Unlike most chronic illnesses which have been declining or stabilizing in prevalence, incidence of type 2 diabetes has been increasing in the Western Hemisphere, and is now increasing at alarming rates in Asia1. While all causes of these increases cannot be identified, without question the increase in adiposity is an important contributor. The latter is due to increased caloric intake and reduced energy expenditure, although other factors may contribute2. Adiposity leads to insulin resistance, which in normal individuals elicits an hyperinsulinemic response, which compensates for the insulin resistance. Unresolved questions relate to whether fat storage in specific depots is particularly egregious, what mechanisms are responsible for the pancreatic islet-cell compensation, and why said compensation can fail, leading to diabetes in some, but not all individuals. Epidemiological studies suggest that visceral fat is particularly detrimental to metabolic health. Direct evidence favoring the importance of visceral fat is the result of surgical extirpation of the superior omentum in the canine model. While eliminating only 7% of total visceral fat in the dog, insulin sensitivity increased over 50%. This study compliments human data from Klein et al that evisceration of subcutaneous fat did not alter insulin resistance3.
Why is visceral fat detrimental? Induction of visceral adiposity by feeding of an hypercaloric high fat diet leads to insulin resistance for several reasons: a) increase of stored visceral fat in adipo-cytes which are themselves insulin resistant resulting in flux of free fatty acids (FFA) from viscera to liver and extrasplanchnic tissues; b) action of the sympathetic nervous system (SNS) which favors lipolysis and causes pulsatile release of FFA from visceral fat into the portal circulation; c) effects of pulsatile release of FFA which results in hepatic insulin resistance, associated with upregulation of liver gluconeogenic enzymes (fig. 1). Interestingly, the resultant insulin resistance of liver can be successfully reversed by antagonism of the cannabanoid system with rimonabant.
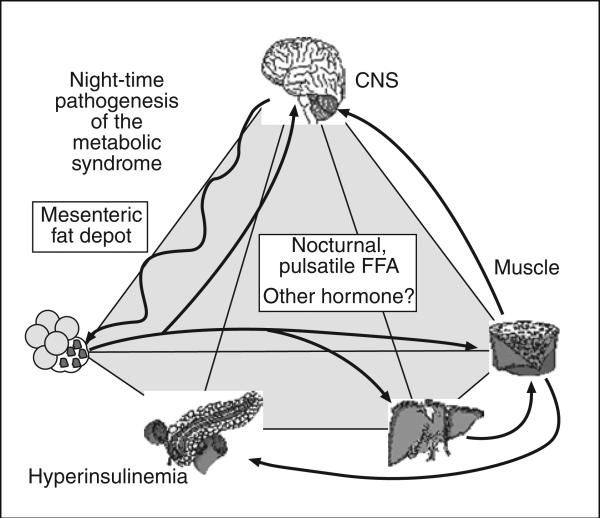
Fig. 1.
Pathogenesis of insulin resistance syndrome. Sympathetic nervous system drives FFA release from visceral fat depot; insulin resistance results as does hyperinsulinemia. FFA: free fatty acids; CNS: central nervous system.
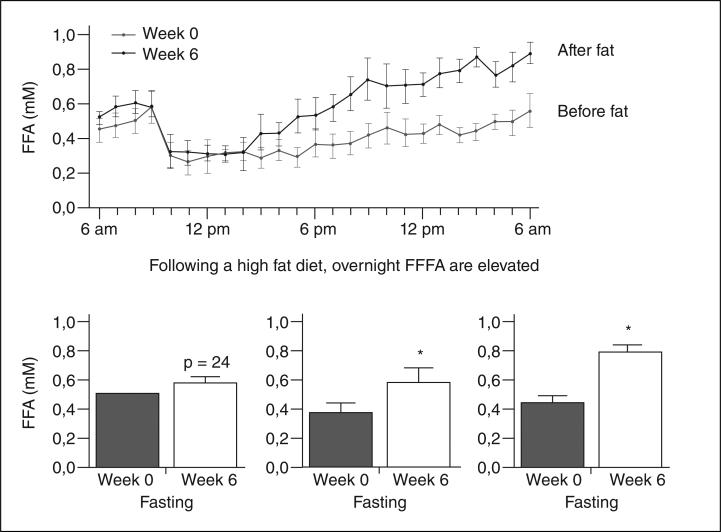
The role of FFA in pathogenesis of insulin resistance has been questioned, as evidence was lacking for increased fasting FFA in obese individuals. Recently we reported a powerful circadian rhythm in FFA levels, with a peak in levels between 2 and 4 AM4. We propose that it is the nocturnal surge in plasma FFA which is responsible for onset of insulin resistance in the overweight subject (fig. 2). This surge is due at least in part to a night-time outpouring of FFA from the visceral fat depot. We propose that omentectomy reduces this outpouring and increases insulin sensitivity.
