Abstract
Five-year survival from childhood acute lymphoblastic leukemia (ALL) approaches 90%, but 40% of survivors experience central nervous system (CNS) treatment-related cognitive problems. Despite considerable evidence for cognitive problems, less is known about mechanisms of neurologic injury. Our purpose was to investigate oxidative stress, measured by lipid peroxidation, as a mechanism of CNS treatment-related neurologic injury. The sample included 55 children (mean age at diagnosis = 6.84 years, SD = 3.40) who received intrathecal and intravenous chemotherapy for CNS-directed treatment according to Children’s Oncology Group protocols. Glycerophospholipids were extracted from cerebrospinal fluid samples (CSF) obtained at diagnosis and during intrathecal chemotherapy administration. Unoxidized and oxidized phosphatidylcholine (PC) and phosphatidylinositol (PI) were measured by normal phase high performance liquid chromatography with diode array detection, and analyzed with a general linear model for repeated measures analysis of variance. Compared to the diagnostic CSF sample, unoxidized and oxidized PC and PI increased significantly across treatment phases. Amount of intravenous methotrexate received was significantly correlated with oxidized PI, and age at time of ALL diagnosis was significantly associated with oxidized PC. These findings support for our hypothesis that oxidative stress is a mechanism of neurologic injury associated with CNS-directed treatment for ALL.
Keywords: oxidative stress, lipid peroxidation, CNS treatment-related toxicity
Introduction
In the United States, acute lymphoblastic leukemia (ALL) is the most prevalent cancer among children and adolescents less than 15 years of age, and disease-free survival is approximately 90%.1 Central nervous system (CNS)-directed treatment is essential for survival because circulating lymphoblasts cross the blood brain barrier and metastasize into brain parenchyma.2, 3 CNS-directed treatment with intrathecal (IT) and escalating or high doses of intravenous (IV) methotrexate is most intense during the first year of therapy (referred to as post-induction), but continues over the 2 ½ to 3 years of ALL treatment. Whole brain radiation was initially associated with cognitive problems and neuroanatomical changes including changes in cortical thickness, decreased hippocampal volume, and loss of white matter integrity4–10. However, more recent studies show that CNS-directed chemotherapy is also linked to diminished cognitive abilities and neuroanatomical changes10–24. In fact, up to 40% of ALL survivors experience cognitive problems in memory, attention, executive function, processing speed, visual-motor and visual-spatial skills.7, 10, 16, 17, 20, 22–27 Despite collective evidence for cognitive problems, far less is known about mechanisms of chemotherapy-induced neurologic injury.
Methotrexate decreases the effectiveness of antioxidant defense systems resulting in an increase in oxidative stress,28, 29 and methotrexate-induced oxidative stress has been observed in tumor tissue, cultured HeLa cells, rat brain, and children with ALL.28–33 Oxidative stress refers to cellular injury and degeneration caused by reactive oxygen species (ROS). ROS include oxygen ions, free radicals, and peroxides that are highly reactive due to the presence of an unpaired electron on an oxygen molecule.34 Compared to other organs, the brain is particularly vulnerable to oxidative stress due to lower antioxidant capacity, greater energy requirements, and a higher lipid concentration.35 Glycerophospholipids are a class of lipids critically important for the integrity of cellular membranes, and lipid peroxidation is a well-established consequence of oxidative stress.
Oxidative stress-induced lipid peroxidation results in deterioration of the membrane permeability barrier,36 loss of membrane functions including neurotransmitter-receptor interactions,37–39 and induction of apoptosis36. Oxidized lipids are markers of and pathologic mechanisms in a variety of disease states including atherosclerosis38, 40, Alzheimer’s disease39, 41, diabetes, chronic renal failure, and retinal degenerative diseases.42, 43 Increased plasma levels of lipid peroxidation have also been observed in children with autism,37, 44 and traumatic brain injury.45
We measured peroxidation of two glycerophospholipids (phosphatidylcholine [PC], and phosphatidylinositol [PI]) in cerebrospinal fluid (CSF) samples obtained during diagnostic and therapeutic lumbar punctures throughout the course of ALL therapy. We also determined if levels of oxidized PC and PI were associated with amount of methotrexate received, age at the time of ALL diagnosis, and gender. PC is the most prevalent phospholipid in brain cellular membranes, and oxidized PC has been observed in neuroinflammatory conditions.46 Less is known about the biological effects of oxidized PI, however PI directly binds to many cytoskeletal proteins and is a precursor for three second messengers (inositol 1,4,5-triphosphate, diacylglycerol, and phosphatidylinositol 3,4,5-triphosphate) involved in cell signaling pathways.47, 48 Further, there is evidence for the role of phosphorylated derivatives of PI in neurotransmission, 49 and for oxidized PI in brain degeneration associated with Alzheimer’s disease.39, 50 We hypothesized that the levels of unoxidized and oxidized PC and PI would increase during CNS-directed treatment.
Materials and Methods
Patients were recruited from two cancer centers in the southwestern United States. Eligibility requirements included children with a recent diagnosis of pre-B or pre-T cell acute lymphoblastic leukemia (ALL) and being treated on a Children’s Oncology Group (COG) protocol for low, standard, high, or very high risk disease. The project was approved by the respective Human Subjects Protection Committee, and consent was obtained from parents and assent from children 7 years of age or older at the time of ALL diagnosis.
A within subject repeated measures design was used to investigate chemotherapy-induced oxidative stress in the CNS. Potential participants with a prior history of other causes of neurologic injury (i.e. seizures, traumatic brain injury, or developmental disabilities such as Down Syndrome or attention deficit disorder) were excluded. Children who experienced a CNS or bone marrow relapse, or who were treated with cranial radiation or a bone marrow transplant were also excluded.
