Abstract
Disentanglement of functional complexity associated with plant mitogen-activated protein kinase (MAPK) signaling has benefited from transcriptomic, proteomic, phosphoproteomic, and genetic studies. Published transcriptomic analysis of a double homozygous recessive anp2anp3 mutant of two MAPK kinase kinase (MAPKKK) genes called Arabidopsis thaliana Homologues of Nucleus- and Phragmoplast-localized Kinase 2 (ANP2) and 3 (ANP3) showed the upregulation of stress-related genes. In this study, a comparative proteomic analysis of anp2anp3 mutant against its respective Wassilevskaja ecotype (Ws) wild type background is provided. Such differential proteomic analysis revealed overabundance of core enzymes such as FeSOD1, MnSOD, DHAR1, and FeSOD1-associated regulatory protein CPN20, which are involved in the detoxification of reactive oxygen species in the anp2anp3 mutant. The proteomic results were validated at the level of single protein abundance by Western blot analyses and by quantitative biochemical determination of antioxidant enzymatic activities. Finally, the functional network of proteins involved in antioxidant defense in the anp2anp3 mutant was physiologically linked with the increased resistance of mutant seedlings against paraquat treatment.
Keywords: mitogen-activated protein kinase kinase kinase, ANP2, ANP3, Arabidopsis, proteomics, signaling antioxidant defense, oxidative stress
Graphical Abstract

INTRODUCTION
Mitogen-activated protein kinase (MAPK) signaling lies at the core of plant growth, development, environmental perception and stress responses.1,2 It is characterized by cross-talk, redundancy and complexity exemplified by the presence of 20 MAPKs (EC 2.7.11.24), 10 MAPKKs (EC 2.7.12.2), and 60–80 MAPKKKs (EC 2.7.11.25) in the Arabidopsis genome and their involvement in heavily interconnected pathways.3
A well characterized example of cross-talk in plant MAPK signaling can be found in pathways initiated by a MAPKKK family called Arabidopsis nucleus- and phragmoplast-localized kinase 1 (ANP1), 2 (ANP2), and 3 (ANP3). These are homologous to the tobacco NPK1,4 a MAPKKK targeting cytokinetic phragmoplast progression.5 Similarly, all three members of the Arabidopsis ANP family regulate cytokinesis and cell expansion by cortical microtubule organization of growing or differentiating plant cells.4,6,7 While single knockout mutants of any ANP member show no phenotype changes, double mutants and particularly anp2anp3 display aberrations related to both cytokinetic defects and cortical microtubule misorganization4,6,7 affecting the overall vegetative growth. Thus, anp2anp3 double mutant seedlings are reduced in size and fresh weight, and they show irregular hypocotyl4 and root7 cell outlines resulting from radial cell swelling.
The full pathway regulating cytokinetic progression downstream of ANPs (MAPKKKs) is mediated by the MKK6 (MAPKK) and results in the activation of the MPK4 (MAPK) while the activation of the entire pathway depends on the interaction of ANPs with kinesin-related protein HINKEL8.
However, earlier studies implicated ANPs in Arabidopsis stress responses, particularly under oxidative stress.9 In this case, oxidative-stress ANP-mediated signaling occurs via the activation of MPK3 and MPK6 in Arabidopsis.9,10 Although MPK4 is also activated by oxidative stress,11 its activation rather occurs through the MEKK1-MKK1/2 pathway.12,13
Therefore, the ANP family of MAPKKKs initiates two different signaling cascades that are functionally separated, while MPK4, which assumes a developmental role downstream of ANPs, is likely excluded from stress-induced ANP pathways.
MAPK cascades are important regulators of antioxidant defense. MKK5, a mitogen-activated protein kinase kinase mediates the high light-induced expression of genes of two copper/zinc SOD isoforms, namely CuZnSOD1 and CuZnSOD2.14 Additionally, the expression of CATALASE 1 is mediated by AtMKK1 in the presence of ABA and stress conditions.15,16 Microarray analysis of gene expression showed transcriptional upregulation of genes related to oxidative stress in anp2anp3 mutants, suggesting negative regulation of the respective stress responses by ANP2 and ANP3.4 However, these results were neither correlated at the respective protein level nor validated by physiological stress responses of the mutant.
For the reasons above, and considering the involvement of ANPs in stress responses through MPK3 and MPK6,9 we analyzed the proteome of whole anp2anp3 seedlings. We found that, by comparison to the Wassilevskaja ecotype (Ws) wild type, proteins involved in photosynthesis and oxidative stress were differentially regulated in the anp2anp3 double mutant. Results from proteomic analyses suggested decreased ROS production in the mutant and increased tolerance to oxidative stress. The above assumptions were validated by appropriate biochemical and physiological approaches.
EXPERIMENTAL PROCEDURES
Plant material and cultivation
Seeds of Arabidopsis ihaliana, ecotype Wassilevskaja (Ws) and double mutant anp2anp3 derived from the same ecotype background4 were surface sterilized and placed on 1/2 Murashige-Skoog solid culture medium17 (pH 5.7) containing 1% (w/v) sucrose and 0.8% (w/v) phytagel. Seeds were stratified at 4 °C for 48 h and grown vertically at environmental conditions (16 h light/8 h dark, 22 °C). Ten days after germination, seedlings were collected for proteomic, biochemical and histochemical analyses as well as chlorophyll fluorescence imaging. Seedlings of anp2anp3 mutant were preselected on the basis of known root and root hair phenotype.7 For oxidative stress resistance evaluation, 3-day-old seedlings were transferred to a 1/2 Murashige-Skoog solid culture medium supplemented with 0.5 µM paraquat. The oxidative stress resistance was evaluated and recorded 7 days later, on 10th day of plants age. For biochemical analyses on paraquat-treated plants, 10 days old Ws and anp2anp3 seedlings were surface treated with liquid 1/2 Murashige-Skoog media (control) or with the same media supplemented with 15 µM paraquat for 5 h. Seedlings were kept horizontally on variable speed rocker under slow shaking to prevent complete submergence of the plants. Proteome mapping of anp2anp3 mutant was performed from four biological replicates while all other analyses were carried out in three independent biological replicates.
Proteomic analysis
Preparation of peptide digests
Arabidopsis wild type and anp2anp3 mutant seedlings were homogenized in liquid nitrogen to fine powder and extracted in buffer containing 0.9 M sucrose, 0.1 M Tris-HCl, pH 8.8, 10 mM EDTA, 100 mM KCl and 0.4% v/v 2-mercaptoethanol. Total proteins were fractionated from the extract using phenol extraction and sequential precipitation in methanolic ammonium acetate (100 mM), 80% v/v aqueous acetone, 70% v/v ethanol, followed by final incubation with 80% v/v acetone.18,19 The final protein pellet was dissolved in 6 M urea in 100 mM Tris-HCl, pH 6.8. Prior to trypsin digestion, proteins were reduced and alkylated as previously described.18 Digested peptides were desalted on C18 cartridges (Sep Pak, Waters, Milford, MA, USA) and vacuum-dried.
2-D LC-MS/MS analysis and quantitation
Immediately prior to the 2-D LC-MS/MS analysis, tryptic digests were dissolved in 20 µL of 0.1% v/v formic acid and 5% v/v acetonitrile. The ion exchange and reverse phase online chromatography and mass spectra collection was carried out according to previously published procedure.18 Briefly, the analysis was performed using the Surveyor auto sampler and the Surveyor HPLC unit linked in-line to LCQ Deca XP Plus – ESI ion trap mass spectrometer (Thermo Scientific, Waltham, MA, USA). The HPLC included a 2-D LC separation on a strong cation exchange column (SCX BioBasic 0.32 × 100 mm), followed by a reverse phase column (BioBasic C18,0.18 × 100 mm; both by Thermo Scientific, Waltham, MA, USA). For both columns, a flow rate of 3.0 µL.min−1 was used. A discontinuous gradient of 0, 10, 15, 20, 25, 30, 35, 40, 45, 50, 57, 64, 90, and 700 mM ammonium acetate in 5% v/v acetonitrile and 0.1% v/v formic acid was applied for SCX column. The reverse phase column was subjected to a 59 min gradient of acetonitrile (in 0.1% v/v formic acid) as follows: 5%–30% v/v for 30 min, 30%–65% v/v for 9 min, 95% v/v for 5 min, 5% v/v for 15 min. The mass spectra were collected in the data dependent mode, with dynamic exclusion applied, in four scan events: one MS scan (m/z range: 300–1700) followed by three MS/MS scans for the three most intense ions detected in MS scan. Other critical parameters were set as given here: Normalized collision energy: 35%, AGC (automatic gain control) “on” with MSn Target 4 × 104, isolation width (m/z): 3.0, capillary temperature 170 °C, spray voltage 2.7 kV, maximum injection time 50 and 400 ms for MS and MSMS mode, respectively.
The .raw files were searched using the SEQUEST algorithm of the Proteome Discoverer 1.1.0 (Thermo Scientific, Waltham, MA, USA) software. The workflow option was utilized with selection of parameters: Lowest and highest charge: +1 and +3, respectively; minimum and maximum precursor mass: 300 and 6,000 Da, respectively; minimum S/N ratio: 3; enzyme: trypsin; maximum missed cleavages: 2; LCQ default (according to manufacturer) precursor and fragment mass tolerance: 2.5 and 0.8 Da, respectively; FDR≤ 0.01; dynamic modifications: cysteine carbamidomethylation (+57.021), methionine oxidation (+15.995), methionine dioxidation (+31.990).
The spectral data were matched against target and decoy databases. The NCBI (www.ncbi.nlm.nih.gov) Arabidopsis genus taxonomy referenced protein database (67,924 entries as of November 2012) served as the target database, while its reversed copy served as a decoy database. The Proteome Discoverer results files (.msf) were uploaded to ProteoIQ 2.1 (NuSep) software for further filtering. Only proteins detected with at least three spectral counts, FDR ≤ 1%, 95% probability and listed as “Top” proteins (defined by ProteoIQ “Within a protein group, each and every respective peptide could be matched to the top protein”) are considered as high confidence matches and are presented in the results.
Label-free quantitative analysis was carried out as previously published.18 Briefly, the unfiltered TurboSEQUEST result files were uploaded to ProtQuant software20 for analysis based on sum of TurboSEQUEST cross correlation factors (Xcorr) of all identified peptides in three replicates of each biological sample. One-way ANOVA analysis was used to identify statistically significant (p < 0.05) differences in protein amount.
Protein extract preparation for immunoblotting, native electrophoresis and enzyme activity assays
Liquid nitrogen powders of wild type and anp2anp3 mutant seedlings were homogenized with 50 mM sodium phosphate buffer (pH 7.8) containing 1 mM EDTA, 10% v/v glycerol and “Complete” EDTA-free protease inhibitor cocktail (Roche, Basel, Switzerland). Subsequently, the extract was centrifuged at 12,000 g for 15 min at 4 °C and the protein concentration of the supernatant was measured using the Bradford assay.21 The extract was used for superoxide dismutase (SOD) isozyme activity detection as well as immunoblotting on native polyacrylamide gels and spectrophotometric measurements of dehydroascorbate reductase (DHAR) and ascorbate peroxidase (APX) activities. For immunoblot analysis on SDS PAGE gels, the extracts containing equal amount of proteins were supplemented with 4 times concentrated Laemmli SDS buffer (to reach final concentration of 10% v/v glycerol, 60 mM Tris/HCl pH 6.8, 2% w/v SDS, 0.002% w/v bromophenol blue and 5% v/v β -mercaptoethanol), boiled for 5 min at 95 °C and the insoluble particles were removed by centrifugation.
