SUMMARY
Acute lung injury (ALI) remains a serious health issue with little improvement in our understanding of the pathophysiology and therapeutic approaches. We investigated the mechanism that lipopolysaccharide (LPS) induces early neutrophil recruitment to lungs and increases pulmonary vascular permeability during ALI. Intratracheal LPS induced release of pro-interleukin-1α (IL-1α) from necrotic alveolar macrophages (AM), which activated endothelial cells (EC) to induce vascular leakage via loss of vascular endothelial (VE)-cadherin. LPS triggered the AM purinergic receptor P2X7(R) to induce Ca2+ influx and ATP depletion, which led to necrosis. P2X7R deficiency significantly reduced necrotic death of AM and release of pro-IL-1α into the lung. CD14 was required for LPS binding to P2X7R, as CD14 neutralization significantly diminished LPS induced necrotic death of AM and pro-IL-1α release. These results demonstrate a key role for pro-IL-1α from necrotic alveolar macrophages in LPS-mediated ALI, as a critical initiator of increased vascular permeability and early neutrophil infiltration.
Keywords: ARDS, ALI, sepsis, lung inflammation, lung injury, MyD88, IL-1α, endothelial cells, alveolar macrophage, necrosis, neutrophil, P2X7R, Ca2+ influx
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a life-threatening condition of acute lung injury (ALI) characterized by bilateral pulmonary infiltrates, severe hypoxemia, and non-cardiogenic pulmonary edema (Matthay et al., 2012). Despite decades of research and numerous clinical trials, the only treatment that reduces mortality in ARDS is mechanical ventilation using low tidal volumes (Matthay et al., 2003). Even with best supportive care, the mortality from ARDS approaches 30–50% (Matthay et al., 2012), emphasizing the need for better understanding of the pathophysiology and new treatments.
Lipopolysaccharide (LPS)-induced ALI is an animal model that replicates several key pathologic processes of ARDS, including loss of vascular integrity, neutrophil infiltration, and accumulation of protein-rich fluid in the airspaces of the lung (Matute-Bello et al., 2011; Orfanos et al., 2004; Tsushima et al., 2009). There is great interest in the mechanisms by which LPS disrupts the endothelial barrier to allow increased lung vascular permeability and alveolar edema. Multiple mechanisms have been postulated, including decreased intracellular cAMP concentrations (Schlegel et al., 2009), modulation of atrial natriuretic peptide receptors (Xing et al., 2012), induction of sphingosine-1-phosphatase (Zhao et al., 2011), and stimulation of RhoA-Rho kinase pathway (Han et al., 2013), among others. However, while experimental evidence indicates that LPS administered to the lung does not signal through toll-like receptor-4 (TLR4) on the endothelium but instead targets hematopoietic and epithelial cells (Andonegui et al., 2009), it remains uncertain as to which secondary mediators, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and other cytokines released from macrophages and/or damaged epithelium, provide the necessary signals on the endothelium for vascular dysfunction and neutrophil influx (Salgado et al., 1994).
Interleukin-1 (IL-1) is a master cytokine of local and systemic inflammation, and the IL-1 family of ligands and receptors is associated with both acute and chronic inflammation induced by bacteria and bacterial products (Dinarello, 2011; Ulich et al., 1991a; Ulich et al., 1991b). There are two related but distinct IL-1 genes, Il1a and Il1b, encoding IL-1α and IL-1β, respectively (Dinarello, 2011). Both IL-1α and IL-1β bind to the same cell surface receptor, IL-1R1, which is present on nearly all cells. While the role of IL-1α in innate immune responses has been reported for Legionella pneumophila (Barry et al., 2013), the role and the mechanism of IL-1α-dependent neutrophil recruitment in vivo in LPS-induced acute lung injury has not been investigated. The “danger” model proposes that the immune system responds to nonphysiological cell death, damage, or stress (Matzinger, 1994). Accordingly, necrotic cell death releases damage-associated molecular patterns (DAMPs), which activate immune pathways (Chen et al., 2007; Chen and Nunez, 2010; Eigenbrod et al., 2008). Since IL-1α can be released by necrotic cells to induce sterile inflammation, it can be considered to function as a damage associated molecular pattern (DAMP) under these circumstances (Chen and Nunez, 2010).
Endothelial cells are critical mediators of the inflammatory response (Pober and Sessa, 2007). However, the importance of endothelium in innate immunity, specifically, as it pertains to LPS-induced ALI and neutrophil recruitment still remains underappreciated. Adherens junction proteins such as VE-cadherin are critically involved in controlling vascular permeability (Dejana et al., 2008), and IL-1β can disrupt VE-cadherin cell-surface localization by promoting its endocytic internalization (London et al., 2010). Mice expressing TLR4 only on their endothelium do not accumulate neutrophils in the lungs following intratracheal administration of LPS, indicating that, at least for LPS-TLR4 signaling, TLR4 is required on bone marrow–derived leukocytes (Andonegui et al., 2009). Additionally, MyD88 adaptor-based signaling in endothelial cells is important for the disruption of endothelial barrier function and vascular permeability (Zhu et al., 2012).
While LPS induces pro-inflammatory signals through TLR4, the P2X7 receptor also can bind bacterial LPS (Denlinger et al., 2001). P2X7 receptors are a family of ATP gated ion channels that make the plasma membrane permeable to small cations such as Ca2+, resulting in cellular depolarization (Costa-Junior et al., 2011), and they have gained recent attention due to their ability to induce Ca2+ mobility (Garcia-Marcos et al., 2006). P2X7R is expressed in most immune cells (Volonte et al., 2012), and its expression rapidly increases in alveolar macrophages (AM) following smoke-induced lung inflammation (Lucattelli et al., 2011). Moreover, P2X7R may function in the pathophysiology of LPS-induced ALI (Moncao-Ribeiro et al., 2011). Indeed, P2X7 receptor-deficient mice show reduced inflammation and lung fibrosis to bleomycin injury (Mishra, 2013).
In our study of LPS-induced ALI, we found that LPS administered intratracheally led to increased Ca2+ influx via a CD14-P2X7R dependent pathway in AMs, which resulted in ATP depletion followed by necrotic death of the AMs. AM necrosis led to the release of pro-IL-1α into the alveolar space, which in turn induced vascular leakage through endothelial IL-1R signaling. Our results reveal a key role for pro-IL-1α released from necrotic alveolar macrophages in LPS-induced ALI as a critical initiator of increased vascular permeability and early neutrophil infiltration.
RESULTS
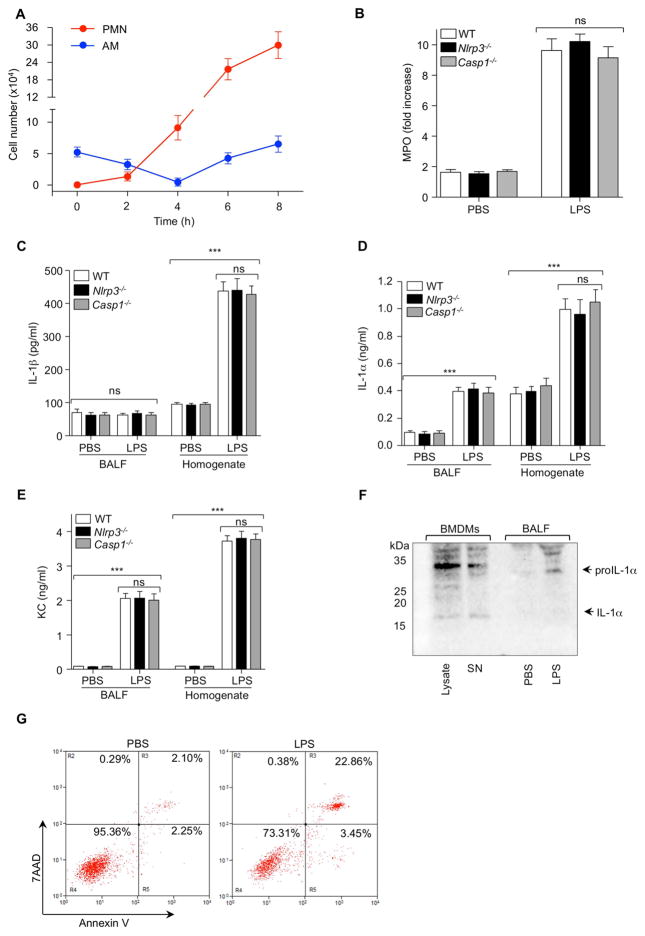
Necrotic Alveolar Macrophages Release IL-1α in LPS-induced ALI
LPS instilled intratracheally (i.t.) induces neutrophil recruitment and vascular leakage into the lungs, but the exact mechanism controlling these responses are unknown. AMs play a major role in LPS-induced neutrophil (PMN) accumulation in the lung (Harmsen, 1988). AM numbers decreased 4 h after LPS instillation, whereas PMN numbers increased over time in the bronchiolar lavage fluid (BALF) (Figure 1A). Thus, we hypothesized that dying AM may trigger neutrophil infiltration into the lung by releasing IL-1β and IL-1α. Secretion of both IL-1β and IL-1α is impaired in macrophages from Casp1−/− or Nlrp3−/− mice (Gross et al., 2012). Therefore, we assessed whether the inflammasome was involved in ALI by using Casp1−/− or Nlrp3−/− mice. WT, Nlrp3−/− and Casp1−/− mice were given LPS (i.t.) and 6 h later myeloperoxidase (MPO) activity, a surrogate of PMN numbers, was measured. MPO activity did not differ among WT Casp1−/− or Nlrp3−/− mice LPS (Figure 1B), suggesting that the inflammasome did not play a role in LPS-induced ALI in this model. Furthermore, we measured the concentrations of IL-1β, IL-1α, and KC chemokine in bronchoalveolar lavage fluid (BALF) and lung homogenates. Corroborating our data that the inflammasome was not involved in LPS induced ALI, IL-1β protein was only detected in the lung homogenate and its concentration was not altered in Nlrp3−/− and Casp1−/− mice (Figure 1C). In contrast, both IL-1α and KC were significantly increased in BALF and lung homogenates of LPS-treated mice but again, these endpoints did not differ among genotypes (Figure 1D and E).