Subjects received ALL treatment according to Children’s Oncology Group (COG) protocols that are based on leukemia risk category. Therapy occurred in three phases: induction, post-induction, and continuation. CNS prophylaxis, most intense during post-induction, was administered throughout therapy with standardized doses of IT methotrexate based on age: 10 mg for children aged 2-2.99 years, 12 mg for children aged 3–8.99 years, and 15 mg for children aged ≥ 9 years. The first phase, induction (29 days in duration) included weekly treatment with systemic chemotherapy, oral corticosteroids, one IT administration of cytarabine, and two IT methotrexate treatments (Days 8 and 29). The second phase, post-induction, included several courses of escalating or high dose IV methotrexate, other drugs depending on the COG protocol (e.g. vincristine, PEG-asparaginase, daunorubicin, mercaptopurine, cyclophosphamide, cytarabine, thioguanine, doxorubicin, and corticosteroids), and IT methotrexate. This phase of therapy was the most intensive and continued over 6–8 months. The last phase of therapy, continuation (2–3 years), included oral chemotherapy (methotrexate and mercaptopurine) and corticosteroids (prednisone or dexamethasone), cycles of IV chemotherapy (methotrexate and vincristine), and IT methotrexate every 12 weeks. The mean number of IT MTX treatments was 7.96 (range = 5–14; SD= 2.4) during post-induction, and 6.4 (range = 2–11, SD = 2.31) during continuation.
Two mls of CSF were collected during diagnostic and therapeutic lumbar punctures. CSF samples were placed on ice immediately after collection, centrifuged for 10 min at 1750×g to remove any cellular debris, and stored at −80° C. Glycerophospholipids were extracted from CSF samples using a modified method developed by Folch and others51, and separated by high performance liquid chromatography (HPLC). Lipids were extracted from the aqueous CSF with a chloroform:methanol extraction procedure, vortexed for 1 minute and centrifuged at 10,000 rpm for 20 minutes. The organic phase was collected and stored on ice. A second extraction was done in order to insure complete recovery of the less polar phospholipids. The organic fractions were combined, evaporated to dryness under nitrogen, and resuspended in hexane-isopropanol. This method recovers 98% of glycerophospholipids.51–53 Unoxidized porcine brain PC and PI bovine liver standards and CSF extracts were then separated by normal phase HPLC using a Model 126 Solvent System and 168 Diode Array Detector.
HPLC is the method of choice for the separation and analysis of a wide variety of compounds, including glycerophospholipids. Separation is accomplished by injecting the compound of interest into a moving stream of liquid (mobile phase) that passes through a stationary phase column. Separation of a compound into individual components and their retention time on the column, depends on reaction with the mobile and stationary phases. In the resulting chromatogram, the area under a peak [peak area count] is a measure of the concentration of the compound it represents. This area value is integrated and calculated automatically by the computer data station.
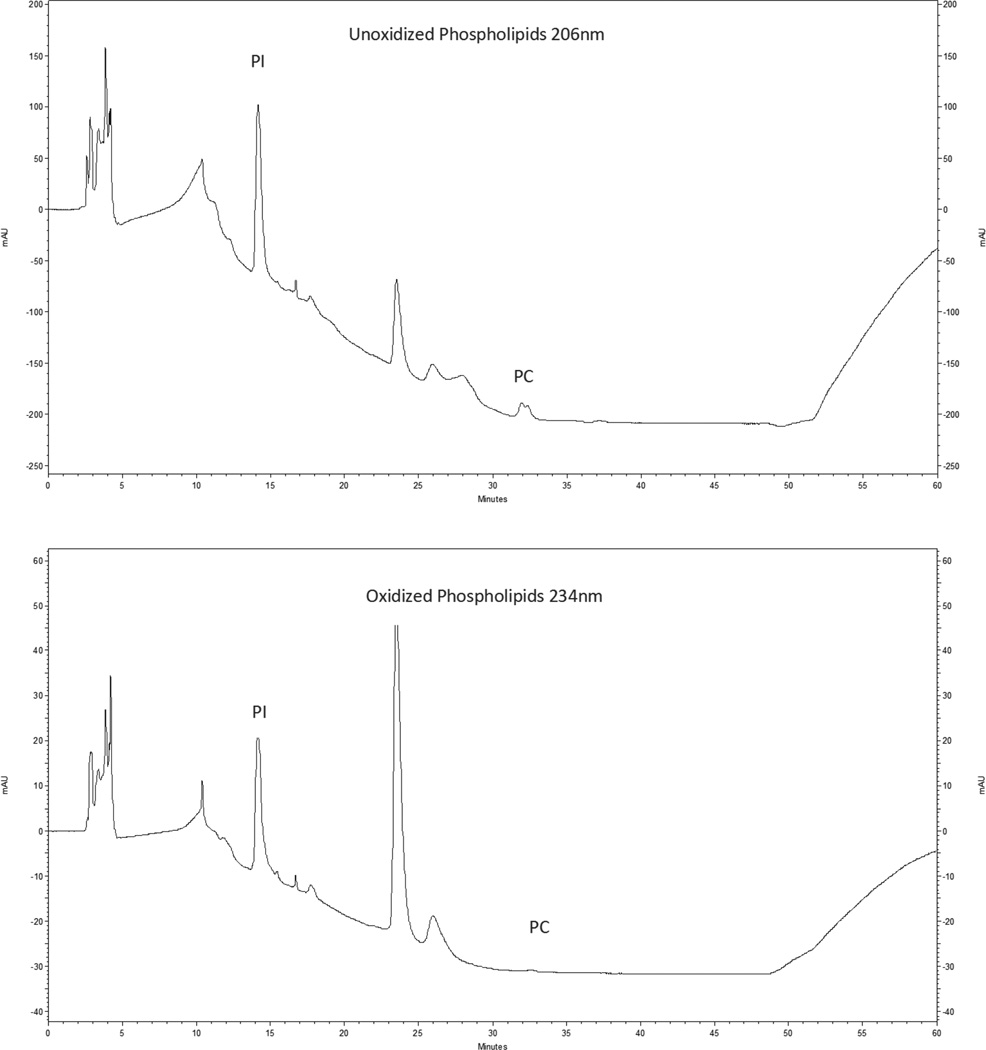
Mawatari and Murakami54 developed a HPLC method for detecting peroxidation of glycerophospholipids in a single chromatic elution. We modified this method to optimize detection for normal phase HPLC separation of glycerophospholipid classes55. Oxidized products and unoxidized glycerophospholipids absorb light at 2 different wavelengths (234 nm and 206 nm, respectively). Therefore, PC and PI can be simultaneously monitored at these two wavelengths.54, 56 Chromatograms showing separation of the components of PI (elution time approximately 14 minutes) and PC (elution time approximately 31.5 minutes) are illustrated in Figure 1.
