Abstract
IMPORTANCE
Persons with type 2 diabetes mellitus (T2DM) are at increased risk for decline in cognitive function, reduced brain volume, and increased white matter lesions in the brain. Poor control of blood pressure (BP) and lipid levels are risk factors for T2DM-related cognitive decline, but the effect of intensive treatment on brain function and structure is unknown.
OBJECTIVE
To examine whether intensive therapy for hypertension and combination therapy with a statin plus a fibrate reduces the risk of decline in cognitive function and total brain volume (TBV) in patients with T2DM.
DESIGN, SETTING, AND PARTICIPANTS
A North American multicenter clinical trial including 2977 participants without baseline clinical evidence of cognitive impairment or dementia and with hemoglobin A1c (HbA1c) levels less than 7.5% randomized to a systolic BP goal of less than 120 vs less than 140 mm Hg (n = 1439) or to a fibrate vs placebo in patients with low-density lipoprotein cholesterol levels less than 100 mg/dL (n = 1538). Participants were recruited from August 1, 2003, through October 31, 2005, with the final follow-up visit by June 30, 2009.
MAIN OUTCOME MEASURES
Cognition was assessed at baseline and 20 and 40 months. A subset of 503 participants underwent baseline and 40-month brain magnetic resonance imaging to assess for change in TBV and other structural measures of brain health.
RESULTS
Baseline mean HbA1c level was 8.3%; mean age, 62 years; and mean duration of T2DM, 10 years. At 40 months, no differences in cognitive function were found in the intensive BP-lowering trial or in the fibrate trial. At 40 months, TBV had declined more in the intensive vs standard BP-lowering group (difference, −4.4 [95% CI, −7.8 to −1.1] cm3; P = .01). Fibrate therapy had no effect on TBV compared with placebo.
CONCLUSIONS AND RELEVANCE
In participants with long-standing T2DM and at high risk for cardiovascular events, intensive BP control and fibrate therapy in the presence of controlled low-density lipoprotein cholesterol levels did not produce a measurable effect on cognitive decline at 40 months of follow-up. Intensive BP control was associated with greater decline in TBV at 40 months relative to standard therapy.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00000620
The prevalence of type 2 diabetes mellitus (T2DM) in older adults has risen in recent decades.1 Older persons with T2DM plus hypertension or, to a lesser extent, dyslipidemia have an increased likelihood of cognitive impairment and dementia compared with persons without T2DM or with T2DM alone.2 Type 2 diabetes mellitus in combination with these comorbidities is also associated with morphologic changes in the brain structure, including brain atrophy,3 increases in white matter lesions4,5 due to small-vessel and microvessel damage, and stroke due to larger-vessel occlusion and hemorrhage. These morphologic changes are also important predictors of impairment in older adults.6,7
No accepted prevention strategies exist at present to slow the effect of hypertension or dyslipidemia on cognitive decline in T2DM. Preliminary studies have suggested hypotheses that intensive therapy to lower blood pressure (BP) and lipid levels may be effective means of preventing T2DM-related cognitive decline.8,9 These hypotheses were tested using measures of cognitive function and magnetic resonance imaging (MRI)–based brain structure in the Memory in Diabetes (MIND) substudy of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial.10–12 The glycemia results for the MIND aspect of the trial have been published.12
Methods
The ACCORD and ACCORD MIND trial designs have been described previously.10–12 Briefly, ACCORD was a randomized, multicenter, double 2 × 2 factorial trial of 10 251 middle-aged and older participants with T2DM at high risk for cardiovascular events because of prevalent cardiovascular disease (CVD) or additional cardiovascular risk factors. All participants in the main ACCORD trial were enrolled in the glycemia trial to compare a therapeutic strategy targeted to a hemoglobin A1c (HbA1c) level of less than 6.0% (intensive therapy arm) vs a strategy that targeted HbA1c levels of 7.0% to 7.9% (standard therapy arm). The lipid trial (53.8% of the total sample) compared masked administration of placebo or fenofibrate in persons with low-density lipoprotein cholesterol (LDL-C) levels of less than 100 mg/dL (to convert to millimoles per liter, multiply by 0.0259) achieved through study-supplied simvastatin. The BP trial included the other 46.2% of participants and compared a therapeutic strategy targeted to systolic BP (SBP) of less than 120 mm Hg (intensive therapy) to one targeting SBP of less than 140 mm Hg (standard therapy). Participants meeting inclusion/exclusion criteria with SBP ranging from 130 to 180 mm Hg and taking 3or fewer antihypertensives were eligible for the BP trial. All others were assigned to the lipid trial. Unique randomization sequences for ACCORD were computer generated centrally at the coordinating center using permuted blocks of 4, 8, or 12 participants. A physical examination was performed, and event data and blood samples were collected at annual visits. In February 2008, the intensive glycemic intervention was stopped because increased risk for mortality was detected in that group.13 All participants in the intervention for intensive glycemic control were transitioned to the standard glycemic intervention protocol. The lipid and BP trials continued to the planned completion date in June 2009.
The MIND substudy within the ACCORD trial (target sample size, 2800 participants) was approved by the institutional review boards of the sponsors and each clinical site to collect additional cognitive and MRI outcomes beginning in August 2003. Immediately after randomization to an ACCORD treatment group, participants were asked to participate in the MIND substudy. Willing participants signed informed consent for collection of additional ACCORD MIND outcomes.
Cognitive Function
Cognitive function was assessed at baseline and 20 and 40 months after randomization using a test battery targeting cognitive functions typically affected inT2DM.10 The cognitive battery assessed verbal memory, processing speed, and executive function.14,15 The primary cognitive outcome was the number of correctly completed symbols in 120 seconds on the Digit Symbol Substitution Test (DSST), an omnibus test of psychomotor function and speed that includes aspects of learning and working memory.16 Secondary cognitive outcomes were verbal memory and executive function. Verbal memory was measured with the Rey Auditory Verbal Learning Test17 and reported as the sum of the number of words recalled (0–15) during the immediate-, short-, and delayed-recall trials. Executive functioning was measured with the modified Stroop Color-Word Test18 and is reported as the interference score; a higher score indicates worse function. To assess global cognitive function and to provide a metric to compare the MIND cohort with other study groups, the Mini-Mental State Examination19 was also administered. In addition to the cognitive tests, the Physician’s Health Questionnaire20 was administered to screen for depression, a frequent comorbidity in T2DM and a potential confounder.
Magnetic Resonance Imaging
For the MRI substudy, total brain volume (TBV) (measured in cubic centimeters), an integrated measure of neurodegenerative processes, was the primary outcome. Substantial evidence suggests that brain volume in nondemented individuals predicts future cognitive disorders.3
Scans were targeted within 45 days after randomization and at 40 months. The standardized MRI scan protocol and image analysis were previously described.21,22 Monthly MRI quality control procedures followed the American College of Radiology’s MRI QC Program (http://www.acr.org/quality-safety/accreditation/mri). Performance of the MRI scanner was stable across MRI sites and throughout the duration of the study as reflected by the stability of intracranial volumes (ICVs) over time (baseline mean ICV, 1132.34 cm3; follow-up mean ICV, 1132.32 cm3; P = .47 by paired t test).