Figure 1. LPS-Induced Acute Lung Injury Is IL-1β Independent.
(A) WT mice were (i.t.) administrated PBS or LPS at time 0 and AM and PMN numbers were assessed in BALF by flow cytometry over time. (B–E) WT, Nlrp3−/− and Casp1−/− mice were (i.t.) challenged with LPS 6 h: (B) MPO in lung homogenates, (C) IL-1β, (D) IL-1α, and (E) KC concentrations were measured by ELISA. (F and G) WT mice were (i.t.) administrated PBS or LPS 2 h: (F) BALF was pooled (3 mice) and concentrated, and IL-1α protein concentrations in BALF were checked by WB. Supernatant and cell lysate of LPS (3 h) primed BMDMs treated with ATP (1 h) was used as a positive control. (G) BAL cells were probed with anti-F4/80 and stained with Annexin V and 7AAD. Necrotic cells were measured by flow cytometry. Results shown are representative of two experiments. Five mice/group were used. Results are shown as mean ± SD. ***p < 0.001.
Previous studies found that pro-IL-1α can be released passively via necrosis from dying macrophages and trigger sterile inflammation (Chen et al., 2007). We wondered whether pro-IL-1α might be released from necrotic AMs in response to LPS. WT mice were given LPS or PBS (i.t.) and 2 h later BALF was collected and analyzed for the presence of IL-1α by immunoblotting. LPS-primed and ATP treated bone marrow derived macrophages (BMDMs) were used as a positive control. We found pro-IL-1α but not mature IL-1α in the BALF of LPS-treated mice (Figure 1F). Next, we examined whether LPS could induce AM necrosis. BAL cells were isolated 2 h post LPS and analyzed by flow cytometry for F4/80 (macrophages), Annexin V, which detects apoptotic and necrotic cells, and 7AAD, which detects necrotic cells. Following LPS administration, AMs underwent increased necrotic cell death (Figure 1G). These data suggested that IL-1β and NLRP3 inflammasome are not required for the early development of LPS-induced ALI, but that instead pro-IL-1α may play an important role.
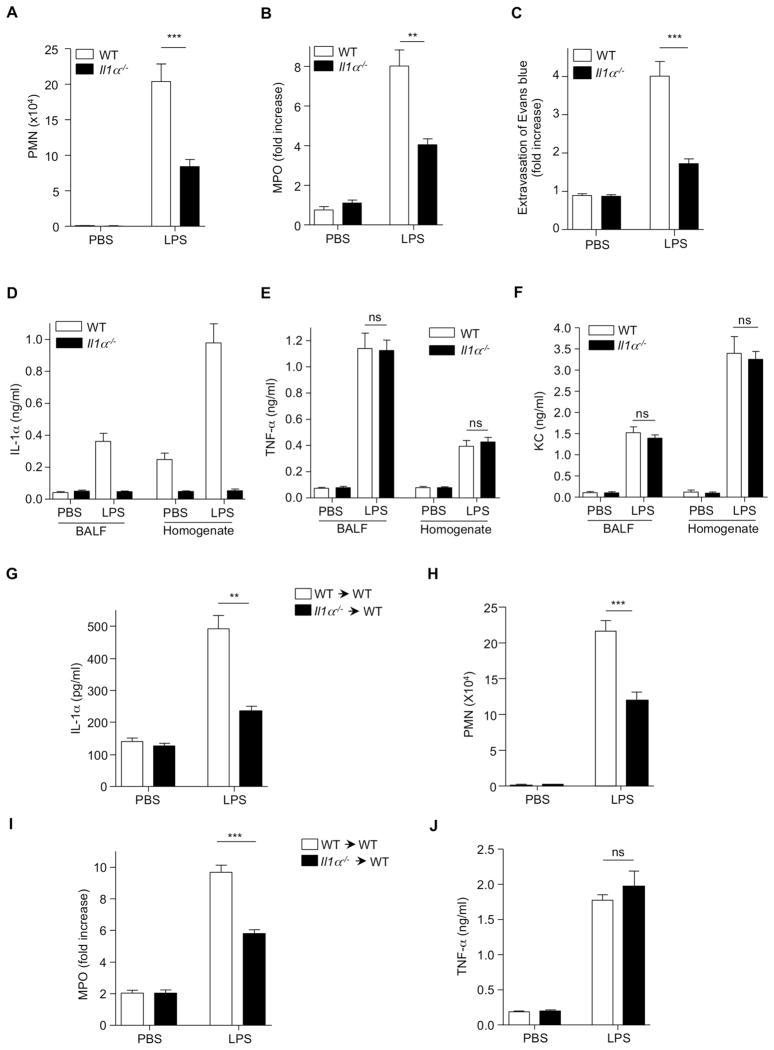
IL-1α Plays a Crucial Role in LPS-Induced Acute Lung Injury
IL-1α can directly increase vascular endothelial cell permeability in vitro and is speculated to be involved in acute vascular endothelial injury associated with endotoxic shock (Royall et al., 1989). To assess the possible direct involvement of IL-1α in LPS-induced ALI, we administered LPS or PBS (i.t.) to WT and Il1α−/− mice, and 6 h later BALF was collected and analyzed. After LPS instillation, PMN recruitment and MPO activity were significantly reduced in Il1α−/− mice compared to WT controls (Figure 2A and B). In addition, we blocked the effects of IL-1α using an IL-1α neutralizing antibody. IL-1α neutralization resulted in a significant reduction in PMN numbers and MPO activity compared to animals receiving control antibody (Figure S1A and B). We next investigated vascular leakage in the lung using Evans blue dye. We observed increased Evans blue leakage from the vascular space into the lung parenchyma in LPS-treated WT mice, which was dramatically reduced in the lungs of Il1α−/− mice. Quantitative analysis of Evans blue extravasation into the lung tissue showed significantly lower dye accumulation into the lungs of LPS treated Il1α−/− mice compared to lung of LPS-treated WT mice (Figure 2C), suggesting that IL-1α plays a pivotal role in LPS-induced ALI. As expected, IL-1α amounts were increased in BALF and lung homogenates from WT mice but not Il1a−/− mice (Figure 2D). Importantly, there was no significant difference in amounts of TNF-α or KC in BALF and lung homogenate from Il1α−/− mice compared to WT mice after LPS treatment (Figure 2E and F).
Figure 2. IL-1α Is Crucial During LPS-Induced Acute Lung Injury.
(A and B) WT and Il1a−/− mice were (i.t.) challenged with PBS or LPS and 6 h later (A) PMN numbers were counted in the BALF and (B) MPO concentrations were measured in the lung homogenate. (C) After 6 h LPS (i.t.) administration, mice were injected (i.v.) with Evans blue dye and 2 h later, Evans blue extravasation was measured by spectrophotometric analysis. (D–F) WT and Il1a−/− mice were (i.t.) challenged with PBS or LPS and 6 h later, (D) IL-1α, (E) TNF-α, and (F) KC concentrations were measured by ELISA. (G–J) WT mice received bone marrow cells from WT or Il1a−/− mice, and 12 weeks later mice were (i.t.) challenged (i.t.) with PBS or LPS 6 h. (G) IL-1α concentrations in BALF by ELISA. (H) PMN numbers were counted in the BALF. (I) MPO activity was measured in the lung homogenate. (J) TNF-α amounts in the BALF were determined by ELISA. Results shown are representative of two experiments. Results are shown as mean ± SD. **p < 0.01, ***p < 0.001. See also Figure S1.