Figure 1.
HPLC Chromatograms showing unoxidized phospholipids at wavelength 206nm and oxidized phospholipids at wavelength 234nm.
Data were analyzed using SPSS version 20. Sample characteristics were summarized with descriptive statistics. General linear model (GLM) for repeated measures analysis of variance (ANOVA) was used to test for significant changes over time in the components of PC and PI. Within group contrasts were used to determine if the components of PC and PI at day 8 (first CSF sample collected during induction but prior to administration of IT MTX), induction, post-induction, and continuation were significantly different from those at diagnosis. More than one CSF sample was obtained during induction, post-induction, and continuation. Therefore, data were analyzed using mean and highest peak area for PC and PI during those treatment phases. Pearson correlation was used to determine if the amount of methotrexate received and child’s age at the time of ALL diagnosis were associated with the levels of the components of PC and PI.
Results
An initial sample of 62 children were consented and enrolled in the study. Seven children received cranial radiation as part of CNS-directed treatment and were excluded. The final sample included 55 children who received IT and IV MTX for CNS-directed treatment. Age at time of ALL diagnosis ranged from 2.32 to 14.74 years (mean = 6.84 SD = 3.40). There were 28 males and 27 females. The majority of participants were Hispanic (n = 26) or Caucasian (n = 24). Remaining subjects were African American (n = 2), Native American (n = 1), or other (n = 2). Maternal education ranged from 7 to 18 years (mean = 13.45; SD = 2.22), and paternal education ranged from 5 to 20 years (mean = 12.85; SD = 2.93) years. Demographic characteristics of subjects, number of IT MTX treatments received, COG ALL protocols, and risk group status are summarized in Table 1.
Table 1.
Demographics and Treatment Characteristics
| Variable | N | M | SD | Min | Max | |
|---|---|---|---|---|---|---|
| Number IT treatments received during each phase | ||||||
| Diagnosis | 55 | 1 | 0 | 1 | 1 | |
| Day 8 | 55 | 1 | 0 | 1 | 1 | |
| Induction | 55 | 1.93 | 0.33 | 1 | 3 | |
| Post-Induction | 55 | 7.96 | 2.44 | 5 | 14 | |
| Continuation | 55 | 6.40 | 2.31 | 2 | 11 | |
| Age at diagnosis | 55 | 6.84 | 3.40 | 2.32 | 14.74 | |
| Maternal education (years) | 47 | 13.45 | 2.22 | 7 | 18 | |
| Paternal education (years) | 47 | 12.85 | 2.93 | 5 | 20 | |
| COG protocol | N | %a | ||||
| AALL0031 | 3 | 5.5 | ||||
| AALL0232 | 16 | 29.1 | ||||
| AALL0622 | 1 | 1.8 | ||||
| AALL0331 | 24 | 43.6 | ||||
| AALL0434 | 2 | 3.6 | ||||
| AALL0932 | 8 | 14.5 | ||||
| AALL1131 | 1 | 1.8 | ||||
| Risk group | ||||||
| Low | 1 | 1.8 | ||||
| Standard | 33 | 60.0 | ||||
| High | 17 | 30.9 | ||||
| Very high | 4 | 7.3 | ||||
Category totals may not equal 100% due to rounding.
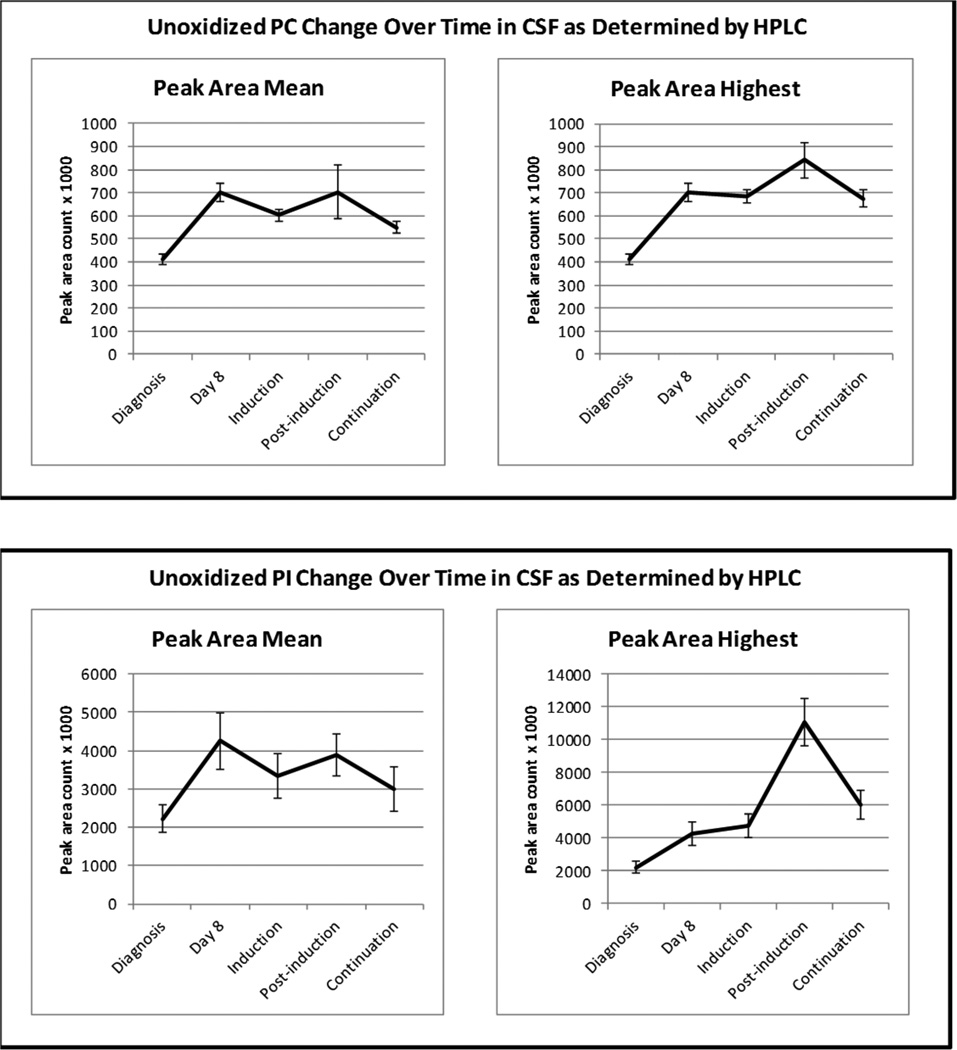
Compared to diagnosis, mean and highest levels of unoxidized glycerophospholipids increased at day 8, and during induction, post-induction, and continuation, with the greatest increase occurring during post-induction (see Figure 2). The increase in unoxidized PC peak area during post-induction (842380) was two-fold greater that at diagnosis (411670), while unoxidized PI peak area was almost 5-fold greater during post-induction (11053670) than at diagnosis (2217370). The main effect for time was significant for mean (F = 5.78; df = 4; p < 0.013) and