Sample Size
Using unpublished data from participants in the Cardiovascular Health Study aged 65 to 75 years,23 we anticipated a 3-point, 40-month decline in the mean DSST score among participants randomized to standard glycemia, standard BP, or placebo fibrate therapy. For comparison of cognitive function between the intensive and standard BP therapy groups (or the fibrate and placebo lipid therapy groups) using a .05 two-sided type I error rate, a sample size of 600 participants per group (300 per cell) provided approximately 80% power to detect a 3% (1.2 DSST units) difference in 40-month means, assuming an underlying 2.5% difference in 40-month means for those participants in the intensive vs standard glycemia therapy groups (Supplement [eAppendix]). Recruitment was targeted at 350 participants per cell to account for an anticipated nonresponse rate of 15%. We ultimately recruited 2977 randomized ACCORD participants from 51 clinics throughout 6 clinical center networks (CCNs) (745 in the intensive BP therapy group, 694 in the standard BP therapy group, 782 in the fibrate therapy group, and 756 in the placebo group). The Veterans Administration CCN opted not to enroll MIND participants.
For the MRI substudy, assuming 200 participants were recruited to each BP intervention, we had 70% power to detect a 40-month difference in mean TBV of 3.3 cm3 under the same dropout and type I error assumptions. Post hoc power calculations for the lipid trial indicated that 100 participants with evaluable data per group would provide 70% power to detect a 40-month difference of 4.1 cm3 in mean TBV.
Statistical Analysis
All analyses were conducted at the ACCORD Coordinating Center, Wake Forest School of Medicine, using commercially available software (SAS, version 9.2; SAS Institute, Inc). All P values are reported as 2-sided tests. Participant characteristics are summarized with means (standard deviations) and percentages. Owing to the requirement to recruit ACCORD MIND participants and obtain consent 1 month after ACCORD randomization, which allowed some randomized participants to choose not to participate in MIND, baseline characteristics were compared (using 2-sided t tests and χ2 tests) between intervention groups within each 2 × 2 factorial trial. Characteristics that differed between groups at baseline were adjusted for in post hoc analyses to explore whether conclusions from unadjusted analyses resulted from baseline imbalances.
Within the BP and lipid trials, to test the effect of the interventions on cognitive function, we used a mixed-effects analysis of covariance model appropriate for 2 × 2 factorial studies that incorporated the 20- and 40-month outcome measures and used an unstructured covariance matrix.24 Each 2 × 2 trial was analyzed separately. Within each trial, the basic model included main-effect terms for the glycemia and BP (or lipid) interventions, a visit effect, a glycemia × visit interaction, a BP (or lipid) × visit interaction, the baseline value of the outcome, and other factors used to stratify randomization (CCN and history of CVD). Contrasts were used to test the primary hypothesis of no difference between BP (or lipid) groups at the 40-month visit.
We investigated the BP and lipid intervention effect on 40-month TBV using analysis of covariance. We analyzed each 2 × 2 trial separately. The model included main-effect terms for glycemia and BP (or lipid) interventions, baseline TBV, ICV (to adjust for head size), and previously described stratification factors.
Within the BP trial, we investigated the sensitivity of the TBV results to missing 40-month observations (including deaths) using multiple-imputation regression methods. The multiple-imputation regression models imputed missing 40-month TBV using baseline TBV, glycemia group assignment, history of CVD, CCN, and ICV. After recommendations for exploring the sensitivity of results to different missing data models,24 we estimated 2 regression-based imputation models. In model 1, the imputation of missing 40-month outcomes was based on fitting the same regression coefficients in both BP groups; in model 2, the imputation was based on allowing the regression coefficients to be estimated within each BP group.
For cognition and MRI outcomes, prespecified subgroup analyses were conducted for sex, history of CVD, glycemia arm in the BP (or lipid) trial, and baseline CCN. We also conducted post hoc exploratory subgroup analyses for baseline age (<60, 60–69, and ≥70 years), T2DM duration (<5, 6–10, 11–15, and ≥16 years), and baseline DSST score (<47, 47–59, and ≥60). As prespecified, the main treatment effects on the primary cognitive (DSST) and MRI (TBV) outcomes were each tested at the .05 significance level. All other hypothesis tests (interactions, subgroup analyses, and analyses of secondary outcomes) were considered to be hypothesis generating and conducted at the .05 level. Because we report 73 tests of secondary hypotheses each at the .05 level, a 98% chance (ie, 1 − [1 − .05]73) that at least 1 test would be significant at an .05 level, assuming independence between tests.25
Results
Participants
Among the 10 251 participants randomized to the ACCORD trial, 5575 were eligible to participate in the MIND substudy. Of the remaining 4676, the major reasons for ineligibility included enrollment during the vanguard period of the ACCORD trial (an initial 12-month period when investigators assessed the feasibility of recruiting and treating participants according to the protocol) or before site institutional review board approval for the ACCORD MIND substudy (79.4%), being younger than 55 years (13.4%), and enrollment in the lipid trial after MIND enrollment from the lipid trial had closed (7.2%). Participants enrolled in the ACCORD MIND substudy were similar to eligible participants who did not enroll (Supplement [eTable 1]). At baseline, 46.6% of participants were female and 30.3% were nonwhite; the mean age was 62 years. The mean HbA1c level was 8.3%, and mean (SD) duration of T2DM was 10.4 (7.4) years (Supplement [eTable 2]). Baseline characteristics of participants in the cognitive (n = 2977) and MRI (n = 614) portions of the ACCORD MIND substudy are presented by intervention group in the Supplement (eTables 3–6). Baseline characteristics were similar in both arms of the BP (or lipid) trial as illustrated by our comparison of baseline characteristics that identified significant (at α = .05) differences in 4 of 84 baseline comparisons (Supplement [eTables 3 and 4]). This result illustrates that despite the opportunity to opt out of the ACCORD MIND substudy after randomization, intervention group differences were consistent with chance alone. Similar results were found for comparisons between groups in the MIND MRI substudy (Supplement [eTables 5 and 6]).
