Abstract
Purpose
Melanoma is a solid tumor that is notoriously resistant to chemotherapy, and its incidence is rapidly increasing. Recently, several signaling pathways have been demonstrated to contribute to melanoma tumorigenesis, including constitutive activation of MAP kinase, Akt and Stat-3. The activation of multiple pathways may account in part for the difficulty in treatment of melanoma. In a recent screen of compounds, we found that an organopalladium compound, tris (dibenzylideneacetone) dipalladium (Tris DBA), demonstrated significant antiproliferative activity against melanoma cells. Studies were carried out to determine the mechanism of action of Tris DBA
Experimental Design
Tris DBA was tested on efficacy on proliferation of human and murine melanoma cells. In order to find the mechanism of action of Tris DBA, we performed Western Blot analysis and gene array analysis. The ability of Tris DBA to block tumor growth in vivo was assessed.
Results
(Tris DBA), has activity against B16 murine and A375 human melanoma in vivo. Tris DBA inhibits several signaling pathways including activation of MAP kinase, Akt, Stat-3 and S6 kinase activation, suggesting an upstream target. Tris DBA was found to be a potent inhibitor of N-myristoyltransferase 1 (NMT-1), which is required for optimal activity of membrane based signaling molecules. Tris DBA demonstrated potent antitumor activity in vivo against melanoma.
Conclusion
Tris DBA is thus a novel inhibitor of NMT-1 with significant antitumor activity and is well tolerated in vivo. Further preclinical evaluation of Tris DBA and related complexes is warranted.
Introduction
Melanoma is one of the most common solid tumors and is notoriously difficult to treat. Recently, constitutive activation of several signaling pathways has been demonstrated in melanoma. Many melanomas carry mutations in B-raf, which cause constitutive activation of MAP kinase (1; 2). Even melanomas that do not carry activated B-raf demonstrate activation of MAP kinase, and constitutive expression of activated MAP kinase kinase is sufficient to transform melanocytes to melanoma (3, 4, 5). Other pathways that are known to be activated in advanced melanoma include phosphoinositol-3 kinase/akt and nuclear factor kappa beta (NFκB) (6, 7, 8, 9, 10). All of these pathways confer survival and proliferative advantages to melanoma, such as induction of angiogenic factors, including vascular endothelial growth factor, interleukin-8, survivin, IAP, and mcl-1 (11, 12, 13).
Platinum compounds have been the mainstay of many solid tumor regimens, especially testicular cancer. However, platinum compounds, including cisplatin and carboplatin, have also shown activity in melanoma and have been incorporated into melanoma treatment regimens (14). Other inhibitors, such as sorafenib, a B-raf inhibitor, have had modest effects on melanoma with B-raf mutation despite robust inhibition of B-raf (15). This may be due to the ability of aggressive tumors to switch signaling pathways (16). We have observed this phenomenon in Burkitt’s lymphoma, in which MAP kinase is activated when NFκB is downregulated (17). Similarly, inhibition of NFκB with velcade has had modest effects in melanoma (18, 19).
In our screens for angiogenesis inhibitors, we have identified a small molecule palladium complex which has structural similarities to curcumin and chalcones, compounds with known chemopreventive activity (20, 21). While chemopreventive agents are effective against preneoplastic lesions in mice and man, they are less effective against established tumors (22, 23). Analysis of Tris DBA treated melanoma cells by gene array revealed reduction of NMT-1, which was confirmed by quantitative RT-PCR. Myristoylation performed by NMT-1 is required for most membrane based signaling molecules. C-src is a candidate molecule that requires myristoylation for optimal activity. Tris DNA reduced expression of c-src, which is a substrate of NMT-1. Consistent with inhibition of c-src/NMT-1, Tris DBA inhibited downstream signaling pathways, including MAPK kinase, phosphoinositol-3 kinase, and Stat-3. Tris DBA has activity in vivo against A375 and B16 melanoma in vivo. Further preclinical evaluation of Tris DBA is warranted.
Materials and Methods
Cells
B16 melanoma cells were cultured in Dulbeccos Modified Eagle Medium (1000mg glucose/L, Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum, L-glutamine (14 ml/L), and antibiotic/antimycotic (14 ml/L, Sigma-Aldrich). A375 cells were cultured in Dulbeccos Modified Eagle Medium (4500mg glucose/L, Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum, L-glutamine (14 ml/L), and antibiotic/antimycotic (14 ml/L, Sigma-Aldrich).