Immunoblot analysis
Protein extracts were separated either by SDS-PAGE or native PAGE (MINI-Protean Tetra Cell, Biorad, Hercules, CA, USA). Identical protein amounts were loaded for each sample. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (GE Healthcare, Little Chalfont, United Kingdom) in a wet tank unit (Bio-Rad) at 100 V for 1.5 h. For immuno-detection of protein bands, the membrane was blocked with 5% w/v low-fat dry milk in Tris-buffer-saline (TBS, 100 mM Tris-HCl; 150 mM NaCl; pH 7.4) for 1 h, and subsequently incubated with anti-FeSOD1 and anti-MnSOD primary antibodies (Agrisera, Vännäs, Sweden) diluted 1:3,000 and 1:2,000 respectively in TBS-T (TBS; 0.1% v/v Tween 20) containing 1% w/v low-fat dry milk at 4 °C overnight. After washing in TBS-T, the membrane was incubated at room temperature for 1.5 h with a horseradish peroxidase conjugated goat anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), diluted 1:5,000 in TBS-T containing 1% w/v BSA Following at least three washing steps in TBST, proteins were detected by incubating the membrane in Clarity Western ECL substrate (Biorad). Luminescence was detected using Chemidoc MP documentation system (Biorad).
Analysis of SOD isozymes
Isozymes of SOD were separated on 10% native PAGE at constant 10 mA/gel. To differentiate activities of different SOD isoforms22, samples were loaded on three separate gels. One of the gels was preincubated for 15 min in 5 mM hydrogen peroxide (H2O2), in 50 mM sodium phosphate buffer (pH 7.8), which inhibits CuZnSOD and FeSOD but does not affect the MnSOD isozymes. The second gel was preincubated in 2 mM KCN (in 50 mM sodium phosphate buffer, pH 7.8) for 15 min, which inhibits CuZnSOD, but has no effect on FeSOD and MnSOD activity. The last gel was preincubated for the same period of time in 50 mM sodium phosphate buffer, pH 7.8 without the addition of an inhibitor. Afterward, the gels were stained according to modified method of Beauchamp and Fridovich23 by two-step incubation in dark in a solution containing 0.6 mM nitroblue tetrazolium (NBT) in 50 mM sodium phosphate buffer (pH 7.8), followed by incubation in a solution containing 0.06 mM riboflavin, 5 mM EDTA and 0.25% v/v TEMED in 50 mM sodium phosphate buffer (pH 7.8). Finally, the gels were exposed to light until the SOD activity appeared as translucent bands on a dark background. The bands intensities were measured using Image Lab software (Biorad). Statistical evaluation of data (3 replicates) was carried out using Student’s t test. As a loading control equal amounts of protein were resolved by SDS-PAGE as described above and stained with colloidal coomassie blue.
Spectrophotometric measurement of APX and DHAR activities, and ascorbate content
APX activity was measured following the H2O2-dependent oxidation of ascorbic acid at 290 nm (extinction coefficient 2.8 mM−1.cm−1) as described by Amako et al24 DHAR was measured by following the increase in absorbance at 265 nm due to glutathione dependent production of ascorbate.25 The ascorbate content was measured in trichloroacetic acid-extracted samples using an α–α′-bipyridyl-based colorimetric assay as described by Gillespie and Ainsworth.26
Measurement of NADPH oxidase activity
NADPH oxidase activity was measured in membrane fraction according to Saghi and Fluhr.27 For extraction of membrane fraction, seedlings were homogenized in ice-cold extraction buffer (50 mM HEPES, pH 7.2, 0.1 mM MgCl2, 3 mM EDTA, 0.25 M sucrose, 1 mM DTT, 0.6% w/v poly(vinylpolypyrrolidone), 3.6 mM L-cysteine, “Complete” EDTA free protease inhibitor cocktail from Roche). The homogenate was centrifuged at 10,000 g for 45 min at 4 °C, and the membrane fraction was isolated by ultracentrifugation of the supernatant at 203,000 g for 5 min. The resulting pellet was resuspended with 10 mM TrisHCL pH 7.4. The NADPH-dependent superoxide (O2•−) - generating activity was determined spectrophotometrically by following the reduction of sodium 3′-[l-[phenylamino-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate (XTT) at 492 nm for 5 min. The rate of NADPH dependent O2•− generation was calculated using an extinction coefficient of 0.26 mM. cm−1.
Measurement of O2•− production
Production of O2•− was measured as described by Able et al.28 by monitoring the reduction of XTT. Equal amount of fresh material for both wild type and mutant seedlings was homogenized with ice-cold 50 mM TrisHCl buffer (pH 7.5) and centrifuged at 10,000 g for 10 min. The reaction mixture contained 50 mM TrisHCl buffer (pH 7.5), 30 µL of sample and 0.5 mM XTT. The reduction of XTT was determined at 470 nm for 1h. Corrections were made for the background absorbance in the presence of 100 units of commercial SOD (Sigma S4636).
Measurement of H2O2 levels
The levels of H2O2 were measured spectrophotometrically using xylenol orange assay based on the oxidation of ferrous (Fe2+) to ferric (Fe3+) ions in the presence of soluble peroxides.29 Ws and anp2anp3 seedlings (100 µg in fresh weight) were homogenized by liquid nitrogen and incubated for 15 min on ice after the addition of cold distilled water (300 µL). Following centrifugation (13,000 g for 5 min), 40 µL of the extract was used for H2O2 level estimation. The reaction mixture contained 250 µM ammonium sulfate, 250 µM ferrous sulfate, 100 µM sorbitol, 1% (v/v) ethanol and 100 µM xylenol orange in 25 mM sulfuric acid. The difference in absorbance between 550 and 800 nm generated after 15 min was used for estimation of H2O2 level according to calibration curve.
Histochemical detection of O2•− and H2O2 production
Seedlings of wild type and anp2anp3 mutant were used for visualization of O2•− production using NBT staining.30 After a brief vacuum infiltration (3 × 30 s) in NBT solution (4.3 mM) in 10 mM potassium phosphate buffer (pH 7.8), whole seedlings were stained for 30 min in the dark. Stained seedlings were boiled in clearing solution containing 20% v/v acetic acid, 20% v/v glycerol and 60% v/v ethanol for 5 min and stored in mixture of 20% glycerol v/v and 80% v/v ethanol. Reduced NBT was visualized as a dark blue-colored formazan deposit.
Seedlings of wild type and anp2anp3 mutant were used for in situ detection of H2O2 with 3,3′-diaminobenzidine (DAB).31 Briefly, seedlings were after brief vacuum infiltration in a solution containing 4.7 mM DAB, Tween 20 (0.05% v/v) and 10 mM sodium phosphate buffer (pH 7.0) placed in the dark for 8 h. The stained seedlings were cleared by boiling into 20% v/v acetic acid, 20% v/v glycerol and 60% v/v ethanol for 15 min and stored in the mixture of 20% glycerol v/v and 80% v/v ethanol. Production of H2O2 was visualized as a brown precipitate of oxidized DAB.
The stained seedlings were observed with a Leica M165FC stereomicroscope (Leica Microsystems, Wetzlar, Germany). For detailed images acquisition, a light microscope Zeiss Axio Imager M2 equipped with DIC optics (Carl Zeiss, Jena, Germany) was used. Settings were identical for all the pictures in the experiment. The ImageJ software was used to assess the mean staining intensity of labeled cotyledons and leaves. The data were statistically evaluated by Student’s t test.
Chlorophyll fluorescence imaging
The chlorophyll fluorescence was monitored using a FluorCam 700 MF imaging system (Photon Systems Instruments, Czech Republic). All measurements were performed in 12–15 replicates. To measure fluorescence signal, short, several microseconds-lasting flashes of red light were applied in 20 ms intervals. The fluorescence signal was calculated by pulse amplitude modulation (the signals measured before the flashes were subtracted from the signal measured during the flashes). Overall integral light intensity of the measuring flashes was low enough to avoid the closure of the reaction centers of photosystem II (PSII). The minimum chlorophyll fluorescence yield (F0) was determined after 30 min of dark adaptation by application of the measuring flashes. For the determination of maximum chlorophyll fluorescence (FM), a saturating pulse of 1.6 s (white light, 2,500 µmol m−2 s−1) was applied in dark adapted state. To determine the maximum fluorescence yield during light adaptation (FM′), plants were exposed to red actinic light (230 µmol m−2 s−1) after next 2 min of dark adaptation. After 3 s, the actinic light was accompanied by series of saturating pulses applied in 24 s intervals for 2 min and additional 8.5 min in 70 s intervals.
The maximum quantum efficiency of PSII photochemistry (FV/FM) was calculated as (FM – F0)/FM; the effective quantum yield of electron transport (ΦPSII) as (FM′ – Ft)/FM′; the nonphotochemical quenching of chlorophyll fluorescence (qN) as 1 – (FM′ – F0′)/(FM – F0) and the excitation pressure on PSII (1 – qP) as 1 – (FM′– Ft)/(FM′ – F0′), where Ft is the fluorescence yield measured just prior the application of saturating pulse and F0′ is the minimal fluorescence for light adapted state calculated as 32.
The two-way t test was performed for statistical analyses using the OriginPro 8.5.1 (OriginLab Corporation, Northampton, MA, USA).
RESULTS
General overview of the differential proteome of anp2anp3 mutant compared to wild type
Global proteome analysis of anp2anp3 seedlings resulted in the identification of 630 proteins. Detailed information pertinent to peptide and protein identification is publicly available at http://www.igbb.msstate.edu/journal_data/Anp_Arabidopsis.html, http://www.igbb.msstate.edu/journal_data/ANP_matched_peptides.csv, and http://www.igbb.msstate.edu/journal_data/ANP_protein&peptide_results.csv, as well as in Tables S1–S3.
Identified proteins in Ws and anp2anp3 were functionally classified using the STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) web-based application,33 which allows functional grouping of proteins according to KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways (Figure 1). Functional classes containing less than 0.3% of the identified proteins in both proteomes are not included in the chart. Complete outputs of the KEGG-based classification of Ws and anp2anp3 proteomes are presented in Table S4 and Table S5. The most abundant functional classes referred to metabolic pathways (24% in both proteomes), biosynthesis of secondary metabolites (11.2% in both proteomes), ribosome assembly (6% in both proteomes) and photosynthesis (3.85% in Ws and 4.5% in anp2anp3). Additionally, some protein classes appear to be overrepresented either in the wild type or in the anp2anp3 mutant. For example, proteins involved in glucosino-late biosynthesis were identified only in the anp2anp3 mutant. Next, functional classes such as proteins involved in galactose metabolism and glycerolipid metabolism were more than 3-fold more abundant in the anp2anp3 mutant as compared to Ws. On the other hand, functional classes of proteins involved in phenylalanine, tyrosine and tryptophan biosynthesis as well as in tyrosine metabolism and RNA transport were underrepresented in the mutant, containing 5-, 4-, and 3-fold less proteins, respectively, as compared to Ws.
Figure 1.
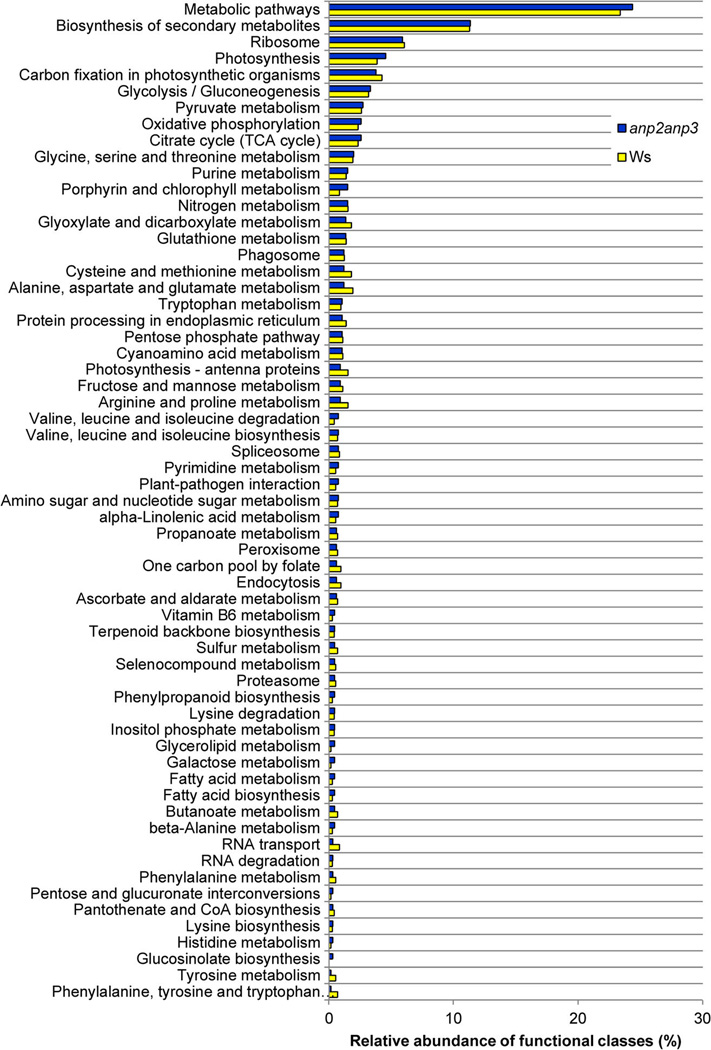
Classification of proteins identified in the Arabidopsis anp2anp3 double mutant and Ws according to KEGG pathways.