As in the main trial, the interventions achieved substantial separations in treatment targets in the MIND component (Supplement [eTable 7] lists unadjusted means). At 40 months, the mean SBP was 119.0 (14.7) vs 133.2 (14.8) mm Hg and mean diastolic BP was 64.0 (10.1) vs 70.2 (9.9) mm Hg in the intensive vs standard BP therapy groups, respectively. At 3 years, mean lipid levels were 156.8 (38.4) vs 152.2 (31.5) mg/dL for total cholesterol level, 82.3 (26.9) vs 82.8 (25.6) mg/dL for LDL-C level, and 43.7 (10.0) vs 45.5 (10.8) mg/dL for high-density lipoprotein cholesterol levels in women and 37.0 (8.0) vs 37.9 (9.5) mg/dL in men for the placebo vs fibrate groups, respectively. Median triglyceride levels were 150 vs 122 mg/dL (to convert to millimoles per liter, multiply by 0.0113). Adverse effects associated with each intervention have been reported previously.13,26–28
Cognitive Function Outcomes
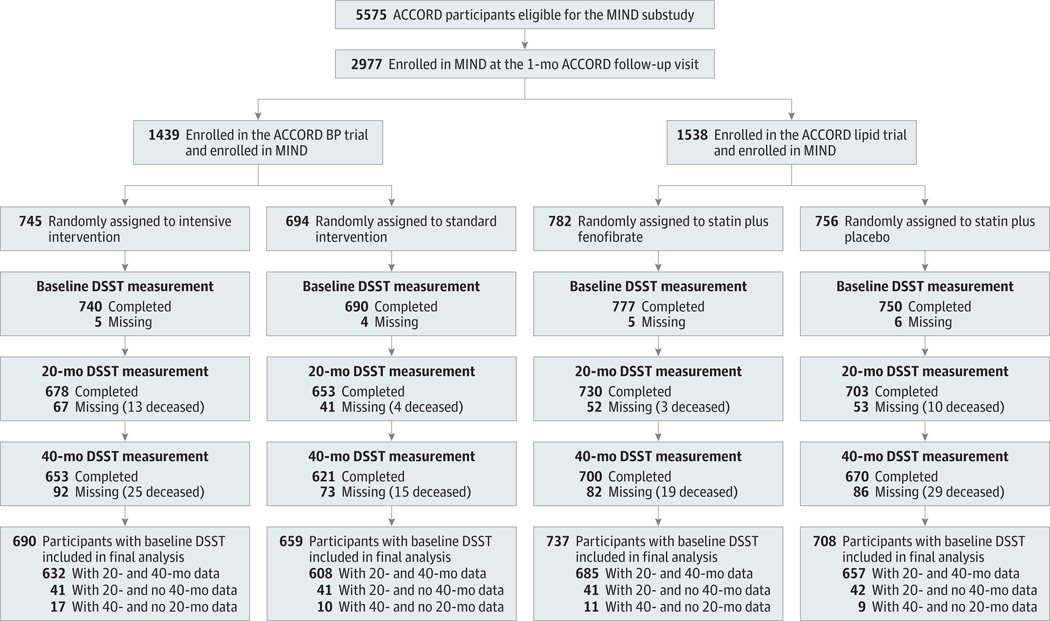
Baseline cognitive function scores were similar for participants in the intensive vs standard BP and fibrate vs placebo lipid trial groups (Supplement [eTables 3 and 4]). Overall, 2957 of the 2977 ACCORD MIND participants (99.3%) had a baseline DSST (Figure 1) and 2794 (93.9%) had at least 1 (20- or 40-month) follow-up measure. In the BP and lipid trials, 1274 and 1370 participants, respectively, completed the 40-month cognitive assessment (Figure 1). For both trials, slight differences existed for baseline characteristics (Supplement [eTables 3 and 4]) between those with and without follow-up data (data not shown). Those participants missing follow-up data were older (mean age, 64.2 vs 62.4 years; P < .001), had higher SBP (mean, 140.2 vs 135.3 mm Hg; P < .001), and had lower DSST scores (mean, 46.7 vs 52.9; P < .001) compared with participants with follow-up data.
Figure 1. Cohort Participation in the Primary Cognitive Outcome of the Blood Pressure (BP) and Lipid Trials.
Participants were enrolled in the Memory in Diabetes (MIND) substudy of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. DSST indicates Digit Symbol Substitution Test.
The primary outcome, DSST score, declined in the BP and lipid intervention groups (Table 1). However, we found no significant difference in the adjusted 40-month DSST mean scores between intensive vs standard BP therapy (BP difference between means, −0.26 [95% CI, −1.11 to 0.59]; P = .55) or between the fibrate vs placebo lipid groups (lipid difference between means, −0.08 [−0.92 to 0.76]; P = .85). Mean 40-month cognitive function did not differ between intervention groups in the BP or the lipid trial for any of the other 3 cognitive tests.
Table 1.
Cognitive Primary and Secondary Outcomes of the BP and Lipid Trials
| End Pointa | Time | BP Trial, Adjusted Mean (95% CI) | Lipid Trial, Adjusted Mean (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| INT Intervention | STD Intervention | Difference in Means |
P Valueb |
Fenofibratec | Placeboc | Difference in Means |
P Valueb |
||
| DSSTd | Baselinee | 52.28 | 52.28 | 52.79 | 52.79 | ||||
| 20 mo | 50.94 (50.36 to 51.53) | 51.00 (50.41 to 51.60) | −0.06 (−0.90 to 0.78) | .89 | 51.39 (50.82 to 51.97) | 51.59 (51.00 to 52.18) | −0.20 (−1.02 to 0.63) | .64 | |
| 40 mo | 50.42 (49.82 to 51.01) | 50.67 (50.07 to 51.28) | −0.26 (−1.11 to 0.59) | .55c | 50.94 (50.35 to 51.53) | 51.02 (50.42 to 51.62) | −0.08 (−0.92 to 0.76) | .85c | |
| 40-mo change | −1.86 (−2.46 to −1.27) | −1.61 (−2.21 to −1.00) | −1.85 (−2.44 to −1.26) | −1.77 (−2.37 to −1.17) | |||||
| RAVLTf | Baselinee | 7.51 | 7.51 | 7.51 | 7.51 | ||||
| 20 mo | 7.77 (7.64 to 7.91) | 7.89 (7.75 to 8.02) | −0.12 (−0.31 to 0.08) | .23 | 7.91 (7.78 to 8.04) | 7.86 (7.73 to 7.99) | 0.05 (−0.13 to 0.23) | .58 | |
| 40 mo | 7.98 (7.85 to 8.12) | 8.04 (7.89 to 8.18) | −0.05 (−0.26 to 0.15) | .58 | 7.98 (7.86 to 8.11) | 7.94 (7.80 to 8.07) | 0.05 (−0.14 to 0.23) | .61 | |
| 40-mo change | 0.47 (0.34 to 0.61) | 0.53 (0.39 to 0.67) | 0.47 (0.35 to 0.60) | 0.43 (0.29 to 0.56) | |||||
| Stroopg | Baselinee | 32.60 | 32.60 | 31.46 | 31.46 | ||||
| 20 mo | 31.50 (30.51 to 32.48) | 31.35 (30.35 to 32.36) | 0.15 (−1.26 to 1.55) | .84 | 31.18 (30.20 to 32.16) | 30.69 (29.70 to 31.68) | 0.48 (−0.91 to 1.88) | .50 | |
| 40 mo | 31.10 (30.08 to 32.12) | 32.14 (31.09 to 33.19) | −1.04 (−2.51 to 0.42) | .16 | 31.62 (30.63 to 32.62) | 32.21 (31.19 to 33.22) | −0.59 (−2.01 to 0.84) | .42 | |
| 40-mo change | −1.50 (−2.52 to −0.48) | −0.46 (−1.51 to 0.59) | 0.16 (−0.83 to 1.16) | 0.75 (−0.27 to 1.76) | |||||
| MMSEh | Baselinee | 27.25 | 27.25 | 27.53 | 27.53 | ||||
| 20 mo | 27.06 (26.89 to 27.23) | 27.17 (27.00 to 27.35) | −0.11 (−0.35 to 0.13) | .35 | 27.35 (27.18 to 27.52) | 27.47 (27.30 to 27.64) | −0.12 (−0.36 to 0.11) | .31 | |
| 40 mo | 27.00 (26.83 to 27.17) | 26.95 (26.77 to 27.11) | 0.05 (−0.20 to 0.29) | .70 | 27.17 (27.00 to 27.34) | 27.10 (26.93 to 27.27) | 0.07 (−0.17 to 0.31) | .58 | |
| 40-mo change | −0.25 (−0.42 to −0.08) | −0.30 (−0.48 to −0.13) | −0.36 (−0.53 to −0.19) | −0.43 (−0.60 to −0.26) | |||||
Abbreviations: BP, blood pressure; DSST, Digit Symbol Substitution Test; INT, intensive; MMSE, Mini-Mental State Examination; RAVLT, Rey Auditory Verbal Learning Test; STD, standard.