Macrophages are major producers of proinflammatory cytokines in response to LPS, and TLR4 on hematopoietic cells (but not stromal cells) is required for ALI following intratracheal administration of LPS (Andonegui et al., 2009). To more specifically assess the role of IL-1α, we generated hematopoietic Il1α−/− chimeric mice. Irradiated WT mice received bone marrow from IL-1α deficient or WT mice. After reconstitution (12 weeks), the mice were given LPS or PBS (i.t.) and BALF was assessed 6 h later. Mice that received Il1α deficient bone marrow showed a significant reduction of IL-1α in BALF compared to WT transplants (Figure 2G). This finding correlated with significantly reduced PMN numbers and MPO activity in IL-1α deficient bone marrow recipients compared to control mice receiving WT bone marrow (Figure 2H and I). TNF-α concentrations in BALF were similar between both groups (Figure 2J). Moreover significantly increased PMN numbers as well as IL-1α concentrations but not KC were observed in BALF from Il1α−/− mice that received WT bone marrow compared to Il1α−/− mice that received Il1a−/− bone marrow (Figure S1C–E). These findings indicate that IL-1α released from hematopoietic cells plays an important role in LPS-induced ALI and neutrophil infiltration.
We also confirmed the requirement for MyD88 signaling in hematopoietic cells by creating bone marrow chimeras. WT mice received bone marrow from Myd88−/− mice, and control mice received bone marrow from WT mice. IL-1α and TNF-α amounts were dramatically reduced in BALF from mice that received Myd88−/− bone marrow compared with BALF from WT mice that received WT bone marrow (Figure S1F and G). As a control, we measured RANTES (TLR4-TRIF pathway) (Tachado et al., 2010) and found no difference in RANTES concentration in the BALF between mice that received Myd88 deficient bone marrow and controls (Figure S1H). These findings suggest that MyD88 signaling in hematopoietic cells is required for the induction of key proinflammatory mediators in response to i.t. LPS.
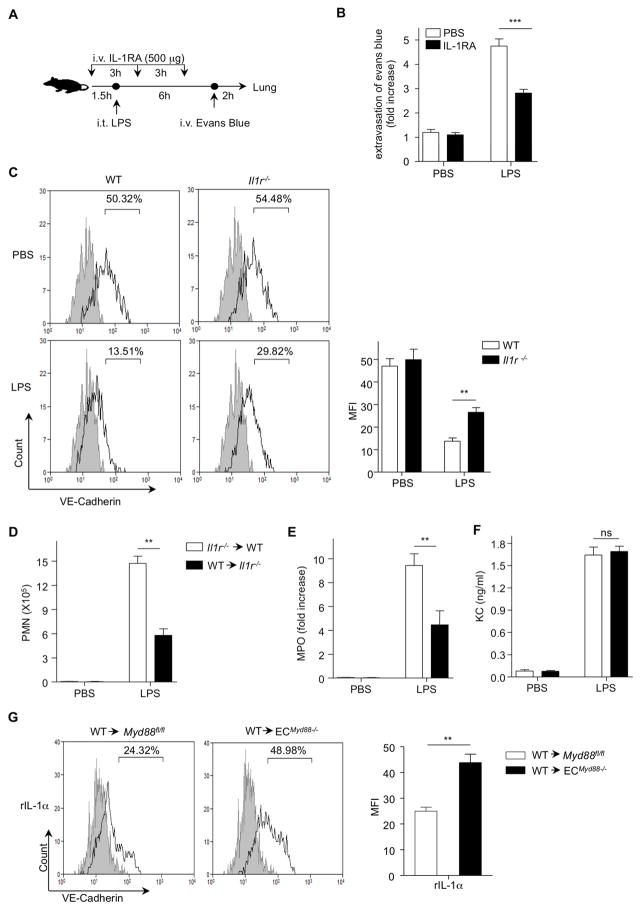
IL-1α Signaling through Endothelial IL-1R Is Resulted in Vascular Leakage
We investigated whether LPS-induced vascular permeability in LPS-induced ALI occurred via a similar mechanism, except using pro-IL-1α. We blocked IL-1 signaling in the LPS model of ALI using the IL-1 receptor antagonist (IL-1RA). WT mice were injected intravenously (i.v.) with IL-1RA or PBS every 3 h and LPS was administered (i.t.) 1.5 h after first injection of IL-1RA, followed 6 h later by Evans blue injected i.v. and analysis 2 h after that (Figure 3A). Inhibition of the IL-1 receptor signaling in mice significantly attenuated Evans blue extravasation compared to control mice (Figure 3B).
Figure 3. IL-1R-MyD88 in Endothelial Cells Is Important for Vascular Permeability.
(A) WT mice were (i.v.) injected with PBS or IL-1RA following indicated scheme. (B) Evans blue dye was (i.v.) injected 2 h before termination of the experiment and Evans blue extravasation was assessed by spectrophotometric analysis. (C) WT and Il1r−/− mice were challenged with PBS or LPS 2 h, lung single cell suspensions were obtained and surface VE-cadherin was measured by flow cytometry. (D–F) WT mice received bone marrow cells from Il1r−/− mice or Il1r−/− mice received bone marrow cells from WT mice, and 12 weeks later mice were (i.t.) challenged with PBS or LPS 6 h. (D) PMN numbers counted in the BALF, (E) MPO activity was measured in the lung homogenate, and (F) KC concentrations in BALF were measured by ELISA. (G) Myd88fl/fl or ECMyD88−/− mice received bone marrow cells from WT mice and 12 weeks later mice were (i.t.) challenged with recombinant IL-1α (rIL-1α). After 1 h, lung single cell suspensions were obtained and VE-cadherin was measured by flow cytometry. Results shown are representative of three experiments. Results are shown as mean ± SD. **p < 0.01, ***p < 0.001. See also Figure S2.
Vascular endothelial (VE)-Cadherin provides endothelial cell barrier integrity (Xiao et al., 2003). Therefore, we measured the amount of VE-cadherin on the surface of EC in lungs of WT and Il1r−/− after LPS administration. VE-cadherin staining was significantly reduced in the lungs of WT mice compared to Il1r−/− mice 2 h after i.t. instillation of LPS (Figure 3C), suggesting that IL-1α may regulate vascular integrity through endothelial IL-1R signaling during LPS-induced ALI. To assess the role of endothelial IL-1R signaling in LPS induced neutrophil recruitment, we generated chimeric mice between WT and Il1r−/− mice. WT mice received bone marrow from Il1r deficient mice or Il1r−/− mice received bone morrow from WT mice. Mice that were deficient for IL-1R only on stromal cells had a significant reduction in pulmonary PMN recruitment and MPO activity (Figure 3D and E) compared to WT mice that received Il1r−/− bone marrow cells. However, no differences were seen in KC concentrations (Figure 3F). These data indicate that IL-1 signaling on stromal cells is required for LPS-induced neutrophil recruitment. Additionally, after i.t administration of LPS, Il1r−/− mice showed significantly fewer PMNs in BALF and less MPO activity in the lungs compared to WT mice (Figure S2A and B). We did not see any difference in TNF-α, MIP-2 and KC protein amounts in BALF and lung homogenate between WT and Il1r−/− mice (Figure S2C–E) suggesting that production of these cytokines is downstream of LPS TLR4 signaling, and not IL-1 IL-1R during LPS-induced ALI.
In order to assess a direct role for IL-1α in endothelial MyD88 signaling, we used a Cre loxP strategy to engineer EC-specific MyD88-deficient mice (ECMyD88−/−). We crossed mice with the MyD88fl allele (Hou et al., 2008) to mice carrying the Tie2-Cre transgene, which is preferentially expressed in ECs (Kisanuki et al., 2001). The deletion of the Myd88fl allele was assessed by quantitative PCR, (Figure S2F), and MyD88 protein expression in aorta EC by Western blot (Figure S2G). Tie-2 has been reported on a small number of hematopoietic stem cells in addition to endothelium (Makinde and Agrawal, 2011), but Tie-2 is turned off at the start of differentiation, making it unlikely that nonendothelial cells would express it (De Palma et al., 2005). Nevertheless, we generated chimeric mice by irradiating ECMyD88−/− or control Myd88fl/fl mice followed by transplantation of WT bone marrow cells. After reconstitution (Figure S2H), the mice were given (i.t.) recombinant IL-1α (rIL-1α) or PBS, and 1 h later the lungs were lavaged and measured for the loss of VE-cadherin on EC. Surface expression of VE-cadherin was significantly reduced on EC from the lungs of LPS treated WT→Myd88fl/fl mice compared to WT→ECMyD88−/− mice (Figure 3G). Collectively, these results suggest that pulmonary endothelial MyD88, downstream of IL-1α signaling, plays a crucial role in vascular permeability via downregulation or internalization of VE-cadherin in vivo. Overall our data reveal a critical role for IL-1R MyD88 signaling in lung ECs in response to i.t. LPS, and corroborates the role of IL-1α as the mediator of LPS signaling on ECs.