Cell proliferation assays
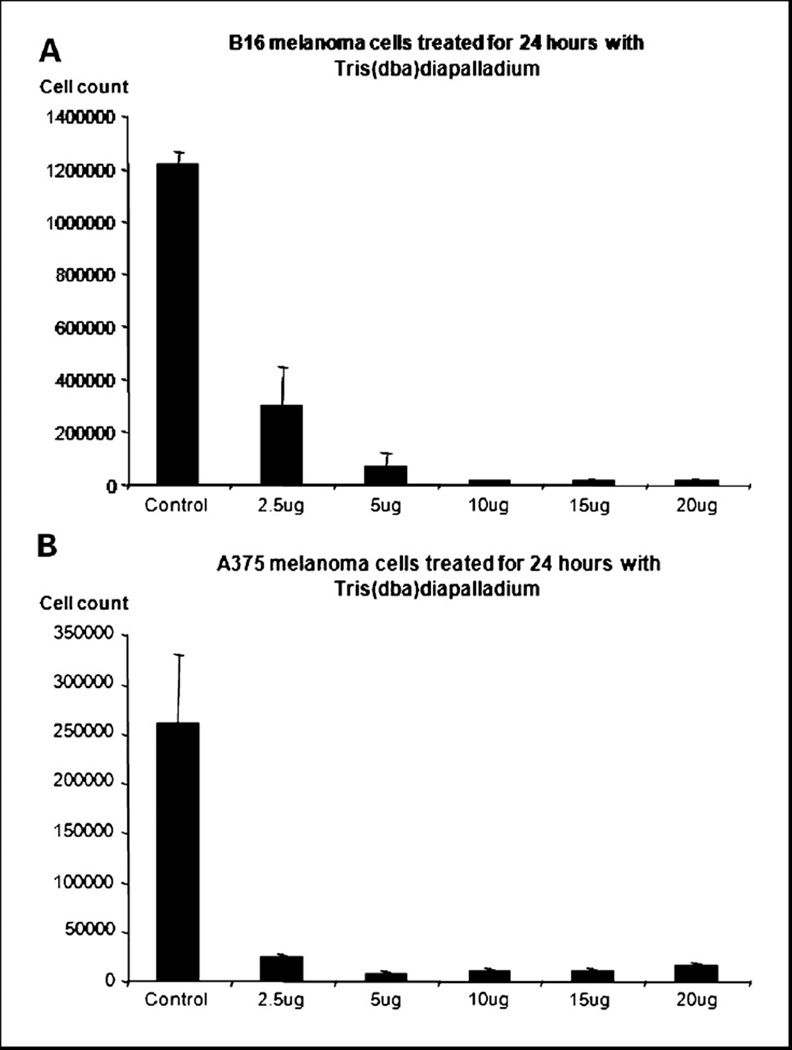
In order to evaluate the potential of Tris DBA as an antitumor agent, a proliferation assay was performed using B16 cells (Figure 1a) and A375 cells (Figure 1b). The assay was performed according to the method previously described by the Arbiser lab (24, 25). Ten thousand cells were plated per well in a 24-well plate. After incubation for twenty-four hours at 37 degrees and 5% CO2, the cells were treated at 2.5, 5, 10, 15 and 20ug/ml from a stock solution of 10mg/ml Tris DBA dissolved in DMSO, also used as the control. Experiments were performed in triplicate. The cells were allowed to incubate for an additional twenty four hours and then counted using a Coulter Counter (Hialeah, FL).
Figure 1. Tris (dibenzylideneacetone) dipalladium inhibits murine melanoma cell proliferation (a) and human melanoma cell proliferation (b) in vitro.
Tris DBA decreases B16 and A375 cell number in a dose dependent fashion. Control is with the vehicle, DMSO. Each bar represents the average of triplicate experiments, and error bars reflect the standard error of the mean.
Western blot analysis
For signal transduction analysis, B16 cells, untreated and treated with 10µg/ml Tris DBA at timed intervals, were lysed in Nonidet P-40 lysis buffer (1% Nonidet P-40, 150 mM NaCl, 10% glycerol, 20 mM HEPES, 1 mM phenylmethylsulfonyl fluoride, 2.5 mM EDTA, 100 µM Na3VO4, and 1% aprotinin). The lysate was spun in microfuge, and the pellet was discarded. Protein concentration of the supernatant was determined by the eppendorf BioPhotometer. Samples were treated with Laemmeli sample buffer and heated to 90°C for 5 min before SDS-PAGE (National Diagnostics, Atlanta, GA) and was transferred to nitrocellulose membranes. The membranes were then blocked with 5% nonfat dry milk in 10 mM Tris/0.1% Tween 20/100 mM NaCl and were subsequently incubated with p42/44 MAP kinase antibody, Phospho-p44/42 MAP kinase (Thr202/Tyr204) antibody, Phospho- Akt (Ser-473) and Phospho-p70 S6 Kinase(Thr421/Ser424) antibody(Cell Signaling Laboratories, Beverly, MA).Monoclonal Anti-β-Tubulin antibody (Catalog number T0198, Sigma) was used as a loading control and detected using horseradish peroxidase-conjugated secondary antibody. The immunoreactive bands were visualized by enhanced chemiluminescence (Amersham Biosciences).