For the DSST, RAVLT, and MMSE, higher values indicate better cognition; a negative change value, a decline in cognitive score. For the Stroop test, lower values indicate better cognition and a positive change value represents a decline in cognitive score.
Calculated as tests of differences between intervention group means at each follow-up visit, adjusted for the baseline level of the outcome. The P value for a comparison of the 40-month change between intervention groups is left blank because, when controlling for the baseline level, it will be identical to that provided for the comparison between means at the 40-month visit.
Calculated as result of test of prespecified primary outcome.
Indicates number of correct cells (possible range, 0–90).
Baseline mean indicates the overall mean for both groups combined as measured before randomization. This value is used to obtain the adjusted mean estimates at follow-up.
Indicates the total number of words recalled (possible range, 0–15).
Measured as the interference score.
Possible range, 0 to 30.
We conducted several participant subgroup analyses for the DSST, Rey Auditory Verbal Learning Test, and Stroop Color-Word Test. For the BP and lipid trials, we found no consistent differences in intervention effects on cognition within the subgroups examined (Supplement [eFigures 1–6]), including BP (or lipid) intervention effects within glycemia intervention groups.
MRI Outcomes
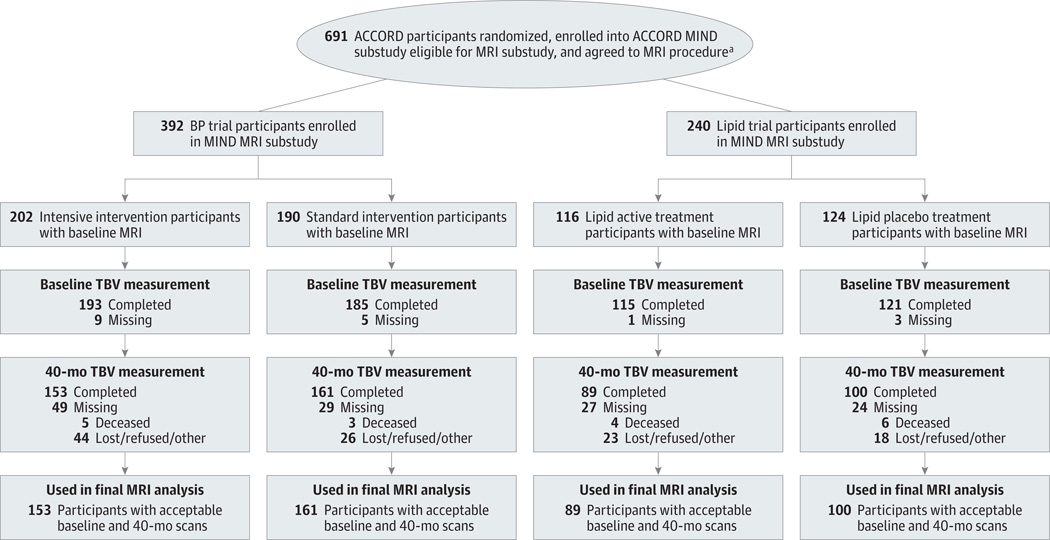
Six hundred thirty-two participants enrolled in the MRI substudy and 614 (378 BP and 236 lipid) had baseline scans usable for analysis (Figure 2). Participants were similar to the ACCORD MIND participants not in the MRI substudy (Supplement [eTable 2]). At 40 months, 503 of the 614 original participants with an acceptable baseline scan (81.9%) also had an acceptable repeated MRI for the final analysis (Figure 2). Participants unable to undergo repeated scanning included 18 (16.2%) who died, 16 (14.4%) who refused, and 27 (24.3%) with new MRI-related reasons (eg, pacemaker, poor image quality). The remainder had a variety of other reasons (Supplement [eTable 8]). Unadjusted means for TBV are listed in the Supplement (eTable 9).
Figure 2. Cohort Participation in the Primary Magnetic Resonance Imaging (MRI) Outcome of the Blood Pressure (BP) and Lipid Trials.
Participants were enrolled in the Memory in Diabetes (MIND) substudy of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Standard and intensive BP interventions and active and placebo lipid treatments are described in the legend to Figure 1. TBV indicates total brain volume.
aAgreeing to the MRI procedure indicates that participants signed a consent for MRI. Enrolled in the MIND MRI substudy indicates that they underwent a baseline MRI.
At 40 months, the intensive BP intervention group had a statistically significant lower TBV compared with the standard BP intervention group (difference between adjusted means, −4.4 [95% CI, −7.8 to −1.1] cm3; P = .01) (Table 2). The fibrate and placebo groups did not differ in TBV (difference between adjusted means, 1.2 [95%CI, −3.1 to 5.5] cm3) (Table 2).
Table 2.
Adjusted Means for 40-Month TBV by Blood Pressure and Lipid Trials
| Trial | Therapy Group | No. of Participants |
Baseline Mean TBV, cm3a |
TBV, Mean (95% CI), cm3b | P Valuec | |
|---|---|---|---|---|---|---|
| 40 mo | Change From Baseline | |||||
| Blood pressure | INT | 153 | 921.5 | 902.6 (900.2 to 905.0) | −18.9 (−21.3 to −16.5) | |
| STD | 161 | 921.5 | 907.0 (904.7 to 909.4) | −14.5 (−16.8 to −12.2) | ||
| 40-mo difference | −4.4 (−7.8 to −1.1) | .01 | ||||
| Lipid | Fenofibrate | 89 | 937.0 | 924.1 (920.9 to 927.2) | −12.9 (−16.1 to −9.8) | |
| Placebo | 100 | 937.0 | 922.9 (919.9 to 925.9) | −14.1 (−17.1 to −11.1) | ||
| 40-mo difference | 1.2 (−3.1 to 5.5) | .59 | ||||
Abbreviations: INT, intensive; STD, standard; TBV, total brain volume.
Baseline mean is the overall mean for both groups combined as measured before randomization. This value is used to obtain the adjusted mean estimates at follow-up.
Indicates adjusted mean obtained from analysis of covariance.
Calculated as a test of difference between the intervention group means at the 40-month visit, adjusted for the baseline level of the outcome. When controlling for the baseline value of the outcome, the P value for a comparison of the 40-month means between intervention groups will be identical to the P value for a comparison of change from baseline (controlling for the baseline level as a covariate) to the 40-month visit.