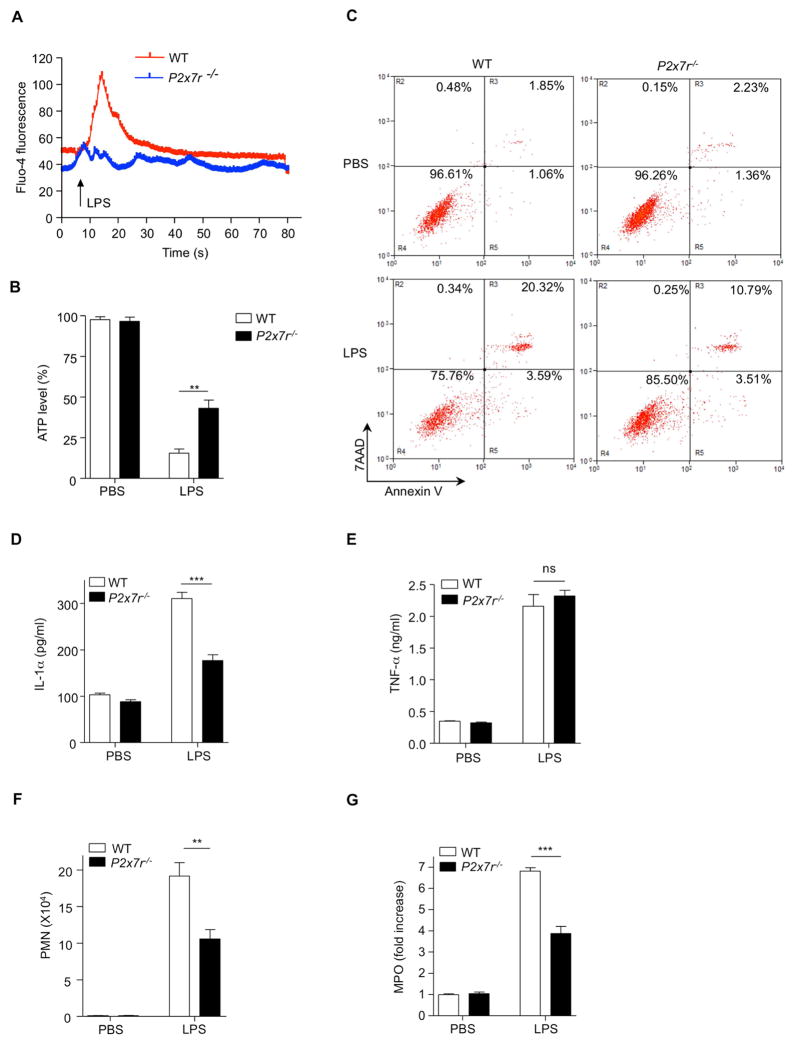
P2X7R Is Involved in Calcium Influx and Necrotic Death of Alveolar Macrophages
While our data clearly shows that LPS induced necrosis of AMs leads to pro-IL-1α release and subsequent signaling on ECs to allow neutrophil influx, the mechanism by which LPS induces AM necrosis to initiate this cascade is still unclear. LPS can initiate a rapid transient increase of cytosolic Ca2+ via calcium release-activated calcium channels in bone marrow derived dendritic cells (BMDC) (Matzner et al., 2008). LPS also leads to ATP depletion in mouse lungs (Aggarwal et al., 2012), and ATP depletion has been linked with necrotic cell death (Ha and Snyder, 1999; Zong and Thompson, 2006). Thus, we investigated whether i.t. LPS would increase calcium influx and deplete ATP in alveolar macrophages in this ALI model. AM were isolated from WT mice and loaded with Fluo-4 (calcium indicator). Upon LPS stimulation, calcium mobilization was increased in AM with Ca2+-containing medium, but not AM in Ca2+-free medium (Figure S3A). Indeed, we observed that after LPS instillation, intracellular ATP concentrations dropped in AM over time (Figure S3B), suggesting that LPS may deplete intracellular ATP through increasing Ca2+ mobilization. It was recently reported that TLR4 is not required for LPS-induced Ca2+ mobilization in DCs (Zanoni et al., 2009). Similar to that study, we also observed that Tlr4−/− AM responded to LPS with Ca2+ mobilization (Figure S3C), indicating that a TLR4-independent pathway is responsible for Ca2+ mobilization after LPS. Finally, these data revealed that LPS-induced Ca2+ influx resulted in intracellular ATP depletion, as one possible mechanism for the necrotic cell death of AM.
Next we sought to understand the signaling mechanism for LPS induced necrotic death of AM. We focused on the purinergic receptor P2X7 (P2X7R), which is expressed on most immune cells, including AM (Lemaire and Leduc, 2003) (Figure S3D), and has gained recent notoriety due to its ability to induce calcium mobilization (Garcia-Marcos et al., 2006). P2X7R also has a potential binding site for bacterial LPS (Denlinger et al., 2001) and was recently revealed to be important for LPS-induced lung injury (Moncao-Ribeiro et al., 2011). Therefore, we examined whether P2X7R was involved in LPS-induced Ca2+ mobility and ATP depletion in AM. AM isolated from WT or P2x7r−/− mice were loaded with the calcium indicator Fluo-4. After LPS stimulation, calcium influx was increased in AM from WT mice, but not in AM from P2x7r−/− mice (Figure 4A). Moreover, while LPS reduced ATP in AM of both genotypes, ATP concentrations were significantly higher in AM from P2x7r−/− mice compared to WT controls after LPS (i.t.) administration (Figure 4B). Consistent with this, P2X7R deficiency resulted in less necrotic cell death (Figure 4C) and a significantly higher number of surviving AM compared to WT controls (Figure S3E), which was associated with reduced leakage of pro-IL-1α into the BALF compared to WT animals (Figure 4D). Importantly, there was no difference in TNF-α concentration in the BALF of both genotypes (Figure 4E). In addition, there was a significant reduction of PMN number and MPO activity in the BALF and lungs of P2x7r−/− mice following (i.t.) LPS administration (Figure 4F and G). Collectively, these data demonstrate that the P2X7R plays an important role in LPS-induced ALI by inducing Ca2+ mobilization, ATP depletion, necrosis, and pro-IL-1α release in response to LPS.
Figure 4. P2X7 Receptor Is Crucial for LPS-induced ATP Depletion and Necrosis in Alveolar Macrophages.
(A) AM from WT or P2x7r−/− mice were loaded with Fluo-4 a calcium indicator (4μM) for 30 min at room temperature and calcium mobility was measured by confocal microscopy. (B and C) WT and P2x7r−/− mice were (i.t.) challenged with PBS or LPS 30 min (B) or 2 h (C). (B) ATP amounts in the AMs were measured by ATP assay kit. (C) BAL cells were probed F4/80 and stained with annexin V and 7AAD and necrotic cells were measured by flow cytometry. (D–G) WT and P2x7r−/− mice were (i.t.) challenged with or without LPS 4 h. (D) IL-1α and (E) TNF-α concentrations in the BALF were determined by ELISA. (F) PMN numbers in the BALF and (G) MPO activity were measured in the lung homogenate. Results shown are representative of two experiments. Results are shown as mean ± SD. **p < 0.01, ***p < 0.001. See also Figure S3.