Quantitative RT-PCR for and VEGF in B16 cells and A375 cells and NMT-1 in A375 cells treated with vehicle control and 10µg/ml Tris (dibenzylideneacetone) dipalladium
B16 cells and A375 cells were seeded equally into 2 T-25 flasks each and 24 hours later were treated with 0 and 10µg/ml Tris DBA (Aldrich 328774) in DMSO for 24 hours. RNA was extracted and purified using Qiagen RNeasy mini kit (Cat No. 74104) and measured using spectrophotometer (Perkin-Elmer UV/VIS). 1µg of RNA was used for DNase Amplification (Invitrogen Cat No. 18068-015) followed by First-Strand Synthesis for RT-PCR (SuperScript Cat No. 12371-019). 96-well Optical Reaction Plate (7500 Fast Real-Time PCR) was used for the RT-PCR reaction. 2.5µl of template, which had been diluted 1:10 in cross-linked water, was used in each well and the experiment was performed in triplicate. Vegfa (Applied Biosystems, Taqman Gene Expression Assay, Mm00437304_ml), NMT-1 (Applied Biosystems, Taqman Gene Expression Assay, Hs00221506_m1) and 18S (Applied Biosystems Taqman Gene Expression Assay, Hs99999901_s1) primers were used along with cross-linked molecular grade water (Cellgro) and master mix (Applied Biosystems Taqman Fast Universal PCR Master Mix (2X)). Reaction was set up at the 7500 Applied Biosystems Reader for Absolute Quantification for 96 well plate. Ct values were analyzed by ΔΔCt method, and the standard error of the mean was calculated (Figure 3 and Figure 5b)
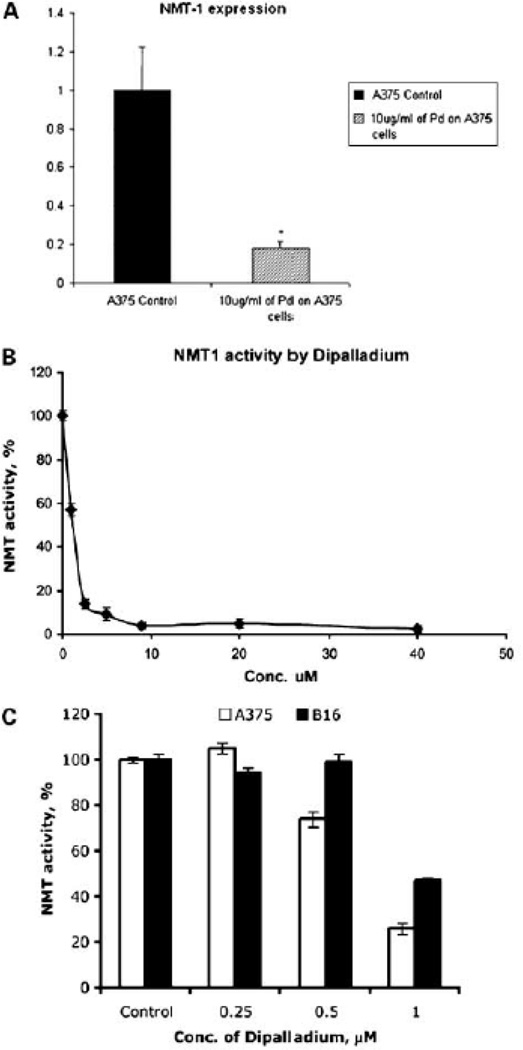
Figure 3. Inhibition of human NMT expression at the level of RNA and protein by Tris(dibenzylidenacetone)dipalladium.
(a) Treatment of A375 cells with 10µg/ml of Tris DBA decreases levels of N-myristoyltransferase-1 mRNA (corrected for 18S RNA). Bars shown represent the average of triplicate experiments, and error bars indicate the standard error of the mean. p value is .0002.
(b) Inhibition of human N-myristoyltransferase-1 by Tris DBA. The purified recombinant human NMT1 (0.2 µg/assay) was incubated with various concentrations of Tris DBA using cAMP-dependent protein kinase derived peptide as a substrate described earlier. Representative data from three independent experiments are shown, with ± SD from three determinations.
(c) Effect of Tris(dibenzylideneacetone)dipalladium on NMT1 and NMT2 in melanoma cells (A375 and B16). Cells were treated with 0.25, 0.5 and 1.0 uM concentrations of Tris(dba)dipalladium. After 48 hours, cells were lyzed and analyzed by using NMT1 and NMT2 antibodies. β-actin was used as the loading control by using monoclonal anti β-actin antibody. Representative data from three independent experiments.
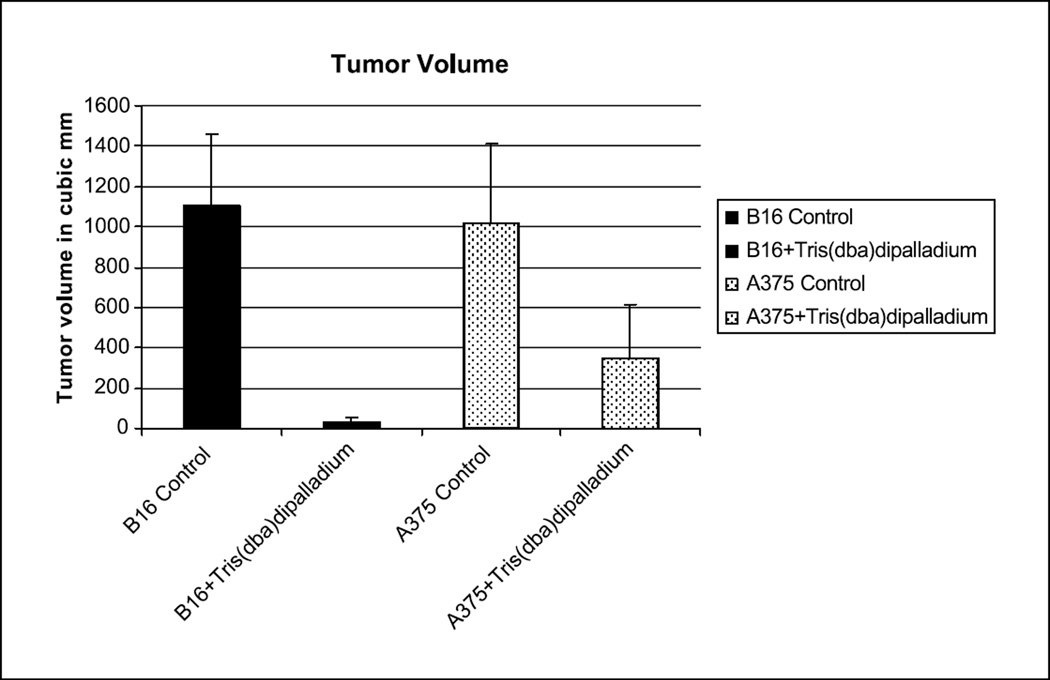
Figure 5. Effect of Tris DBA in melanomas in vivo.
Nude mice were injected with one million B16 or A375 cells and received intraperitoneal injection with Tris DBA and vehicle control. Animals were euthanized on day fifteen, secondary to tumor burden in the control animals. Figure depicts average tumor volume (mm3) in each of the B16 and A375 treated and control groups. Error bars represent the standard error of the mean. Differences are significant p<0.05.