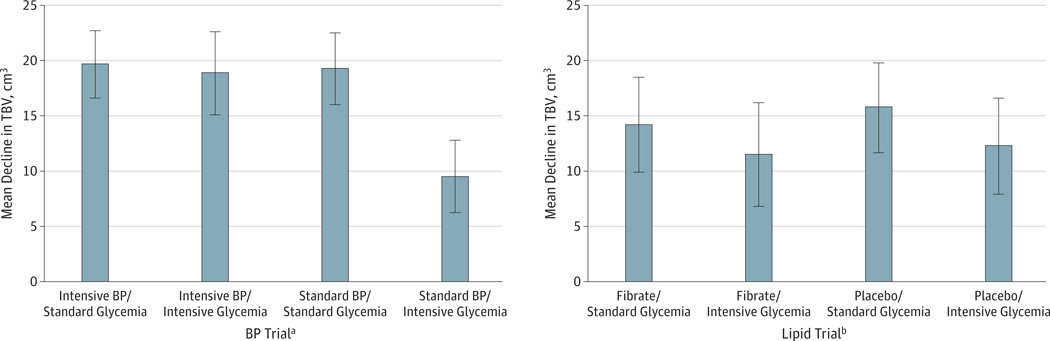
Analysis of subgroups identified a significant interaction between BP and glycemia effects on TBV (P = .009). Figure 3 confirms the earlier finding of the glycemic intervention in the ACCORD MIND substudy12 that the strategy of intensive glycemia control preserved TBV across the BP and lipid trials. Figure 3 also illustrates that participants receiving the combination of standard antihypertensive therapy and intensive glycemic control experienced approximately 50% of the decline in TBV observed in the other BP trial groups. This interaction P value remained significant or approached significance under both imputation models (model 1, P = .07; model 2, P = .03) and when controlling for baseline covariates that differed between groups (Supplement [eTables 5 and 6]). Tests for different BP (or lipid) effects on TBV within predefined participant subgroups showed no differences (sex, CCN, baseline CVD, or cognitive function).
Figure 3. 40-Month Decline in Total Brain Volume (TBV) in the Blood Pressure (BP) and Lipid Trials.
Whiskers mark 95% confidence intervals. Intensive and standard glycemia therapy groups are described in the Supplement (eAppendix). Standard and intensive BP interventions and active and placebo lipid treatments are described in the legend to Figure 1.
aP < .001 for heterogeneity of glycemia effect.
bP = .86 for heterogeneity of glycemia effect.
Discussion
The previous ACCORD MIND glycemia results12 showed that intensive glycemia control does not preserve cognitive function as measured by the same battery used in the present study. The present results extend these findings to show that intensive BP management to a target SBP of less than 120 mm Hg and fibrate therapy in the context of LDL-C level control are not effective in reducing cognitive decline in persons with poorly controlled T2DM at high risk for CVD. Memory loss and its most dire consequence, dementia, are proven complications of T2DM. We implemented the MIND substudy within the ACCORD trial because effective treatments for prevention of cognitive decline in persons with T2DM are lacking, and recent studies suggested potential benefit from intensive BP and lipid therapy on cognitive decline.29–31
The previous report12 found that maintaining TBV is best achieved in patients with T2DM by applying a strategy of intensive glycemia therapy with an HbA1c treatment goal of less than 6.0%. These results add to the previous findings by showing that preservation of TBV is greatest when used in combination with treatment to current recommended SBP targets of 135 to 140 mm Hg. Although a greater decline in TBV is associated with early cognitive impairment, a precursor to dementia,31 the long-term implications of the imaging findings in the ACCORD MIND substudy are unknown and remain a focus of ongoing investigation and analyses. The ACCORD MIND substudy was designed with 2 primary outcomes, cognition and TBV, and not to test whether MRI measures were adequate “surrogate markers” for treatment-related preservation of cognitive function. Our finding, however, suggests that TBV and white matter lesion burden alone cannot, to date, be used as surrogate markers for cognitive outcomes.
Strengths of this study include its prospective design within a randomized clinical trial. This design allows for balance between randomized groups of factors such as genetics. Other strengths include the high degree of data capture, attainment of substantial BP separation between the treatment groups, and the ability to capture functional and anatomic brain outcomes. The study also has several limitations. First, cognitive decline is a slow process, and 40 months of follow-up may be an inadequate time to ascertain subtle differences in cognitive function.32 A 5-year extension of the ACCORD MIND substudy with MRI scanning is under way. Second, our findings are generalizable only to people with long-term T2DM at high risk of CVD and with relatively poorly controlled HbA1c levels (minimal level, 7.5%). These results do not apply to persons with newly diagnosed T2DM or to individuals with longstanding glycemic control to an HbA1c level of less than 7.5%. Third, the ACCORD trial tested overall strategies for achieving treatment goals. Dosages and medications used to achieve goals differed within interventions; thus, attempts to attribute effects to individual medications or doses are hampered by confounding between patient characteristics and medication choice.28–30 Fourth, some of the advantages of randomization may have been lost because of the necessity to obtain consent from participants in the ACCORD MIND substudy immediately after randomization, thus allowing some participants to opt out of participation. Last, we acknowledge that with the large number of hypothesis tests that were performed, these results could result from chance alone.
Conclusions
During the past 2 decades, the belief that more intensive treatment strategies for controlling T2DM-related comorbidities, such as hyperglycemia, hyperlipidemia, and hypertension, would reduce clinical complications has driven large investment in new medications for this disease syndrome. However, these results from the ACCORD MIND substudy, along with the other recent ACCORD results, make clear the decreasing returns of intensive medication-based therapy for advanced T2DM and add further evidence to the need for increased investment in disease prevention and early intervention.
These results do not negate other evidence that intensive strategies to control BP and lipid levels may be indicated for other conditions such as stroke or coronary heart disease. However, this randomized clinical trial in 2977 older adults with a mean baseline Mini-Mental State Examination score higher than 27, a mean HbA1c level of 8.3%, and long-term T2DM shows no overall reduction of the rate of T2DM-related cognitive decline through intensive BP therapy or adding a fibrate to well-controlled LDL-C levels.
Supplementary Material
Acknowledgments
Dr Gerstein received consulting fees from sanofi-aventis, GlaxoSmithKline, Eli Lilly, Novo Nordisk, AstraZeneca, Bristol-Myers Squibb, Roche, Medtronic, Merck, Bayer, Bioavail, and Janssen-Ortho; received institutional grant support to McMaster University from sanofi-aventis, GlaxoSmithKline, Novo Nordisk, Merck, Pronova, Roche, Eli Lilly, and Boehringer Ingelheim; received lecture fees from sanofi-aventis, GlaxoSmithKline, Solvay, Boehringer Ingelheim, Servier, Bayer, Eli Lilly, Novo Nordisk, and Takeda; is a member of the board of Merck and Schering-Plough and of the global advisory board of Bristol-Myers Squibb/AstraZeneca; receives consulting fees from GlaxoSmithKline, Merck, Bristol-Myers Squibb, AstraZeneca, Regeneron/sanofi-aventis, Abbott, Roche, Isis/Genzyme, Novartis, and Pfizer; received institutional grant support to the Columbia University College of Physicians and Surgeons from Merck, Roche, Isis/Genzyme, and AstraZeneca; and received payment from Pfizer for development of an educational presentation. Dr Miller received consulting fees from Roche.