CD14 Is Required for P2X7R Dependent Ca2+ Mobilization by LPS
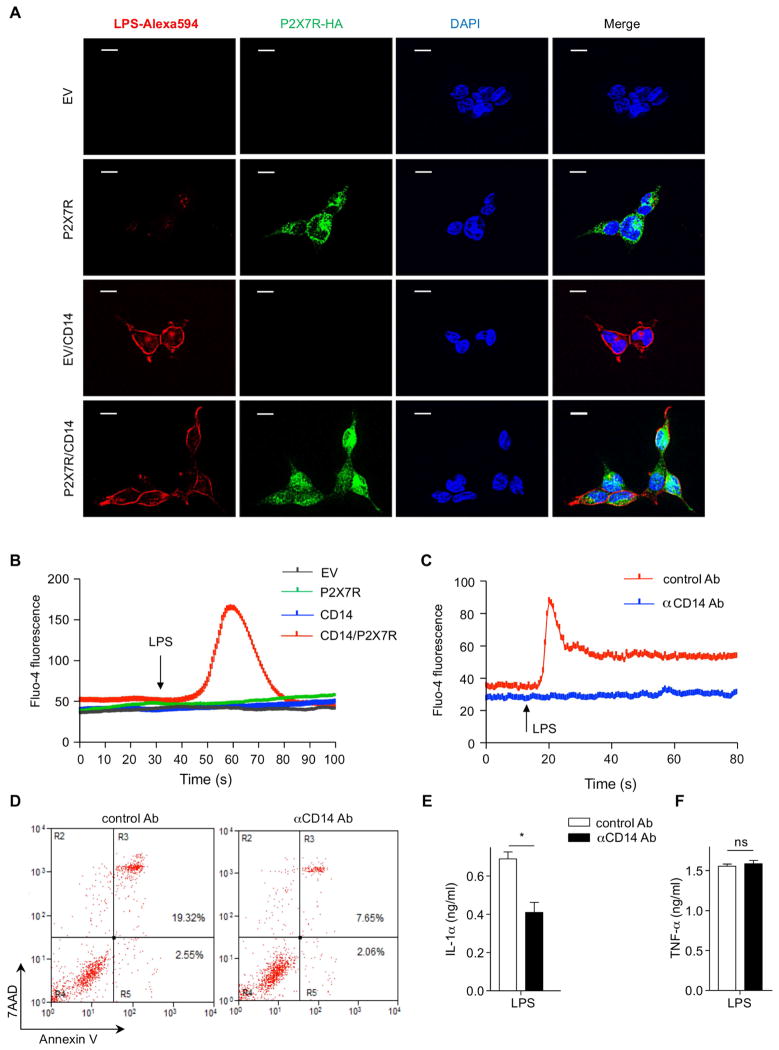
A recent report suggested that LPS does not induce P2X7R-related activity in P2X7R expressing HEK293 cells (Leiva-Salcedo et al., 2011). Therefore, we tested whether LPS directly binds to P2X7R. Empty vector (EV) or P2X7R stably-expressing HEK293 cells were stimulated with LPS-Alexa594 for 10 min and fixed cells were stained with anti-HA for P2X7R. However, we observed extremely weak co-localization between LPS and P2X7R (Figure 5A upper panel) in P2X7R stably-expressing HEK293 cells. According to the literature, the LPS binding site on P2X7R is located within the cytoplasmic C-terminal tail of P2X7R (Denlinger et al., 2001). Therefore we hypothesized that LPS uptake might be important for LPS binding to P2X7R. Previous studies revealed that CD14 plays an active role during LPS internalization (Kitchens and Munford, 1998; Kitchens et al., 1998). In a recent study, CD14 was also found to be required for LPS induced Ca2+ influx in BMDC (Zanoni et al., 2009). For this reason, EV or P2X7R stably-expressing HEK293 cells were transfected with CD14, stimulated with LPS-Alexa594, and stained with anti-HA for the C-terminal tail of P2X7R. Consistent with the hypothesis, CD14 transfection internalized LPS, which resulted in strong colocalization between LPS and P2X7R in P2X7R and CD14 stably-expressing cells (Figure 5A lower panel). Moreover, LPS increased Ca2+ influx only in P2X7R and CD14-expressing HEK293 cells (Figure 5B and Movies S1–4), suggesting that CD14 may be important for LPS-induced channel activity of P2X7R by internalizing LPS. CD14 is known as a coreceptor for TLR4 and is important for its downstream signaling by LPS (Wright et al., 1990). However, CD14 is not required for TLR4-MyD88 dependent signaling when stimulated with a high dose of LPS (Jiang et al., 2005; Zanoni et al., 2009) (Figure S4A). Next, we tested whether CD14 is required for LPS-induced Ca2+ influx and necrotic death of AM by using in ex vivo and in vivo models. CD14 neutralizing antibody completely inhibited LPS induced Ca2+ mobilization in mouse AM compared to control antibody (Figure 5C). In vivo, WT mice were given i.t. anti-CD14 antibody (10 μg/mouse) or control antibody 1 h before i.t. LPS instillation. CD14 neutralization significantly reduced necrotic death of AM and IL-1α concentrations in BALF compared to control mice (Figure 5D and E). Importantly, TNF-α concentrations in BALF were comparable between these two groups (Figure 5F). Additionally, CD14 was expressed equally in both AM of WT and P2x7r−/− mice (Figure S4B). Collectively, these data indicate that CD14 plays an important role in LPS-induced Ca2+ mobilization and necrotic death of AM.
Figure 5. CD14 Is Required for LPS-P2X7R Dependent Calcium Influx.
(A) HEK293 cells were stimulated with LPS-Alexa594 (2.5μg/ml) for 10 min and probed for P2X7R (anti-HA) followed by and followed by Alexa-488 (green) conjugated anti-rabbit secondary. LPS (red) and P2X7R (green) localization were measured by fluorescence microscopy. Nuclei are counterstained with DAPI (blue). (B) EV, CD14, P2X7R and P2X7R and CD14 stably expressing HEK293 cells were loaded with Fluo-4 (4 μM) for 30 min and calcium mobility was measured by confocal microscopy. (C) AM from WT mice were incubated with anti-mouse CD14 antibody 2 h and loaded with Fluo-4 (4 μM) and calcium mobility was measured by confocal microscopy. (D–F) WT mice were (i.t.) treated with control or anti-mouse CD14 antibody (1 h) prior to LPS challenge 2 h. (D) BAL cells were stained with annexin V and 7AAD and necrotic cells were measured by flow cytometry. (E) IL-1α and (F) TNF-α concentration in the BALF were determined by ELISA. Results shown are representative of two experiments. Results are shown as mean ± SD. *p < 0.05. Scale bar represents 10 μm. See also Figure S4 and Movies S1–4.
CD14 Interacts with P2X7R
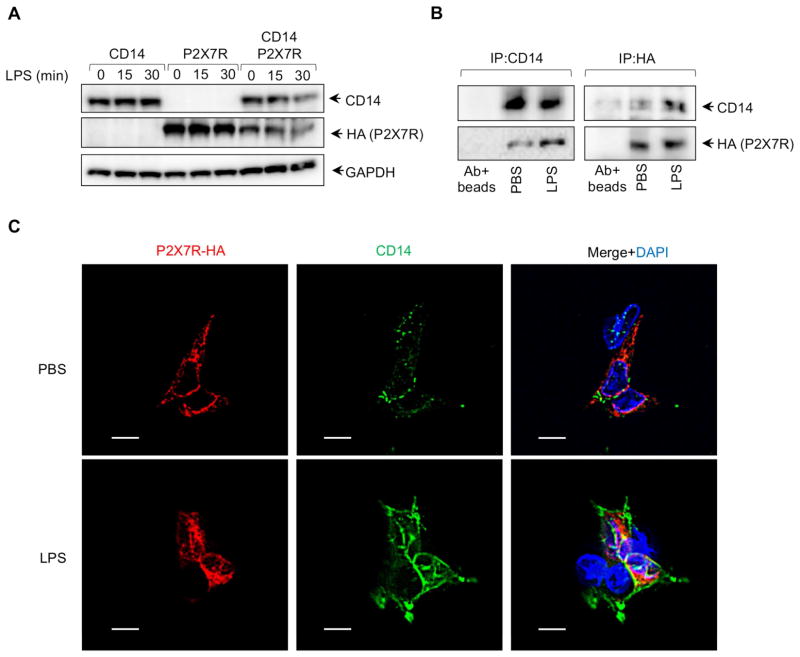
We and others have shown that LPS is internalized into cells by a CD14-dependent pathway. Therefore we hypothesized that CD14 facilitated LPS internalization where it could bind to P2X7R. CD14 and P2X7R expression were not altered after LPS stimulation in CD14 or P2X7R stably-expressing HEK293 cells. However, CD14 and P2X7R amounts were time-dependently reduced after LPS stimulation in P2X7R and CD14 stably expressing cells (Figure 6A) suggesting that the LPS-CD14-P2X7R receptor complex might be rapidly degraded by a negative regulatory mechanism.
Figure 6. CD14 Interacts with P2X7R.
(A) CD14 and P2X7R expression was detected by WB at various time points after LPS stimulation. (B and C) P2X7R and CD14 stably expressing HEK293 cells were stimulated with PBS or LPS for 10 min. CD14 and P2X7R was immunoprecipitated from whole cell extracts using anti-CD14 or anti-HA (for P2X7R) antibodies and antibodies+beads served as negative control. (C) P2X7R and CD14 stably expressing HEK293 cells were fixed and incubated with anti-CD14 and anti-HA (for P2X7R) antibodies for fluorescence microscopy. Nuclei are counterstained with DAPI (blue). Scale bar represents 10 μm.
CD14 is located within lipid rafts of the plasma membrane following LPS stimulation (Olsson and Sundler, 2006; Triantafilou et al., 2002). P2X7R is distributed among both raft and nonraft domains of the plasma membrane (Garcia-Marcos et al., 2006). Therefore we carried out coimmunoprecipitiation experiments for interactions between CD14 and P2X7R. CD14 and P2X7R-HA were immunoprecipitated with anti-CD14 or anti-HA antibody from P2X7R and CD14 stably expressing HEK293 cells stimulated with or without LPS for 10 min. In the absence of LPS, CD14 weakly interacted with P2X7R, whereas strong interaction between those two receptors was seen after LPS stimulation (Figure 6B). Additionally, we looked for colocalization of CD14 and P2X7R by confocal microscopy. Similar to the coprecipitation findings, colocalization of CD14 and P2X7R was increased after LPS stimulation compared to PBS control (Figure 6C), suggesting that CD14 is a coreceptor for P2X7R.