highest (F = 16.981; df = 4, p < 0.001) unoxidized PC peak area. Results from univariate ANOVA for within group contrasts (Table 2) show that mean peak area at day 8, as well as mean and highest peak area during induction, post-induction, and continuation were significantly greater than at diagnosis. There was also a significant time effect for mean (F = 2.666; df = 4, p = 0.043) and highest (F = 17.621; df = 4, p < 0.001) unoxidized PI peak area. Within group contrasts (Table 2) show that highest peak areas at all CNS treatment phases were significantly greater than at diagnosis however, only mean PI peak area at day 8 and during post-induction were significantly greater than at diagnosis.
Figure 2.
Changes in CSF Unoxidized Gylcerophospholipids by Treatment Phase.
Table 2.
| Biomarker | Phase | Peak Area Mean | Peak Area Highestc |
|---|---|---|---|
| F | F | ||
| Unoxidized PC | Day 8 | 73.685** | |
| Induction | 84.006** | 123.504** | |
| Post-Induction | 7.998* | 35.170** | |
| Continuation | 56.251** | 64.141** | |
| Unoxized PI | Day 8 | 7.981** | |
| Induction | 3.523 | 11.941* | |
| Post-Induction | 7.836** | 36.863** | |
| Continuation | 1.847 | 20.382** | |
| Oxidized PC | Day 8 | 22.297** | |
| Induction | 19.034** | 54.763** | |
| Post-Induction | 14.536** | 66.346** | |
| Continuation | 3.910* | 60.901** | |
| Oxidized PI | Day 8 | 9.929* | |
| Induction | 2.690 | 10.569* | |
| Post-Induction | 6.295* | 32.394** | |
| Continuation | 0.280 | 19.632** |
Within group contrast is compared to level at diagnosis
n=55, df=1,54 for all variables
Highest peak area for diagnosis and day 8 not available because there is only one CSF sample at these data collection times
p<.05,
p<.001
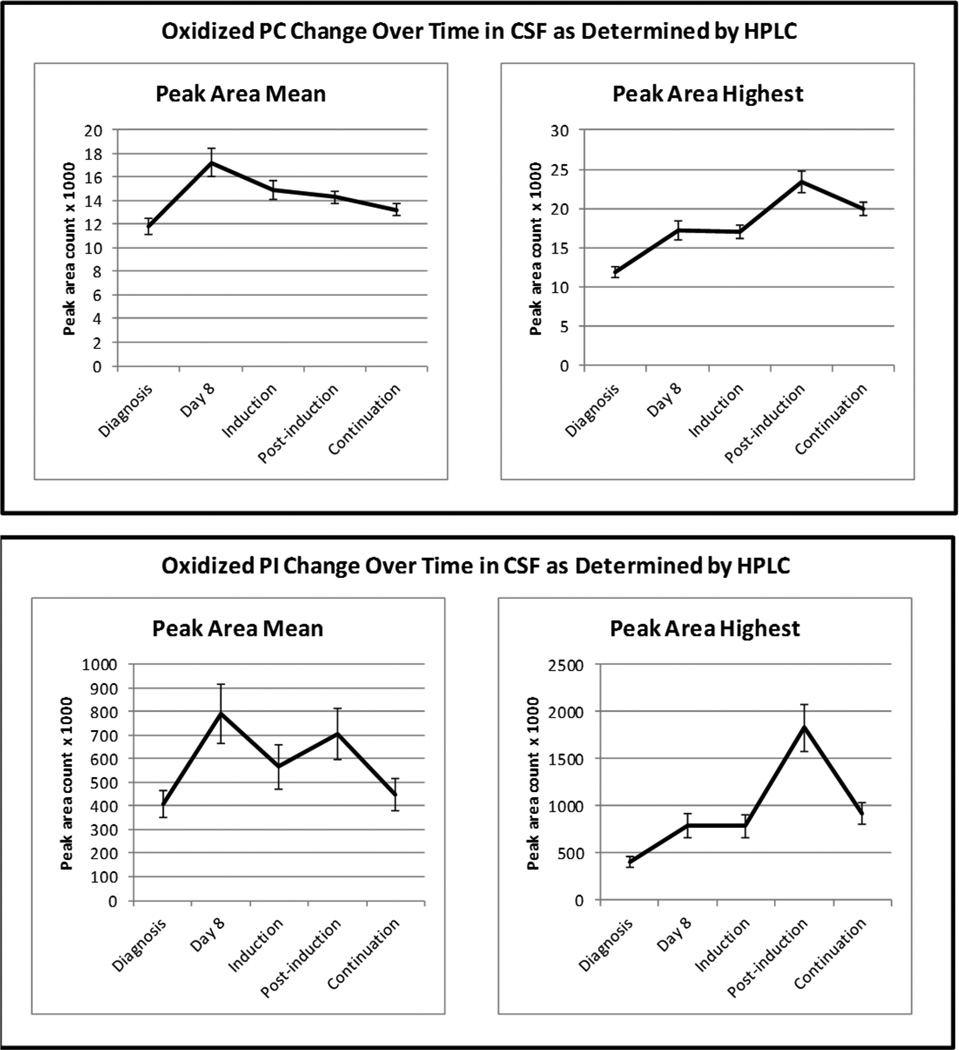
Levels of oxidized glycerophospholipids also increased at all treatment phases, and again the greatest increase occurring during post-induction. As shown in Figure 3, oxidized PC peak area was almost two-fold greater during post-induction (23370) than at diagnosis (11840). However, oxidized PI peak area during post-induction (1827470) was 4.5 times greater than at diagnosis (406730). There was a significant main effect for time for mean (F = 11.188; df = 4; p < 0.001) and highest (F = 22.135; df = 4; p < 0.001) oxidized PC peak area, and all within group contrasts were significant. For oxidized PI, the main effect for time was also significant for mean (F = 4.192; df = 4; p = 0.006) and highest (F = 16.747; df = 4; p < 0.001) peak area. Highest oxidized PI peak areas during induction, post-induction, and continuation were all significantly greater than at diagnosis, however, only mean peak areas at day 8 and post-induction were significantly greater than at diagnosis.
Figure 3.
Changes in CSF Oxidized Gylcerophospholipids by Treatment Phase.
Mean cumulative amount of IV methotrexate was 7977.86 mg/M2 (SD = 10,297.65) during post-induction and 7,833.38 mg/M2 (SD = 10563.53) during continuation. Amount of IV methotrexate received during post-induction and continuation was significantly correlated with oxidized PI peak areas during those treatment phases (r = 0.397, p < 0.01 and r = 0.317, p < 0.01, respectively). Amount of IV methotrexate received was not significantly associated with oxidized PC at any time point. Child’s age at the time of ALL diagnosis was significantly and positively correlated with oxidized PC peak area at diagnosis (r = 0.260, p = 0.024), during induction (r = 0.304, p = 0.006), post-induction (r = 0.279, p = 0.010), and continuation (r = 0.240, p = 0.025). There were no gender differences in the level of oxidized glycerophospholipids at any time period.