Funding/Support: This study was supported by the National Institute of Aging and the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Role of the Sponsors: The funding sources contributed to the design and conduct of the study; the analysis and interpretation of the data; and preparation, review, and approval of the manuscript.
Group Information
The full trial protocol is given at https://www.accordtrial.org. Members of the ACCORD MIND Study Group include the following: Lenore J. Launer (principal investigator [PI]), and Robert Kramer, Intramural Research Program, National Institute on Aging, Bethesda, Maryland; Jeff D. Williamson (PI), Michael E. Miller, Laura H. Coker, James Lovato, Laura Lovato, John Hepler, Deborah Felton, Leanne Andrews, S. Mayer, C. Carter, and Nancy Woolard (Wake Forest School of Medicine, Winston-Salem, North Carolina [coordinating center]); R. Nick Bryan (PI), Lisa Desiderio, Christos Davatzikos, Michael Bilello, Elias R. Melhem, D. Koka, Evi Parmpi, Harsha Battapady, and Guray Erus (Department of Radiology, Hospital of the University of Pennsylvania, Philadelphia [MRI Reading Center]); Hertzel C. Gerstein (MIND PI), Salim Yusuf, Sarah Capes, Zubin Punthakee, Tali Cukierman-Yaffe, (MIND investigator), Michael Vallis, Stephanie Hall, P. Mackie, V. Reiding, Rosalie Russo, Beth Tadeson, and Kim Thompson (Canadian Diabetes Outcome Researchers, Population Health Research Institute, Hamilton Health Sciences/McMaster University, Hamilton, Ontario [Canadian CCN]); Sarah Capes, Zubin Punthakee, Ada Smith, and Teresa Valla (McMaster Medical Centre [Canadian clinical site]); Carol Joyce, Minnie Parsons, and Bernadette Rowe (Memorial University of Newfoundland, St John’s [Canadian clinical site]); Irene Hramiak, Melissa Gehring, and Sue Tereschyn (St Joseph’s Health Care London, London, Ontario [Canadian clinical site]); Heather Lochnan, Julie Maranger, Martha McLean, and Ron Sigal (Division of Endocrinology and Metabolism, Ottawa Hospital, Ottawa, Ontario [Canadian clinical site]); Vincent Woo, Lori Berard, Teresa Anderlic, Adrian Bernard, and Al-Noor Mawani (Winnipeg Regional Health Authority, Health Science Center, Diabetes Research Group, Winnipeg, Manitoba [Canadian clinical site]); Carl Abbott, Ehud Ur, Glenda McCarthy, Melanie Yuille, Heather Murdock, Tabitha Palmer, and Anne Marie Patterson (Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia [Canadian clinical site]); Richard H. Grimm, Jr, Brenda Kirpach, Marian M. Bartkoske, Colleen M. Boyce, Nicole Druckman, Arlene M. Gillett, Julie A. Levin, Gloria J. Livingston (MIND investigator), Anne M. Murray (MIND PI), Karen L. Margolis (MIND investigator), and Charles Truwit (MIND MRI investigator) (Berman Center for Outcomes & Clinical Research, Minneapolis, Minnesota [Minnesota-Iowa CCN]); Sara Kempainen, Marcia Madden, Kathleen Hall, and Kim Wood (Hennepin ACCORD Clinic, Minneapolis [Minnesota-Iowa clinical site]); Richard Bergenstal, R. Cuddihy, Bradley Davick, Susan List, Diane Whipple, Charlotte Ashanti, and D. Kendall (International Diabetes Center, Minneapolis [Minnesota-Iowa clinical site]); Elizabeth R. Seaquist, Michael V. Mech, Luke E. Benedict, Debra J. Demmon, Anjali F. Kumar, Shaina M. Martinson, Sherry A. Miller, C. Pease, Jyothi P. Rao, J. Bruce Redmon, Joyce E. Swanson, Julie K. Wimmer, Yolanda Okorocha, M. Stiles, C. Kodl, and C. Chadha (University of Minnesota, Minneapolis [Minnesota-Iowa clinical site]); Kevin A. Peterson, Lea A. Seaquist, Christy Boese, Tai J. Mendenhall, Andrea M. Peterson, Amanda Rudelt, and Terri M. Schrock (Phalen Village Clinic, University of Minnesota, St Paul [Minnesota-Iowa clinical site]); JoAnn Sperl-Hillen, Patrick J. O’Connor, Maureeen E. Busch, A. Chung, Becky K. Klein, Nicole Krugen, Theresa Bunkers-Lawson, Heidi L. Ekstrom, Heidi S. Gunderson, Bonnie M. Johnson, John H. MacIndoe, Donna J. Prewedo, Janet L. Rawl, Colleen M. Roethke, Mary Quinlan, Carol R. Fox, Beverly M. Bate, Q. T. Cao, Melissa M. Ohnstad, and P. J. Meyers (Department of Endocrinology, Riverside Health Partners Clinic, Minneapolis [Minnesota-Iowa clinical site]); William I. Sivitz, Sheila M. Wayson, Theresa A. Lower, Kurt A. Ochs, and Denise Wells (Health Care Diabetes Clinical Research and Programs, University of Iowa, Iowa City [Minnesota-Iowa clinical site]); Ronald M. Lazar (MIND PI), Kevin Slane (MIND investigator), and Carlos R. Lopez-Jimenez (MIND investigator) (Columbia University College of Physicians and Surgeons, New York, New York [Northeastern CCN]); Joy Hirsch, Truman R. Brown, Stephen M. Dashnaw, Jamie Cruz-Lobo (Columbia University Medical Center, New York [MIND MRI Center]); Ulrich K. Schubart, Jeanne Russo, Nilda Vincenty, and Katherine Ibarrando (Jacobi Medical Center, Bronx, New York [Northeastern clinical site]); Michael H. Alderman, Lillian Carroll, Mary Jo Sanguily, Anna C. Mayer, and Lee Ramos (Albert Einstein General Clinical Research Center, Bronx, New York [Northeastern clinical site]); David Brillon, Juan Cordero, Raymond Chiong, Karen Hyams, and Cassia Charles (Cornell Internal Medicine Associates, New York, New York [Northeastern clinical site]); Arnaud Bastien, Susan Grudzinski, Patrick Niblack, L. Abreu, Karen Brown, Monica Casale, Denise Dougherty, Dawn Linneman, Miriam A. Salvador, Pamela Zee, Daniel Hyman, Diane Atabek, Jenifer Miranda, Indira Kumar, and Charlotte Baker (The Cooper Health System, Cherry Hill, New Jersey [Northeastern clinical site]); Daniel F. Brautigam, Rosemary Fischer, Deanna M. Scharf, E. Brautigam, Barbara Nunn, June M. Chiarot, and Chris Flanders (Great Lakes Medical Clinic Research, Westfield, New York [Northeastern clinical site]); Robin Goland, Patricia Kringas, Jennifer Montes, and Janiel Belle (Naomi Berrie Diabetes Center, New York, New York [Northeastern clinical site]); Asqual Getaneh, Jennifer Ramirez, G. Kranwinkel, and Erida Vasquez (Ambulatory Care Network at Columbia University [Northeastern clinical site]); Daniel S. Donovan, Gerardo Febres, Clara Hernandez, Mary Anne Jonaitis, and Carlos Lopez-Jimenez (Irving Diabetes Research Unit, New York, New York [Northeastern clinical site]); Mary Ann Banerji, Margaret Norton, Priti Patel, and Sondra Hirsch (State University of New York Downstate Medical Center, Brooklyn, and New York Kings County, Brooklyn [Northeastern clinical sites]); Saul Genuth, Faramarz Ismail-Beigi, Mark Thibonnier, Laura Vargo, Carol