In conclusion, we have identified a mechanism by which IL-1α participates in LPS-induced early neutrophil recruitment to the lungs and increased pulmonary vascular permeability during ALI, through the release of pro-IL-1α from necrotic AMs via a CD14-P2X7R dependent pathway. The down-regulation of VE-cadherin on endothelial tight junctions follows in response to pro-IL-1α in an IL-1R-MyD88-dependent fashion. These data establish IL-1α as a critical initiator of neutrophil recruitment and inflammatory responses during LPS induced acute lung injury.
DISCUSSION
It is well documented that both IL-1β and IL-1α can be processed by NLRP3-Caspase-1 (Gross et al., 2012) and are important regulators for neutrophil recruitment into the lung during inflammation. Therefore, we hypothesized that dying AM release IL-1 via the NLRP3 inflammasome. However, both Nlrp3 and Caspase 1 deficient mice had similar amounts of neutrophil recruitment as WT mice and no IL-1β secretion at the early time points investigated. IL-1α amounts were elevated in BALF after i.t. LPS administration despite the lack of Caspase-1 or NLRP3. In contrast to IL-1β, IL-1α has been considered an endogenous danger signal, functioning as an alarmin. Uncleaved IL-1α can be passively released upon necrotic cell death, triggering neutrophilic sterile inflammation (Chen et al., 2007; Rider et al., 2011). This form of IL-1α is capable of binding to IL-1R1 and is biologically active (Mosley et al., 1987). In our study, we detected only pro-IL-1α in the BALF of LPS-treated mice, and our data reveal that pro-IL-1α released from necrotic AMs is the main driver of the early inflammation in LPS-induced ALI.
IL-1α deficiency significantly reduced neutrophil recruitment after i.t administration of LPS. Our results revealed that IL-1α, not IL-1β, is required for early events of LPS-induced ALI. However, because we saw constitutive expression of pro-IL-1α in lung homogenates, and because others show that lung endothelial cells can also constitutively express pro-IL-1α (Brunn et al., 2008), we created bone marrow chimeric mice (WT mice with Il1α−/− bone marrow, and Il1α−/− mice with WT bone marrow) to determine which cells are responsible for pro-IL-1α production in response to i.t. LPS instillation. We found that mice with Il1α−/− hematopoietic cells had significantly reduced pro-IL-1α production consistent with our hypothesis that pro-IL-1α is generated by AM in response to LPS in this ALI model. In contrast to our model, Noulin et al finds that IL-1R deficient mice display normal neutrophil recruitment in the lungs of mice given LPS intranasally (Noulin et al., 2005). However, in their study, they give substantially more LPS (50 μg compared to 10 μg) and made observations at later time points (24 hr versus 8 hr). In contrast to their study, IL-1 receptor antagonist is required to prevent deleterious inflammation during a Klebsiella pneumoniae infection (Herold et al., 2011). Moreover, Tanabe et al find that Il1a−/− Il1b−/− double knockout mice have increased amounts of TNF-α, thereby compensating for the loss of IL-1 signaling during K. pneumoniae infection (Tanabe et al., 2005).
Endothelium must be activated either directly by LPS or indirectly by mediators released by other cells to become more permeable and express adhesion molecules, facilitating PMN transmigration into tissues. Since ECs express TLR4 as well as receptors for TNF-α and IL-1, either scenario was thought to be plausible. However, while endothelial TLR4 is able to recognize and respond to systemically administered LPS, it is unable to induce neutrophil migration into the lungs when LPS is given locally by intratracheal installation (Andonegui et al., 2009). Both IL-1α and IL-1β can trigger the MyD88 signaling cascade in endothelial cells and break down endothelial cell junctions (Royall et al., 1989; Zhu et al., 2012). Indeed, our current data demonstrated that AM respond directly to LPS, undergo necrosis, and release pro-IL-1α, which then acts on lung vascular endothelium triggering the IL-1R-MyD88 signaling pathway to downregulate VE-cadherin, resulting in increased vascular permeability and neutrophil infiltration. While it is always possible that ex vivo handling of macrophages in the BALF may result in them dying more easily before flow cytometric analysis, we believe that this is highly unlikely as this process is extremely quick (1hr), and our PBS control group did not show any macrophage necrosis.
While our data indicated that necrotic death of AMs led to the release of pro-IL-1a and its subsequent downstream effects, the mechanism for the induction of necrosis by LPS was not understood. LPS can induce Ca2+ mobilization in BMDC (Matzner et al., 2008; Zanoni et al., 2009) but there are conflicting results for LPS-induced Ca2+ mobilization in macrophages (Seabra et al., 1998; Zanoni and Granucci, 2010; Zhou et al., 2006). However, given the similarities between AM and DCs, we hypothesized that LPS could induce Ca2+ influx to trigger necrotic death of AM. Increased intracellular Ca2+ can affect mitochondrial inner membrane integrity, abrogating ATP synthesis and even triggering ATP hydrolysis by mitochondria (Brookes et al., 2004), eventually culminating in necrosis (Proskuryakov et al., 2003).
In ex vivo experiments, LPS-induced Ca2+ mobilization is TLR4-independent in AM as well as in BMDC (Zanoni et al., 2009). Therefore, we focused on P2X7R, a purinergic receptor which contains multiple protein and lipid interaction motifs, including a potential binding site for bacterial LPS (Denlinger et al., 2001), and which plays an essential role for LPS-induced acute lung injury (Moncao-Ribeiro et al., 2011). P2X7R is thought to act as a trigger for cell necrosis (Jun et al., 2007) and AM express functional P2X7 receptors which are required for ATP-induced Ca2+ mobilization (Lemaire and Leduc, 2003; Myrtek et al., 2008). Therefore, we examined whether P2X7R was involved in LPS-induced necrotic death of AM. Indeed, we found greater amounts of ATP in AM of P2X7R-deficient mice compared to WT mice. This was associated with less necrotic cell death and reduced pro-IL-1α secretion into the BALF. However, LPS alone is unable to induce any P2X7R-related activity, suggesting that the P2X7R is not directly activated by endotoxin in vitro (Leiva-Salcedo et al., 2011). In our in vitro experiments, we also detected only weak fluorescence for LPS-Alexa594 in P2X7R-expressing HEK293 cells and we did not see any Ca2+ mobilization in response to LPS in those cells. However, since the LPS binding site of P2X7R is located in its C-terminal cytosolic tail (Denlinger et al., 2001), we hypothesized that LPS may require internalization for LPS binding to P2X7R. We focused on CD14 as it is required for LPS uptake (Kitchens and Munford, 1998) and plays a role in LPS-induced Ca2+ mobilization in BMDC (Zanoni et al., 2009). For this reason, we made P2X7R and CD14 stably-expressing HEK293 cells to address whether CD14 is important for P2X7R-dependent Ca2+ mobilization by LPS. Our data suggested that LPS was internalized in a CD14-dependent manner and bound to P2X7R which led to Ca2+ mobilization in CD14 and P2X7R stably-expressing HEK293 cells. This was confirmed by that fact that CD14 neutralization significantly blocked LPS-induced Ca2+ mobilization in AM. Moreover, CD14 blockade significantly reduced necrotic death of AM and pro-IL-1α secretion into the BALF without altering TNF-α. In a similar vein, CD14 interacts with TLR7 and TLR9 ligands, facilitating their uptake, and serving as a coreceptor required for proper activation (Baumann et al., 2010). We also found that CD14 interacts with P2X7R following LPS stimulation, suggesting that CD14 may act as a coreceptor for P2X7R as well as other TLRs.
In summary, our data reveal an unappreciated and key role for IL-1α in the complex cascade of events that are triggered upon exposure of the airways to LPS, leading to neutrophil recruitment to the lungs as an early response during ALI and ARDS. LPS provides a dual signal on alveolar macrophages that induces cytokine production (TLR4-MyD88) and necrosis (P2X7R-CD14) that results in the release of pro-IL-1α, which subsequently activates endothelial cells (IL-1R-MyD88), inducing tight junction opening to allow neutrophils to infiltrate into the lungs. Understanding the complex and sequential molecular mechanisms by which immune cells are recruited to the lungs in response to LPS or bacteria remains an important goal and may provide potential novel targets to modulate immune responses and treat ALI and ARDS.
EXPERIMENTAL PROCEDURES
Mice
Myd88fl/fl mice were obtained from Dr. Anthony DeFranco (University of California, San Francisco, CA). Casp1−/− mice (Kuida et al., 1995) and TekCre mice (Tie2-Cre) were kindly provided by Dr. Richard Flavell (Yale Univ, New Haven, CT). Nlrp3−/− mice (Mariathasan et al., 2006) were generously provided by Dr. Katherine Fitzgerald (University of Massachusetts Medical School, Worcester, MA). Il1a−/− mice were kindly provided by Dr. Yoichiro Iwakura (Univ. Tokyo, Japan). C57BL/6, Tlr4−/−, P2rx7−/− (P2X7 receptor) mice were obtained from Jackson Labs, Maine, USA. Myd88−/− mice (Naiki et al., 2005) were maintained according to Cedars-Sinai Medical Center Institutional Animal Care and Use Committee guidelines. All mice were used at 8–9 weeks of age.