We recently reported increased CSF concentrations of F2-Isoprostanes, a well- established biomarker of oxidative stress, over the first 18 months of ALL treatment.57 To further confirm oxidized glycerophospholipids as a biomarker of oxidative stress in the brain, we correlated the concentration of F2-Isoprostanes with levels of oxidized PC and PI. F2-Isoprostanes and oxidized PI were significantly correlated at day 8 (r = 0.507 p < 0.001), during post-induction (r = 0.274, p = 0.021), and continuation (r = 0.358, p = 0.004). F2-Isoprostanes were also significantly associated with oxidized PC during induction (r = 0.245, p = 0.040), post-induction (r = 0.427, p =0.001) and continuation (r = 0.358, p = 0.004).
Discussion
As hypothesized, we found a significant increase over time in the CSF levels of unoxidized and oxidized glycerophospholipids. This finding supports our hypothesis that CNS treatment disrupts membrane integrity in brain tissue, and increases oxidative stress. Protas and colleagues58 reported a significant increase in 8-isoprostane concentration (a measure of lipid peroxidation) and decrease in total antioxidant capacity in CSF samples obtained from 38 children with ALL on day 59 of treatment and at 4 time points during intensive CNS-directed treatment which also supports the role of oxidative stress in neurological injury. We reported increased CSF concentrations of F2-Isoprostanes during the first 18 months of treatment.57 In this study, the number of doses of IT methotrexate during post-induction was significantly associated with the mean and highest concentrations of F2-Isoprostanes during post-induction and continuation therapy, and acute increases in F2-Isoprostanes were highest among children who received more than six doses of IT methotrexate during post-induction.57 Collectively, these findings support chemotherapy-induced oxidative stress as a mechanism of neurologic injury.
The increase in unoxidized and oxidized PI was greater than PC during ALL treatment. Further, only oxidized PI was significantly correlated with the total amount of IV methotrexate received during post-induction and continuation. These findings suggest that PI may be a more sensitive biomarker of chemotherapy-induced oxidative stress, while PC may be a more general marker of neuroinflammation associated with ALL treatment. Qin and colleagues46 found significantly more oxidized PC in plaque regions of post-mortem brain tissue from patients with multiple sclerosis (MS) compared to normal brain and unaffected MS white matter regions. Post-translational protein modification (glycation) of oxidized PC was also found in affected brain tissue. These investigators propose that oxidized PC is a marker for neuroinflammation in MS and could play a role in disease progression. While we did not examine associations among glycerophospholipids and cognitive abilities, we recently reported that the level of oxidized PC was significantly associated with total number of treatment-related symptoms, symptom distress, and symptom severity experienced during induction and post-induction in a subset (n = 36) of the same sample of children with ALL reported here55. This finding provides support for the proposed role of oxidized PC in neuroinflammation which may be manifested as treatment-related symptoms.