Fig. 2.
Nocturnal increase in plasma fatty free acids. FFFA: flux of free fatty acids.
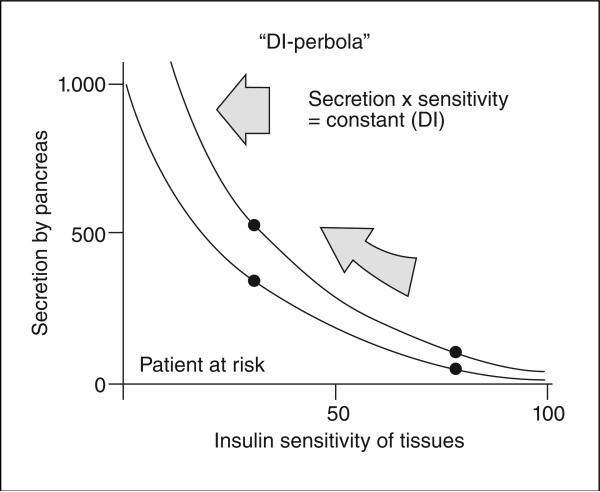
Not all insulin obese, insulin resistant individuals develop Type 2 diabetes, and induction of insulin resistance per se does not cause diabetes5. Type 2 diabetes occurs when the ability of the beta-cells of the pancreatic islets fail to compensate for diet-induced insulin resistance. It is only recently that it has been widely accepted that type 2 diabetes is usually due to a combination of insulin resistance and failed pancreatic compensation. This failure of compensation can be most well understood in terms of the hyperbolic relationship between insulin resistance and islet compensation (fig. 3). Normally resistance results in upregu-lation of beta-cell function which is described by a rectangular hyperbola6: insulin sensitivity x insulin secretion = constant (disposition index, “DI”). A higher value of DI is protective, a reduced DI portends conversion to type 2 diabetes mellitus. In fact, at this juncture, the value of DI is the most powerful predictor of conversion from prediabetes to frank diabetes mellitus, and this predictive power of DI far outweighs predictability of genes for type 2 diabetes so far identified. Interestingly, all genes for type 2 diabetes so far identified are related to beta-cell failure, rather than insulin resistance.
Fig. 3.
DI: disposition index.
What is responsible for the hyperbolic relationship between insulin action and insulin secretion? It is widely believed that insulin resistance results in increased glycemia, which in turn increases secretory potential of the pancreatic islets. But, recent evidence from our laboratory shows that hyperinsulinemic compensation for insulin resistance can occur even in the absence of increased fasting glucose levels. Thus, it is unknown what the signal or signals are which account for hype-rinsulinemic compensation. Because it is such compensation which fails as an initial step in progress to diabetes, it is important to understand beta-cell compensation. We hypothesize that nocturnal FFA, not only responsible for insulin resistance, may also mediate islet cell compensation. Thus, we are reminded of the importance of battle by sword here in El Escorial; by analogy, nocturnal FFA secondary to visceral lipolysis may not only cause insulin resistance, but may function as a two-edged sword, also mediating the islet cell response to compensate for insulin resistance. It is only in the modern world with a plethora of foodstuffs that such a two-edged sword has turned towards the owner to result in an international epidemic of type 2 diabetes.
Footnotes
Conflict of interest
The author declares he has no conflict of interest.
REFERENCES
- 1.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–8. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 2.Ludvigsson J. Why diabetes incidence increases—a unifying theory. Ann NY Acad Sci. 2006;1079:374–82. doi: 10.1196/annals.1375.058. [DOI] [PubMed] [Google Scholar]
- 3.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–57. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 4.Kim SP, Catalano IR, Hsu JD, Chiu JM, Richey J, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperin-sulinemia. Am J Physiol. 2007;292:E1590–8. doi: 10.1152/ajpendo.00669.2006. [DOI] [PubMed] [Google Scholar]
- 5.Yechoor VK, Patti ME, Ueki K, Laustsen PG, Saccone R, Rau-niyar R, et al. Distinct pathways of insulin-regulated versus diabetes-regulated gene expression: an in vivo analysis in MIRKO mice. Proc Natl Acad Sci USA. 2004;101:16525–30. doi: 10.1073/pnas.0407574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–20. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]