Statistically significant quantitative results were obtained via comparative proteomic analysis of anp2anp3 double mutant vs wild type Arabidopsis plants. Twenty-five differentially abundant proteins were detected (Table 1). These proteins were categorized into seven different functional classes (Table 1). An overview of functional protein networks affected in the anp2anp3 mutant as suggested by present comparative proteomic analysis is shown in Figure S1. Proteins differentially abundant in the anp2anp3 mutant vs wild type seedling plants are involved in calcium ion signaling (e.g., calmodulin isoform CAM434 and chaperonin 2035), vesicular trafficking and cytokinesis (e.g., RabEld36 ), photosynthesis, and finally oxidative stress (see next section). Notably, lipoxygenase 2 (LOX2) was the most upregulated protein in the mutant (14.57-fold) when compared to the wild type. LOX2 is a 13S linolenic acid peroxidase involved in wound-induced jasmonic acid biosynthesis.37 Jasmonic acid signaling pathway is interconnected with MAPK signaling via the MKK3/MPK6 pathway.38 Our data indicate that perturbed function of ANPs in the anp2anp3 double mutant leads to LOX2 accumulation, and therefore, ANPs likely play a negative role in the regulation of jasmonic acid signaling.
Table 1.
Proteins Differentially Abundant in the anp2anp3 Mutant as Compared to the Ws Wild Type
| Sequence ID | Protein | Protein Weight (kDa) |
Protein Isoelectric Point (pI) |
Total Scorea |
Total % Seq Coverage |
Total Peptides |
Total Spectra |
Epxression in anp2anp3 vs Ws |
anp2anp3/ Ws ratiob | 1-way ANOVA p factor |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Photosyntesis and Carbon Fixation | |||||||||||
| NP_186799.2 | At3g01S00 | CA1 (carbonic anhydrase 1) | 37.41 | 5.67 | 26.42 | 37.46 | 8 | 45 | down | 0.68 | 0.025 |
| NP_176291.1 | Atlg609S0 | FED A (ferredoxin 2) | 15.51 | 4.18 | 8.26 | 54.05 | 2 | 32 | down | 0.58 | 0.016 |
| NP_051084.1 | NA | photosystem II 47 kDa protein | 55.98 | 6.43 | 11.92 | 9.06 | 4 | 8 | down | 0.29 | 0.036 |
| NP_5638S2.1 | Atlg09780 | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase 1 |
60.52 | 5.20 | 18.29 | 20.83 | 6 | 21 | down | 0.27 | 0.039 |
| NP_172153.1 | Atlg06680 | PSBP-1 (oxygen-evolving enhancer protein 2) |
28.11 | 8.38 | 13.46 | 19.85 | 4 | 44 | down | 0.65 | 0.002 |
| NP_180637.2 | At2g30790 | PSBP-2 (photosystem II subunit P-2) | 28.15 | 5.88 | 15.87 | 21.07 | 4 | 82 | down | 0.53 | 6.04 × 10−04 |
| NP_051067.1 | AtCg00490 | ribulose-1,5-bisphosphate carboxylase/ oxygenase large subunit |
52.90 | 5.85 | 109.16 | 57.62 | 33 | 652 | down | 0.81 | 1.85 × 10−05 |
| NP_192186.1 | At4g02770 | PSAD-1 (photosystem I subunit D-1) | 22.57 | 10.17 | 30.05 | 50.00 | 10 | 89 | up | 2.14 | 2.67 × 10−04 |
| NP_192772.1 | At4gl0340 | LHCB5 (light harvesting complex of photosystem II 5); |
30.12 | 5.97 | 23.12 | 40.71 | 8 | 24 | down | 0.13 | 0.0158 |
| NP_173459.1 | Atlg20340 | DRT112 (DNA-damage-repair/toleration protein 112) |
16.96 | 4.92 | 6.34 | 38.92 | 2 | 6 | down | 0.48 | 0.0023 |
| Stress Response | |||||||||||
| NP_001190417.1 | AtSg28840 | GME (GDP-D-mannose 3’,5’-epimerase) | 42.71 | 5.81 | 15.05 | 18.57 | 6 | 16 | up | 2.03 | 0.0253 |
| NP_173387.1 | Atlg19570 | DHAR1 (dehydroascorbate reductase) | 23.61 | 5.52 | 31.77 | 59.62 | 9 | 77 | up | 1.53 | 0.049 |
| NP_181934.1 | At2g44060 | late embryogenesis abundant domain- containing protein |
36.00 | 4.51 | 6.15 | 12.00 | 3 | 4 | down | 0.47 | 7.27 × 10−05 |
| NP_001189847.1 | At3g09440 | heat shock cognate 70 kDa protein 3 (HSC70-3) |
71.09 | 4.82 | 36.67 | 32.20 | 14 | 35 | down | 0.51 | 0.014 |
| NP_194098.1 | At4g23670 | major latex protein-related/MLP-related | 17.49 | 5.91 | 22.06 | 50.99 | 7 | 111 | down | 0.66 | 0.014 |
| NP_188267.1 | At3gl6460 | jacalin-like lectin domain-containing protein |
72.41 | 5.20 | 6.94 | 9.50 | 3 | 3 | down | 0.58 | 0.047 |
| NP_194240.1 | At4g25100 | FeSODl (Fe superoxide dismutase 1) | 21.07 | 6.51 | 18.22 | 46.24 | 5 | 52 | up | 2.21 | 0.019 |
| Amino Acid Metabolism | |||||||||||
| NP_195506.1 | At4g37930 | SHM1 (serine hydroxymethyltransferase 1) | 57.35 | 8.28 | 41.30 | 33.27 | 13 | 67 | up | 1.32 | 0.045 |
| NP_194927.1 | At4g31990 | ASP5 (aspartate aminotransferase 5) | 50.92 | 7.65 | 6.79 | 6.93 | 2 | 10 | up | anp2anp3 unique | 1.77 × 10−06 |
| NP_177876.1 | Atlg77520 | O-methyltransferase family 2 protein | 29.12 | 5.00 | 9.22 | 15.06 | 3 | 10 | up | anp2anp3 unique | 1.29 × 10−07 |
| Calcium Signaling | |||||||||||
| NP_176814.1 | Atlg66410 | CAM4 (calmodulin 4) | 16.83 | 3.82 | 12.22 | 46.31 | 3 | 31 | down | 0.57 | 0.027 |
| Protein Folding | |||||||||||
| NP_197572.1 | AtSg20720 | CPN20 (chaperonin 20) | 26.77 | 9.38 | 29.66 | 46.64 | 9 | 24 | up | 3.41 | 0.027 |
| Transport | |||||||||||
| NP_186777.1 | At3g01280 | porin, putative | 29.39 | 9.20 | 8.27 | 20.29 | 3 | 7 | up | 3.84 | 0.009 |
| NP_193769.1 | At4g20360 | AtRABE1b/AtRab8D (Arabidopsis Rab GTPase homologue E1b) |
51.58 | 5.78 | 55.53 | 54.83 | 17 | 99 | up | 1.32 | 0.035 |
| Lipid Metabolism | |||||||||||
| NP_S66875.1 | At3g45140 | LOX2 (lipoxygenase 2) | 101.96 | 5.33 | 31.99 | 22.99 | 11 | 24 | up | 14.57 | 3.90 × 10−05 |
Total score represents sum of Xcorr parameters for each protein as calculated by the SEQUEST algorithm91 of the Proteome Discoverer software (Thermo Scientific, Waltham, MA, USA).
Ratios of sums of Xcorr values for particular proteins across three replicates.
Upregulation of ROS detoxifying enzymes in the anp2anp3 mutant
Four proteins with essential roles in ROS detoxification were upregulated in the anp2anp3 mutant when compared to wild type (Table 1). Among them, the abundance of FeSOD1 was more than 2-fold increased. This plastid-localized enzyme is responsible for the decomposition of O2•− radicals.39 In addition, chaperonin 20 (CPN20), a recently identified regulator of FeSOD1 activity,35 was also increased. The MnSOD isozyme (At3gl0920) was also upregulated (2.36-fold), however, the confidence in this result is not satisfactory (p = 0.19). Furthermore, DHAR1 was upregulated as well. DFIAR is an enzyme involved in ascorbate-glutathione (asc-glu) cycle and is responsible for maintenance of cellular reduced ascorbate pool which is crucial for the favorable redox state in the cell.40,41
Finally, a protein with GDP-D-mannose 3′,5′-epimerase activity was also more abundant and it is known to be involved in ascorbate biosynthesis.42 Together, these proteomic data indicated that anp2anp3 mutant may have increased capacity to decompose ROS eventually leading to better tolerance of oxidative stress conditions.
anp2anp3 mutant show accelerated antioxidant defense
We performed immunoblot analysis in order to validate the increased FeSOD1 and MnSOD abundances in anp2anp3 seedlings compared to wild type. This approach consistently showed upregulation of FeSOD1 and MnSOD in the mutant (Figure 2). To examine whether the higher FeSOD1 and MnSOD abundance resulted also in increased activity of these enzymes in the anp2anp3 mutant, we performed a native PAGE followed by specific SOD activity staining. Based on the sensitivity of individual SOD isozymes to KCN and H2O2 (Figure S2), we have detected three isozymes of SOD in both wild type and anp2anp3 mutant corresponding to MnSOD, FeSOD and CuZnSOD accordingly (Figure 3A, B). Comparison between the wild type and the anp2anp3 mutant revealed substantial increase in the total FeSOD and MnSOD activities in the mutant seedlings, which was supported also by quantification of the band densities (Figure 3C). Immunoblotting following native PAGE was carried out in order to correlate the activity of FeSOD and MnSOD with their abundance. This analysis showed, that the FeSOD1 and MnSOD levels (Figure 3D – I) correlated with total FeSOD and MnSOD activities visualized on native PAGE gels (Figure 3A – C).
Figure 2.
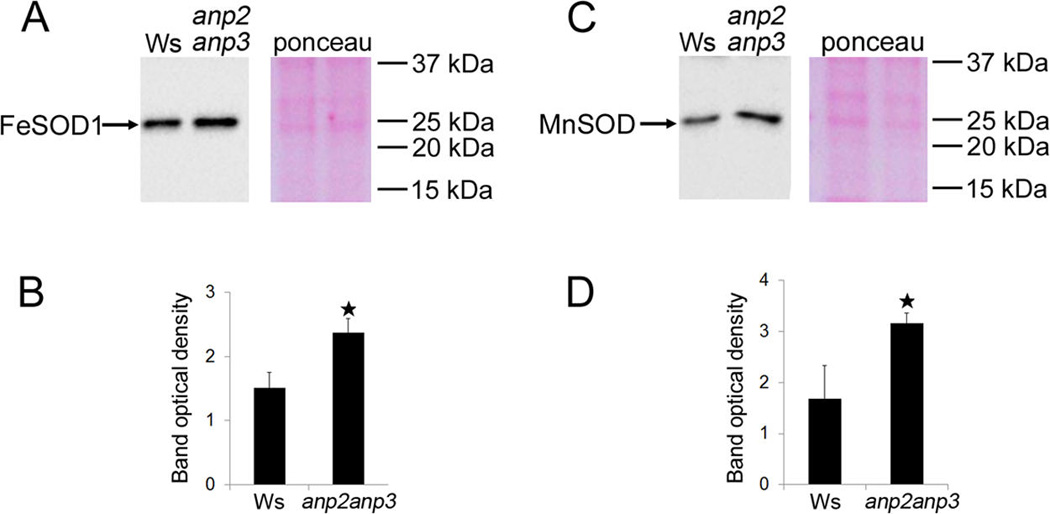
Immunoblotting analysis of superoxide dismutase (SOD) isozymes in anp2anp3 mutant and wild type (Ws) seedlings. (A, B) Immunoblot detection (A) and band optical density quantification (B) of FeSOD1 (mean ± SD, N = 3); (C, D) Immunoblot detection (C) and band optical density quantification (D) of MnSOD (mean ± SD, N = 3). The right panels in A and C show Ponceau S staining of respective PVDF membranes. * in B, D indicates statistical significance of optical density difference at p < 0.05.