Kelly, Theresa Bongorno, Amanda Dolish (MIND investigator), Laura Pavlik (MIND investigator), Margaret Tiktin (MIND investigator), Souzan Isteitieh, Andrew Bartlett, Kimberly Yee, Tanya Kulow, and Karen A. Horowitz (MIND PI); (Division of Clinical and Molecular Endocrinology, Case Western Reserve University, Cleveland, Ohio [Ohio-Michigan CCN]); Jeffery Sunshine and Mark Clampitt (University Hospitals of Cleveland, Department of Radiology, Cleveland [MIND MRI Center]); Faramarz Ismail-Beigi, A. Krikorian, L. Moore, Lynn Richardson, E. Coles-Herman, Jamie Frankino, Ajay Sood, Leighanne Hustak, Mary Julius, Laura Pavlik, Toni Ross, Lucy Long, Mary K. Sullivan, MD, Louise Strauss, Kim Behm, Farideh Eskandari, Cynthia Hall, Debbie Hayes, Karen A. Horowitz, Souzan Isteitieh, Zuhayr Madhun, Eileen Seeholzer, Julie Shina, Harris Taylor, and Adrian Schnall (Division of Endocrinology, University Hospitals of Cleveland, and University Hospitals Westlake Medical, Cleveland [Ohio-Michigan clinical site]); Laurie S. Sadler, Mary Griffith, Ann Hornsby, Karen Klyn, Ellen Ospelt, Lucy Long, Mariellen DeSmit, Peggy McCann, Nicole Pero Schmidt, Tanya Kulow, and Tania Zaletal (Lipid Research Center, St Vincent Charity Hospital, Cleveland [Ohio-Michigan clinical site]); Adrian. M. Schnall, Lori Dragmen, Renee Ellert, Jonathon Smith, J. Leksan, and T. Sussman (University Suburban Health Center, South Euclid, Ohio [Ohio-Michigan clinical site]); Faramarz Ismail-Beigi, Leighanne Hustak, Mary Julius, William Schwing, Cynthia Hall, Karen A. Horowitz, Souzan Isteitieh, Cynthia Johnson, Elizabeth Kern, Mary Ann Richmond, Lynn Richardson, Kimberly Roberts, Julie Shina, Pam Suhan, Harris Taylor, Sharon Watts, J. Martin, and G. J. Strauss (Department of Medicine, Cleveland Veterans Affairs Medical Center, and Ravenna Community Based Outpatient Clinic, Cleveland [Ohio-Michigan clinical site]); Basil Akpunonu, Roberto Franco-Saenz, Jenny Gilmore, Maureen Gilmore, Patricia Ross, Becky Bauer, Rodica Pop-Busui, Jason Roman, and Zachary Blust (Department of Medicine, Medical University of Ohio, Ruppert Health Center, Toledo [Ohio-Michigan clinical site]); A. Thomas, Dorothy M. Kahkonen, Terra Cushman, Melissa Roman, Ann M. Stys, Karen White, Mary Austin, Cindy Chatterton, J. Kimberly Francis, Charlene Jones, David Kruger, Amanda McLellan, Fred Whitehouse, E. Higgins, Shiri Levy, and A. Schoenherr (Henry Ford Health System–New Center One, Detroit, Michigan [Ohio-Michigan clinical site]); David C. Goff, Jr, John H. Summerson, Lenore Crago, Caroline S. Blackwell, Alain Bertoni, Rhonda L. Blaine, Julienne K. Kirk, Rhonda L. Spach, Jeff Williamson, Jorge Calles, Jeff Katula, Dorothy B. Wishnietsky, Leslie Gordineer, and Jingzhong Ding (MIND investigator); (Divison of Public Health Sciences, Wake Forest School of Medicine [Southeastern CCN]); Sandy Kaminsky and Debra Fuller (Center for Biomolecular Imaging, Wake Forest School of Medicine [MIND MRI Center]); Mark N. Feinglos, Jennifer Jones, and Georgianne Gedon-Lipscomb (Duke University Medical Center, Durham, North Carolina [Southeastern clinical site]); Hal H. Atkinson, Judy Stanfield, and Thania Delvalle-Fagan (Department of Geriatrics/Gerontology, Wake Forest School of Medicine [Southeastern clinical site]); Carolyn F. Pedley and Sally Mauney (Downtown Health Plaza, Winston-Salem, North Carolina [Southeastern clinical site]); John B. Buse, Michelle D. Duclos, Ruth E. Kirby, A. Goley, and K. Vukojicik (Diabetes Care Center, University of North Carolina, Chapel Hill [Southeastern clinical site]); Jerry L. Miller, W. Besterman, Susan M. Norton, and M. Surgener (Holston Medical Group, Kingsport, Tennessee [Southeastern clinical site]); Michael Dulin, Tom Barringer, Carol Morris, and S. Norton (Carolinas Medical Center Family Practice, Charlotte, North Carolina [Southeastern clinical site]); Robin Peace, Dennis O. Stuart, Janice Strickland, Lynn Cummings, and Judy Stanfield (Robeson Health Care Corporation, Fairmont Clinic, Fairmont, North Carolina [Southeastern clinical site]); Robin Peace, Janice Strickland, Lynn Cummings, and Judy Stanfield (Robeson Health Care Corporation, Julian T. Pierce Clinic, Pembroke, North Carolina [Southeastern clinical site]); John R. Crouse III and Julie Ellis (Departments of Internal Medicine and Endocrinology, Wake Forest School of Medicine [Southeastern clinical site]); Vivian Fonseca, J. John-Kalarickal, Sharice M. Leger, and Kartik Munshi (Tulane University Health Science Center, New Orleans, Louisiana [Southeastern clinical site]); Joshua Barzilay, Katrina Bader, and Melanie Eley (Kaiser Permanente, Crescent Center Medical office [Southeastern clinical site]); Jeffery L. Probstfield, Mark D. Sullivan (MIND PI), Connie Kingry, Janice M. Johnson (MIND investigator), A. S. Line, Marshall A. Corson, R. Knopp, Edward Lipkin, MD, C. Griswold, K. Liebert, Ashley Brown, Dawn Juliano, Ella Mae Kurashige, Stephanie Moberg, and J. Leader (University of Washington, Seattle [Western CCN]); Kevin Ariani and Haydee Gutierrez (Cardiovascular Center, Northridge Hospital Medical Center, Northridge, California [Western MIND site]); Richard Failor, Allan Ellsworth, Nikki Jackson, and Kim Capoccia (University of Washington Medical Center at Roosevelt, Family Medical Center, Seattle [Western MIND site]); Rex Force, Mimi Macdonald, Shannon Koester, and Krysti Pettingill (Department of Family Medicine, Idaho State University, Pocatello [Western MIND site]); Peter E. Linz, Patricia V. Pepper, Susan Griffin, Janet Kozlowski, Marty Engle, and Paige Gutierrez (Cardiology Division, Naval Medical Center San Diego, San Diego, California [Western MIND site]); Matthew C. Riddle, Patricia A. McDaniel, Sarah C. Gammell-Matthews, Brittany MacNeil, Sharlene K. DesRochers, and Reyna Swift (Section of Diabetes, Oregon Health and Science University, Portland [Western MIND site]); Carol Wysham, Debbie Weeks, and Linda Kuntsmann (Washington State University, Spokane [Western MIND site]); Jim Dudl, Patricia Wu, Laura Lyons, Barbara House, Margaret Murray, Anton Palma, and Rachel Stevenson (Kaiser Endocrine Clinic, San Diego, California [Western MIND site]); and George Dailey, Athena Philas-Tsimikas, Anna Giannella, A. Bravo-Medina, Melissa Jacobson, Estela Farro, S. Cruz, Noemi Juarez, and Connie Dadkhah (Clinical Trials Department, Whittier Institute for Diabetes, La Jolla, California [Western MIND site]).
