induced change in Tb was greater in animals tested during a trough than in animals tested during a peak (E). Data shown are mean ± SEM. *p < 0.05, n indicates the number of tests in a Tb peak or in a Tb trough.
DISCUSSION
Hibernation is a robust seasonal phenomenon, but mechanisms that regulate the transition into the hibernation phenotype displayed during the hibernation season are poorly understood. A1AR activation within the central nervous system is necessary and sufficient to induce torpor in AGS and other species (Tamura et al., 2005; Iliff and Swoap, 2012). However, in AGS, stimulating A1AR induces torpor only during the hibernation season, indicating that a higher order of regulation controls the ability of adenosine to induce torpor (Jinka et al., 2011). Our results support the hypothesis that this higher order of regulation involves increased sensitivity to A1AR-induced cooling. We show that a seasonal minimum in euthermic Tb reflects increased sensitivity to A1AR-induced cooling that waxes and wanes between the summer and the hibernation seasons. This seasonal rhythm in euthermic Tb predicts and is fundamental to the expression of spontaneous torpor.
Field data collected using data loggers implanted in the abdominal cavity in the fall and retrieved in the spring show a decrease in core Tb to 34 to 35 °C beginning 45 days prior to entrance into the first torpor bout. Body temperature during interbout arousals does not exceed this lowered basal temperature (Sheriff et al., 2012). A decrease in euthermic Tb precedes onset of torpor in other hibernating species as well (Arai et al., 2005; Levesque and Tattersall, 2010). Surprisingly, predrug body temperature in CHA-treated AGS in summer and winter was similar and did not predict CHA-induced torpor. This may be because baseline Tb prior to CHA administration did not accurately reflect Tb of animals at rest. Although animals were administered CHA at the same time each day, handling and movement from the home cage to the test cage likely resulted in an increase in activity and predrug Tb.
A seasonal increase in sensitivity to A1AR signaling may underlie seasonal rhythms in sleep drive as well as Tb. The seasonal decrease in euthermic Tb reported here coincides with a seasonal increase in sleep duration observed in ground squirrels housed under conditions similar to the present study (Walker et al., 1980). Hibernation is an extension of sleep (Walker et al., 1977, 1979), where homeostatic sleep drive is mediated in part through the activation of A1AR (Benington et al., 1995; Porkka-Heiskanen et al., 1997). Golden mantled ground squirrels sleep more during the hibernation season (Walker et al., 1980), and sleep is associated with lower euthermic Tb (Berger and Phillips, 1988). Indeed, the lower euthermic Tb noted in the present study could be due to the animals sleeping.
The gradual transition into the hibernation season is consistent with a gradual decrease in the irritability of hibernating animals as the hibernation season progresses and a gradual increase in irritability as spring approaches (Twente and Twente, 1968). Waxing and waning in the sensitivity to A1AR signaling were expected based on the observed gradual increase in the effectiveness of CHA to induce torpor in the midhibernation season compared with the early hibernation season (Jinka et al., 2011).
Although it was surprising to see the period in Tb rhythm decrease under constant L:D conditions, the results are consistent with shortening of circannual rhythm period in spontaneous torpor that we have noted previously (Jinka et al., 2011). Constant daylight is known to phase advance the circannual cycle of obligate hibernators (Pengelley et al., 1976). In the present study, group synchrony in euthermic Tb rhythm subsided while individual euthermic Tb began to free-run on a 7- to 9-month cycle. The rhythm was clearly associated with the hibernation season since animals displayed torpor only during troughs in Tb. These observations are consistent with other observations of circannual rhythms in captive ground squirrels housed under constant L:D conditions where the circannual cycle persists but begins to free-run, that is, oscillate on a cycle slightly shorter than 12 months (Lee and Zucker, 1991; Hiebert et al., 2000). Surprisingly, absolute euthermic Tb did not predict the magnitude of response to CHA except in animals expressing a maximum or minimum in euthermic Tb. If altered sensitivity in A1AR signaling leads to a gradual decrease in euthermic Tb and seasonal torpor, we would expect to see a correlation between Tb and the magnitude of response to CHA. The lack of a statistically significant correlation in euthermic Tb and response to CHA could be due to the relatively small sample size studied and other factors that influence Tb.