Figure 3.
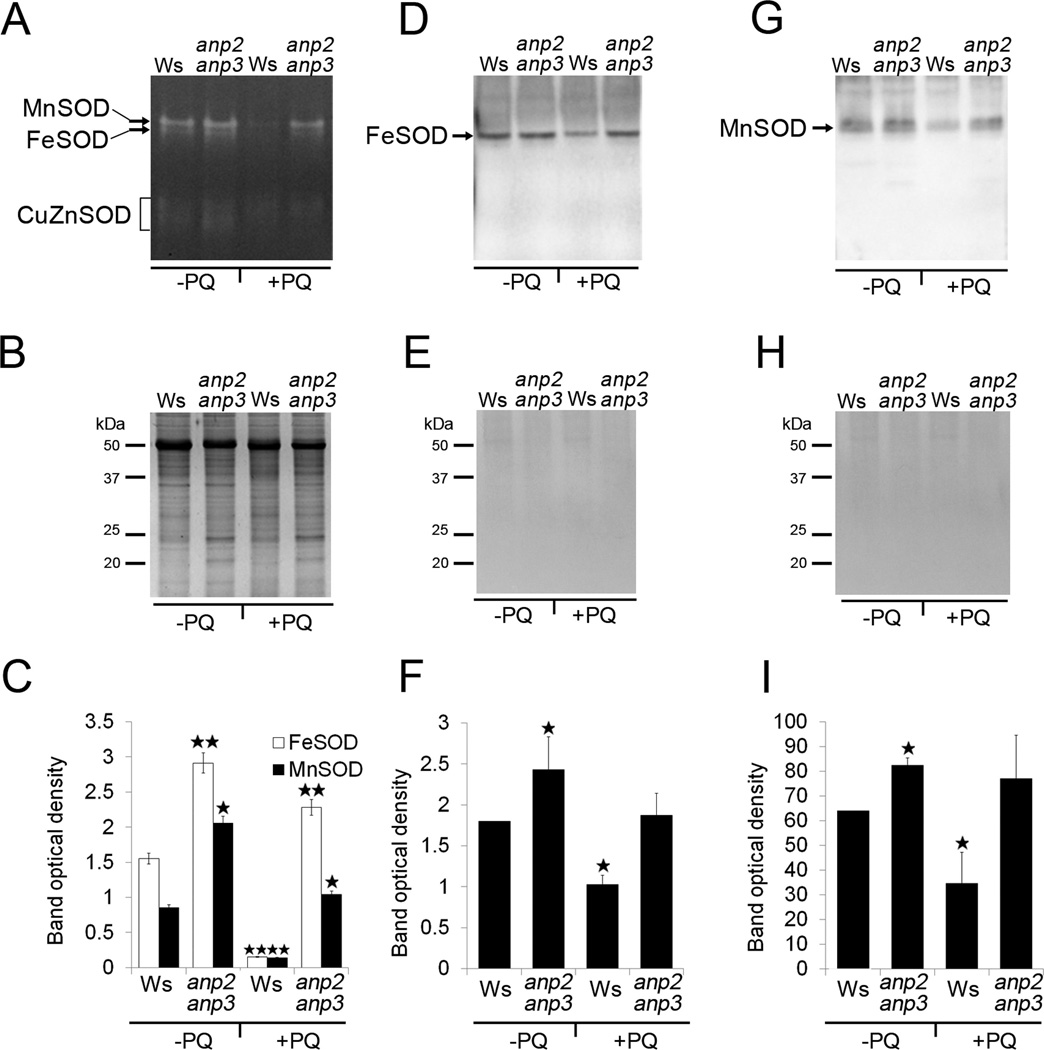
Isozyme pattern and immunoblotting on native PAGE gels of superoxide dismutase (SOD) in wild type (Ws) and anp2anp3 double mutant in control conditions (−PQ) and after paraquat treatment (+PQ). (A) SOD specific activity staining and (B) respective loading control represented by colloidal coomassie blue-stained SDS PAGE gels. Arrows in A indicate MnSOD and FeSOD, and the bracket shows CuZn SOD isozymes. (C) Quantification of the band densities in A (mean ± SD, N = 3). Asterisks indicate a statistically significant difference between Ws and anp2anp3 double mutant in control conditions as well as after paraquat treatment and between Ws in control conditions and after paraquat treatment as revealed by Student’s t test (* indicates statistical significance at p < 0.0S, ** indicates statistical significance at p < 0.01). (D and G) Immunoblots of FeSOD1 and MnSOD prepared on native PAGE gels using anti FeSOD1 and anti MnSOD antibodies. (E, H) Ponceau S staining of proteins (respective to D and G) transferred to PVDF membrane. (F, I) Quantification of the band densities in D and G (mean ± SD, N = 3). Asterisks indicate statistically significant differences in optical densities as revealed by Student’s t test (p < 0.05) between Ws and anp2anp3 double mutant in control conditions, as well as between Ws in control conditions and after paraquat treatment.
The observed increase in MnSOD and FeSOD activities and abundances implies that the anp2anp3 has a higher ability to remove O2•− radicals. The examination of SOD activities and abundances after short-term paraquat treatment (15 µM for 5 min) showed significant decrease in FeSOD and MnSOD isozyme activities in Ws, while they remained at the same level in anp2anp3 seedlings (Figure 3). These results show that anp2anp3 mutant possesses increased capacity to remove O2•− radicals under oxidative stress conditions.
Concomitantly to the upregulation of SOD levels and activities, our proteomic analysis revealed the significant upregulation of enzymes involved in ascorbate metabolism and regeneration in the anp2anp3 mutant (Table 1). The enzymatic activity of DHAR was addressed spectrophotometri-cally,25 in order to validate the significance of DHAR1 upregulation in the anp2anp3 mutant identified by proteomic analysis. In this case, DHAR activity in the mutant was increased by 1.3-fold compared to the wild type (Figure 4A). Consistently with a role of increased ascorbate turnover to scavenge H2O214 in the anp2anp3 mutant, we found a similarly increased enzymatic activity of APX when compared to the wild type (Figure 4B). Next, upregulation of GDP-D-mannose 3′,5′-epimerase (GME; Table 1), an enzyme involved in ascorbate biosynthesis,43 suggested elevated ascorbate levels in the anp2anp3 mutant. For this reason, ascorbate levels were quantitated in both wild type and mutant seedlings.26 The spectrophotometric assay of ascorbate levels validated the increased efficiency of ascorbate production by GME in the anp2anp3 mutant (Figure 4C).
Figure 4.
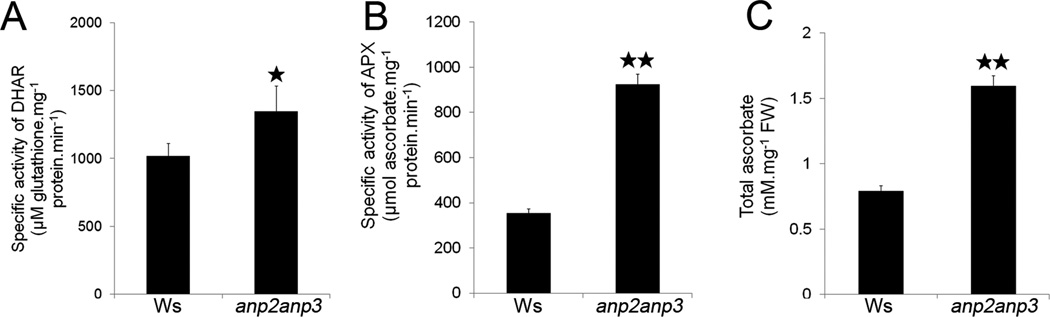
Quantitative demonstration of enzymatic activities within the ascorbate-glutathione cycle and ascorbate content in anp2anp3 double mutant. (A) Specific activity of dehydroascorbate reductase (DHAR; mean ± SD, N = 3). (B) Specific activity of ascorbate peroxidase (APX, mean ± SD, N = 3) and (C) total ascorbate content (mean ± SD, N = 3). * in A indicates statistical significance of enzymatic activities at p < 0.05, ** in B and C indicates statistical significance at p < 0.01.
Determination of ROS levels in the anp2anp3 seedlings
The increased antioxidant capacity in the anp2anp3 mutant should be reflected in alleviated levels of ROS. To prove this hypothesis, we aimed to monitor the production of O2•− and H2O2, both in whole seedlings according to standard histochemical detection (Figures 5 and 6). We also analyzed the activity of NADPH oxidase (generating O2•−) as well as the levels of O2•− and H2O2 spectrophotometrically (Figure 7).
Figure 5.
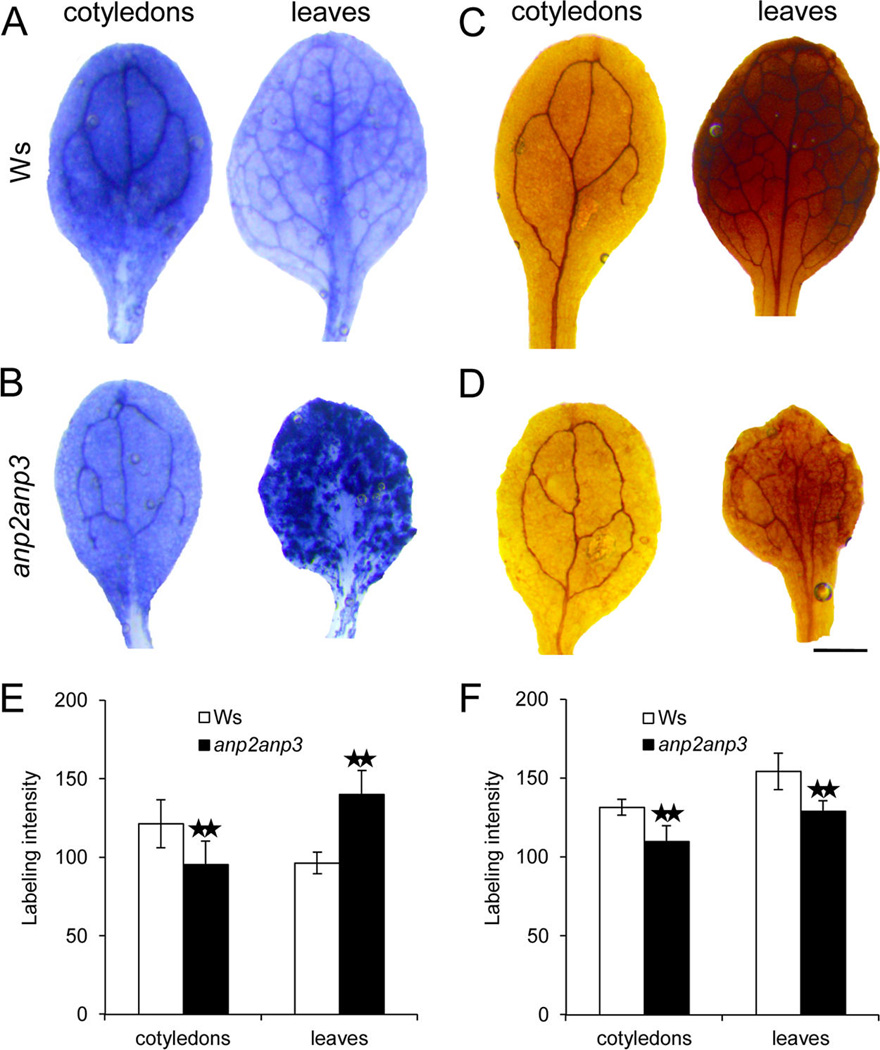
Generation and distribution of superoxide (O2•−) and hydrogen peroxide (H2O2) in leaves and cotyledons of 10-day-old Arabidopsis plants of wild type Ws (A, C) and anp2anp3 double mutant (B, D). O2•− production was visualized as dark blue coloration by nitroblue tetrazolium (NBT) staining (A, B), and H2O2 production was visualized as dark brown coloration by 3,3′-diaminobenzidine (DAB) staining (C, D). Semiquantitative analysis of the intensity of NBT (E) and DAB (F) staining in cotyledons and leaves of wild type plants and anp2anp3 mutants. ** indicates statistical difference significant at a p value <0.01 as determined by Student’s t test (n = 34 for cotyledons and N = 76 for leaves in E, N = 30 for cotyledons and N = 58 for leaves in F). Bar represents 0.S mm for A–D.