Battisti and colleagues59 measured biomarkers of oxidative damage in serum samples obtained from 80 patients with ALL and 50 healthy controls. Measures of lipid peroxidation were increased in ALL patients who were newly diagnosed, in treatment, or out-of-treatment compared to healthy controls. Compared to controls, catalase and superoxide dismutase (the most efficient enzymatic antioxidants) were decreased in newly diagnosed patients and those in induction treatment.59 The investigators proposed that the increase in oxidative stress may be related to the pathogenesis of leukemia rather than chemotherapy, however, their study was limited to measures of oxidative stress in serum. In the study reported here, we measured lipid peroxidation in CSF as a measure of oxidative stress in brain tissue. However, we acknowledge that microscopic disease in the brain could also contribute to lipid peroxidation.
Unoxidized and oxidized glycerophospholipids were higher at day 8 (prior to administration of IT MTX) compared to levels in the diagnostic sample which suggests that systemically administered drugs or IT cytarabine could also increase oxidative stress in the brain. Papageorgious found that the level of serum antioxidant capacity was similar between children with a new cancer diagnosis and healthy children. However, chemotherapy induced a significant decrease in serum antioxidant capacity and an increase in ROS60. Children in the study reported here received other antineoplastic agents some of which could cross the blood brain barrier, although in smaller percentages than methotrexate. Therefore, systemically administered drugs in addition to methotrexate could contribute to the observed increase in oxidized glycerophospholipids.
Age at time of ALL diagnosis was moderately correlated with oxidized PC. PC is the most prevalent lipid in brain tissue, and therefore most vulnerable to peroxidation. Post-natal brain development, involving myelination as well as dendritic and axonal aborization, is most rapid during the first 2 years of life. By the age of 2 years, the brain has achieved 80% of adult brain weight and by 5 years the brain is approximately 90% of adult size61. At an earlier age there may be a stronger relationship between age and PC peak area, however, the sample in our study ranged from 2.32 to 14.74 years of age, with a median age of 6.2 years. Since the majority of post-natal brain development occurs within the first 2 years of life, the moderate correlations are not surprising.
A limitation of this study is the lack of a healthy control group to compare levels of unoxidized and oxidized glycerophospholipids with our sample of children with ALL receiving CNS treatment. Collecting CSF samples from healthy children was not feasible because of the invasiveness of lumbar punctures. However, the within subjects repeated measures design does allow us to test for changes in PC and PI over the course of treatment. There is evidence that compared to chemotherapy, the addition of cranial radiation exacerbates deficits in cognitive performance and loss of white matter5. However, we did not have a sufficient number of children (n = 7) who received cranial radiation to compare with our sample of children who received only chemotherapy for CNS-directed treatment.
In summary, the significant increase in oxidized PC and PI reported here, as well as findings from previous studies showing increased F2-isoprostanes57, 58 provide collective evidence for oxidative stress as a mechanism associated with intrathecal and intravenous chemotherapy. Future studies are needed to investigate novel neuroprotective strategies that could protect healthy brain tissue from oxidative stress while not compromising the anti-leukemic effects of chemotherapeutic agents.
Acknowledgments
Funding support from: This work was supported by R01NR010889 from the National Institute of Nursing Research
Footnotes
Conflict of Interest: None Declared
Contributor Information
Ida M. (Ki) Moore, Anne Furrow Professor and Biobehavioral Health Science Division Director, University of Arizona College of Nursing, Tucson, AZ.
Patricia Gundy, Principal Research Specialist, University of Arizona College of Nursing, Tucson, AZ.
Alice Pasvogel, Assistant Research Scientist, University of Arizona College of Nursing, Tucson, AZ.
David W. Montgomery, Research Professor, University of Arizona College of Nursing and Research Service, Southern Arizona VA Health Care System, Tucson, AZ.
Olga A. Taylor, Senior Research Coordinator, Baylor College of Medicine, Houston, TX.
Kari M. Koerner, Research Specialist, University of Arizona College of Nursing, Tucson, AZ.
Kathy McCarthy, Senior Research Nurse, Baylor College of Medicine, Houston TX.
Marilyn J. Hockenberry, Bessie Baker Professor, Duke School of Nursing, Durham, NC.
References
- 1.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the Children's Oncology Group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH. Toward optimal central nervous system-directed treatment in childhood acute lymphoblastic leukemia. J Clin Oncol. 2003;21:179–181. doi: 10.1200/JCO.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 4.Halberg FE, Kramer JH, Moore IM, et al. Prophylactic cranial irradiation dose effects on late cognitive function in children treated for acute lymphoblastic leukemia. Int J Radiat Oncol Biol Phys. 1992;22:13–16. doi: 10.1016/0360-3016(92)90976-o. [DOI] [PubMed] [Google Scholar]
- 5.Reddick WE, Shan ZY, Glass JO, et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer. 2006;106:941–949. doi: 10.1002/cncr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iuvone L, Mariotti P, Colosimo C, et al. Long-term cognitive outcome, brain computed tomography scan, and magnetic resonance imaging in children cured for acute lymphoblastic leukemia. Cancer. 2002;95:2562–2570. doi: 10.1002/cncr.10999. [DOI] [PubMed] [Google Scholar]