Imaging
Alveolar macrophages were cultured on glass coverslip and loaded with 4μM of Fluo-4 AM and equal volume Pluronic F-127 (Invitrogen) in HBSS for 30 min at room temperature then were washed two times in PBS containing 2% FBS, 1 mM sulfinpyrazone (Santa Cruz), and RPMI (2% FBS) was added. For HEK293 cells were cultured in complete DMEM on the glass coverslip coated with collagen type I (BD Biosciences) for overnight and loaded 4μM of Fluo-4 in serum free DMEM for 30 min at 37°C, followed by one wash with PBS cells were cult ured DMEM (2% FBS) for 20 min at 37°C. The fluorescence of Fluo-4 was measured by using confocal laser scanning microscopy (Leica Mycrosystems, IL, USA).
Detection of Intracellular ATP
The ATP concentrations in total BAL cells (AM) were determined with the ATPlite luminescence assay kit from PerkinElmer (MA, USA) according to the manufacturers’ instructions. BAL cells were assayed at various time points after intratracheal LPS administration.
Immunoprecipitation
CD14 and P2X7R-HA stably expressing HEK293 (2×106) cells were lysed with a buffer containing HEPES (pH 7.4), 150 mM NaCl, 10% glycerol, 0.5% NP-40, 1mM Na3VO4 and protease inhibitor (Sigma) for 30 min and whole cell extracts were pre-cleaned by using anti-mouse or anti-rabbit beads (Rockland, Trueblot) for 30 min at 4°C. After centrifugation at 3000 x rpm for 5 min at 4°C, CD14 or P2X7R was immunoprecipated overnight by using 5 μg of mouse anti-hCD14 (R&D) or rabbit anti-HA (Cell signaling) followed by addition with anti-mouse or anti-rabbit beads for 1 h on rocking platform at 4°C. The fractions were then subjected to SDS-PAGE and immunoblotting was performed with anti-hCD14 and anti-HA antibodies according to standard procedures.
Immunocytochemistry
HEK293 cells were cultured on glass coverslips coated with collagen type I (BD Biosciences) overnight. LPS-594 or LPS was diluted in complete DMEM medium for 15 min at 37°C. After stimulation with LPS (2.5 μg/ml) for 10 min, HEK293 cells were fixed and incubated with anti-HA and anti-CD14 antibodies for 2 h at room temperature. Followed by Alexa-488 and Alexa-594 secondary antibodies, localization was measured by fluorescence microscopy with BZ-II analyzer (KEYENCE, Osaka, Japan)
Transfection
HEK293 cells were cultured with DMEM complete medium (CORNING cellgro) and transfected with pLenti-GIII-CMV-hP2RX7-HA (Applied Biological Materials Inc.) or pcDNA3-hCD14 (Addgene) by using Lipofectamine 2000 (Invitrogen). The cells were positively selected by 1 mg/ml of G418 (Gemini Bio-Products) and 5 μg/ml of Puromycin (InvivoGen, CA, USA). For co-expressing cells, hP2X7R stably expressing HEK293 cells were transfected with pcDNA3-hCD14 and selected with neomycin.
Supplementary Material
Highlights.
Intratracheal LPS induces alveolar macrophage necrosis and pro-IL-1a release
Pro-IL-1a activates EC via IL-1/MyD88 signaling leading to loss of VE-Cadherin
VE Cadherin loss facilitates neutrophil recruitment and vascular leakage
LPS induces AM necrosis via Ca2+ influx triggered by CD14/P2X7R signaling
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants RO1AI-067995 and 2R56AI067995-06 to M.A. KS is supported by the American Heart Association 13BGIA17220050. The authors would like to thank Ganghua Huang and Polly Sun for technical support. We would like to thank Bill Parks for his help in the editing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal S, Dimitropoulou C, Lu Q, Black SM, Sharma S. Glutathione supplementation attenuates lipopolysaccharide-induced mitochondrial dysfunction and apoptosis in a mouse model of acute lung injury. Frontiers in physiology. 2012;3:161. doi: 10.3389/fphys.2012.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andonegui G, Zhou H, Bullard D, Kelly MM, Mullaly SC, McDonald B, Long EM, Robbins SM, Kubes P. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. The Journal of clinical investigation. 2009;119:1921–1930. doi: 10.1172/JCI36411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry KC, Fontana MF, Portman JL, Dugan AS, Vance RE. IL-1alpha signaling initiates the inflammatory response to virulent Legionella pneumophila in vivo. Journal of immunology. 2013;190:6329–6339. doi: 10.4049/jimmunol.1300100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CL, Aspalter IM, Sharif O, Pichlmair A, Bluml S, Grebien F, Bruckner M, Pasierbek P, Aumayr K, Planyavsky M, et al. CD14 is a coreceptor of Toll-like receptors 7 and 9. The Journal of experimental medicine. 2010;207:2689–2701. doi: 10.1084/jem.20101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. American journal of physiology. Cell physiology. 2004;287:C817–833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Saadi S, Platt JL. Constitutive repression of interleukin-1alpha in endothelial cells. Circulation research. 2008;102:823–830. doi: 10.1161/CIRCRESAHA.107.165332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nature medicine. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nature reviews Immunology. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Junior HM, Sarmento Vieira F, Coutinho-Silva R. C terminus of the P2X7 receptor: treasure hunting. Purinergic signalling. 2011;7:7–19. doi: 10.1007/s11302-011-9215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. Journal of cell science. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- Denlinger LC, Fisette PL, Sommer JA, Watters JJ, Prabhu U, Dubyak GR, Proctor RA, Bertics PJ. Cutting edge: the nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. Journal of immunology. 2001;167:1871–1876. doi: 10.4049/jimmunol.167.4.1871. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrod T, Park JH, Harder J, Iwakura Y, Nunez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. Journal of immunology. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Marcos M, Perez-Andres E, Tandel S, Fontanils U, Kumps A, Kabre E, Gomez-Munoz A, Marino A, Dehaye JP, Pochet S. Coupling of two pools of P2X7 receptors to distinct intracellular signaling pathways in rat submandibular gland. Journal of lipid research. 2006;47:705–714. doi: 10.1194/jlr.M500408-JLR200. [DOI] [PubMed] [Google Scholar]
- Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13978–13982. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Ding R, Zhao D, Zhang Z, Ma X. Unfractionated heparin attenuates lung vascular leak in a mouse model of sepsis:Role of RhoA/Rho kinase pathway. Thrombosis research. 2013;132:e42–47. doi: 10.1016/j.thromres.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Harmsen AG. Role of alveolar macrophages in lipopolysaccharide-induced neutrophil accumulation. Infection and immunity. 1988;56:1858–1863. doi: 10.1128/iai.56.8.1858-1863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold S, Tabar TS, Janßen H, Hoegner K, Cabanski M, Lewe-Schlosser P, Albrecht J, Driever F, Vadasz I, Seeger W, et al. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. American Journal of Respiratory and Critical Care Medicine. 2011;183:1380–1390. doi: 10.1164/rccm.201009-1431OC. [DOI] [PubMed] [Google Scholar]
- Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, et al. CD14 is required for MyD88-independent LPS signaling. Nature immunology. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- Jun DJ, Kim J, Jung SY, Song R, Noh JH, Park YS, Ryu SH, Kim JH, Kong YY, Chung JM, Kim KT. Extracellular ATP mediates necrotic cell swelling in SN4741 dopaminergic neurons through P2X7 receptors. The Journal of biological chemistry. 2007;282:37350–37358. doi: 10.1074/jbc.M707915200. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Developmental biology. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kitchens RL, Munford RS. CD14-dependent internalization of bacterial lipopolysaccharide (LPS) is strongly influenced by LPS aggregation but not by cellular responses to LPS. Journal of immunology. 1998;160:1920–1928. [PubMed] [Google Scholar]
- Kitchens RL, Wang P, Munford RS. Bacterial lipopolysaccharide can enter monocytes via two CD14-dependent pathways. Journal of immunology. 1998;161:5534–5545. [PubMed] [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Leiva-Salcedo E, Coddou C, Rodriguez FE, Penna A, Lopez X, Neira T, Fernandez R, Imarai M, Rios M, Escobar J, et al. Lipopolysaccharide inhibits the channel activity of the P2X7 receptor. Mediators of inflammation. 2011;2011:152625. doi: 10.1155/2011/152625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire I, Leduc N. Purinergic P2X(7) receptor function in lung alveolar macrophages: Pharmacologic characterization and bidirectional regulation by Th1 and Th2 cytokines. Drug Develop Res. 2003;59:118–127. [Google Scholar]