N-Myristoyltransferase Assay
[3H]Myristic acid (39.3 Ci/mmol) was obtained from NEN Life Science Products. Pseudomonas acyl CoA synthetase and coenzyme A were obtained from Sigma-Aldrich Canada. The peptide based on the NH2-terminal sequence of the type II catalytic subunit of cAMP-dependent protein kinase (GNAAAAKKRR) was obtained from Alberta Peptide Institute, University of Alberta, Edmonton, Canada. The expression and purification of recombinant human NMT-1 were undertaken as described previously (26). The NMT activity was measured as previously described (27; 28). For the standard enzyme assays, the reaction mixture contained 0.4 µM [3H] myristoyl-CoA, 50 mM Tris-HCl, pH 7.8, 0.5 mM EGTA, 0.1% Triton X-100, 500 µM synthetic peptide and purified human NMT-1 in a total volume of 25 µl. The reaction was initiated by the addition of radiolabeled [3H] myristoyl-CoA and incubated at 30 °C for 10–30 min. The reaction was terminated by spotting aliquots of incubation mixture onto P81 phosphocellulose paper discs and drying them under a stream of warm air. The P81 phosphocellulose paper discs were washed in three changes of 40 mM Tris-HCl, pH 7.3, for 90 min. The radioactivity was quantified in 7.5 ml of Beckman Ready Safe Liquid Scintillation mixture using a Beckman Liquid Scintillation Counter. One unit of NMT activity was expressed as 1 pmol of myristoyl-peptide formed per min per mg protein. The human NMT-1 inhibitory assay was carried out using Tris DBA according to the method described earlier (Figure 5a) (28). A control experiment was performed in the absence of Tris DBA and the human NMT-1 activity was considered as 100%.
In vivo tumor growth
In order to determine if a compound which inhibits melanoma growth in vitro would also inhibit tumor formation in vivo, we injected one million B16 melanoma cells and one million A375 cells subcutaneously into six nude mice respectively. Beginning two days later, the mice received intraperitoneal injections three times per week of either Tris DBA or control. 40mg/kg/d Tris DBA was suspended in 0.3ml peanut oil, and control was 0.3ml peanut oil alone. Neither local nor systemic toxicity was observed in any of the nude mice as a result of treatment. A total of six rounds of injections were given over a period of two weeks, after which the mice were sacrificed due to overwhelming tumor burden in the control group. Animals were euthanized on day fifteen, secondary to tumor burden in the control animals. Graph represents average tumor volume (mm3) in each of the two groups with controls (Figure 5). Error bars represent the standard error of the mean.
Results
Tris (dibenzylidenacetone) dipalladium inhibits melanoma proliferation
Tris DBA was used to treat at five different concentrations on B16 and A375 cells1. Treatment with Tris DBA for twenty-four hours significantly reduced the number of viable cells. A 10ug/ml concentration of Tris DBA resulted in a 99% decrease in B16 cell count (Figure 1a) and a 96% decrease in A375 cell count (Figure 1b).
Tris DBA inhibits activation of MAPK, Akt, Stat3, and phospho-S6 kinase in melanoma cells
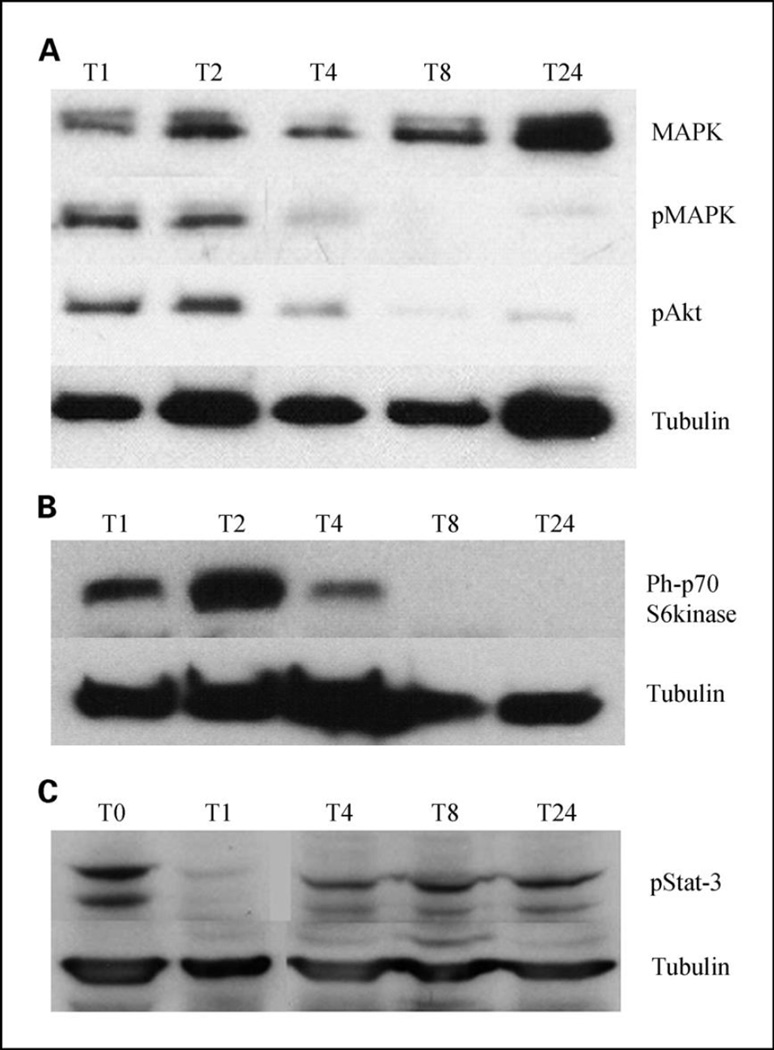
To examine this effect, we tested 10µg/ml Tris DBA on MAPK and Akt signaling pathway using B16 melanoma cells. Using a time course pattern, we found that it inhibited the phosphorylated forms of both downstream (Figure 2a). We further tested Tris DBA on human melanoma cell line and found that it inhibits phosphorylated forms of S6 kinase downstream (Figure 2b) and down regulates phospho-Stat-3 at shorter time intervals (1 and 4 hours) than after 24 hours of treatment (Figure 2c)
Figure 2. Western blot analysis using Phosphorylated forms of MAPK, Akt (a), p70 S6kinase (b), Stat-3 (c).