- 7.Moore BD., 3rd Neurocognitive outcomes in survivors of childhood cancer. J Pediatr Psychol. 2005;30:51–63. doi: 10.1093/jpepsy/jsi016. [DOI] [PubMed] [Google Scholar]
- 8.Moore BD, 3rd, Copeland DR, Ried H, et al. Neurophysiological basis of cognitive deficits in long-term survivors of childhood cancer. Arch Neurol. 1992;49:809–817. doi: 10.1001/archneur.1992.00530320033009. [DOI] [PubMed] [Google Scholar]
- 9.Moore IM, Kramer J, Ablin A. Late effects of central nervous system prophylactic leukemia therapy on cognitive functioning. Oncol Nurs Forum. 1986;13:45–51. [PubMed] [Google Scholar]
- 10.Ki Moore IM, Hockenberry MJ, Krull KR. Cancer-related cognitive changes in children, adolescents and adult survivors of childhood cancers. Semin Oncol Nurs. 2013;29:248–259. doi: 10.1016/j.soncn.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Reddick WE, Glass JO, Helton KJ, et al. Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. AJNR Am J Neuroradiol. 2005;26:1263–1269. [PMC free article] [PubMed] [Google Scholar]
- 12.Reddick WE, Glass JO, Helton KJ, et al. A quantitative MR imaging assessment of leukoencephalopathy in children treated for acute lymphoblastic leukemia without irradiation. AJNR Am J Neuroradiol. 2005;26:2371–2377. [PMC free article] [PubMed] [Google Scholar]
- 13.Hill DE, Ciesielski KT, Hart BL, et al. MRI morphometric and neuropsychological correlates of long-term memory in survivors of childhood leukemia. Pediatr Blood Cancer. 2004;42:611–617. doi: 10.1002/pbc.20004. [DOI] [PubMed] [Google Scholar]
- 14.Hill DE, Ciesielski KT, Sethre-Hofstad L, et al. Visual and verbal short-term memory deficits in childhood leukemia survivors after intrathecal chemotherapy. J Pediatr Psychol. 1997;22:861–870. doi: 10.1093/jpepsy/22.6.861. [DOI] [PubMed] [Google Scholar]
- 15.Kaemingk KL, Carey ME, Moore IM, et al. Math weaknesses in survivors of acute lymphoblastic leukemia compared to healthy children. Child Neuropsychol. 2004;10:14–23. doi: 10.1076/chin.10.1.14.26240. [DOI] [PubMed] [Google Scholar]
- 16.Espy KA, Moore IM, Kaufmann PM, et al. Chemotherapeutic CNS prophylaxis and neuropsychologic change in children with acute lymphoblastic leukemia: A prospective study. J Pediatr Psychol. 2001;26:1–9. doi: 10.1093/jpepsy/26.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Carey ME, Hockenberry MJ, Moore IM, et al. Brief report: Effect of intravenous methotrexate dose and infusion rate on neuropsychological function one year after diagnosis of acute lymphoblastic leukemia. J Pediatr Psychol. 2007;32:189–193. doi: 10.1093/jpepsy/jsj114. [DOI] [PubMed] [Google Scholar]
- 18.Buizer AI, De Sonneville LM, van den Heuvel-Eibrink MM, et al. Visuomotor control in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. J Int Neuropsychol Soc. 2005;11:554–565. doi: 10.1017/S1355617705050666. [DOI] [PubMed] [Google Scholar]
- 19.Buizer AI, de Sonneville LM, van den Heuvel-Eibrink MM, et al. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: Effect of treatment intensity. Pediatr Blood Cancer. 2005;45:281–290. doi: 10.1002/pbc.20397. [DOI] [PubMed] [Google Scholar]
- 20.Buizer AI, de Sonneville LM, Veerman AJ. Effects of chemotherapy on neurocognitive function in children with acute lymphoblastic leukemia: A critical review of the literature. Pediatr Blood Cancer. 2009;52:447–454. doi: 10.1002/pbc.21869. [DOI] [PubMed] [Google Scholar]
- 21.Waber DP, Tarbell NJ. Toxicity of CNS prophylaxis for childhood leukemia. Oncology (Williston Park) 1997;11:259–264. discussion 264-5. [PubMed] [Google Scholar]
- 22.Hockenberry M, Krull K, Moore K, et al. Longitudinal evaluation of fine motor skills in children with leukemia. J Pediatr Hematol Oncol. 2007;29:535–539. doi: 10.1097/MPH.0b013e3180f61b92. [DOI] [PubMed] [Google Scholar]
- 23.Krull KR, Hockenberry MJ, Miketova P, et al. Chemotherapy-related changes in central nervous system phospholipids and neurocognitive function in childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2013;54:535–540. doi: 10.3109/10428194.2012.717080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krull KR, Okcu MF, Potter B, et al. Screening for neurocognitive impairment in pediatric cancer long-term survivors. J Clin Oncol. 2008;26:4138–4143. doi: 10.1200/JCO.2008.16.8864. [DOI] [PubMed] [Google Scholar]
- 25.Walsh KS, Paltin I, Gioia GA, et al. Everyday executive function in standard-risk acute lymphoblastic leukemia survivors. Child Neuropsychol. 2014 doi: 10.1080/09297049.2013.876491. [DOI] [PubMed] [Google Scholar]
- 26.Montour-Proulx I, Kuehn SM, Keene DL, et al. Cognitive changes in children treated for acute lymphoblastic leukemia with chemotherapy only according to the Pediatric Oncology Group 9605 protocol. J Child Neurol. 2005;20:129–133. doi: 10.1177/08830738050200020901. [DOI] [PubMed] [Google Scholar]
- 27.Moore IM, Espy KA, Kaufmann P, et al. Cognitive consequences and central nervous system injury following treatment for childhood leukemia. Semin Oncol Nurs. 2000;16:279–290. doi: 10.1053/sonu.2000.16582. discussion 291-9. [DOI] [PubMed] [Google Scholar]
- 28.Jahovic N, Cevik H, Sehirli AO, et al. Melatonin prevents methotrexate-induced hepatorenal oxidative injury in rats. J Pineal Res. 2003;34:282–287. [PubMed] [Google Scholar]
- 29.Oktem F, Yilmaz HR, Ozguner F, et al. Methotrexate-induced renal oxidative stress in rats: The role of a novel antioxidant caffeic acid phenethyl ester. Toxicol Ind Health. 2006;22:241–247. doi: 10.1191/0748233706th265oa. [DOI] [PubMed] [Google Scholar]
- 30.Babiak RM, Campello AP, Carnieri EG, et al. Methotrexate: Pentose cycle and oxidative stress. Cell Biochem Funct. 1998;16:283–293. doi: 10.1002/(SICI)1099-0844(1998120)16:4<283::AID-CBF801>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 31.Cetinkaya A, Bulbuloglu E, Kurutas EB, et al. N-acetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med Sci Monit. 2006;12:BR274–BR278. [PubMed] [Google Scholar]