Euthermic Tb is lower during interbout arousal than during summer euthermy in free-living as well as captive AGS (Barnes, 1989; Karpovich et al., 2009; Sheriff et al., 2012). However, the gradual waxing and waning in Tb are not evident in AGS housed under natural conditions (Barnes, 1989). Rather, Tb during interbout arousal remains at about 35 °C throughout the hibernation season (Karpovich et al., 2009). The range of euthermic Tb in this study was greater than the range of euthermic Tb reported in free-living AGS during the summer season (Williams et al., 2012b). Although the Tb highs near 40 °C are similar to field data, lows near 30 °C are lower than Tb of summer, free-living AGS. We expect that in free-living animals, ambient temperatures near 0 °C promote onset of torpor so that circannual fluctuation in euthermic Tb is masked by torpid Tb.
Daily Tb readings did not allow for analysis of circadian variation in Tb, so it remains unclear whether circannual rhythm in euthermic Tb reflects a vertical shift in the entire circadian Tb curve and thus a decrease in maximum and minimum Tb or a change in the amplitude of the Tb rhythm. Daily Tb readings also cannot rule out the possibility that a circannual influence on the period of the circadian Tb rhythm explains the seasonal fluctuation in daily measures of Tb. In free-living AGS, body temperature rhythms persist during the summer season but are not detected during hibernation at Tb near 0 °C (Williams et al., 2012a). Diurnal Tb rhythms in AGS entrain to approximately 24-h periods in fall and spring (Williams et al., 2012b) and a period of 24-h persists throughout the summer season (Williams et al., 2012a). Since it is not clear what AGS entrain to under the midnight sun and since AGS in the present study were housed at constant 12:12 L:D and ambient temperature, it is difficult to extrapolate between these observations and observations made in free-living AGS.
A decrease in Tb was found to precede or decline at the same time as the decrease in food intake. These results do not support our original hypothesis that voluntary dietary restriction prior to onset of torpor increases the response to CHA. Instead, they suggest that lower Tb may reflect a seasonal decrease in thermogenesis and thus metabolic rate that leads to, or is associated with, a decrease in food intake. Thus, it appears that the mechanism regulating seasonal fluctuation in Tb in AGS differs from the mechanism that sensitizes A1AR during dietary restriction in rats (Jinka et al., 2010).
In conclusion, the present results suggest that an increase in sensitivity of A1AR signaling is correlated with a decrease in euthermic Tb and predicts torpor onset. This finding is significant because a seasonal rhythm in A1AR signaling represents a molecular mechanism that, in part, defines the hibernation season. More work will be required to determine the molecular basis of the seasonal fluctuation in A1AR signaling to determine where in the brain changes occur, whether changes occur in extracellular levels of adenosine and/or at the receptor level, and what processes underlie these changes. Whether the decrease in euthermic Tb reflects an increase in sleep remains to be determined, but both sleep and Tb likely result from increased sensitivity in A1AR signaling. The ability of a decrease in euthermic Tb to predict the onset of the hibernation season is also significant because secondary influences of torpor confound interpretation of seasonal differences in gene and protein expression.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Neurological Disorders and Stroke NS041069-06 and R15NS070779 and Flint Hills Undergraduate Research Grant. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Anderson R, Sheehan MJ, Strong P. Characterization of the adenosine receptors mediating hypothermia in the conscious mouse. Br J Pharmacol. 1994;113:1386–1390. doi: 10.1111/j.1476-5381.1994.tb17151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Hanaya T, Sakurai T, Ikeda M, Kurimoto M. A novel phenomenon predicting the entry into a state of hibernation in Syrian hamsters (Mesocricetus auratus). J Vet Med Sci. 2005;67:215–217. doi: 10.1292/jvms.67.215. [DOI] [PubMed] [Google Scholar]
- Barnes BM. Freeze avoidance in a mammal: body temperatures below 0 degree C in an Arctic hibernator. Science. 1989;244:1593–1595. doi: 10.1126/science.2740905. [DOI] [PubMed] [Google Scholar]