Figure 6.
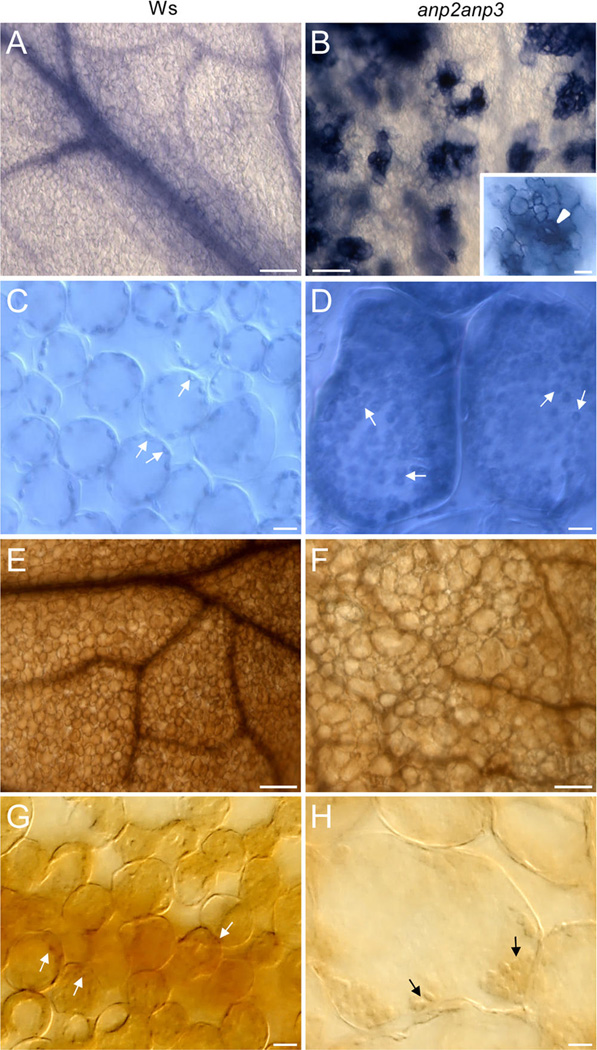
Cellular pattern of superoxide (O2•−) and hydrogen peroxide (H2O2) distribution in leaves of wild type (Ws) plants (A, C, E, G) and anp2anp3 mutants (B, D, F, H). Nitroblue tetrazolium (NBT) staining revealed generation of O2•− in leaf vascular tissue and uniform distribution in leaf mesophyll cells in Ws plants (A), while in leaves of anp2anp3 mutant the staining showed a clustered pattern (B). Intensive staining in these isolated islands was localized to stomata (arrowhead), surrounding epidermal cells (inset in B), and mesophyll cells close to the stomatal cavity (B). In both Ws plants and anp2anp3 mutants, NBT staining in mesophyll cells was restricted mainly to chloroplasts (arrows in C, D). 3,3′-Diaminobenzidine (DAB) staining of Ws and anp2anp3 mutant leaves (E, F) revealed generation of H2O2 in vascular tissue and in mesophyll cells, which was more intensive in leaves of Ws plants (E). Staining in leaf mesophyll cells was localized mainly to chloroplasts (arrows in G, H). Bar = 100 µm (A, B, E, F), 20 µm (insert in B), and 10 µm(C, D, G, H).
Figure 7.
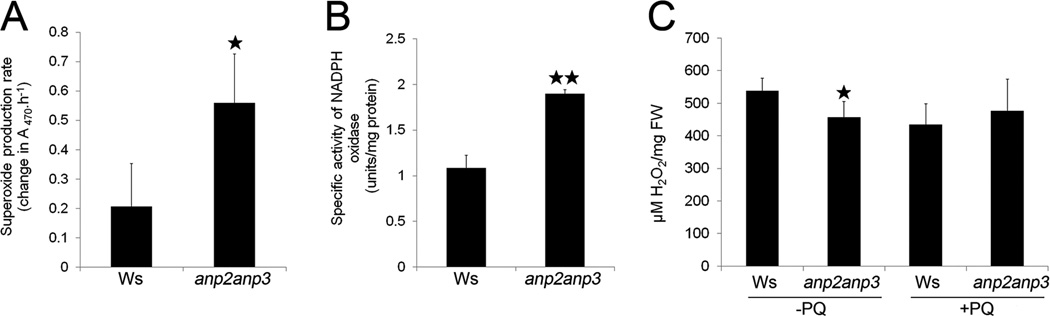
Quantitative demonstration of superoxide (O2•−) production and H2O2 level in anp2anp3 double mutant. (A) Level of O2•− production in wild type (Ws) and anp2anp3 seedlings, presented as change in absorbance at 470 nm caused by O2•− -induced XTT reduction (mean ± SD, N = 3). (B) Specific activity of NADPH oxidase in anp2anp3 mutant and wild type seedlings (mean ± SD, N = 3). (C) H2O2 concentration in control (−PQ) and paraquat treated (+PQ) seedlings of Ws and anp2anp3. Asterisks indicate statistically significant difference between Ws and anp2anp3 double mutant (* indicates statistical significance at p < 0.0S, ** indicates statistical significance at p < 0.01).
The production of O2•− was examined directly in wild type and anp2anp3 seedlings through the reduction of nitroblue tetrazolium (NBT) to formazan precipitate.30 NBT staining revealed decreased formazan accumulation in cotyledons but increased accumulation in leaves, thus reflecting lower O2•− production in cotyledons but higher in leaves of the anp2anp3 seedlings as compared to the wild type ones (Figure 5A, B, E). Next, the anp2anp3 seedlings accumulated significantly lower levels of H2O2 in their cotyledons and leaves in comparison to wild type seedlings, as determined by histochemical DAB staining (Figure 5C, D, F).
NBT staining in wild type leaves was weak and rather diffuse with prevalent localization at the vasculature (Figure 6A). However, strong NBT staining of anp2anp3 leaves was observed in clustered pattern restricted to stomatal complexes and mesophyll cells surrounding the stomatal pore (Figure 6B inset, arrowhead). In all cases, the most prominent NBT staining of mesophyll cells in both wild type and anp2anp3 seedlings showed plastidic localization while the apoplast was also stained (Figures 6C, D, arrows).
DAB staining of H2O2 followed similar pattern in leaves of both wild type and anp2anp3 seedlings (Figures 6E, F), differing only in staining intensity. Again, chloroplasts were the most prominent sites where DAB deposits accumulated (Figures 6G, H). Leaf cells of anp2anp3 seedlings were much bigger by comparison to those of Ws seedlings (Figures 6C – H) due to disturbed cell growth and development caused by microtubule-related defects.7
By assaying the O2•− production rate spectrophotometrically, it was found that its levels increased by 2.5-fold in the anp2anp3 mutant compared to the wild type (Figure 7A).
This increase was further corroborated by direct measurement of NADPH oxidase activity, one of the main sources of O2•− production, which was nearly 2-fold increased in the anp2anp3 mutant (Figure 7B). These results suggest that the increased O2•− level in the anp2anp3 mutant is, at least partially, linked to higher NADPH oxidase activity.
We assayed H2O2 levels by using a method based on xylenol orange.29 Consistent with the histochemical observations, spectrophotometric assessments showed lower levels of H2O2 in mutant seedlings (Figure 7C). The short-term paraquat treatment had no significant effect on H2O2 levels in neither Ws nor anp2anp3 mutant seedlings (Figure 7C).
Increased paraquat resistance of anp2anp3 mutant
The question of whether the increased abundance and activity of antioxidant enzymes would affect the response of anp2anp3 mutants to oxidative stress was addressed by comparing the growth of anp2anp3 and wild type seedlings in medium containing paraquat, a methyl viologen inducing the production of O2•− radicals in photosynthetic tissues.44 The effects of paraquat on growth and viability were recorded after 7 days of continuous exposure of seedlings of anp2anp3 and wild type (seedlings were 10 days old). Exposure to paraquat caused chlorophyll bleaching in wild type seedlings, which was not the case in the mutant (Figure 8). Paraquat treatment hampered seedling growth more effectively in the case of wild type, while anp2anp3 mutants exhibited significant resilience to continuous paraquat presence (Figure 8 C , D).
Figure 8.

Effect of oxidative stress on seedlings of anp2anp3 double mutant. (A, B) Arabidopsis seedlings of wild type (Ws) (A) and anp2anp3 mutant (B) grown under control conditions (−PQ). (C, D) Arabidopsis seedlings of Ws (C) and anp2anp3 double mutant (D) grown on 1/2 MS media supplemented with 0.S µM paraquat (+PQ). Three-day-old seedlings (wild type and mutant) were transferred to control and paraquat containing media and grown for 7 days. Note inhibition of the seedling growth and development as well as chlorophyll bleaching (arrows in C) in the leaves of Ws plants in comparison to the mutant plants. Bar represents 1 cm.
Downregulation of several photosynthetic proteins in the anp2anp3 mutant
ROS production is intimately linked to aerobic metabolism, particularly during the photosynthesis.45 Meanwhile, the differential proteomic analysis of anp2anp3 and Ws seedlings revealed significant downregulation of photo-synthetic proteins in the mutant (Table 1). For example, ferredoxin 2, a protein responsible for the electron delivery to various substrates, was substantially downregulated. The DNA-damage-repair/toleration protein 112, otherwise known as plastocyanin 2, a photosynthetic electron carrier participating in electron transfer between P700 and the cytochrome b6f complex in photosystem I,46 was also downregulated in the mutant seedlings. The decreased abundance of constituents of photosystems I and II, such as PsBP-1 and 2, and LHCB5, may relate to their possible instability in the mutant seedlings.47 On the other hand, a major subunit of photosystem I (PSAD1)48 was upregulated. The data above indicate that double mutation of ANP2 and ANP3 genes lead to the lower abundance of crucial photosynthetic proteins in Arabidopsis thaliana.
In order to prove, whether the decreased abundance of photosynthetic proteins lead to lower photosynthetic activity in the anp2anp3 mutant, we performed a chlorophyll fluorescence imaging analysis. Measurement of chlorophyll fluorescence induction did not reveal significant differences between anp2anp3 mutant and wild type in either maximum quantum efficiency of photosystem II (PSII) photochemistry in the dark adapted plants (FV/FM) (Figure 9A) nor in parameters of the light adapted state such as the effective quantum yield of electron transport through PSII (ΦPSII) and nonphotochemical quenching of chlorophyll fluorescence (qN) (Figure 9B). The above results indicate that the anp2anp3 mutant exhibits similar photosynthetic efficiency as the wild type.
Figure 9.