Western blot analysis of B16 and A375 cells treated with 10µg/ml Tris DBA at T0, T1, T2, T4, T8, T24. Cells were lysed and analyzed by using antibodies specific for the unphosphorylated form of MAPK, phosphorylated forms of MAPK, Akt, p70-S6kinase and Stat3. Tubulin was used as the loading control by using monoclonal anti β Tubulin antibody.
Inhibition of human N-myristoyltransferase-1 activity and N-myristoyltransferase-1 expression by Tris DBA
Gene array was performed on A375 melanoma cells treated with Tris-DBA palladium compared with control cells. Among the genes that was strongly downregulated as a result of treatment was N-myristoyltransferase 1 (NMT1). We confirmed this downregulation by RT-PCR (Figure 3a).
When purified human NMT was incubated in the presence of various concentrations of Tris DBA, the human NMT was inhibited in a concentration-dependent manner, with maximal inhibition at a concentration of 2.5 ± 0.97µM and half-maximal inhibition at 1.0 ± 0.26µM (Figure 3a). Further testing of 10µg/ml Tris DBA on NMT-1 expression on A375 cells, we found an 80% decrease compared to control (Figure 3b,c). Thus, Tris DBA is a novel and potent inhibitor of NMT-1 activity.
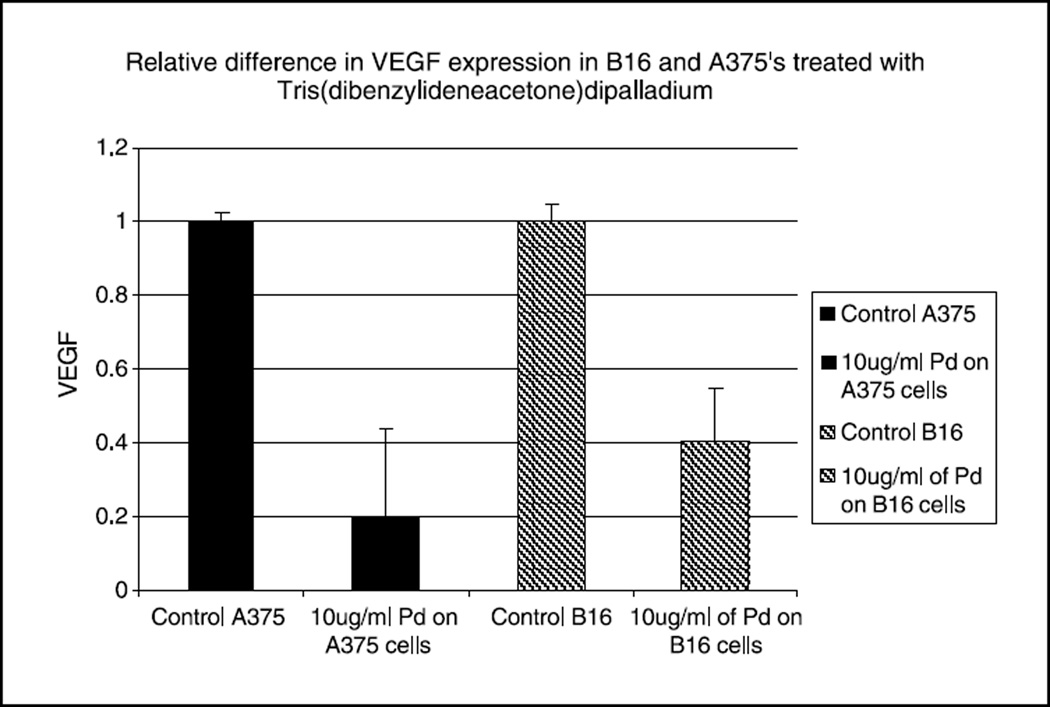
Tris DBA inhibits VEGF expression in murine and human melanoma cells in vitro
We tested 10µg/ml Tris DBA on VEGF expression on B16 cells and found a 60% decrease in VEGF expression compared to control. We further tested 10µg/ml of the same compound on A375 cells and found an 80% decrease compared to control (Figure 4).
Figure 4. Treatment of B16 and A375 cells with 10µg/ml of Tris DBA decreases levels of VEGF mRNA (corrected for 18S RNA).
Bars shown represent the average of triplicate experiments, and error bars indicate the standard error of the mean. Differences are significant p<0.05.
Tris DBA inhibits B16 and A375 melanoma growth in vivo
In order to determine if compounds that inhibit VEGF, phosphorylated forms of MAPK, Akt, Stat 3 and p-70 S6 kinase in vitro would affect melanoma formation in vivo, we injected one million B16 cells subcutaneously into six nude mice and one million A375 cells subcutaneously into six nude mice. Intraperitoneal treatment with Tris DBA resulted in a 97% decreased tumor volume compared to control when using the B16 murine melanoma model. In the A375 human melanoma model there was a 65% reduction in tumor volumes as compared to control (Figure 5). Neither local nor systemic toxicity was observed in any of the nude mice as a result of treatment.