- London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Science translational medicine. 2010;2:23ra19. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucattelli M, Cicko S, Muller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Durk T, et al. P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. American journal of respiratory cell and molecular biology. 2011;44:423–429. doi: 10.1165/rcmb.2010-0038OC. [DOI] [PubMed] [Google Scholar]
- Makinde TO, Agrawal DK. Increased expression of angiopoietins and Tie2 in the lungs of chronic asthmatic mice. American journal of respiratory cell and molecular biology. 2011;44:384–393. doi: 10.1165/rcmb.2009-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. The Journal of clinical investigation. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. American journal of respiratory and critical care medicine. 2003;167:1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM Acute Lung Injury in Animals Study G. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. American journal of respiratory cell and molecular biology. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annual review of immunology. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Matzner N, Zemtsova IM, Nguyen TX, Duszenko M, Shumilina E, Lang F. Ion channels modulating mouse dendritic cell functions. Journal of immunology. 2008;181:6803–6809. doi: 10.4049/jimmunol.181.10.6803. [DOI] [PubMed] [Google Scholar]
- Mishra A. New insights of P2X7 receptor signaling pathway in alveolar functions. Journal of biomedical science. 2013;20:26. doi: 10.1186/1423-0127-20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncao-Ribeiro LC, Cagido VR, Lima-Murad G, Santana PT, Riva DR, Borojevic R, Zin WA, Cavalcante MC, Rica I, Brando-Lima AC, et al. Lipopolysaccharide-induced lung injury: role of P2X7 receptor. Respiratory physiology & neurobiology. 2011;179:314–325. doi: 10.1016/j.resp.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Mosley B, Urdal DL, Prickett KS, Larsen A, Cosman D, Conlon PJ, Gillis S, Dower SK. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. The Journal of biological chemistry. 1987;262:2941–2944. [PubMed] [Google Scholar]
- Myrtek D, Muller T, Geyer V, Derr N, Ferrari D, Zissel G, Durk T, Sorichter S, Luttmann W, Kuepper M, et al. Activation of human alveolar macrophages via P2 receptors: coupling to intracellular Ca2+ increases and cytokine secretion. Journal of immunology. 2008;181:2181–2188. doi: 10.4049/jimmunol.181.3.2181. [DOI] [PubMed] [Google Scholar]
- Naiki Y, Michelsen KS, Schroder NW, Alsabeh R, Slepenkin A, Zhang W, Chen S, Wei B, Bulut Y, Wong MH, et al. MyD88 is pivotal for the early inflammatory response and subsequent bacterial clearance and survival in a mouse model of Chlamydia pneumoniae pneumonia. The Journal of biological chemistry. 2005;280:29242–29249. doi: 10.1074/jbc.M503225200. [DOI] [PubMed] [Google Scholar]
- Noulin N, Quesniaux VFJ, Schnyder-Candrian S, Schnyder B, Maillet I, Robert T, Vargaftig BB, Ryffel B, Couillin I. Both hemopoietic and resident cells are required for MyD88-dependent pulmonary inflammatory response to inhaled endotoxin. Journal of immunology (Baltimore, Md: 1950) 2005;175:6861–6869. doi: 10.4049/jimmunol.175.10.6861. [DOI] [PubMed] [Google Scholar]
- Olsson S, Sundler R. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Molecular immunology. 2006;43:607–612. doi: 10.1016/j.molimm.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Orfanos SE, Mavrommati I, Korovesi I, Roussos C. Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive care medicine. 2004;30:1702–1714. doi: 10.1007/s00134-004-2370-x. [DOI] [PubMed] [Google Scholar]
- Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nature reviews Immunology. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Experimental cell research. 2003;283:1–16. doi: 10.1016/s0014-4827(02)00027-7. [DOI] [PubMed] [Google Scholar]
- Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. Journal of immunology. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- Royall JA, Berkow RL, Beckman JS, Cunningham MK, Matalon S, Freeman BA. Tumor necrosis factor and interleukin 1 alpha increase vascular endothelial permeability. The American journal of physiology. 1989;257:L399–410. doi: 10.1152/ajplung.1989.257.6.L399. [DOI] [PubMed] [Google Scholar]
- Salgado A, Boveda JL, Monasterio J, Segura RM, Mourelle M, Gomez-Jimenez J, Peracaula R. Inflammatory mediators and their influence on haemostasis. Haemostasis. 1994;24:132–138. doi: 10.1159/000217093. [DOI] [PubMed] [Google Scholar]
- Schlegel N, Baumer Y, Drenckhahn D, Waschke J. Lipopolysaccharide-induced endothelial barrier breakdown is cyclic adenosine monophosphate dependent in vivo and in vitro. Critical care medicine. 2009;37:1735–1743. doi: 10.1097/CCM.0b013e31819deb6a. [DOI] [PubMed] [Google Scholar]
- Seabra V, Stachlewitz RF, Thurman RG. Taurine blunts LPS-induced increases in intracellular calcium and TNF-alpha production by Kupffer cells. Journal of leukocyte biology. 1998;64:615–621. doi: 10.1002/jlb.64.5.615. [DOI] [PubMed] [Google Scholar]
- Tachado SD, Li X, Bole M, Swan K, Anandaiah A, Patel NR, Koziel H. MyD88-dependent TLR4 signaling is selectively impaired in alveolar macrophages from asymptomatic HIV+ persons. Blood. 2010;115:3606–3615. doi: 10.1182/blood-2009-10-250787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe M, Matsumoto T, Shibuya K, Tateda K, Miyazaki S, Nakane A, Iwakura Y, Yamaguchi K. Compensatory response of IL-1 gene knockout mice after pulmonary infection with Klebsiella pneumoniae. J Med Microbiol. 2005;54:7–13. doi: 10.1099/jmm.0.45736-0. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. Journal of cell science. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- Tsushima K, King LS, Aggarwal NR, De Gorordo A, D’Alessio FR, Kubo K. Acute lung injury review. Internal medicine. 2009;48:621–630. doi: 10.2169/internalmedicine.48.1741. [DOI] [PubMed] [Google Scholar]
- Ulich TR, Yin S, Guo K, Yi ES, Remick D, del Castillo J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. The American journal of pathology. 1991a;138:1097–1101. [PMC free article] [PubMed] [Google Scholar]
- Ulich TR, Yin SM, Guo KZ, del Castillo J, Eisenberg SP, Thompson RC. The intratracheal administration of endotoxin and cytokines. III. The interleukin-1 (IL-1) receptor antagonist inhibits endotoxin- and IL-1-induced acute inflammation. The American journal of pathology. 1991b;138:521–524. [PMC free article] [PubMed] [Google Scholar]
- Volonte C, Apolloni S, Skaper SD, Burnstock G. P2X7 receptors: channels, pores and more. CNS & neurological disorders drug targets. 2012;11:705–721. doi: 10.2174/187152712803581137. [DOI] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Xiao K, Allison DF, Kottke MD, Summers S, Sorescu GP, Faundez V, Kowalczyk AP. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. The Journal of biological chemistry. 2003;278:19199–19208. doi: 10.1074/jbc.M211746200. [DOI] [PubMed] [Google Scholar]
- Xing J, Yakubov B, Poroyko V, Birukova AA. Opposite effects of ANP receptors in attenuation of LPS-induced endothelial permeability and lung injury. Microvascular research. 2012;83:194–199. doi: 10.1016/j.mvr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Granucci F. Differences in lipopolysaccharide-induced signaling between conventional dendritic cells and macrophages. Immunobiology. 2010;215:709–712. doi: 10.1016/j.imbio.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G, et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Gorshkova IA, Berdyshev E, He D, Fu P, Ma W, Su Y, Usatyuk PV, Pendyala S, Oskouian B, et al. Protection of LPS-induced murine acute lung injury by sphingosine-1-phosphate lyase suppression. American journal of respiratory cell and molecular biology. 2011;45:426–435. doi: 10.1165/rcmb.2010-0422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yang W, Li J. Ca2+- and protein kinase C-dependent signaling pathway for nuclear factor-kappaB activation, inducible nitric-oxide synthase expression, and tumor necrosis factor-alpha production in lipopolysaccharide-stimulated rat peritoneal macrophages. The Journal of biological chemistry. 2006;281:31337–31347. doi: 10.1074/jbc.M602739200. [DOI] [PubMed] [Google Scholar]
- Zhu W, London NR, Gibson CC, Davis CT, Tong Z, Sorensen LK, Shi DS, Guo J, Smith MC, Grossmann AH, et al. Interleukin receptor activates a MYD88-ARNO-ARF6 cascade to disrupt vascular stability. Nature. 2012;492:252–255. doi: 10.1038/nature11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong WX, Thompson CB. Necrotic death as a cell fate. Genes & development. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.