- 32.Rouse K, Nwokedi E, Woodliff JE, et al. Glutamine enhances selectivity of chemotherapy through changes in glutathione metabolism. Ann Surg. 1995;221:420–426. doi: 10.1097/00000658-199504000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sener G, Eksioglu-Demiralp E, Cetiner M, et al. L-carnitine ameliorates methotrexate-induced oxidative organ injury and inhibits leukocyte death. Cell Biol Toxicol. 2006;22:47–60. doi: 10.1007/s10565-006-0025-0. [DOI] [PubMed] [Google Scholar]
- 34.Farooqui AA, Horrocks LA. Lipid peroxides in the free radical pathophysiology of brain diseases. Cell Mol Neurobiol. 1998;18:599–608. doi: 10.1023/A:1020625717298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 36.Volinsky R, Kinnunen PKJ. Oxidized phosphatidylcholines in membrane-level cellular signaling: From biophysics to physiology and molecular pathology. FEBS J. 2013;280:2806–2816. doi: 10.1111/febs.12247. [DOI] [PubMed] [Google Scholar]
- 37.Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology. 2006;13:171–181. doi: 10.1016/j.pathophys.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Bochkov VN, Oskolkova OV, Birukov KG, et al. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasad MR, Lovell MA, Yatin M, et al. Regional membrane phospholipid alterations in Alzheimer's disease. Neurochem Res. 1998;23:81–88. doi: 10.1023/a:1022457605436. [DOI] [PubMed] [Google Scholar]
- 40.McIntyre TM, Zimmerman GA, Prescott SM. Biologically active oxidized phospholipids. J Biol Chem. 1999;274:25189–25192. doi: 10.1074/jbc.274.36.25189. [DOI] [PubMed] [Google Scholar]
- 41.Butterfield DA, Drake J, Pocernich C, et al. Evidence of oxidative damage in Alzheimer's disease brain: Central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 42.Babizhayev MA. Failure to withstand oxidative stress induced by phospholipid hydroperoxides as a possible cause of the lens opacities in systemic diseases and ageing. Biochim Biophys Acta. 1996;1315:87–99. doi: 10.1016/0925-4439(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 43.Leitinger N. The role of phospholipid oxidation products in inflammatory and autoimmune diseases: Evidence from animal models and in humans. Subcell Biochem. 2008;49:325–350. doi: 10.1007/978-1-4020-8830-8_12. [DOI] [PubMed] [Google Scholar]
- 44.Chauhan A, Chauhan V, Brown WT, et al. Oxidative stress in autism: Increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin--the antioxidant proteins. Life Sci. 2004;75:2539–2549. doi: 10.1016/j.lfs.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 45.Bayir H, Kagan VE, Tyurina YY, et al. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr Res. 2002;51:571–578. doi: 10.1203/00006450-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Qin J, Goswami R, Balabanov R, et al. Oxidized phosphatidylcholine is a marker for neuroinflammation in multiple sclerosis brain. J Neurosci Res. 2007;85:977–984. doi: 10.1002/jnr.21206. [DOI] [PubMed] [Google Scholar]
- 47.Raucher D, Stauffer T, Chen W, et al. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100:221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- 48.Liscovitch M, Chalifa V, Pertile P, et al. Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase d. J Biol Chem. 1994;269:21403–21406. [PubMed] [Google Scholar]
- 49.Frere SG, Chang-Ileto B, Di Paolo G. Role of phosphoinositides at the neuronal synapse. Subcell Biochem. 2012;59:131–175. doi: 10.1007/978-94-007-3015-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 51.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 52.Miketova P, Moore I, Pasvogel A, et al. Determination of phospholipids as biomarkers of brain tissue damage in acute lymphoblastic leukemia patients. Scientific Papers of the University of Pardubice, Series A Faculty of Chemical Technology. 2003:73–91. [Google Scholar]
- 53.Watson BD. Evaluation of the concomitance of lipid peroxidation in experimental models of cerebral ischemia and stroke. Prog Brain Res. 1993;96:69–95. doi: 10.1016/s0079-6123(08)63259-8. [DOI] [PubMed] [Google Scholar]
- 54.Mawatari S, Murakami K. Analysis of membrane phospholipid peroxidation by isocratic high-performance liquid chromatography with ultraviolet detection. Anal Biochem. 1998;264:118–123. doi: 10.1006/abio.1998.2830. [DOI] [PubMed] [Google Scholar]
- 55.Hockenberry MJ, Taylor OA, Pasvogel A, et al. The influence of oxidative stress on symptom occurrence, severity, and distress during childhood leukemia treatment. Oncol Nurs Forum. 2014;41:E238–E247. doi: 10.1188/14.ONF.E238-E247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miketova P, Kaemingk K, Hockenberry M, et al. Oxidative changes in cerebral spinal fluid phosphatidylcholine during treatment for acute lymphoblastic leukemia. Biol Res Nurs. 2005;6:187–195. doi: 10.1177/1099800404271916. [DOI] [PubMed] [Google Scholar]
- 57.Hockenberry MJ, Taylor OA, Gundy PM, et al. F2-isoprostanes: A measure of oxidative stress in children receiving treatment for leukemia. Biol Res Nurs. 2013 doi: 10.1177/1099800413498507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Protas PT, Muszynska-Roslan K, Holownia A, et al. Cerebrospinal fluid oxidative stress during chemotherapy of acute lymphoblastic leukemia in children. Pediatr Hematol Oncol. 2010;27:306–313. doi: 10.3109/08880011003639960. [DOI] [PubMed] [Google Scholar]
- 59.Battisti V, Maders LD, Bagatini MD, et al. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin Biochem. 2008;41:511–518. doi: 10.1016/j.clinbiochem.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 60.Papageorgiou M, Stiakaki E, Dimitriou H, et al. Cancer chemotherapy reduces plasma total antioxidant capacity in children with malignancies. Leuk Res. 2005;29:11–16. doi: 10.1016/j.leukres.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]