DISCUSSION
Melanoma is a common solid tumor notorious for its high rate of metastasis and resistance to chemotherapy and radiation. Several factors may account for the resistance of melanoma to current therapies. First, melanomas are derived from melanocytes, specialized neural crest cells that are specialized to produce melanin. The production of melanin results in the generation of toxic reactive oxygen species and cytotoxic phenol derivatives, thus melanocytes are equipped with mechanisms to resist these insults. Recently, the microopthalmia gene (MITF), which is a master transcriptional switch of melanocytes, has been shown to possess antiapoptotic activity and is found in metastatic lesions at a high frequency (29, 30). Second, multiple signaling pathways are activated in melanoma. B-raf is mutated in many melanomas, resulting in constitutive activation of MAP kinase signaling (1, 29). N-ras is also mutated frequently in melanoma, resulting in activation of MAP kinase, phosphoinositol-3 kinase/Akt signaling, and S6 kinase activation (30, 31). While B-raf and constitutive MAP kinase activation is sufficient to cause transformation of melanocytes into melanoma (3, 4), other signal transduction events are frequently observed in B-raf mutant melanomas, such as loss of the tumor suppressor PTEN (31, 32). The consequences of PTEN loss is activation of phosphoinositol-3 kinase/Akt activation.
Multiple regimens have been tried for the treatment of locally advanced and metastatic melanoma. Initial trials several decades ago used agents such as hydroxyurea, and more recent agents used against melanoma include dacarbazine and platinum based therapies including cisplatin and carboplatin. Other therapies, including biochemotherapy, have included interleukin-2 infusion and infusion of lymphocytes which are present in melanoma lesions and have been expanded ex vivo (33). All of these therapies have had modest success in a minority of patients, but with significant toxicity, including pulmonary leak syndrome (34, 35, 36, 37, 38). Currently, interferon alpha is employed in high risk patients, and prolonged therapy results in a 10% long term survival benefit.
Targeted therapies have been attempted in melanoma. Sorafenib was developed as a B-raf inhibitor based upon the observation that B-raf mutation is common in melanoma. However, results from initial trials of sorafenib in melanoma have been disappointing (14). Everolimus has also been tried against human melanoma, and has not been successful as a single agent (39). Current knowledge of signaling may provide an explanation of why previous therapies have failed. Phosphoinositol-3 kinase activation has been shown to mediate against extrinsic pathways of apoptosis, which include apoptosis due to TRAIL, TNF alpha, and interferons (10). Monotherapies of these cytokines may be frustrated in the face of phosphoinositol-3 kinase activation. Similarly, apoptosis induced by tumor infiltrating lymphocytes may be frustrated by phosphoinositol-3 kinase activation. Phosphoinositol-3 kinase also activates VEGF expression, and in addition to stimulating angiogenesis, VEGF inhibits dendritic cell function, impairing immune responses to melanoma (41; 42; 43, 44;45).
Targeting MAP kinase as monotherapy in melanoma is clearly insufficient to eliminate melanoma in most patients. MAP kinase is activated in a majority of human melanomas, including those that lack B-raf mutation (3). In a previous study of human melanomas, we demonstrated that a subset of advanced melanomas had decreased MAP kinase activation, implying that additional signaling pathways are operative in vivo (3). Further support of this hypothesis is our previous finding that treatment of EBV-induced Burkitt’s lymphomas with antioxidants resulted in compensatory MAP kinase activation (17). It is likely that treatment of melanoma patients with sorafenib results in compensatory activation of non-MAP kinase pathways. Similarly, mTOR inhibition due to rapamycin and derivatives has been shown to result in compensatory Akt activation (46). Tris DBA has the advantage that it inhibits several pathways required for melanoma tumorigenesis, including MAP kinase activation, phosphoinositol-3 kinase/Akt activation, stat-3 activation, S6 kinase activation and downregulates NMT-1 at the level of enzyme activity and the level of mRNA. Downregulation of these pathways may lead to diminished transcription of NMT-1. While no drug is likely to be completely effective as monotherapy in melanoma, Tris DBA is well tolerated systemically in mice, and has a novel profile of action compared with other clinically used chemotherapeutic agents. Its ability to inhibit phosphoinositol-3 kinase activation may enhance the activity of cytokines which require Akt inactivation for optimal activity, and may enhance the activity of other chemotherapeutic agents. Our studies provide a rationale for the further investigation of Tris DBA in the treatment of malignant melanoma.
Acknowledgments
JLA was supported by the grant RO1 AR47901and P30 AR42687 Emory Skin Disease Research Core Center Grant from the National Institutes of Health as well as a Veterans Administration Merit Award and funding from the Jamie Rabinowitch-Davis and Minsk Foundations. RKS is supported by Canadian Institutes of Health Research, Canada; Grant Number: MOP-36484.