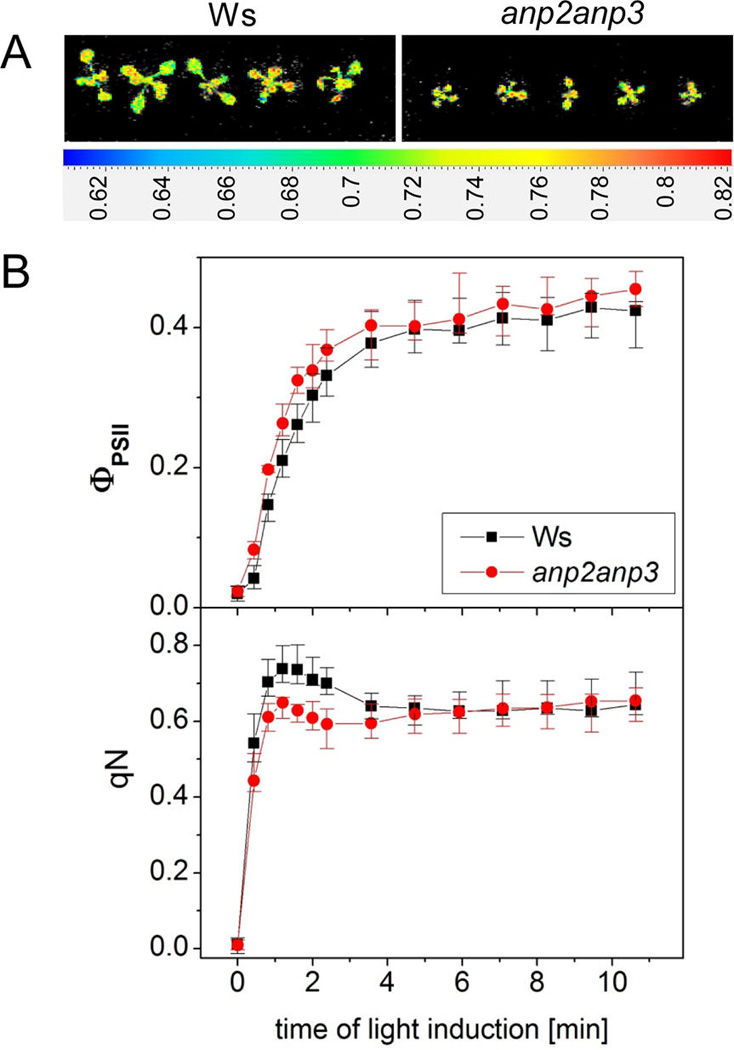
(A) Spatial distribution of the maximum quantum yield of photosystem II photochemistry (FV/FM) within Arabidopsis seedlings of wild type (Ws) and anp2anp3 double mutant obtained by chlorophyll fluorescence imaging. (B) Actual quantum yield of photosystem II electron transport (ΦPSII upper graph) and nonphotochemical chlorophyll fluorescence quenching (qN, bottom graph) during chlorophyll fluorescence induction in Arabidopsis seedlings of Ws (black, mean ± SD, N = 3) and anp2anp3 mutant (red, mean ± SD, N = 3).
DISCUSSION
Differential proteomic studies on MAPK mutants may help to elucidate MAPK functions leading to adaptation of plants to prolonged or continuous environmental stresses.49
Although there have been many studies addressing proteome-wide responses following the exposure of Arabidopsis or crop plants to prolonged abiotic stresses,50–53 there has not been a complementary work linking such responses to specific signaling pathways. Here, we addressed the proteome of anp2anp3 mutant in the light of the previously nondescribed role of ANP MAPKKKs in reaction to oxidative stress. In this respect, the present study provides such quantitative information on the differential protein abundance between the double MAPKKK mutant anp2anp3 and the respective wild type, and it complements previous knowledge on the proteome-wide effects of stress conditions such as oxidative,54 UVB,55 salt56 and heavy metal50 stresses that involve MAPK signaling.
Oxidative stress tolerance of the anp2anp3 mutant
Oxidative stress through the metabolic accumulation of ROS is an unavoidable consequence of aerobic metabolism and particularly photosynthesis,57 but it is also related to various abiotic stress conditions including heavy metal toxicity, salinity, ultraviolet irradiation and temperature stresses, all of which culminate in the accumulation of various ROS.58 Although ROS may have signaling attributes with developmental and stress priming consequences,59 their overproduction may lead to toxic effects including lipid peroxidation60 and protein oxidation,61 ultimately leading to challenging of cellular integrity and finally to cell death.62
To cope with these harmful effects of ROS, plants exposed to oxidative stress (through exposure to high irradiance or high energy UV illumination,63 excessive salinity,64 or heavy metals in the rhizosphere65) show upregulation of stress-responsive genes encoding proteins with protective and ROS-detoxifying functions.59
In this sense, the protein complement, the biochemical and physiological properties of anp2anp3 mutant are suggestive of a constitutive antioxidative response and imply a negative role of the ANP family in the regulation of oxidative stress tolerance. Similar traits were reported for mpk3 and mpk6 knockout mutants against UV–B irradiation66 and oxidative stress67. It is interesting that deactivation of ozone-induced MPK3 and MPK6 activity by the MAPK phosphatase MKP2 has also a positive regulatory role in antioxidant defense since knocking out MKP2 results in constitutive overactivation of MPK3 and MPK6 and hypersensitization against ozone.68 Notably, a recent study10 reported decreased capacity of anp2anp3 mutant to generate elicitor-induced oxidative burst and elevated sensitivity against the necrotrophic fungus Botrytis cinerea.
Increased activity and abundance of FeSOD and MnSOD
In addition to other changes, the comparative proteome analysis of the anp2anp3 mutant essentially unveiled the upregulation of proteins involved in ROS detoxification (Table 1), which is consistent with the oxidative stress tolerance of anp2anp3 seedlings.
First, we observed increased capacity for O2•− removal in the mutant, which is an immediate consequence of the activity of three isozymes of SODs, differing in metal cofactor.69 Proteomic analysis showed more than 2-fold increase in the abundance of FeSOD1 in the mutant as compared to the wild type. FeSOD is a chloroplastic SOD, mostly responsible for the removal of O2•− produced during photosynthesis. The increased abundance of FeSOD1 in anp2anp3 seedlings was validated by immunoblot analysis. Consistently, the FeSOD1 abundance positively correlated with the total FeSOD activity in the anp2anp3 seedlings, showing almost 2-fold increase as revealed by native PAGE SOD activity staining. The heterologous overexpression of Arabidopsis FeSOD1 confers increased oxidative stress tolerance to transgenic maize, suggesting that the upregulation of FeSOD 1 expression70 may suffice to explain the increased tolerance of anp2anp3 seedlings to oxidative stress. Moreover, a chloroplast-localized CPN20 was recently identified as FeSOD1 interacting partner and regulator of FeSOD1 activation. Alongside FeSOD1, CPN20 was also found to be upregulated in the anp2anp3 mutant. On the basis of this knowledge we propose, that the increased FeSOD activity in the anp2anp3 mutant might be a combined consequence of increased FeSOD1 and CPN20 protein levels. In addition, proteomics also showed increased abundance of mitochondrial MnSOD isozyme decomposing O2•− originating from the respiratory electron transport chain.71 This was validated by immunoblot analysis using anti-MnSOD antibody, as well as by MnSOD specific activity staining on native PAGE gels. Importantly, anp2anp3 mutant showed increased SOD activities also under oxidative stress conditions.
The increased O2•− production in the anp2anp3 mutant
Histochemical and spectrophotometric assays showed significant increase of O2•− production in the mutant seedlings. Obviously, O2•− is produced constitutively in photosynthetic reactions by direct photoreduction of O2 by reduced electron transport components. This constitutively produced O2•− is effectively scavenged by SODs in chloroplast (Cu/Zn SOD and FeSOD), serving as an alternative electron acceptor in photoprotection.59 Generation of O2•− during photosynthesis is substantially increased under photoinhibition of photosynthesis induced by abiotic stimuli such as light and cold stress.72 Another source of O2•− is a plasma membrane localized NADPH oxidase, which generates extracellular O2•− using NADPH as an electron donor. NADPH oxidase activity is induced by pathogen infection,73 exogenous abscisic acid,74 or heavy metal stress.75 It is also required for developmental processes such as the ABA-induced stomatal closure76 or for cell expansion.77
Judging from the similar photosynthetic activity in the anp2anp3 mutant and in the wild type, it is unlikely that increased O2•− levels in the anp2anp3 reflect differences in photosynthesis. Since the increase in total O2•− level was similar to the increase of NADPH oxidase, we suggest that the increased total O2•− production in anp2anp3 mutant accounts for plasma membrane NADPH oxidase, which has elevated activities in this mutant. Similarly, elevated O2•− levels were detected also in Arabidopsis mkk4 mutant.78 MKK4 is a MAP kinase kinase which was suggested to act downstream of ANP1,79 and it was proposed to be redundantly targeted by both ANP2 and ANP3. MKK4/MKK5 are upstream activators of MPK3/MPK6 in response to external H2O2.9 ANP1, ANP2 and ANP3 were shown to be redundant in MPK3/MPK6 activation in response to external H2O2.9 Therefore, we hypothesize that ANPs may negatively affect NADPH oxidase activity in Arabidopsis, likely through MKK4-dependent activation of MPK3 and MPK6. Similar regulation was recently proposed for MPK8, acting downstream of MKK3, which suppresses the expression of RbohD gene, encoding NADPH oxidase.80
Accelerated ascorbate-glutathione cycle in the anp2anp3 mutant
The anp2anp3 mutant exhibited upregulation of DHAR1 at the protein level (Table 1). The higher abundance of DHAR1 was also reflected in its increased activity in the anp2anp3 mutant. This enzyme is responsible for recycling of the ascorbate.81 DHAR overexpression leads to elevated foliar ascorbate levels82 and more efficient protection against ROS.83
The reduction of dehydroascorbate to ascorbate by DHAR occurs within the asc-glu cycle.84 Within this cycle, the reduced ascorbate serves as electron donor for efficient removal of H2O2 by APX. The increased activities of APX and DHAR in the anp2anp3 mutant found by the present study support the idea of an accelerated asc-glu cycle resulting in rapid H2O2 decomposition. We measured also the total ascorbate content, showing increased levels in the mutant. This is consistent with the increased abundance of GME, involved in ascorbate synthesis,85 which was found by our proteomic analysis. Except for the important role of ascorbate in the maintenance of redox homeostasis, this abundant nonenzymatic antioxidant itself has an ability to scavenge ROS such as O2•− and hydroxyl radical.84
The histochemical and spectrophotometric examination showed decreased levels of H2O2 in anp2anp3 mutant. The major mechanism of H2O2 production involves the dismutation of the O2•−, which can either be catalyzed by SODs or occur spontaneously.86 At high concentrations, H2O2 causes serious damaging effects leading to cell death.87 The lower levels of H2O2 detected by DAB staining and spectrophotometric measurement in anp2anp3 mutant are in agreement with the accelerated antioxidative defense and redox homeostasis.
Together, our proteomic and biochemical analyses suggest that anp2anp3 double mutant possesses a functional network of proteins (Figure 10), ensuring favorable cellular redox conditions as well as accelerated defense against overproduction of ROS.
Figure 10.
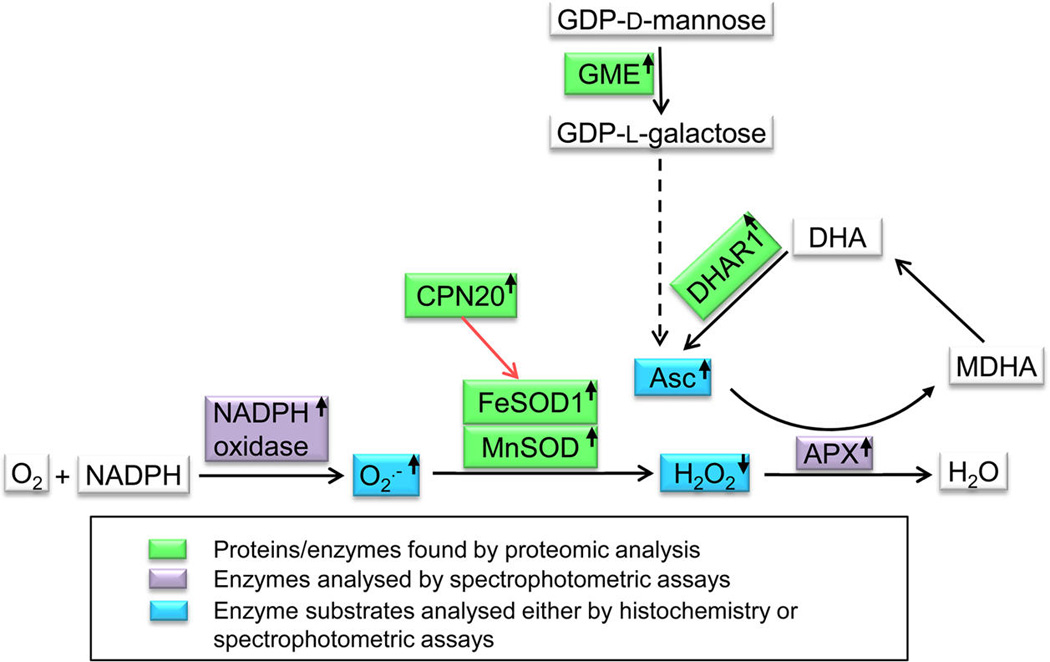
Functional network of proteins/enzymes, reactive oxygen species, and redox-active compounds found by the current study to be modified in the anp2anp3 mutant (depicted in colored boxes). Red arrow shows activation, dashed arrow means metabolic pathway. The small arrows in boxes indicate upregulation or downregulation of individual components. Abbreviations: Asc = ascorbate, APX = ascorbate peroxidase, CPN20 = Chaperonin 20, DHAR 1 = dehydroascorbate reductase, GME = GDP-d-mannose 3′S′-epimerase, FeSOD = Fe-superoxide dismutase, MDHA = monodehydroascorbate, MnSOD = Mn-superoxide dismutase.