Footnotes
Author Contributions: Drs Williamson and Miller had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Williamson, Launer, Bryan, Coker, Lazar, Gerstein, Murray, Sullivan, Marcovina, Margolis, Miller.
Acquisition of data: Williamson, Bryan, Coker, Murray, Sullivan, Horowitz, Ding, Marcovina, Margolis, Barzilay, Ginsberg, Linz, Miller.
Analysis and interpretation of data: Williamson, Launer, Bryan, Lazar, Murray, Horowitz, L. Lovato, J. Lovato, Margolis, Davatzikos, Barzilay, Miller.
Drafting of the manuscript: Williamson, Launer, L. Lovato, J. Lovato, Miller.
Critical revision of the manuscript for important intellectual content: Williamson, Bryan, Coker, Lazar, Gerstein, Murray, Sullivan, Horowitz, Ding, Marcovina, Margolis, Davatzikos, Barzilay, Ginsberg, Linz, Miller.
Statistical analysis: L. Lovato, J. Lovato, Davatzikos, Miller.
Obtained funding: Williamson, Launer, Coker, Gerstein, Miller.
Administrative, technical, or material support: Williamson, Bryan, Coker, Lazar, Horowitz, Marcovina, L. Lovato, J. Lovato, Davatzikos, Barzilay, Ginsberg, Linz, Miller.
Study supervision: Williamson, Bryan, Coker, Sullivan, Davatzikos, Linz, Miller.
Conflict of Interest Disclosures: No other disclosures were reported.
REFERENCES
- 1.McBean AM, Li S, Gilbertson DT, Collins AJ. Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: whites, blacks, Hispanics, and Asians. Diabetes Care. 2004;27(10):2317–2324. doi: 10.2337/diacare.27.10.2317. [DOI] [PubMed] [Google Scholar]
- 2.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R, Anstey KJ, Cherbuin N, Wen W, Sachdev PS. Association of type 2 diabetes with depression, brain atrophy, and reduced fine motor speed in a 60- to 64-year-old community sample. Am J Geriatr Psychiatry. 2008;16(12):989–998. doi: 10.1097/JGP.0b013e31818b40fc. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt R, Launer LJ, Nilsson LG, et al. CASCADE Consortium. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes. 2004;53(3):687–692. doi: 10.2337/diabetes.53.3.687. [DOI] [PubMed] [Google Scholar]
- 5.Jongen C, van der Grond J, Kappelle LJ, Biessels GJ, Viergever MA, Pluim JP Utrecht Diabetic Encephalopathy Study Group. Automated measurement of brain and white matter lesion volume in type 2 diabetes mellitus. Diabetologia. 2007;50(7):1509–1516. doi: 10.1007/s00125-007-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guttmann CR, Benson R, Warfield SK, et al. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54(6):1277–1283. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- 7.van der Flier WM, van Straaten EC, Barkhof F, et al. LADIS Study Group. Medial temporal lobe atrophy and white matter hyperintensities are associated with mild cognitive deficits in non-disabled elderly people: the LADIS study. J Neurol Neurosurg Psychiatry. 2005;76(11):1497–1500. doi: 10.1136/jnnp.2005.064998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elias PK, Elias MF, D’Agostino RB, et al. NIDDM and blood pressure as risk factors for poor cognitive performance: the Framingham Study. Diabetes Care. 1997;20(9):1388–1395. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- 9.DeKosky ST. Statin therapy in the treatment of Alzheimer disease: what is the rationale? Am J Med. 2005;118(12A) suppl 12A:48–53. doi: 10.1016/j.amjmed.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Williamson JD, Miller ME, Bryan RN, et al. ACCORD Study Group. The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol. 2007;99(12A):112i–122i. doi: 10.1016/j.amjcard.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Buse JB, Bigger JT, Byington RP, et al. ACCORD Study Group. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99(12A):21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Launer LJ, Miller ME, Williamson JD, et al. ACCORD MIND Investigators. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10(11):969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes: systematic overview of prospective observational studies. Diabetologia. 2005;48(12):2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 15.Coker LH, Shumaker SA. Type 2 diabetes mellitus and cognition: an understudied issue in women’s health. J Psychosom Res. 2003;54(2):129–139. doi: 10.1016/s0022-3999(02)00523-8. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler D, editor. Wechsler Adult Intelligence Scale–Revised. New York, NY: Psychological Corp; 1988. [Google Scholar]
- 17.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 18.Houx PJ, Jolles J, Vreeling FW. Stroop interference: aging effects assessed with the Stroop Color-Word Test. Exp Aging Res. 1993;19(3):209–224. doi: 10.1080/03610739308253934. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldszal AF, Davatzikos C, Pham DL, Yan MX, Bryan RN, Resnick SM. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22(5):827–837. doi: 10.1097/00004728-199809000-00030. [DOI] [PubMed] [Google Scholar]
- 22.Lao Z, Shen D, Liu D, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol. 2008;15(3):300–313. doi: 10.1016/j.acra.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 24.Molenberghs G, Kenward M, editors. Missing Data in Clinical Studies. Chichester, England: Wiley & Sons; 2007. [Google Scholar]
- 25.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine: reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 26.Ginsberg HN, Elam MB, Lovato LC, et al. ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerstein HC, Miller ME, Genuth S, et al. ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godin O, Tzourio C, Maillard P, Mazoyer B, Dufouil C. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the Three-City (3C)-Dijon Magnetic Resonance Imaging Study. Circulation. 2011;123(3):266–273. doi: 10.1161/CIRCULATIONAHA.110.961052. [DOI] [PubMed] [Google Scholar]
- 30.de Leeuw F-E, de Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125(pt 4):765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- 31.Launer LJ. Diabetes and brain aging: epidemiologic evidence. Curr Diab Rep. 2005;5(1):59–63. doi: 10.1007/s11892-005-0069-1. [DOI] [PubMed] [Google Scholar]
- 32.Bateman RJ, Xiong C, Benzinger TL, et al. Dominantly Inherited Alzheimer Network. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.