References
- 1.Pollock PM, Meltzer PS. A genome-based strategy uncovers frequent BRAF mutations in melanoma. Cancer Cell. 2002;2:5–7. doi: 10.1016/s1535-6108(02)00089-2. [DOI] [PubMed] [Google Scholar]
- 2.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, Salem G, Pohida T, Heenan P, Duray P, Kallioniemi O, Hayward NK, Trent JM, Meltzer PS. High frequency of BRAF mutations in nevi. Nat. Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 3.Cohen C, Zavala-Pompa A, Sequeira JH, Shoji M, Sexton DG, Cotsonis G, Cerimele F, Govindarajan B, Macaron N, Arbiser JL. Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin. Cancer Res. 2002;8:3728–3733. [PubMed] [Google Scholar]
- 4.Govindarajan B, Bai X, Cohen C, Zhong H, Kilroy S, Louis G, Moses M, Arbiser JL. Malignant transformation of melanocytes to melanoma by constitutive activation of mitogen-activated protein kinase kinase (MAPKK) signaling. J. Biol. Chem. 2003;278:9790–9795. doi: 10.1074/jbc.M212929200. [DOI] [PubMed] [Google Scholar]
- 5.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–2421. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 6.Dhawan P, Richmond A. A novel NF-kappa B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B activity in melanoma cells. J. Biol. Chem. 2002;277:7920–7928. doi: 10.1074/jbc.M112210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selzer E, Thallinger C, Hoeller C, Oberkleiner P, Wacheck V, Pehamberger H, Jansen B. Betulinic acid-induced Mcl-1 expression in human melanoma-mode of action and functional significance. Mol. Med. 2002;8:877–884. [PMC free article] [PubMed] [Google Scholar]
- 8.Satyamoorthy K, Li G, Vaidya B, Patel D, Herlyn M. Insulin-like growth factor-1 induces survival and growth of biologically early melanoma cells through both the mitogen-activated protein kinase and beta-catenin pathways. Cancer Res. 2001;61:7318–7324. [PubMed] [Google Scholar]
- 9.Dhawan P, Singh AB, Ellis DL, Richmond A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer Res. 2002;62:7335–7342. [PubMed] [Google Scholar]
- 10.Soengas MS, Gerald WL, Cordon-Cardo C, Lazebnik Y, Lowe SW. Apaf-1 expression in malignant melanoma. Cell Death. Differ. 2006;13:352–353. doi: 10.1038/sj.cdd.4401755. [DOI] [PubMed] [Google Scholar]
- 11.Bhoumik A, Gangi L, Ronai Z. Inhibition of melanoma growth and metastasis by ATF2-derived peptides. Cancer Res. 2004;64:8222–8230. doi: 10.1158/0008-5472.CAN-04-0714. [DOI] [PubMed] [Google Scholar]
- 12.Shattuck-Brandt RL, Richmond A. Enhanced degradation of I-kappaB alpha contributes to endogenous activation of NF-kappaB in Hs294T melanoma cells. Cancer Res. 1997;57:3032–3039. [PubMed] [Google Scholar]
- 13.Huang S, DeGuzman A, Bucana CD, Fidler IJ. Level of interleukin-8 expression by metastatic human melanoma cells directly correlates with constitutive NF-kappaB activity. Cytokines Cell Mol. Ther. 2000;6:9–17. doi: 10.1080/13684730050515868. [DOI] [PubMed] [Google Scholar]
- 14.Karakousis CP, Getaz EP, Bjornsson S, Henderson ES, Irequi M, Martinez L, Ospina J, Cavins J, Preisler H, Holyoke E, Holtermann O. cis-Dichlorodiammineplatinum(II) and DTIC in malignant melanoma. Cancer Treat. Rep. 1979;63:2009–2010. [PubMed] [Google Scholar]
- 15.Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, Gibbens I, Hackett S, James M, Schuchter LM, Nathanson KL, Xia C, Simantov R, Schwartz B, Poulin-Costello M, O'dwyer PJ, Ratain MJ. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br. J. Cancer. 2006;95:581–586. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arbiser JL. Molecular regulation of angiogenesis and tumorigenesis by signal transduction pathways: evidence of predictable and reproducible patterns of synergy in diverse neoplasms. Semin. Cancer Biol. 2004;14:81–91. doi: 10.1016/j.semcancer.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Cerimele F, Battle T, Lynch R, Frank DA, Murad E, Cohen C, Macaron N, Sixbey J, Smith K, Watnick RS, Eliopoulos A, Shehata B, Arbiser JL. Reactive oxygen signaling and MAPK activation distinguish Epstein-Barr Virus (EBV)-positive versus EBV-negative Burkitt's lymphoma. Proc. Natl. Acad. Sci. U. S. A. 2005;102:175–179. doi: 10.1073/pnas.0408381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markovic SN, Geyer SM, Dawkins F, Sharfman W, Albertini M, Maples W, Fracasso PM, Fitch T, Lorusso P, Adjei AA, Erlichman C. A phase II study of bortezomib in the treatment of metastatic malignant melanoma. Cancer. 2005;103:2584–2589. doi: 10.1002/cncr.21108. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton AL, Eder JP, Pavlick AC, Clark JW, Liebes L, Garcia-Carbonero R, Chachoua A, Ryan DP, Soma V, Farrell K, Kinchla N, Boyden J, Yee H, Zeleniuch-Jacquotte A, Wright J, Elliott P, Adams J, Muggia FM. Proteasome inhibition with bortezomib (PS-341): a phase I study with pharmacodynamic end points using a day 1 and day 4 schedule in a 14-day cycle. J. Clin. Oncol. 2005;23:6107–6116. doi: 10.1200/JCO.2005.01.136. [DOI] [PubMed] [Google Scholar]
- 20.Arbiser JL, Klauber N, Rohan R, van LR, Huang MT, Fisher C, Flynn E, Byers HR. Curcumin is an in vivo inhibitor of angiogenesis. Mol. Med. 1998;4:376–383. [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson TP, Ehlers T, Hubbard RB, IV, Bai X, Arbiser JL, Goldsmith DJ, Bowen JP. Design, synthesis, and biological evaluation of angiogenesis inhibitors: aromatic enone and dienone analogues of curcumin. Bioorg. Med. Chem. Lett. 2003;13:115–117. doi: 10.1016/s0960-894x(02)00832-6. [DOI] [PubMed] [Google Scholar]