There is some previous evidence suggesting an increased oxidative stress resistance in plants overexpressing either CuZnSOD, MnSOD, or FeSOD but also H2O2 scavenging enzyme APX.70,88–90 Consistently with these reports, the accelerated antioxidant defense represented by FeSOD, MnSOD, APX, and DHAR as well as ascorbate confers oxidative stress resistance to the anp2anp3 mutant. The MAPK signaling toward antioxidant enzymes (CuZnSOD and catalase) was shown to be mediated by a H2O2-inducible MAPK cascade consisting of MEKK1 (MAPKKK), MKK1, MKK2, and MKK5 (MAPKK) and downstream MPK3 and MPK6.14–16 Our results indicate involvement of ANP2 and ANP3 in the regulation of redox homeostasis and antioxidant defense.
Supplementary Material
ACKNOWLEDGMENTS
We thank Linda Breazeale for English editing and Patrick Krysan for seeds of anp2anp3 mutant. This research was supported by Grant No. P501/11/1764 from the Czech Science Foundation GACR; by the National Program for Sustainability I (Grant No. LO1204) provided by the Czech Ministry of Education; by POSTUP II at Palacký University, Olomouc, Czech Republic (Grant CZ.1.07/2.3.00/30.0041 to P.V.), student project PfF_2013_011 of the Palacký University; by the Operational Program Education for Competitiveness–European Social Fund (project CZ.1.07/2.3.00/20.0165); and Genomics for Southern Crop Stress and Disease, USDA CSREES 2009-34609-20222.
Footnotes
ASSOCIATED CONTENT
Figure S1 - Prediction of functional networks affected in anp2anp3 mutant compared to wild type using STRING 9.0 applied on proteomic data. Figure S2 - Identification of SOD isozymes on native PAGE gels on the basis of inhibitor sensitivity. Table S1 - Data pertinent to proteins and peptides identified in the anp2anp3 mutant. Table S2 - Proteome Discoverer datasheet export related to protein and peptide identification in the anp2anp3 mutant. Table S3 - Proteome Discoverer datasheet export of matched peptides. Table S4 - KEGG-based classification of proteins identified in Arabidopsis, ecotype Ws seedlings. Table S5 - KEGG-based classification of proteins identified in the Arabidopsis anp2anp3 mutant. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Rodriguez MCS, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010;61:621–649. doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- 2.Šamajová O, Plíhal O, Al-Yousif M, Hirt H, Šamaj J. Improvement of stress tolerance in plants by genetic manipulation of mitogen-activated protein kinases. Biotechnol. Adv. 2013;31:118–128. doi: 10.1016/j.biotechadv.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Andreasson E, Ellis B. Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci. 2010;15:106–113. doi: 10.1016/j.tplants.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Krysan PJ, Jester PJ, Gottwald JR, Sussman M. R An Arabidopsis mitogen-activated protein kinase kinase kinase gene family encodes essential positive regulators of cytokinesis. Plant Cell. 2002;14:1109–1120. doi: 10.1105/tpc.001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasabe M, Soyano T, Takahashi Y, Sonobe S, Igarashi H, Itoh TJ, Hidaka M, Machida Y. Phosphorylation of NtMAP6S–l by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev. 2006;20:1004–1014. doi: 10.1101/gad.1408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi Y, Soyano T, Kosetsu K, Sasabe M, Machida Y. HINKEL kinesin, ANP MAPKKKs and MKK6/ANQ MAPKK, which phosphorylates and activates MPK4 MAPK, constitute a pathway that is required for cytokinesis in Arabidopsis thaliana . Plant Cell Physiol. 2010;51:1766–1776. doi: 10.1093/pcp/pcq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck M, Komis G, Muller J, Menzel D, Šamaj J. Arabidopsis homologs of nucleus- and phragmoplast-localized kinase 2 and 3 and mitogen-activated protein kinase 4 are essential for microtubule organization. Plant Cell. 2010;22:755–771. doi: 10.1105/tpc.109.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasabe M, Machida Y. Regulation of organization and function of microtubules by the mitogen-activated protein kinase cascade during plant cytokinesis. Cytoskeleton (Hoboken) 2012;69:913–918. doi: 10.1002/cm.21072. [DOI] [PubMed] [Google Scholar]
- 9.Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savatin DV, Bisceglia NG, Marti L, Fabbri C, Cervone F, Lorenzo GD. The Arabidopsis Nucleus- And Phragmoplast-Localized Kinasel-Related Protein Kinases Are Required for Elicitor-Induced Oxidative Burst and Immunity. Plant Physiol. 2014;165:1188–1202. doi: 10.1104/pp.114.236901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gawroński P, Witoń D, Vashutina K, Bederska M, Betliński B, Rusaczonek A, Karpiński S. Mitogen-Activated Protein Kinase 4 Is a Salicylic Acid-Independent Regulator of Growth But Not of Photosynthesis in Arabidopsis. Mol. Plant. 2014;7:1151–1166. doi: 10.1093/mp/ssu060. [DOI] [PubMed] [Google Scholar]
- 12.Pitzschke A, Djamei A, Bitton F, Hirt H. A major role of the MEKK1-MKK1/2-MPK4 pathway in ROS signalling. Mol. Plant. 2009;2:120–137. doi: 10.1093/mp/ssn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagami H, Soukupová H, Schikora A, Zárský V, Hirt H. A Mtogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis . J. Biol. Chem. 2006;281:38697–38704. doi: 10.1074/jbc.M605293200. [DOI] [PubMed] [Google Scholar]
- 14.Xing Y, Cao Q, Zhang Q, Qin L, Jia W, Zhang J. MKK5 regulates high light-induced gene expression of Cu/Zn superoxide dismutase 1 and 2 in Arabidopsis . Plant Cell Physiol. 2013;54:1217–1227. doi: 10.1093/pcp/pct072. [DOI] [PubMed] [Google Scholar]
- 15.Xing Y, Jia W, Zhang J. AtMEK1 mediates stress-induced gene expression of CAT1 Catalase by triggering H2O2 production in Arabidopsis . J. Exp. Bot. 2007;58:2969–2981. doi: 10.1093/jxb/erm144. [DOI] [PubMed] [Google Scholar]
- 16.Xing Y, Jia W, Zhang J. AtMKKl mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis . Plant J. Cell Mol. Biol. 2008;54:440–451. doi: 10.1111/j.1365-313X.2008.03433.x. [DOI] [PubMed] [Google Scholar]
- 17.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- 18.Takáč T, Pechan T, Richter H, Müller J, Eck C, Böhm N, Obert B, Ren H, Niehaus K, Šamaj J. Proteomics on brefeldin A-treated Arabidopsis roots reveals profilin 2 as a new protein involved in the cross-talk between vesicular trafficking and the actin cytoskeleton. J. Proteome Res. 2011;10:488–501. doi: 10.1021/pr100690f. [DOI] [PubMed] [Google Scholar]
- 19.Hurkman WJ, Tanaka C. K Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986;81:802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridges SM, Magee GB, Wang N, Williams WP, Burgess SG, Nanduri B. ProtQuant: a tool for the label-free quantification of MudPIT proteomics data. BMC Bioinformatics. 2007;8(Suppl 7):S24. doi: 10.1186/1471-2105-8-S7-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Pan SM, Yau YY. Characterization of superoxide dismutase in Arabidopsis . Plant Cell Physiol. 1998;37:58–66. [Google Scholar]
- 23.Beauchamp G, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 24.Amako K, Chen GX, Asada K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994;35:497–504. [Google Scholar]
- 25.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- 26.Gillespie KM, Ainsworth EA. Measurement of reduced, oxidized and total ascorbate content in plants. Nat. Protoc. 2007;2:871–874. doi: 10.1038/nprot.2007.101. [DOI] [PubMed] [Google Scholar]
- 27.Sagi M, Fluhr R. Superoxide production by plant homologues of the gp91 (phox) NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol. 2001;126:1281–1290. doi: 10.1104/pp.126.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Able AJ, Guest DI, Sutherland MW. Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var nicotianae . Plant Physiol. 1998;117:491–499. doi: 10.1104/pp.117.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheeseman JM. Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 2006;57:2435–2444. doi: 10.1093/jxb/erl004. [DOI] [PubMed] [Google Scholar]
- 30.Ramel F, Sulmon C, Bogard M, Couée I, Gouesbet G. Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol. 2009;9:28. doi: 10.1186/1471-2229-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell. 2012;24:275–287. doi: 10.1105/tpc.111.093039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oxborough K, Baker N. R Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components-calculation of qP and Fv′/Fm′ without measuring Fo′. Photosynth. Res. 1997;54:135–142. [Google Scholar]
- 33.Jensen LJ, Kuhn M, Stark M, Chaffron S, Creevey G, Muller J, Doerks T, Julien P, Roth A, Simonovic M, Bork P, von Mering C. STRING 8-a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37:D412–D416. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormack E, Tsai Y-G, Braam J. Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 2005;10:383–389. doi: 10.1016/j.tplants.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Kuo WY, Huang GH, Liu AG, Cheng GP, Li SH, Chang WC, Weiss C, Azem A, Jinn TL. CHAPERONIN 20 mediates iron superoxide dismutase (FeSOD) activity independent of its co-chaperonin role in Arabidopsis chloroplasts. New Phytol. 2013;197:99–110. doi: 10.1111/j.1469-8137.2012.04369.x. [DOI] [PubMed] [Google Scholar]
- 36.Camacho L, Smertenko AP, Perez-Gomez J, Hussey PJ, Moore I. Arabidopsis Rab-E GTPases Exhibit a Novel Interaction with a Plasma-membrane Phosphatidylinositol-4-phosphate 5-kinase. J. Cell Sci. 2009;122:4383–4392. doi: 10.1242/jcs.053488. [DOI] [PubMed] [Google Scholar]
- 37.Seltmann MA, Stingl NE, Lautenschlaeger JK, Krischke M, Mueller MJ, Berger S. Differential impact of lipoxygenase 2 and jasmonates on natural and stress-induced senescence in Arabidopsis . Plant Physiol. 2010;152:1940–1950. doi: 10.1104/pp.110.153114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi F, Yoshida R, Ichimura K, Mzoguchi T, Seo S, Yonezawa M, Maruyama K, Yamaguchi-Shinozaki K, Shinozaki K. The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis . Plant Cell. 2007;19:805–818. doi: 10.1105/tpc.106.046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilon M, Ravet K, Tapken W. The biogenesis and physiological function of chloroplast superoxide dismutases. Biochim. Biophys. Acta BBA-Bioenerg. 2011;1807:989–998. doi: 10.1016/j.bbabio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Gallie D. R The Role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 2013;64:433–443. doi: 10.1093/jxb/ers330. [DOI] [PubMed] [Google Scholar]
- 41.Smirnoff N. Ascorbate biosynthesis and function in photo-protection. Philos. Trans. R. Soc. London, B: Biol. Sci. 2000;355:1455–1464. doi: 10.1098/rstb.2000.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolucka BA, Van Montagu M. GDP-mannose 3′,5′-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem. 2003;278:47483–47490. doi: 10.1074/jbc.M309135200. [DOI] [PubMed] [Google Scholar]