- Barros RC, Branco LG, Carnio EC. Respiratory and body temperature modulation by adenosine A1 receptors in the anteroventral preoptic region during normoxia and hypoxia. Respir Physiol Neurobiol. 2006;153:115–125. doi: 10.1016/j.resp.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692:79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- Berger RJ, Phillips NH. Comparative aspects of energy metabolism, body temperature and sleep. Acta Physiol Scand Suppl. 1988;574:21–27. [PubMed] [Google Scholar]
- Hiebert SM, Thomas EM, Lee TM, Pelz KM, Yellon SM, Zucker I. Photic entrainment of circannual rhythms in golden-mantled ground squirrels: role of the pineal gland. J Biol Rhythms. 2000;15:126–134. doi: 10.1177/074873040001500207. [DOI] [PubMed] [Google Scholar]
- Iliff BW, Swoap SJ. Central adenosine receptor signaling is necessary for daily torpor in mice. Am J Physiol Regul Integr Comp Physiol. 2012;303:R477–484. doi: 10.1152/ajpregu.00081.2012. [DOI] [PubMed] [Google Scholar]
- Jinka TR, Carlson ZA, Moore JT, Drew KL. Altered thermoregulation via sensitization of A1 adenosine receptors in dietary restricted rats. Psychopharmacology (Berl) 2010;209:217–224. doi: 10.1007/s00213-010-1778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinka TR, Toien O, Drew KL. Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors. J Neurosci. 2011;31:10752–10758. doi: 10.1523/JNEUROSCI.1240-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpovich SA, Toien O, Buck CL, Barnes BM. Energetics of arousal episodes in hibernating arctic ground squirrels. J Comp Physiol B. 2009;179:691–700. doi: 10.1007/s00360-009-0350-8. [DOI] [PubMed] [Google Scholar]
- Lee TM, Zucker I. Suprachiasmatic nucleus and photic entrainment of circannual rhythms in ground squirrels. J Biol Rhythms. 1991;6:315–330. doi: 10.1177/074873049100600403. [DOI] [PubMed] [Google Scholar]
- Levesque DL, Tattersall GJ. Seasonal torpor and normothermic energy metabolism in the Eastern chipmunk (Tamias striatus). J Comp Physiol B. 2010;180:279–292. doi: 10.1007/s00360-009-0405-x. [DOI] [PubMed] [Google Scholar]
- Pengelley ET, Asmundson SJ, Barnes B, Aloia RC. Relationship of light intensity and photoperiod to circannual rhythmicity in the hibernating ground squirrel, Citellus lateralis. Comp Biochem Physiol A. 1976;53:273–277. doi: 10.1016/s0300-9629(76)80035-7. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff MJ, Kenagy GJ, Richter M, Lee T, Toien O, Kohl F, Buck CL, Barnes BM. Phenological variation in annual timing of hibernation and breeding in nearby populations of Arctic ground squirrels. Proc Biol Sci. 2011;278:2369–2375. doi: 10.1098/rspb.2010.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff MJ, Williams CT, Kenagy GJ, Buck CL, Barnes BM. Thermoregulatory changes anticipate hibernation onset by 45 days: data from free-living arctic ground squirrels. J Comp Physiol B. 2012:841–847. doi: 10.1007/s00360-012-0661-z. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Shintani M, Nakamura A, Monden M, Shiomi H. Phase-specific central regulatory systems of hibernation in Syrian hamsters. Brain Res. 2005;1045:88–96. doi: 10.1016/j.brainres.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Twente JW, Twente JA. Progressive irritability of hibernating Citellus lateralis. Comp Biochem Physiol. 1968;25:467–474. doi: 10.1016/0010-406x(68)90355-1. [DOI] [PubMed] [Google Scholar]
- Wacker CB, Rojas AD, Geiser F. The use of small subcutaneous transponders for quantifying thermal biology and torpor in small mammals. J Therm Biol. 2012;37:250–254. [Google Scholar]
- Walker JM, Garber A, Berger RJ, Heller HC. Sleep and estivation (shallow torpor): continuous processes of energy conservation. Science. 1979;204:1098–1100. doi: 10.1126/science.221974. [DOI] [PubMed] [Google Scholar]
- Walker JM, Glotzbach SF, Berger RJ, Heller HC. Sleep and hibernation in ground squirrels (Citellus spp): electrophysiological observations. Am J Physiol. 1977;233:R213–221. doi: 10.1152/ajpregu.1977.233.5.R213. [DOI] [PubMed] [Google Scholar]
- Walker JM, Haskell EH, Berger RJ, Heller HC. Hibernation and circannual rhythms of sleep. Physiol Zool. 1980;53:8–11. [Google Scholar]
- Williams CT, Barnes BM, Buck CL. Daily body temperature rhythms persist under the midnight sun but are absent during hibernation in free-living arctic ground squirrels. Biol Lett. 2012a;8:31–34. doi: 10.1098/rsbl.2011.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CT, Barnes BM, Richter M, Buck CL. Hibernation and circadian rhythms of body temperature in free-living Arctic ground squirrels. Physiol Biochem Zool. 2012b;85:397–404. doi: 10.1086/666509. [DOI] [PubMed] [Google Scholar]