- 22.Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62:3868–3875. [PubMed] [Google Scholar]
- 23.Khor TO, Keum YS, Lin W, Kim JH, Hu R, Shen G, Xu C, Gopalakrishnan A, Reddy B, Zheng X, Conney AH, Kong AN. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res. 2006;66:613–621. doi: 10.1158/0008-5472.CAN-05-2708. [DOI] [PubMed] [Google Scholar]
- 24.Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, Murad E, Dubiel W, Soff G, Arbiser JL. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J. Biol. Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 25.Arbiser JL, Panigrathy D, Klauber N, Rupnick M, Flynn E, Udagawa T, D'Amato RJ. The antiangiogenic agents TNP-470 and 2-methoxyestradiol inhibit the growth of angiosarcoma in mice. J Am Acad Dermatol. 1999;40:925–929. doi: 10.1016/s0190-9622(99)70080-0. [DOI] [PubMed] [Google Scholar]
- 26.Raju RV, Moyana TN, Sharma RK. Overexpression of human N-myristoyltransferase utilizing a T7 polymerase gene expression system. Protein Expr. Purif. 1996;7:431–437. doi: 10.1006/prep.1996.0064. [DOI] [PubMed] [Google Scholar]
- 27.King MJ, Sharma RK. N-myristoyltransferase assay using phosphocellulose paper binding. Anal Biochem. 1991;199:149–153. doi: 10.1016/0003-2697(91)90082-5. [DOI] [PubMed] [Google Scholar]
- 28.King MJ, Sharma RK. Identification, purification and characterization of a membrane-associated N-myristoyltransferase inhibitor protein from bovine brain. Biochem. J. 1993;291:635–639. doi: 10.1042/bj2910635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuveson DA, Weber BL, Herlyn M. BRAF as a potential therapeutic target in melanoma and other malignancies. Cancer Cell. 2003;4:95–98. doi: 10.1016/s1535-6108(03)00189-2. [DOI] [PubMed] [Google Scholar]
- 30.Haluska FG, Tsao H, Wu H, Haluska FS, Lazar A, Goel V. Genetic alterations in signaling pathways in melanoma. Clin. Cancer Res. 2006;12:2301s–2307s. doi: 10.1158/1078-0432.CCR-05-2518. [DOI] [PubMed] [Google Scholar]
- 31.Tsao H, Zhang X, Benoit E, Haluska FG. Identification of PTEN/MMAC1 alterations in uncultured melanomas and melanoma cell lines. Oncogene. 1998;16:3397–3402. doi: 10.1038/sj.onc.1201881. [DOI] [PubMed] [Google Scholar]
- 32.Teng DH, Hu R, Lin H, Davis T, Iliev D, Frye C, Swedlund B, Hansen KL, Vinson VL, Gumpper KL, Ellis L, El-Naggar A, Frazier M, Jasser S, Langford LA, Lee J, Mills GB, Pershouse MA, Pollack RE, Tornos C, Troncoso P, Yung WK, Fujii G, Berson A, Steck PA. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997;57:5221–5225. [PubMed] [Google Scholar]
- 33.Legha SS. Durable complete responses in metastatic melanoma treated with interleukin-2 in combination with interferon alpha and chemotherapy. Semin Oncol. 1997;24:S39–S43. [PubMed] [Google Scholar]
- 34.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maker AV, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Hughes M, Yellin MJ, Haworth LR, Levy C, Allen T, Mavroukakis SA, Attia P, Rosenberg SA. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J. Immunother. 2006;29:455–463. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Margolin KA, Liu PY, Unger JM, Fletcher WS, Flaherty LE, Urba WJ, Hersh EM, Hutchins LE, Sosman JA, Smith JW, Weiss GR, Sondak VK. Phase II trial of biochemotherapy with interferon alpha, dacarbazine, cisplatin and tamoxifen in metastatic melanoma: a Southwest Oncology Group trial. J. Cancer Res. Clin. Oncol. 1999;125:292–296. doi: 10.1007/s004320050276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis KD, Gibbs P, O'Day S, Richards J, Weber J, Anderson C, Zeng C, Baron A, Russ P, Gonzalez R. A phase II study of biochemotherapy for advanced melanoma incorporating temozolomide, decrescendo interleukin-2 and GM-CSF. Cancer Invest. 2005;23:303–308. doi: 10.1081/cnv-58832. [DOI] [PubMed] [Google Scholar]
- 38.Atkins MB. Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin Cancer Res. 2006;12:2353s–2358s. doi: 10.1158/1078-0432.CCR-05-2503. [DOI] [PubMed] [Google Scholar]
- 39.Margolin K, Longmate J, Baratta T, Synold T, Christensen S, Weber J, Gajewski T, Quirt I, Doroshow JH. CCI-779 in metastatic melanoma: a phase II trial of the California Cancer Consortium. Cancer. 2005;104:1045–1048. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- 40.Larribere L, Khaled M, Tartare-Deckert S, Busca R, Luciano F, Bille K, Valony G, Eychene A, Auberger P, Ortonne JP, Ballotti R, Bertolotto C. PI3K mediates protection against TRAIL-induced apoptosis in primary human melanocytes. Cell Death. Differ. 2004;11:1084–1091. doi: 10.1038/sj.cdd.4401475. [DOI] [PubMed] [Google Scholar]
- 41.Burdelya L, Kujawski M, Niu G, Zhong B, Wang T, Zhang S, Kortylewski M, Shain K, Kay H, Djeu J, Dalton W, Pardoll D, Wei S, Yu H. Stat3 activity in melanoma cells affects migration of immune effector cells and nitric oxide-mediated antitumor effects. J Immunol. 2005;174:3925–3931. doi: 10.4049/jimmunol.174.7.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arbiser JL, Moses MA, Fernandez CA, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, Byers HR, Folkman J. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 45.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 46.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]