- 43.Valpuesta V, Botella MA. Biosynthesis of L-ascorbic acid in plants: new pathways for an old antioxidant. Trends Plant Sci. 2004;9:573–577. doi: 10.1016/j.tplants.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Bus JS, Aust SD, Gibson JE. Superoxide- and singlet oxygen-catalyzed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem. Biophys. Res. Commun. 1974;58:749–755. doi: 10.1016/s0006-291x(74)80481-x. [DOI] [PubMed] [Google Scholar]
- 45.Karpiński S, Szechyńska-Hebda M, Wituszyńska W, Burdiak P. Light acclimation, retrograde signalling, cell death and immune defenses in plants. Plant Cell Environ. 2013;36:736–744. doi: 10.1111/pce.12018. [DOI] [PubMed] [Google Scholar]
- 46.Pesaresi P, Scharfenberg M, Weigel M, Granlund I, Schroder WP, Finazzi G, Rappaport F, Masiero S, Furini A, Jahns P, Leister D. Mutants, overexpressors, and interactors of Arabidopsis plastocyanin isoforms: revised roles of plastocyanin in photosynthetic electron flow and thylakoid redox state. Mol. Plant. 2009;2:236–248. doi: 10.1093/mp/ssn041. [DOI] [PubMed] [Google Scholar]
- 47.Allahverdiyeva Y, Suorsa M, Rossi F, Pavesi A, Kater MM, Antonacci A, Tadini L, Pribil M, Schneider A, Wanner G, Leister D, Aro EM, Barbato R, Pesaresi P. Arabidopsis plants lacking PsbQ and PsbR subunits of the oxygen-evolving complex show altered PSII super-complex organization and short-term adaptive mechanisms. Plant J. 2013;75:671–684. doi: 10.1111/tpj.12230. [DOI] [PubMed] [Google Scholar]
- 48.Ihnatowicz A, Pesaresi P, Varotto G, Richly E, Schneider A, Jahns P, Salamini F, Leister D. Mutants for photosystem I subunit D of Arabidopsis thaliana: effects on photosynthesis, photosystem I stability and expression of nuclear genes for chloroplast functions. Plant J. 2004;37:839–52. doi: 10.1111/j.1365-313x.2004.02011.x. [DOI] [PubMed] [Google Scholar]
- 49.Sinha AK, Jaggi M, Raghuram B, Tuteja N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal. Behav. 2011;6:196–203. doi: 10.4161/psb.6.2.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li G, Peng X, Xuan H, Wei L, Yang Y, Guo T, Kang G. Proteomic analysis of leaves and roots of common wheat (Triticum aestivum L.) under copper-stress conditions. J. Proteome Res. 2013;12:4846–4861. doi: 10.1021/pr4008283. [DOI] [PubMed] [Google Scholar]
- 51.Hossain Z, Khatoon A, Komatsu S. Soybean proteomics for unraveling abiotic stress response mechanism. J. Proteome Res. 2013;12:4670–4684. doi: 10.1021/pr400604b. [DOI] [PubMed] [Google Scholar]
- 52.Singh R, Jwa NS. Understanding the responses of rice to environmental stress using proteomics. J. Proteome Res. 2013;12:4652–4669. doi: 10.1021/pr400689j. [DOI] [PubMed] [Google Scholar]
- 53.Amme S, Matros A, Schlesier B, Mock HP. Proteome analysis of cold stress response in Arabidopsis thaliana using DIGE-technology. J. Exp. Bot. 2006;57:1537–1546. doi: 10.1093/jxb/erj129. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Wang S, Lu Y, Alvarez S, Hicks LM, Ge X, Xia Y. Proteomic analysis of early-responsive redox-sensitive proteins in Arabidopsis . J. Proteome Res. 2012;11:412–424. doi: 10.1021/pr200918f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du H, Liang Y, Pei K, Ma K. UV radiation-responsive proteins in rice leaves: a proteomic analysis. Plant Cell Physiol. 2011;52:306–316. doi: 10.1093/pcp/pcq186. [DOI] [PubMed] [Google Scholar]
- 56.Jiang Y, Yang B, Harris NS, Deyholos MK. Comparative proteomic analysis of NaCl stress-responsive proteins in Arabidopsis roots. J. Exp. Bot. 2007;58:3591–3607. doi: 10.1093/jxb/erm207. [DOI] [PubMed] [Google Scholar]
- 57.Foyer GH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxidants Redox Signal. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki N, Koussevitzky S, Mttler R, Miller G. ROS and Redox Signalling in the Response of Plants to Abiotic Stress. Plant Cell Environ. 2012;35:259–270. doi: 10.1111/j.1365-3040.2011.02336.x. [DOI] [PubMed] [Google Scholar]
- 59.Apel K, Hirt H. REACTIVE OXYGEN SPECIES: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 60.Farmer EE, Mueller MJ. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013;64:429–450. doi: 10.1146/annurev-arplant-050312-120132. [DOI] [PubMed] [Google Scholar]
- 61.Jacques S, Ghesquière B, Van Breusegem F, Gevaert K. Plant proteins under oxidative attack. Proteomics. 2013;13:932–940. doi: 10.1002/pmic.201200237. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Lin A, Loake GJ, Chu G. H2O2-induced leaf cell death and the crosstalk of reactive nitric/oxygen species. J. Integr. Plant Biol. 2013;55:202–208. doi: 10.1111/jipb.12032. [DOI] [PubMed] [Google Scholar]
- 63.Hideg E, Jansen MA, Strid A. UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends Plant Sci. 2013;18:107–115. doi: 10.1016/j.tplants.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Jaspers P, Kangasjärvi J. Reactive oxygen species in abiotic stress signaling. Physiol. Plant. 2010;138:405–413. doi: 10.1111/j.1399-3054.2009.01321.x. [DOI] [PubMed] [Google Scholar]
- 65.Hossain Z, Komatsu S. Contribution of proteomic studies towards understanding plant heavy metal stress response. Front. Plant Sci. 2013;3:310. doi: 10.3389/fpls.2012.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.González Besteiro MA, Bartels S, Albert A, Ulm R. Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. Plant J. 2011;68:727–737. doi: 10.1111/j.1365-313X.2011.04725.x. [DOI] [PubMed] [Google Scholar]
- 67.Lee JS, Ellis BE. Arabidopsis MAPK phosphatase 2 (MKP2) positively regulates oxidative stress tolerance and inactivates the MPK3 and MPK6 MAPKs. J. Biol. Chem. 2007;282:25020–25029. doi: 10.1074/jbc.M701888200. [DOI] [PubMed] [Google Scholar]
- 68.Xing Y, Jia W, Zhang J. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis . Plant J. 2008;54:440–451. doi: 10.1111/j.1365-313X.2008.03433.x. [DOI] [PubMed] [Google Scholar]
- 69.Kliebenstein DJ, Monde RA, Last RL. Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant Physiol. 1998;118:637–650. doi: 10.1104/pp.118.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Breusegem F, Slooten L, Stassart JM, Moens T, Botterman J, Van Montagu M, Inzé D. Overproduction of Arabidopsis thaliana FeSOD confers oxidative stress tolerance to transgenic maize. Plant Cell Physiol. 1999;40:515–523. doi: 10.1093/oxfordjournals.pcp.a029572. [DOI] [PubMed] [Google Scholar]
- 71.Morgan MJ, Lehmann M, Schwarzlander M, Baxter CJ, Sienkiewicz-Porzucek A, Williams TCR, Schauer N, Fernie AR, Fricker MD, Ratcliffe RG, et al. Decrease in manganese superoxide dismutase leads to reduced root growth and affects tricarboxylic acid cycle flux and mitochondrial redox homeostasis. Plant Physiol. 2008;147:101–114. doi: 10.1104/pp.107.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hodgson RA, Raison JK. Superoxide production by thylakoids during chilling and its implication in the susceptibility of plants to chilling-induced photoinhibition. Planta. 1991;183:222–228. doi: 10.1007/BF00197792. [DOI] [PubMed] [Google Scholar]
- 73.Torres MA, Dangl JL, Jones JDG. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. U. S. A. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang M, Zhang J. Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta. 2002;215:1022–1030. doi: 10.1007/s00425-002-0829-y. [DOI] [PubMed] [Google Scholar]
- 75.Hao F, Wang X, Chen J. Involvement of plasma-membrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seedlings. Plant Sci. 2006;170:151–158. [Google Scholar]
- 76.Desikan R, Cheung M-K, Bright J, Henson D, Hancock JT, Neill SJ. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J. Exp. Bot. 2004;55:205–212. doi: 10.1093/jxb/erh033. [DOI] [PubMed] [Google Scholar]
- 77.Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee G, Jones JDG, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 78.Kim S-H, Woo D-H, Kim J-M, Lee S-Y, Chung WS, Moon Y-H. Arabidopsis MKK4 mediates osmotic-stress response via its regulation of MPK3 activity. Biochem. Biophys. Res. Commun. 2011;412:150–154. doi: 10.1016/j.bbrc.2011.07.064. [DOI] [PubMed] [Google Scholar]
- 79.Colcombet J, Hirt H. Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem. J. 2008;413:217–226. doi: 10.1042/BJ20080625. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi F, Mizoguchi T, Yoshida R, Ichimura K, Shinozaki K. Calmodulin-Dependent Activation of MAP Kinase for ROS Homeostasis in Arabidopsis . Mol. Cell. 2011;41:649–660. doi: 10.1016/j.molcel.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida S, Tamaoki M, Shikano T, Nakajima N, Ogawa D, Ioki M, Aono M, Kubo A, Kamada H, Inoue Y, Saji H. Cytosolic dehydroascorbate reductase is important for ozone tolerance in Arabidopsis thaliana . Plant Cell Physiol. 2006;47:304–308. doi: 10.1093/pcp/pci246. [DOI] [PubMed] [Google Scholar]
- 82.Chen Z, Young TE, Ling J, Chang S-G, Gallie DR. Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3525–3530. doi: 10.1073/pnas.0635176100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Z, Gallie D. R Dehydroascorbate Reductase Affects Leaf Growth, Development, and Function. Plant Physiol. 2006;142:775–787. doi: 10.1104/pp.106.085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Foyer GH, Noctor G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4198–4203. doi: 10.1073/pnas.96.7.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Breusegem F, Vranova E, Dat JF, Inzé D. The role of active oxygen species in plant signal transduction. Plant Sci. 2001;161:405–414. [Google Scholar]
- 87.Yao N, Tada Y, Park P, Nakayashiki H, Tosa Y, Mayama S. Novel evidence for apoptotic cell response and differential signals in chromatin condensation and DNA cleavage in victorin-treated oats. Plant J. 2001;28:13–26. doi: 10.1046/j.1365-313x.2001.01109.x. [DOI] [PubMed] [Google Scholar]
- 88.Gupta AS, Webb RP, Holaday AS, Allen RD. Overexpression of Superoxide Dismutase Protects Plants from Oxidative Stress (Induction of Ascorbate Peroxidase in Superoxide Dismutase-Overexpressing Plants) Plant Physiol. 1993;103:1067–1073. doi: 10.1104/pp.103.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwon SY, Jeong YJ, Lee HS, Kim JS, Cho KY, Allen RD, Kwak SS. Enhanced tolerances of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen-mediated oxidative stress. Plant, Cell Environ. 2002;25:873–882. doi: 10.1007/s00299-006-0199-1. [DOI] [PubMed] [Google Scholar]
- 90.Camp WV, Capiau K, Montagu MV, Inze D, Slooten L. Enhancement of Oxidative Stress Tolerance in Transgenic Tobacco Plants Overproducing Fe-Superoxide Dismutase in Chloroplasts. Plant Physiol. 1996;112:1703–1714. doi: 10.1104/pp.112.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eng J, McCormack A, Yates J. R An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass. Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


